Abstract
The synthesis and antibiofilm activities of sulfonamide, urea, and thiourea oroidin analogues are described. The most active derivative was able to selectively inhibit P. aeruginosa biofilm development and is also shown to be non-toxic upwards of 1 mM to the development of C. elegans in comparison to other similar isosteric analogues and the natural product oroidin.
Bacterial biofilm formation is often described as a developmental process initiated when free floating (planktonic) bacteria adhere to a surface suitable for growth and initiate the formation of a microcolony.1 Occupation of a biofilm growth state confers to the bacteria a unique set of phenotypic traits which include resistance to microbicides and antibiotics that would often lead to eradication.2-4 In a medical setting, biofilms pose a serious threat to individuals who suffer from a myriad of diseases. Recent estimates have attributed biofilm-associated infections as being responsible for upwards of 75% of microbial infections in the human body.5 This problem is further exacerbated by the increased spread of antibiotic resistance. Additionally, biofilms are known to infect patients with indwelling medical devices (IMDsa) such as catheters and heart stents.6-8 Remediation of biofilm infected IMDs is traditionally accomplished by device removal due to the lack of antibiotic efficacy.
As the medical community works towards new approaches aimed at combating the deleterious effects of biofilms, one area that has garnered significant attention is the identification of non-microbicidal modulators of biofilm growth and maintenance (Figure 1).9-13 By not directly killing bacteria, it is postulated that development of resistance to these molecules would be mitigated or significantly impaired. Implementation of remediation therapies which focus on the co-dosing of an antibiofilm modulator with an antibiotic also provides an attractive avenue for treatment. One of the few naturally occurring scaffolds shown to possess non-microbicidal antibiofilm properties are compounds derived from the oroidin class of natural products. Oroidin 5 has been reported to be a moderate inhibitor of Pseudomonas aeruginosa PAO1/PA14 biofilm growth (PAO1 IC50 = 190 μM, PA14 IC50 = 166 μM).14
Figure 1.
Non-microbicidal biofilm modulators.
We have recently begun to focus on the development of methodologies to access oroidin analogues for antibiofilm screening.15-17 Of these approaches, development of conditions for a generic reductive acylation reaction has allowed access to previously unattainable oroidin derivatives through the use of acid chlorides, anhydrides, succinimide esters, and trichloromethylketone pyrroles.18 In a continued effort, we sought to further apply this approach in generating isosteric analogues possessing sulfonamide, urea and thiourea functionalities to further probe the structure-activity relationships (SAR) of the oroidin family in the context of antibiofilm activity and preliminary toxicity.
The Gram-negative γ-proteobacteria Pseudomonas aeruginosa PA14 and Acinetobacter baumannii were selected as the biofilm-forming bacteria employed in the study. Pseudomonas aeruginosa is one of the most well studied organisms in respect to biofilm behavior.19 It is the second most commonly isolated pathogen in cases of nosocomial acquired pneumonia.20 Additionally, the inability to treat cystic fibrosis patients with chronic P. aeruginosa infections has been directly correlated with the emergence and pathogenicity of P. aeruginosa biofilms.21 Acinetobacter baumannii has been identified in recent hospital outbreaks throughout healthcare systems in both Europe and the USA.22, 23 The speed and prevalence with which multi-drug resistance (MDR) is occurring in this bacterium has posed as a significant impediment to A. baumannii remediation therapies. In addition to examining the antibiofilm properties of these new derivatives, it was also a major goal of this study to further delineate the toxicity associated with oroidin-derived compounds. This was accomplished by investigating how the most active member of the newly formed library and previously synthesized isosteric analogues affected larvae development in the eukaryote Caenorhabditis elegans in comparison to the natural product oroidin.
