Abstract
Objective
Mechanisms underlying the association between depression and increased cardiovascular risk remain poorly understood. Central adiposity is one potential pathway. Prior research has shown an association between depression and central adiposity but few studies have examined whether depressive symptoms are differentially associated with visceral adipose tissue (VAT), which is more metabolically active and confers greater cardiovascular risk than subcutaneous fat (SAT).
Methods
We investigated the cross-sectional association between depressive symptoms, assessed by the Center for Epidemiological Studies Depression Scale (CES-D), and VAT and SAT, assessed by computed tomography, in a sample of 409 middle-aged women (44.7% African-Americans, 55.3% whites; mean age=50.4 years) participating in the Chicago site of the Study of Women's Health Across the Nation (SWAN).
Results
With adjustments for age, race, total percent fat and sex hormone binding globulin (SHBG), each 1-point higher score on the CES-D was associated with 1.03 cm2 greater VAT (p<.001). Women with a CES-D score of 16 or greater, indicative of clinically relevant depressive symptomatology, had 24.5% more VAT than women with lower CES-D scores (p<.001). Further adjustment for Framingham Risk Score and physical activity did not alter the findings, and associations did not vary by race. Associations were strongest in obese and overweight women. Depressive symptoms were unrelated to SAT.
Conclusions
Increased visceral fat may be one pathway by which depression contributes to excess risk for cardiovascular disease and diabetes. Further research is needed to examine whether depressive symptoms influence accumulation of VAT over time.
Keywords: Abdominal obesity, depressive symptoms, African-American, women's health, cardiovascular disease
Consistent evidence has accumulated over the past two decades showing that depression or depressive symptoms are related to increased risk of cardiovascular disease (CVD) morbidity and mortality (1) and diabetes (2,3). The pathways or mechanisms that underlie or mediate the association of depression or depressive symptoms with CVD and diabetes – though widely studied – are incompletely understood. The purpose of this study was to examine the relation of depressive symptoms with central adiposity, one pathway by which depression and depressive symptoms may contribute to CVD and diabetes risk.
Central adiposity is more atherogenic than gynoid adiposity and total body fat (4-6). Many studies use waist circumference or waist-to-hip ratio to represent central adiposity but research shows it is not just fat distribution but type of fat that matters. Compared with subcutaneous fat (SAT), visceral fat (VAT) is more metabolically active and a better indicator of CVD risk (6-8) and may be particularly atherogenic for women. In the Dynamics of Health, Aging and Body Composition (Health ABC) Study, VAT significantly predicted 5-year risk of incident MI in elderly women but not men (9). Among healthy, non-obese women and men, increased VAT is associated with decreased insulin sensitivity and increased plasma lipids (10-12).
Few studies on the health effects of depressive symptoms have distinguished among specific types of adipose tissue; however, the evidence currently available is suggestive. One British study and two German studies, all with very small samples, reported that depressed women had higher mean levels of VAT assessed by either CT or MRI compared with non-depressed controls (13-15). One of the German studies included depressed women with comorbid borderline personality disorder and it was this group that had the greatest amount of VAT relative to healthy controls (15). A larger cross-sectional study of 101 overweight, premenopausal women also found that those with depressed mood had higher levels of CT-assessed VAT but not SAT, adjusting for age, physical fitness and obesity (16). Very little longitudinal data have been published. A German study reported that elderly depressed adults (n=29) experienced greater accumulation of VAT mass than did age- and sex-matched controls (n=17) over an average of 14 months of follow-up (17). A new report from the Health ABC Study indicates that among otherwise healthy elderly adults, those with high baseline depressive symptoms experienced significantly greater increases in VAT over 5 years (18). That study included 2,088 elderly adults, only 4% of whom met study criteria for high depressive symptoms, and associations were strongest among white men and not evident in black women.
In the present study, we examined the association between depressive symptoms and central adiposity in a community-based sample of more than 400 middle-aged African-American and white women participating in an ongoing longitudinal study of the menopausal transition. We used state-of-the-art CT assessments of VAT, and tested the hypothesis that increasing depressive symptoms would be significantly associated with more visceral but not subcutaneous fat, independent of known risk factors for VAT.
Methods
Participants
Subjects were 409 women (44.7% African-Americans; 55.3% whites) from the Chicago site of the Study of Women's Health Across the Nation (SWAN) participating in an ancillary study of the impact of menopause on accumulation of VAT (SWAN Fat Patterning Study). SWAN is an ongoing longitudinal study of women transitioning through menopause conducted at 7 clinical sites (Chicago, IL; Pittsburgh, PA; Detroit, MI; Boston, MA; Los Angeles, CA; Oakland, CA; Newark, NJ). Each site recruited white women and either African-American (4 sites, including Chicago), Hispanic, Japanese-American, or Chinese-American women (1 site each). Eligibility was determined and initial data on socioeconomic, demographic, lifestyle and health factors were collected via telephone and in-person screening surveys at all sites beginning in November 1995 through October 1997. Screening surveys were completed by 16,065 women. Eligibility for the longitudinal SWAN cohort included self-identification as one of the targeted ethnicities, aged 42-52 years, not pregnant or breastfeeding, an intact uterus and at least 1 ovary, reported menstrual bleeding within the prior 3 months, not currently using medications affecting pituitary or ovarian function and no use of exogenous hormones within 3 months preceding the baseline interview. A total of 3,302 women (72% of those eligible) participated in the SWAN baseline study, which was conducted in 1996-7. Details of SWAN recruitment have been reported (19, 20).