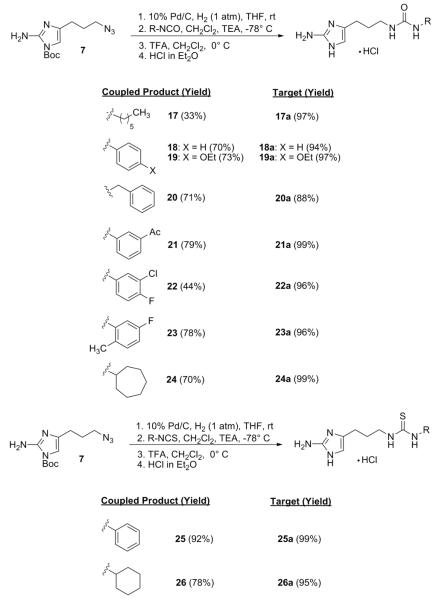
A total of 19 analogues (9 prepared from sulfonyl chlorides, 8 prepared from isocyanates, 2 prepared from isothiocyanates) were synthesized by slightly varying the reaction conditions previously reported (Schemes 1 and 2).18 While no base was used under the initial acylation conditions, it was found that addition of a single equivalent of triethylamine prior to addition of the acylating reagent and keeping the solution at −78 °C throughout the course of the reaction led to optimum yields. In general, the generation of sulfonamide derivatives proceeded smoothly (57-88%) while the corresponding yields for the formation of the ureas and thioureas were less consistent (33-92%). The commercial availability of a wide array of these acylating reagents also made it possible to probe the effect that larger ring systems (15, 16, and 24) had on antibiofilm activity. Coupled products generated through the application of the methodology were then exposed to TFA-mediated deprotection in dichloromethane. The resulting TFA salts were exchanged for the corresponding hydrochloride salts which were then used for biological assessment.
Scheme 1.
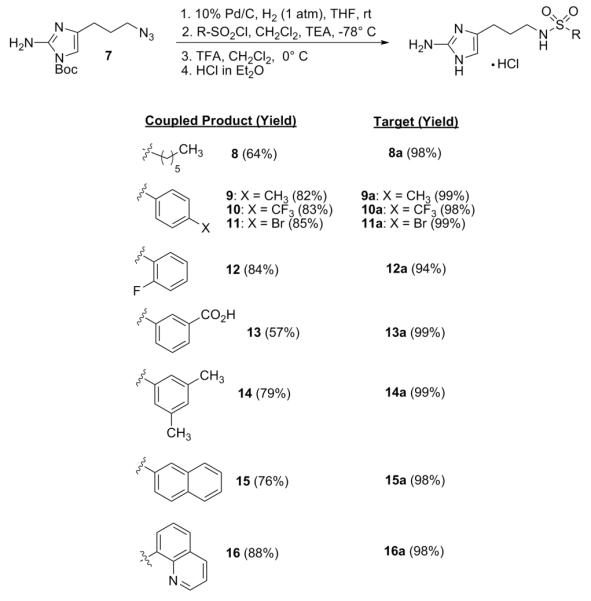
Synthesis of sulfonamide analogues.
Scheme 2.
Synthesis of urea and thiourea analogues.
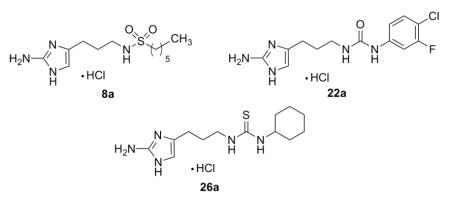
All newly synthesized derivatives were first subjected to static biofilm inhibition assays performed at 100 μM utilizing a crystal violet reporter assay (supporting information).15, 24 Compounds whose inhibition values exceeded 80% in the initial screen were subsequently assayed for IC50 (biofilm inhibition) values. The most active compound in each class of molecules (sulfonamide, urea, thiourea) was then analyzed for biofilm dispersion activity. Surprisingly, none of the newly developed sulfonamides or ureas/thioureas were able to effectively inhibit the formation of A. baumannii biofilms greater than 30% at 100 μM. This is in contrast to previous studies where the biological activity of most analogues could be conserved over γ-proteobacteria.25, 26 However, many of the derivatives were determined to be potent inhibitors of P. aeruginosa PA14 biofilm development with 11 out of the 19 analogues exhibiting PA14 IC50 values under 50 μM. In general, the sulfonamides were the most active (IC50 value range = 10 – 46 μM), followed closely by the two thioureas (IC50 value range = 22 – 26 μM) and lastly the ureas (IC50 value range = 25 – 50 μM). The most active analogues from each class are summarized in Table 1. Non-microbicidal behavior of 8a, 22a, and 26a against PA14 was validated by performing growth curve experiments at the calculated IC50 values. No change in bacterial cell density was observed for all compounds throughout a 24-hour time period (supporting information).
Table 1.