Women enrolled in the SWAN Fat Patterning Study between August, 2002 and December, 2005 coincident with their annual SWAN follow-up visits 04 through 09. Women eligible for this ancillary study had no history of diabetes, chronic liver disease or renal disease, no self-reported history of anorexia nervosa, no alcohol or drug abuse, were not currently pregnant or planning to become pregnant, and had not undergone hysterectomy and/or bilateral oophorectomy. Equipment limitations precluded participation of women with breast implants, hip replacements or weighing 299 pounds or more. Seventy-seven percent of eligible Chicago SWAN participants enrolled in the Fat Patterning Study. Many SWAN participants were post-menopausal by the time this ancillary study began. Because the primary goal of the Fat Patterning Study was to investigate the impact of the menopausal transition on VAT accumulation, it was necessary to recruit additional women to the study who had yet to complete the menopausal transition. Thus, 138 pre- and perimenopausal women (65% of eligible) who were screened for the original SWAN recruitment but were too young to participate in 1996 were recruited to the Fat Patterning Study. These newly recruited women were younger but did not differ in depressive symptoms, body mass index (BMI), education, or age-adjusted total fat or VAT from previously recruited women. The final cohort included 435 women (199 African-Americans; 236 white). Missing data on depressive symptoms (n=22), reproductive hormones (n=1), and VAT (n=3, due to equipment malfunction), left 409 women (183 African-Americans, 226 whites) for the current analyses.
Procedures
At entry into SWAN and annually thereafter, all participants completed a standard protocol, with self- and interviewer-administered questionnaires about psychosocial and lifestyle factors, health status, medical history and medication use, menstrual status and symptoms. Standard protocols for phlebotomy and anthropometry were completed annually. Each fasting blood draw was targeted to days 2-5 of the menstrual cycle among menstruating women and before 10:00 a.m. Blood was maintained at 4 degrees C until separated, then frozen at -80 degrees C and shipped on dry ice to a central laboratory. Full details of the SWAN protocol are reported (19). Covariates of interest for the present analyses were measured during the annual SWAN assessment coincident with recruitment to the Fat Patterning Study for the 286 SWAN participants. If data was missing on a covariate from their SWAN visit coincident to the Fat Patterning visit, then data from the most recently available prior SWAN visit was used. (This approach was needed only for two variables, physical activity and sex hormone binding globulin and details are provided below.) The 138 women recruited specifically for this study completed the SWAN protocol, including measurements obtained for the annual SWAN visit, assessment of physical activity (described below), and specific assessments of total fat and VAT for the Fat Patterning Study. Data presented here represent the baseline visit for the Fat Patterning Study for all participants.
All aspects of the study were approved by the Rush University Medical Center Institutional Review Board and all women provided written, informed consent.
Study Measures
Depressive symptoms
Depressive symptoms were assessed annually in SWAN with the 20-item Center for Epidemiological Studies Depression Scale (CES-D) (21), which is well-validated, has good test-retest reliability in ethnically diverse samples (22) and is used extensively in epidemiological studies (23,24).
Visceral and subcutaneous fat
Visceral and subcutaneous fat in the abdominal cavity were assessed via CT at High Tech Medical Park located within 9 miles of the Chicago SWAN study site. CT scans were completed during the first 12 days of a participant's menstrual cycle, with the participant lying supine with her arms folded across her chest. All CT scans were performed by a trained technician using a General Electric Lightspeed VCT scanner (General Electric Medical Systems, Milwaukee, WI). Following a scout view, a single 10-mm thick abdominal image at the L4-L5 vertebral space was obtained. Images were stored on optical disks and transferred to the reading center at the University of Colorado Health Sciences Center for analysis. Scans were read by a trained radiologist, blind to the participants' clinical or demographic characteristics, using software developed by the reading center (RSI Inc, Boulder, CO) that is used in several large cohort studies (9,25,26). The radiologist defined total abdominal fat area using a cursor to delineate the area within the muscle wall surrounding the abdominal cavity and defined VAT as all adipose tissue within this area with an attenuation range between -190 and -30 Hounsfeld Units (27,28). SAT was quantified as total abdominal fat minus VAT (28).
Total body fat
Total body fat mass was assessed with whole body dual energy x-ray absorptiometry (DXA) scans using a General Electric Lunar Prodigy scanner (GE-Lunar, Madison, WI). DXA scans utilize two X-ray energy sources enabling separation of body mass into fat mass, lean tissue mass and bone mineral content. DXA scans, completed the same day as the CT scans and at the same location, were performed with a participant lying supine with both arms by her side, wearing a hospital gown with all clothing, jewelry and shoes with metal objects removed. Scans were analyzed using GE-Lunar enCORE software (Madison, WI). Total body fat was quantified as percent of fat in the total body habitus to represent the amount of fat for a given body size and was calculated as total fat mass divided by total mass (which includes total fat mass, total lean mass, and bone mineral content). A DXA scan was not completed on 12 women due to equipment malfunction. Because body mass index and total fat are highly correlated (r = 0.93) in our sample, a regression equation was estimated to predict total body fat from BMI and this value was used in analyses for these 12 women.
Sociodemographics
Age was calculated as the difference between exam date and self-reported date-of-birth. Race was self-reported as African-American or white. Education was self-reported highest level of education completed: high school or less, some college, college degree, or graduate school. Parity was self-reported number of live births.
Physical Activity
Physical activity was measured at the SWAN baseline visit and follow-ups 03, 05 and 06 via self-report with an adapted version of the Kaiser Physical Activity Survey (KPAS) (29,30) assessing frequency of sports, non-sports leisure time, and household/childcare activities; an activity score was created by summing across domains, with a higher score indicative of greater activity. For the current analyses, 21% of women did not have KPAS data from their SWAN visit concurrent to their Fat Patterning Study baseline visit so KPAS values were obtained from their most recently available prior visit at which the KPAS was administered.
Sex hormone binding globulin (SHBG)
SHBG, a marker of androgen-to-estrogen balance that binds preferentially to androgens (31), is measured annually and assayed with an ACS-180 automated analyzer (Bayer Diagnostics Corp, Norwood, MA) using a competitive chemiluminescent assay. For 8% of participants without an SHBG value from the concurrent SWAN visit, the SHBG value from the most recent previous SWAN visit was used.
Menopausal status
Bleeding criteria were used to characterize menopausal status as pre-menopausal (normal cycling), early peri-menopausal (irregular cycles with bleeding in the past 3 months), late peri-menopausal (irregular cycles with bleeding in the past 11 months but not in the last 3 months), post-menopausal (no menses for at least 12 months); a fifth category of ‘undetermined’ was included for women who began using hormone replacement prior to cessation of menses and for whom exact menopausal status based on bleeding criteria cannot be ascertained.
Framingham risk score (FRS)
FRS values were calculated based on age, smoking status, total cholesterol and high-density lipoprotein cholesterol (HDL-C), resting blood pressure and use of anti-hypertensive medications (32). Smoking was assessed by annual questions on ever smoking, amount smoked and quit date. Total cholesterol and HDL-C were analyzed on EDTA-treated plasma using standard methods, previously described (33,34). Blood pressure was measured manually in the right arm, with an appropriately sized cuff. Two sequential readings were obtained, two minutes apart, following a 5-minute rest with participants seated. Use of anti-hypertensive medications, self-reported annually in SWAN, was confirmed via medication review.
Anthropometric measures
BMI was calculated as weight (kilograms) divided by height (meters) squared. Weight and height are measured annually in SWAN to the nearest 0.01 kg and 0.01 cm, respectively, using calibrated digital scales and a stadiometer and/or a metric folding wooden ruler. Waist circumference was measured annually over non-restrictive undergarments at the narrowest part of the torso seen from the anterior aspect.
Data Analyses
We used descriptive statistics to characterize participants on depressive symptoms, fat measurements, age, race, education, parity, smoking, physical activity, SHBG, menopausal status, FRS, BMI, and waist circumference. For our primary analyses, CES-D scores were modeled continuously. A CES-D score of 16 or higher is considered indicative of clinically significant symptomatology (24); therefore, in secondary analyses we modeled CES-D scores dichotomously (CES-D <16 as referent). To evaluate our primary hypotheses, we estimated linear regression models, with adjustments for age, race, total percent body fat, and SHBG. Age was included as a standard demographic covariate and race was included because prior research shows that African-Americans have lower levels of VAT than whites even controlling for total body fat (35-37). Prior studies of VAT typically have controlled for body size, using various measures (e.g., BMI, total body fat, total percent fat); we were able to measure total body fat with good precision using the DXA methods described above and chose to quantify this as total percent fat for statistical models as described above. SHBG, which declines over the menopausal transition, was included as a covariate as previous analyses from the Fat Patterning Study show an inverse association with VAT in this sample (38). Education and parity have not been consistently related to VAT; in our preliminary analyses, they were unrelated to depressive symptoms and all fat measurements so we chose not to include them as covariates but they are used to describe our sample (Table 1). Additional models included covariates for physical activity and FRS to control for these known indicators of cardiovascular risk. Finally, we tested the interaction between race and depressive symptoms to see if the association with adiposity measures varied for blacks and whites. All analyses were conducted using PC-SAS ® (SAS Institute Inc., Cary, NC), version 8.2. Age, physical activity, SHBG values and FRS were modeled continuously whereas race (with white as the referent) was modeled as a binary variable.
Table 1.
Baseline participant characteristics in the SWAN Fat Patterning Study.