Antibiofilm activity of select analogues.
 | ||
|---|---|---|
| Analogue | PA14 IC50 ± SE (μM) |
PA14 Dispersion at 100 μM ± SE (%) |
| 8a | 10.1 ± 1.96 | 42 ± 4 |
| 22a | 25.7 ± 2.19 | 55 ± 3 |
| 26a | 22.6 ± 3.24 | 25 ± 5 |
Paralleling previous observations of other oroidin derivatives, there was a decrease in the activities observed in the dispersion experiments.14, 15, 18, 27 Analogues 8a, 22a, and 26a were only modest dispersal agents against pre-formed PA14 biofilms at 100 μM (42%, 55%, and 25%, respectively). Despite the lack of biofilm dispersal activity, the inhibition results further strengthened the argument that inhibition activity of the oroidin scaffold against PA14 can be modulated to deliver molecules even more potent than those found with an amide bond in a similar arrangement to the natural products.15, 25 Also, activity can be tuned to display selectivity against biofilm forming proteobacteria which reside in the same class through the incorporation of sulfonamide or urea/thiourea functionalities.
The continued investigation into the development of antibiofilm analogues derived from the oroidin scaffold has inevitably led us to further question the possible utilization of these molecules as therapeutic treatments in biofilm remediation efforts. Ultimately, the molecules must retain their non-cytotoxic biofilm-modulating properties without affecting the surrounding cellular environments. Preliminary cytotoxicity experiments involving GH4C1 rat pituitary cells and N2A mouse neuroblastoma cells have shown that select 2-aminoimidazole (2-AI) analogues have possessed non-toxic activity at concentrations up to 600 μM.27, 28 To further investigate the toxicity profiles of a specific class of 2-AI derivatives, we sought to analyze the effects they had on the development of the multicellular eukaryote Caenorhabditis elegans.
The overall goal of the assay was to examine what effect various concentrations of 2-AI molecules would have on late larval stage C. elegans development (supporting information for detailed assay). The National Institute of Environmental Health and Safety (NIEHS) has validated preliminary screens for eukaryotic toxicity in model organisms such as C. elegans as a means for the strategic testing of possible toxic substances.29, 30 Briefly, in 96-well microtiter plates, the nematodes were monitored for their ability to develop into egg-laying adults, for the eggs to hatch into early stage larvae, and for these larvae to subsequently feed on a bacterial suspension. Observations were made through visualization under a dissecting microscope, with the results being interpreted in a binary manner. Either the wells remained turbid and opaque signaling that the worms had not fed on the bacterial suspension at the given concentration of the 2-AI compound (toxic) or the wells became transparent indicating that the 2-AI derivative had not interfered with developmental activities at that particular concentration (non-toxic). Ivermectin, a potent nematocide, was employed as a positive control. Using this experimental design, thresholds of toxicity towards C. elegans development were then determined.
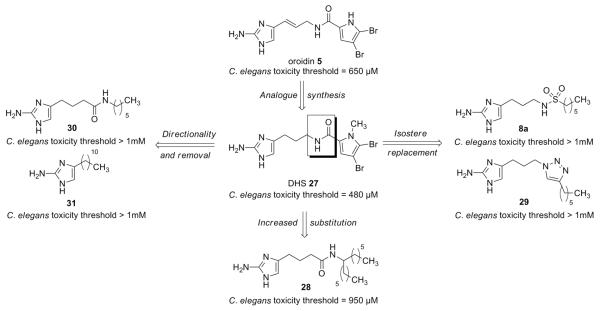
A logical starting point for the assay was to first assess the toxicity of oroidin and dihydrosventrin (DHS) 27, which was one of the first 2-AI analogues assembled from the oroidin skeleton that was found to possess biofilm modulating properties.14, 26 In addition to assaying the most active member of the present isosteric library (8a), it was decided to also examine a number of other hexyl aliphatic chain 2-AI analogues in which the amide bond was modified in an analogous fashion (Figure 2).25 The results from the toxicity screens are summarized in Figure 2. Oroidin 5 was found to display a threshold of 650 μM, meaning that any concentration over that mark was found to be toxic to the worms. DHS 27 was slightly more toxic, exhibiting a detrimental effect at concentrations greater than 480 μM. However, many of the aliphatic derivatives chosen for analysis were found to be non-toxic at much higher concentrations (8a, 29, 30, 31), with most even exceeding the maximum concentration of 1 mM used in the study. Analogue 28 was close to the 1 mM mark, eliciting a toxic effect at concentrations greater than 950 μM. These results are the first to demonstrate the associated toxicity of oroidin and related analogues to the development of C. elegans. The relative lack of toxicity at such high concentrations undoubtedly provides a suitable foundation for the continued exploration of the affects of these compounds in other model organisms.