| % | Mean (SD) | Range | |
|---|---|---|---|
| Age (years) | 50.4 (3.8) | 42-61 | |
| CES-D score | 6.9 (7.2) | 0-33 | |
| CES-D ≥16 | 13.5 | ||
| Visceral fat (cm2) | 94.6 (52.5) | 9.4-312.4 | |
| Subcutaneous fat (cm2) | 389.5 (162.9) | 94.4-879.0 | |
| Total percent fat (%) | 43.0 (8.5) | 20.1-60.3 | |
| SHBG (nM) | 55.4 (32.5) | 6.8-229.9 | |
| Current smoker | 20.3 | ||
| Physical activitya | 7.7 (1.6) | 3.5-12.5 | |
| Framingham Risk Score | 10.2 (4.1) | -1.0-22.0 | |
| Education | |||
| High school or less | 10.9 | ||
| Some college | 27.3 | ||
| College degree | 24.3 | ||
| Graduate school | 37.5 | ||
| Parity | |||
| 0 | 13.8 | ||
| 1-2 children | 46.7 | ||
| > 2 children | 39.5 | ||
| Menopausal status | |||
| Pre-menopausal | 11.7 | ||
| Early Peri-menopausal | 33.0 | ||
| Late Peri-menopausal | 8.6 | ||
| Post-menopausal | 35.9 | ||
| Undetermined | 10.8 | ||
| Waist circumference (cm) | 90.1 (13.7) | 61.0-134.4 | |
| Body Mass Index (kg/m2) | 29.2 (6.3) | 18.4-52.9 | |
| Normal weight (BMI < 25 kg/m2) | 28.9 | ||
| Overweight (BMI 25 to < 30 kg/m2) | 31.8 | ||
| Obese (BMI of 30 kg/m2 or greater) | 39.4 |
Note. N = 409 (55.3% white; 44.7% African-American); due to missing data, sample size for individual characteristics varies from 384 to 409.
A higher score on the measure of physical activity indicates greater activity levels.
Results
Participant Characteristics
Women were approximately 50 years old, well-educated, the majority were overweight or obese, 20% were current smokers, and most women were either early peri-menopausal or post-menopausal (Table 1). The mean CES-D score was 6.9 (SD, 7.2) and more than 13% of women reported a CES-D score of 16 or higher.
Depressive Symptoms and Visceral and Subcutaneous Fat
Table 2 presents estimates and p-values from the regression models testing our primary hypotheses. Each 1-point higher CES-D score was associated with 1.03 cm2 greater VAT (p<.001), adjusting for age, race, total percent fat and SHBG. The magnitude of the association of depressive symptoms with VAT was nearly twice as great as its association with SAT, which was nonsignificant (p=.15) (row 1, table 2). Depressive symptoms remained significantly associated with VAT after adjusting for physical activity and FRS (p=.014). The depressive symptoms by race interaction was nonsignificant for both VAT (p>.87) and SAT (p>.98).
Table 2.
Results of Adjusted Linear Regression Models Examining Depressive Symptoms in Relation to Visceral Adipose Tissue (VAT) and Subcutaneous Adipose Tissue (SAT).
| VAT (cm2) | SAT (cm2) | |||
|---|---|---|---|---|
| MODEL 1 | Estimate (S.E.) | p-value | Estimate (S.E.) | p-value |
| CES-D score | 1.03 (0.27) | <.001 | 0.76 (0.53) | .15 |
| Age | 1.55 (0.53) | .004 | -2.00 (1.03) | .05 |
| Black race | -11.55 (4.05) | .004 | 35.25 (7.80) | <.001 |
| Total percent fat | 3.15 (0.25) | <.001 | 16.29 (0.48) | <.001 |
| SHBG | -0.41 (0.06) | <.001 | -0.282 (0.12) | .02 |
| MODEL 2 | ||||
| CES-D score | 0.70 (0.28) | .01 | 0.49 (0.58) | .39 |
| Age | 1.17 (0.57) | .04 | -2.13 (1.17) | .07 |
| Black race | -15.53 (4.05) | <.001 | 32.00 (8.30) | <.001 |
| Total percent fat | 2.73 (0.26) | <.001 | 16.291 (0.53) | <.001 |
| SHBG | -0.43 (0.06) | <.001 | -0.25 (0.13) | .05 |
| FRS | 2.48 (0.53) | <.001 | 0.31 (1.09) | .78 |
| Physical activity | -4.69 (1.30) | <.001 | -2.78 (2.67) | .30 |
Note. N = 409 for Model 1 for both VAT and SAT; due to missing data on covariates added in Model 2 the N decreased to 384 for both VAT and SAT.
Covariates in our models had differing associations with the adiposity measures (Table 2). Age was positively related to VAT but negatively related to SAT. African-Americans had less VAT but more SAT than whites. Total percent fat was strongly related to both types of adipose tissue but the coefficient for SAT was more than 5 times larger than the coefficient for VAT. SHBG, physical activity and FRS were associated with VAT but unrelated to SAT.
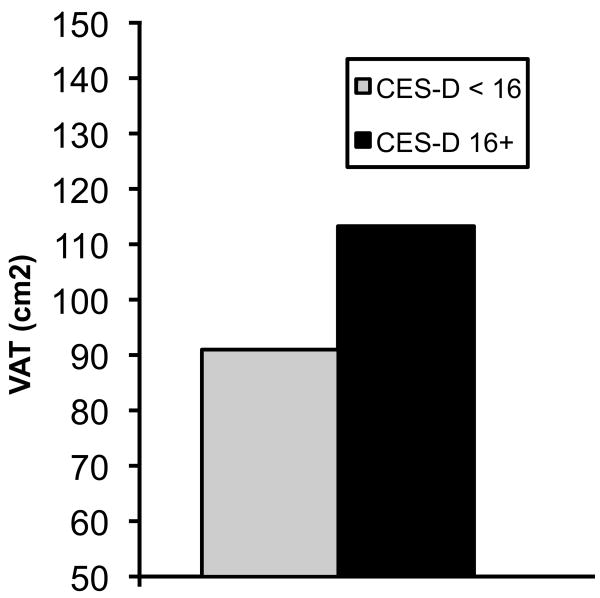
In our secondary analyses with dichotomous CES-D scores, women scoring 16 or greater had 24.5% more VAT than women with fewer depressive symptoms (p<.001) with adjustments for age, race, total percent fat, and SHBG (Figure 1). This association was little changed and remained significant after adjusting for physical activity and FRS (p=.007). Women with high CES-D scores did not have more SAT than women with few depressive symptoms (p>.13).
Figure 1.
Values shown are mean levels of visceral adipose tissue, adjusted for age, race, total percent fat and SHBG, for women with a CES-D score of 16 or higher (black bar) and women with a CES-D score < 16 (hatched bar). The difference between groups is significant (p<.001).
Additional Analyses
We repeated the models for VAT shown in Table 2, substituting BMI for total percent fat, and included a categorical BMI × CES-D interaction, which was significant in both models (p<.05). Therefore, we stratified by BMI to examine whether the association of depressive symptoms with VAT differed for normal weight (BMI<25 kg/m2), overweight (BMI 25 to 29.9 kg/m2) and obese (BMI≥30 kg/m2) women. Depressive symptoms were associated with VAT among overweight (estimate=1.26, p=.007) and obese women (estimate=1.29, p=.006), adjusting for age, race, total percent fat and SHBG; with adjustment for FRS and physical activity, the relation of depressive symptoms with VAT in overweight women was slightly diminished (estimate=.824, p=.08) but unchanged in obese women (estimate=1.30, p=.015). Among normal weight women (who comprise <29% of our sample), depressive symptoms were not related to VAT (p>.40).
Waist circumference frequently is used as a simple, inexpensive indicator of central adiposity; thus, we examined the correlation of waist with VAT and SAT and estimated the association of depressive symptoms with waist circumference. Waist circumference correlated highly with SAT (r=0.83) and VAT (r=0.77) (both p<.001). However, CES-D scores were unrelated to waist circumference in a linear regression model with covariates for age, race, total percent fat, SHBG, FRS and physical activity (estimate=0.086, t=1.28, p>.20). A depressive symptoms by race interaction was nonsignificant (p>.6) with waist circumference as the outcome.
Discussion
We observed a strong association between depressive symptoms and VAT in middle-aged women, particularly among overweight and obese women. These data suggest central adiposity may be an important pathway by which depression contributes to risk for diabetes and CVD. What this study adds to the literature is clear epidemiologic evidence from a well-characterized cohort of middle-aged women that depressive symptoms are differentially related to the type of adipose tissue (i.e., VAT) considered metabolically active and atherogenic (4-6). Depressive symptoms were strongly related to VAT after taking into account other risk factors, and the effect was similar among African-American and white women.
Some studies have quantified the amount of VAT beyond which CVD risk is elevated. In the Health ABC Study, a VAT area of 106 cm2 or greater in post-menopausal women was associated with a more adverse lipid profile (low HDL-C, hypertriglyceridemia, high low-density lipoprotein cholesterol to HDL-C ratio), impaired glucose tolerance and hyperinsulinemia (39). In that study, women in the top quintile of the VAT distribution (≥163 cm2) were 4-5 times more likely to have an adverse lipid profile and impaired glucose tolerance than women in the bottom VAT distribution quintile (≤105 cm2). Of interest, other studies investigating VAT levels associated with elevated cardiovascular risk have identified a VAT area between 100 and 110 cm2 as the critical value in premenopausal women also (40,41). Given these reports in the literature, we examined the VAT distribution in our sample. More than one-third of our study participants (144 women) had a VAT area equal to or greater than 106 cm2 or greater and 27.8% of these had a VAT area of 163 cm2 or greater. Of note, the mean VAT area among our women with a CES-D score of 16 or higher was 113.3 cm2 (Figure 1). As can be seen in Table 2, FRS values were positively associated with VAT; moreover, depressive symptoms were significantly associated with FRS scores (p<.002; data not shown). Together, these findings indicate that a substantial proportion of our women, including those with the most depressive symptoms, may be at marked risk for CVD and diabetes.
How might depressive symptoms contribute to greater visceral adiposity? Depression is associated with hypothalamic-pituitary-adrenal (HPA) axis alterations, often manifest as excess cortisol secretion or altered diurnal cortisol patterning (42). Chronic HPA arousal could lead to differentially greater VAT deposition because glucocorticoid receptor density is higher in VAT than in other types of adipose tissue (43) and the actions of cortisol thus may be more pronounced in VAT. Animal and human studies indicate increased glucocorticoid production is associated with increased deposition of VAT (43,44). Depression and depressive symptoms also are associated with increased inflammation, including higher hs-CRP, fibrinogen, IL-6 and tumor necrosis factor alpha (45-47), all of which are elevated in obesity, diabetes, and atherosclerotic vascular disease - conditions characterized as states of chronic, low-grade inflammation (48-51). Adipose tissue, particularly VAT, secretes a host of inflammatory markers and is associated with increased systemic inflammation (52). It also should be noted that the associations of depression with inflammation as well as obesity are bidirectional (45,53). Although addressing specific glucocorticoid or inflammatory mechanisms is beyond the scope of the present study, future studies are planned to explore whether cortisol or inflammatory markers influence the association of depressive symptoms with VAT.