Figure 2.
C. elegans toxicity thresholds of selected hexyl chain based isosteric oroidin analogues.
In conclusion, employment of a reductive acylation strategy to incorporate sulfonamide, urea, or thiourea functionalities into the oroidin backbone has led to the discovery that other amide isosteres have the potential to be potent and selective modulators of P. aeruginosa biofilm development. Furthermore, related isosteric analogues which bear aliphatic side chains are reported to be highly non-toxic in nature to the development of C. elegans in comparison to the natural product oroidin. Ultimately, it is envisioned that the reductive acylation reaction will prove amenable for the synthesis of a variety of biotinylated conjugates to help further probe the mechanism of action of these biofilm modulating non-toxic compounds.
Supplementary Material
Acknowledgements
Financial support for this work was graciously provided by the NIH (R01 GM55769), the Jimmy V. NCSU Cancer Therapeutics Training Program, the UNCGA Competiveness Research Fund, and the North Carolina Biotechnology Center.
Footnotes
- IMD
- indwelling medical device
- IC50
- biofilm inhibitory concentration of 50%
- SAR
- structure-activity relationship
- MDR
- multi-drug resistance
- Pd/C
- palladium on carbon
- THF
- tetrahydrofuran
- rt
- room temperature
- TEA
- triethylamine
- TFA
- trifluoroacetic acid
- SE
- standard error
- 2-AI
- 2-aminoimidazole
- NIEHS
- National Institute of Environmental Health and Safety
- DHS
- dihydrooroidin.
Supporting Information Available: Complete antibiofilm activity charts, growth curves, C. elegans toxicity assay procedures, characterization data, and representative spectra from each class of compounds is provided. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Musk DJ, Hergenrother PJ. Chemical countermeasures for the control of bacterial biofilms: Effective compounds and promising targets. Curr. Med. Chem. 2006;13(18):2163–2177. doi: 10.2174/092986706777935212. [DOI] [PubMed] [Google Scholar]
- (2).Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marrie TJ. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- (3).Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284(5418):1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- (4).Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- (5).Davies D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2(2):114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- (6).Darouiche RO. Device-associated infections: A macroproblem that starts with microadherence. Clin. Infect. Dis. 2001;33(9):1567–1572. doi: 10.1086/323130. [DOI] [PubMed] [Google Scholar]
- (7).Darouiche RO, Raad II, Heard SO, Thornby JI, Wenker OC, Gabrielli A, Berg J, Khardori N, Hanna H, Hachem R, Harris RL, Mayhall G, Grp CS. A comparison of two antimicrobial-impregnated central venous catheters. N. Engl. J. Med. 1999;340(1):1–8. doi: 10.1056/NEJM199901073400101. [DOI] [PubMed] [Google Scholar]
- (8).Donlan RM, Costerton JW. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Geske GD, Wezeman RJ, Siegel AP, Blackwell HE. Small molecule inhibitors of bacterial quorum sensing and biofilm formation. J. Am. Chem. Soc. 2005;127(37):12762–12763. doi: 10.1021/ja0530321. [DOI] [PubMed] [Google Scholar]
- (10).Junker LM, Clardy J. High-throughput screens for small-molecule inhibitors of Pseudomonas aeruginosa biofilm development. Antimicrob. Agents Chemother. 2007;51(10):3582–3590. doi: 10.1128/AAC.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Manefield M, Kjelleberg S, Givskov M. Controlling bacterial infection by inhibiting intercellular signalling. Curr. Med. Chem. Anti Infect. Agents. 2003;2(3):213–218. [Google Scholar]
- (12).Musk DJ, Banko DA, Hergenrother PJ. Iron salts perturb biofilm formation and disrupt existing biofilms of Pseudomonas aeruginosa. Chem. Biol. 2005;12(7):789–796. doi: 10.1016/j.chembiol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- (13).Ren DC, Zuo RJ, Barrios AF, Bedzyk LA, Eldridge GR, Pasmore ME, Wood TK. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005;71(7):4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Richards JJ, Ballard TE, Huigens RW, Melander C. Synthesis and screening of an oroidin library against Pseudomonas aeruginosa biofilms. ChemBioChem. 2008;9(8):1267–1279. doi: 10.1002/cbic.200700774. [DOI] [PubMed] [Google Scholar]