Our study shows that waist circumference is not a good proxy for visceral adiposity. Measurement of waist circumference is simpler and less expensive than CT-assessments to determine relative amounts of visceral and subcutaneous fat – important considerations in typically expensive epidemiological studies with lengthy study protocols. However, our data indicate that null associations between putative risk factors and waist circumference may be masking important effects of these risk factors on visceral adiposity. More precise measurement of visceral and subcutaneous fat provides unique information useful in assessing risk for diabetes or CVD.
Though we did not observe differences in the association of depressive symptoms with VAT by race, we found that African-American women had lower levels of VAT and higher levels of SAT compared with white women. The relation of race/ethnicity to measures of central adiposity is not a focus of the present study; however, we note that our observations are consistent with several reports in the literature (35-37). Also, as noted, a recently reported longitudinal study (18) did note some differences in the strength of the association of depression with change in VAT by both race and sex, so more detailed examination of these relations is warranted.
This study is limited by its cross-sectional design, which cannot distinguish temporality of the depressive symptoms—VAT association. This is an important issue. Comorbidity of depression and obesity is widely documented, even in youth, and the relation between these disorders is bidirectional (53-57). However, few studies of depression and obesity have included specific assessments of VAT. As noted, depressed elderly patients had increased VAT compared to non-depressed, age- and sex-matched controls over 14 months of follow-up in one study limited to a very small sample (17). The newly reported Health ABC findings indicate that depression is related to greater VAT accumulation in a healthy elderly cohort of men and women (18). That study also used the CES-D to measure depressive symptoms; greater 5-year increases in VAT were seen amongst the small proportion of participants (4%) with CES-D scores ≥16, relative to those with scores <16. Modeled continuously, the CES-D was not significantly related to VAT change in that study, but reasons for this are unclear. Thus, further research is needed to more fully explore whether depressive symptoms contribute to greater increases in visceral adiposity over time and in younger cohorts such as ours. With repeat assessments of visceral and subcutaneous adipose tissue over 4 years in the SWAN Fat Patterning Study, we plan to address this issue in future work.
It is unknown whether depressive symptoms are similarly related to VAT in other populations. Our findings are consistent with findings by Lee and colleagues (160) who reported a positive association between depressed mood and VAT in a sample of overweight, premenopausal Korean women. It is unknown whether associations are similar or possibly stronger in women with major depressive disorder. Information on current and lifetime history of diagnosed depression was unavailable for our sample. There is strong debate whether the adverse cardiovascular effects of depression are greater with major depressive disorder compared with elevated depressive symptoms identified via a self-reported checklist, as was used here. Clinically assessed major depression significantly influences later health outcomes (58,59); however, depressive symptoms, assessed by various checklist-type measures, also are associated with greater morbidity and worse health outcomes across a wide variety of populations (60,61).
Strengths of this study include a large biracial sample of women from a well-characterized cohort study, CT assessments for quantifying visceral and subcutaneous fat, DXA scans to assess total body fat mass, and adjustment for important covariates of visceral adiposity. Depressive symptoms were independently related to VAT but not SAT or waist circumference, suggesting depressive symptoms may contribute to excess risk of diabetes and CVD via increased visceral adiposity. Research is needed to examine mechanisms underlying this association and to determine whether depressive symptoms lead to greater accumulation of visceral adiposity over time.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The SWAN Fat Patterning Study is supported by the National Heart, Lung and Blood Institute (NHLBI) (Grant HL067128) and the Charles J. and Margaret Roberts Trust. In addition, Dr. Lewis was supported by the National Institute of Mental Health (NIMH) (Grant MH075625) and Dr. Everson-Rose received support from the Program in Health Disparities Research, University of Minnesota Medical School. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIMH, NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor - MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA - Robert Neer, PI 1994 - 1999; Joel Finkelstein, PI 1999- present; Rush University, Rush University Medical Center, Chicago, IL - Lynda Powell, PI; University of California, Davis/Kaiser - Ellen Gold, PI; University of California, Los Angeles - Gail Greendale, PI; University of Medicine and Dentistry - New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; Nanette Santoro, PI 2004 – present; and the University of Pittsburgh, Pittsburgh, PA - Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD - Marcia Ory 1994 – 2001; Sherry Sherman 1994 – present; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor - Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001; University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, PI 2001 – present.
Steering Committee: Chris Gallagher, Chair, Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN and the SWAN Fat Patterning Study. We also thank Elizabeth Avery, M.S. for statistical analyses.
Acronyms
- BMI
body mass index
- CES-D
Center for Epidemiological Studies Depression Scale
- CT
computed tomography
- CVD
cardiovascular disease
- DXA
dual energy x-ray absorptiometry
- FRS
Framingham risk score
- Health ABC
Dynamics of Health, Aging and Body Composition Study
- HDL-C
high-density lipoprotein cholesterol
- HPA
hypothalamic-pituitary-adrenal axis
- KPAS
Kaiser Physical Activity Survey
- MRI
Magnetic resonance imaging
- SAT
subcutaneous adipose tissue
- SHBG
sex hormone binding globulin
- SWAN
Study of Women's Health Across the Nation
- VAT
visceral adipose tissue
Footnotes
Dr. Everson-Rose was affiliated with Rush University Medical Center when this work was initiated but completed the work after relocation to the University of Minnesota.
Portions of this work were presented at the 64th annual meeting of the American Psychosomatic Society, Denver, CO, 3/4/06 and are published in abstract form (Psychosom Med 2006;68:A-27).