- (15).Richards JJ, Ballard TE, Melander C. Inhibition and dispersion of Pseudomonas aeruginosa biofilms with reverse amide 2-aminoimidazole oroidin analogues. Org. Biomol. Chem. 2008;6(8):1356–1363. doi: 10.1039/b719082d. [DOI] [PubMed] [Google Scholar]
- (16).Richards JJ, Melander C. Synthesis of a 2-aminoimidazole library for antibiofilm screening utilizing the Sonogashira reaction. J. Org. Chem. 2008;73(13):5191–5193. doi: 10.1021/jo800618q. [DOI] [PubMed] [Google Scholar]
- (17).Rogers SA, Melander C. Construction and screening of a 2-aminoimidazole library identifies a small molecule capable of inhibiting and dispersing bacterial biofilms across order, class, and phylum. ACIE. 2008;47(28):5229–5231. doi: 10.1002/anie.200800862. [DOI] [PubMed] [Google Scholar]
- (18).Ballard TE, Richards JJ, Aquino A, Reed CS, Melander C. Antibiofilm activity of a diverse oroidin library generated through reductive acylation. J. Org. Chem. 2009;74(4):1755–1758. doi: 10.1021/jo802260t. [DOI] [PubMed] [Google Scholar]
- (19).Bjarnsholt T, Givskov M. The role of quorum sensing in the pathogenicity of the cunning aggressor Pseudomonas aeruginosa. Anal. Bioanal. Chem. 2007;387(2):409–414. doi: 10.1007/s00216-006-0774-x. [DOI] [PubMed] [Google Scholar]
- (20).Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs. 2007;67(3):351–368. doi: 10.2165/00003495-200767030-00003. [DOI] [PubMed] [Google Scholar]
- (21).Costerton JW. Cystic fibrosis pathogenesis and the role of biofilms in persistent infection. Trends Microbiol. 2001;9(2):50–52. doi: 10.1016/s0966-842x(00)01918-1. [DOI] [PubMed] [Google Scholar]
- (22).Moro M, Nizzero P, Biancardi A, Baldan R, Scarpellini P, Curti C, Cichero P, Ossi C, Mancini N, Colombo S, Marazzi M, Mazzuconi R, Cirillo D. An outbreak caused by multidrug-resistant OXA-58-positive Acinetobacter baumannii in an intensive care unit in Italy. J. Hosp. Infect. 2008;68(1):97–99. doi: 10.1016/j.jhin.2007.10.007. [DOI] [PubMed] [Google Scholar]
- (23).Shelburne S, Singh K, White C, Byrne L, Carmer A, Austin C, Graviss E, Stager C, Murray B, Atmar R. Sequential outbreaks of infections by distinct Acinetobacter baumannii strains in a public teaching hospital in Houston, Texas. J. Clin. Microbiol. 2008;46(1):198–205. doi: 10.1128/JCM.01459-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 1998;28(3):449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- (25).Ballard TE, Richards JJ, Wolfe AL, Melander C. Synthesis and Antibiofilm Activity of a Second-Generation Reverse-Amide Oroidin Library: A Structure-Activity Relationship Study. Chem. Euro. J. 2008;14(34):10745–10761. doi: 10.1002/chem.200801419. [DOI] [PubMed] [Google Scholar]
- (26).Richards JJ, Huigens RW, Ballard TE, Basso A, Cavanagh J, Melander C. Inhibition and dispersion of proteobacterial biofilms. Chem. Commun. 2008;(14):1698–1700. doi: 10.1039/b719802g. [DOI] [PubMed] [Google Scholar]
- (27).Huigens RW, Ma LY, Gambino C, Moeller PDR, Basso A, Cavanagh J, Wozniak DJ, Melander C. Control of bacterial biofilms with marine alkaloid derivatives. Mol. BioSyst. 2008;4(6):614–621. doi: 10.1039/b719989a. [DOI] [PubMed] [Google Scholar]
- (28).Melander C, Moeller PDR, Ballard TE, Richards JJ, Huigens RW, Cavanagh J. Evaluation of dihydrooroidin as an antifouling additive in marine paint. Inter. Biodeter. & Biodeg. 2009 doi: 10.1016/j.ibiod.2008.08.009. doi:10.1016/j.ibiod.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Collins FS, Gray GM, Bucher JR. Toxicology - transforming environmental health protection. Science. 2008;319(5865):906–907. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Cui YX, McBride SJ, Boyd WA, Alper S, Freedman JH. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. 2007;8(6):R122. doi: 10.1186/gb-2007-8-6-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.