References
- 1.Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- 2.Everson-Rose SA, Meyer PM, Powell LH, Pandey D, Torrens JI, Kravitz HM, Bromberger JT, Matthews KA. Depressive symptoms, insulin resistance, and risk of diabetes in women at midlife. Diabetes Care. 2004;27:2856–62. doi: 10.2337/diacare.27.12.2856. [DOI] [PubMed] [Google Scholar]
- 3.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 4.Albu JB, Kovera AJ, Johnson JA. Fat distribution and health in obesity. Ann N Y Acad Sci. 2000;904:491–501. doi: 10.1111/j.1749-6632.2000.tb06505.x. [DOI] [PubMed] [Google Scholar]
- 5.Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–8. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 6.Peiris AN, Sothmann MS, Hoffmann RG, Hennes MI, Wilson CR, Gustafson AB, Kissebah AH. Adiposity, fat distribution, and cardiovascular risk. Ann Intern Med. 1989;110:867–72. doi: 10.7326/0003-4819-110-11-867. [DOI] [PubMed] [Google Scholar]
- 7.Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, Theriault G, Pinault S, Bouchard C. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–9. doi: 10.2337/diab.38.3.304. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto WY, Bergstrom RW, Boyko EJ, Chen KW, Leonetti DL, Newell-Morris L, Shofer JB, Wahl PW. Visceral adiposity and incident coronary heart disease in Japanese-American men. The 10-year follow-up results of the Seattle Japanese-American Community Diabetes Study. Diabetes Care. 1999;22:1808–12. doi: 10.2337/diacare.22.11.1808. [DOI] [PubMed] [Google Scholar]
- 9.Nicklas BJ, Penninx BW, Cesari M, Kritchevsky SB, Newman AB, Kanaya AM, Pahor M, Jingzhong D, Harris TB. Association of visceral adipose tissue with incident myocardial infarction in older men and women: the Health, Aging and Body Composition Study. Am J Epidemiol. 2004;160:741–9. doi: 10.1093/aje/kwh281. [DOI] [PubMed] [Google Scholar]
- 10.DeNino WF, Tchernof A, Dionne IJ, Toth MJ, Ades PA, Sites CK, Poehlman ET. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925–32. doi: 10.2337/diacare.24.5.925. [DOI] [PubMed] [Google Scholar]
- 11.DiPietro L, Katz LD, Nadel ER. Excess abdominal adiposity remains correlated with altered lipid concentrations in healthy older women. Int J Obes Relat Metab Disord. 1999;23:432–6. doi: 10.1038/sj.ijo.0800848. [DOI] [PubMed] [Google Scholar]
- 12.Park KS, Rhee BD, Lee KU, Kim SY, Lee HK, Koh CS, Min HK. Intra-abdominal fat is associated with decreased insulin sensitivity in healthy young men. Metabolism. 1991;40:600–3. doi: 10.1016/0026-0495(91)90050-7. [DOI] [PubMed] [Google Scholar]
- 13.Thakore JH, Richards PJ, Reznek RH, Martin A, Dinan TG. Increased intra-abdominal fat deposition in patients with major depressive illness as measured by computed tomography. Biol Psychiatry. 1997;41:1140–2. doi: 10.1016/S0006-3223(97)85394-2. [DOI] [PubMed] [Google Scholar]
- 14.Weber-Hamann B, Hentschel F, Kniest A, Deuschle M, Colla M, Lederbogen F, Heuser I. Hypercortisolemic depression is associated with increased intra-abdominal fat. Psychosom Med. 2002;64:274–7. doi: 10.1097/00006842-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Kahl KG, Bester M, Greggersen W, Rudolf S, Dibbelt L, Stoeckelhuber BM, Gehl HB, Sipos V, Hohagen F, Schweiger U. Visceral fat deposition and insulin sensitivity in depressed women with and without comorbid borderline personality disorder. Psychosom Med. 2005;67:407–12. doi: 10.1097/01.psy.0000160458.95955.f4. [DOI] [PubMed] [Google Scholar]
- 16.Lee ES, Kim YH, Beck SH, Lee S, Oh SW. Depressive mood and abdominal fat distribution in overweight premenopausal women. Obes Res. 2005;13:320–5. doi: 10.1038/oby.2005.43. [DOI] [PubMed] [Google Scholar]
- 17.Weber-Hamann B, Werner M, Hentschel F, Bindeballe N, Lederbogen F, Deuschle M, Heuser I. Metabolic changes in elderly patients with major depression: evidence for increased accumulation of visceral fat at follow-up. Psychoneuroendocrinology. 2006;31:347–54. doi: 10.1016/j.psyneuen.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Vogelzangs N, Kritchevsky SB, Beekman AT, Newman AB, Satterfield S, Simonsick EM, Yaffe K, Harris TB, Penninx BW. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65:1386–93. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sowers MF, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 20.Bromberger JT, Meyer PM, Kravitz HM, Sommer B, Cordal A, Powell L, Ganz PA, Sutton-Tyrrell K. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health. 2001;91:1435–42. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 22.Roberts RE. Reliability of the CES-D Scale in different ethnic contexts. Psychiatry Res. 1980;2:125–34. doi: 10.1016/0165-1781(80)90069-4. [DOI] [PubMed] [Google Scholar]
- 23.Davidson K, Jonas BS, Dixon KE, Markovitz JH. Depression symptoms predict early hypertension in young adults from the CARDIA study? Coronary artery risk development in young adults. Arch Intern Med. 2000;160:1495–1500. doi: 10.1001/archinte.160.10.1495. [DOI] [PubMed] [Google Scholar]
- 24.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–4. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 25.Wagenknecht LE, Langefeld CD, Scherzinger AL, Norris JM, Haffner SM, Saad MF, Bergman RN. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–6. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 26.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr. 1999;69:381–7. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 27.Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, Kotani K, Funahashi T, Yamashita S, Matsuzawa Y. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–6. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 28.Seidell JC, Oosterlee A, Thijssen MA, Burema J, Deurenberg P, Hautvast JG, Ruijs JH. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr. 1987;45:7–13. doi: 10.1093/ajcn/45.1.7. [DOI] [PubMed] [Google Scholar]
- 29.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 30.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 31.Rosner W. The binding of steroid hormones in human serum. Prog Clin Biol Res. 1976;5:377–95. [PubMed] [Google Scholar]
- 32.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 33.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 34.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the lipid clinics' methodology. J Clin Chem. 1981;19:850. [Google Scholar]
- 35.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–71. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 36.Stanforth PR, Jackson AS, Green JS, Gagnon J, Rankinen T, Despres JP, Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH. Generalized abdominal visceral fat prediction models for black and white adults aged 17-65 y: the HERITAGE Family Study. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28:925–32. doi: 10.1038/sj.ijo.0802563. [DOI] [PubMed] [Google Scholar]
- 37.Carroll JF, Chiapa AL, Rodriquez M, Phelps DR, Cardarelli KM, Vishwanatha JK, Bae S, Cardarelli R. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity. 2008;16:600–7. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 38.Janssen I, Powell LH, Lewis T, Dugan S, Chen Z. Reproductive hormones are related to intra-abdominal fat in women in mid-life: P184 [abstract] Circulation. 2006;113:e348. [Google Scholar]
- 39.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care. 2003;26:1413–20. doi: 10.2337/diacare.26.5.1413. [DOI] [PubMed] [Google Scholar]
- 40.Williams MJ, Hunter GR, Kekes-Szabo T, Trueth MS, Snyder S, Berland L, Blaudeau T. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in women. Int J Obes Relat Metab Disord. 1996;20:613–7. [PubMed] [Google Scholar]
- 41.Despres JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev. 1993;6:137–59. doi: 10.1079/NRR19930010. [DOI] [PubMed] [Google Scholar]
- 42.Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580–92. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- 43.Bjorntorp P. The regulation of adipose tissue distribution in humans. Int J Obes Relat Metab Disord. 1996;20:291–302. [PubMed] [Google Scholar]
- 44.Rebuffe-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol Behav. 1992;52:583–90. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- 45.Matthews KA, Schott LL, Bromberger J, Cyranowski J, Everson-Rose SA, Sowers MF. Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosom Med. 2007;69:124–30. doi: 10.1097/01.psy.0000256574.30389.1b. [DOI] [PubMed] [Google Scholar]
- 46.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–83. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 47.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–24. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 48.Ross R. Atherosclerosis - an inflammatory process. New Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 49.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 50.Rana JS, Nieuwdorp M, Jukema JW, Kastelein JJ. Cardiovascular metabolic syndrome - an interplay of, obesity, inflammation, diabetes and coronary heart disease. Diabetes Obes Metab. 2007;9:218–32. doi: 10.1111/j.1463-1326.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 51.Pietropaolo M, Barinas-Mitchell E, Kuller LH. The heterogeneity of diabetes: unraveling a dispute: is systemic inflammation related to islet autoimmunity? Diabetes. 2007;56:1189–97. doi: 10.2337/db06-0880. [DOI] [PubMed] [Google Scholar]
- 52.You T, Ryan AS, Nicklas BJ. The metabolic syndrome in obese postmenopausal women: relationship to body composition, visceral fat, and inflammation. J Clin Endocrinol Metab. 2004;89:5517–22. doi: 10.1210/jc.2004-0480. [DOI] [PubMed] [Google Scholar]
- 53.Markowitz S, Friedman MA, Arent SM. Understanding the relation between obesity and depression: Causal mechanisms and implications for treatment. Clin Psychol Sci Prac. 2008;15:1–20. [Google Scholar]
- 54.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003;27:514–21. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 55.Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53:891–5. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- 56.Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J Epidemiol. 2000;152:163–70. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- 57.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain Behav Immun. 2003;17:276–85. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 58.Carney RM, Rich MW, Freedland KE, Saini J, teVelde A, Simeone C, Clark K. Major depressive disorder predicts cardiac events in patients with coronary artery disease. Psychosom Med. 1988;50:627–33. doi: 10.1097/00006842-198811000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Pulska T, Pahkala K, Laippalla P, Kivela SL. Major depression as a predictor of premature deaths in elderly people in Finland: a community study. Acta Psychiatr Scand. 1998;97:408–11. doi: 10.1111/j.1600-0447.1998.tb10023.x. [DOI] [PubMed] [Google Scholar]
- 60.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 61.Everson SA, Roberts RE, Goldberg DE, Kaplan GA. Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med. 1998;158:1133–8. doi: 10.1001/archinte.158.10.1133. [DOI] [PubMed] [Google Scholar]