Abstract
An imbalance in the redox-state of the brain may be part of the underlying pathophysiology in schizophrenia. Inflammatory mediators, such as IL-6, which can tip the redox balance into a pro-oxidant state, have been consistently found to be altered in schizophrenia patients. However, the relationship of altered redox-state to altered brain functions observed in the disease has been unclear. Recent data from a pharmacological model of schizophrenia suggest that redox and inflammatory imbalances may be directly linked to the pathophysiology of the disease by alterations in fast-spiking interneurons. Repetitive adult-exposure to the NMDA-R antagonist ketamine increases the levels of the proinflammatory cytokine interleukin-6 in brain which, through activation of the superoxide-producing enzyme NADPH-oxidase (Nox2), leads to the loss of the GABAergic phenotype of PV-interneurons and to decreased inhibitory activity in prefrontal cortex. This effect is not observed after a single exposure to ketamine, suggesting that the first exposure to the NMDA-R antagonist primes the brain such that deleterious effects on PV-interneurons appear upon repetitive exposures. The effects of activation of the IL-6/Nox2 pathway on the PV-interneuronal system are reversible in the adult brain, but permanent in the developing cortex. The slow development of PV-interneurons, while essential for shaping of neuronal circuits during postnatal brain development, increases their vulnerability to deleterious insults that can permanently affect their maturational process. Thus, in individuals with genetic predisposition, the persistent activation of the IL-6/Nox2 pathway may be an environmental factor that tips the redox-balance leading to schizophrenia symptoms in late adolescence and early adulthood.
Keywords: redox, parvalbumin, fast-spiking, gamma oscillations, GABAergic, Interleukin-6, NADPH oxidase, schizophrenia
Introduction
The pathophysiology of schizophrenia is complex and involves many different cortical and subcortical systems. In particular, fast-spiking parvalbumin (PV)-positive inhibitory neurons, which represent 5% of all cortical neurons, are strongly affected. Reduced expression of GAD67, the main isoform synthesizing GABA in brain, is one of the most replicated findings in schizophrenia post mortem brain studies (Benes and Berretta, 2001; Lewis et al., 2005), and single nucleotide polymorphisms in the regulatory region of Gad1 (the gene coding for GAD67) are associated with childhood onset of schizophrenia (Rapoport et al., 2005). The decrease in GAD67 occurs primarily in the subset of inhibitory interneurons expressing the calcium binding protein parvalbumin (Beasley and Reynolds, 1997; Hashimoto et al., 2003). This apparent “loss of GABAergic phenotype” in PV-interneurons led to the suggestion that dysfunction of these fast-spiking inhibitory interneurons may be a core feature of the disease (Lewis et al., 2005). Whether these deficiencies are a consequence or a cause of the disorder is, however, a matter of debate.
PV-interneurons are involved in the generation of gamma oscillations, which regulate working memory and information transmission between cortical areas (Salinas and Sejnowski, 2001; Bartos et al., 2007). In particular, synaptic inhibition from PV-interneurons controls the firing rates of pyramidal neurons, synchronizes spikes within populations of neurons, and participates in the development of executive functions associated with prefrontal brain regions (Kawaguchi and Kubota, 1993; Goldman-Rakic, 1999; Markram et al., 2004). PV-interneurons are a part of the network that generates oscillatory activity in the gamma range (Sohal et al., 2009; Cardin et al., 2009), suggesting that their dysfunction may account for the disruption in evoked gamma-frequency oscillations and as well as the cognitive deficits observed in schizophrenia (Gonzalez-Burgos and Lewis, 2008; Roopun et al., 2008; Uhlhaas et al., 2008).
Adult levels of executive function emerge relatively late in the postnatal development of primates (Alexander and Goldman, 1978) and probably of rodents (Bachevalier and Beauregard, 1993; Ba and Seri, 1995). In primates and rodents, the delay in achieving mature performance on executive function tasks correlates with the maturation of oscillatory activity in the gamma range and with the maturation of PV-interneuronal networks (Wilson et al., 1994; Rao et al., 2000; Doischer et al., 2008; Uhlhaas et al., 2009), consistent with the delayed maturation of PV-inhibitory circuits. Development of PV-synaptic contacts, which sculpt this inhibitory network throughout childhood and adolescence, is dependent on GAD67 synthesis and activity in rodents (Chattopadhyaya et al., 2007). Environmental insults affecting the development of this inhibitory network, e. g. by affecting GAD67 expression, may lead to the abnormal formation of synaptic contacts by these interneurons. Thus, the dependence on GAD67 expression could make the developing brain vulnerable to environmental inputs that through disruption of the normal development of this inhibitory circuit lead to psychiatric diseases in adulthood.
The NMDA receptor antagonist model of schizophrenia
Several animal models recapitulating aspects (endophenotypes) of schizophrenia have been developed. Among these, exposure to NMDA receptor (NMDA-R) antagonists such as phencyclidine (PCP), ketamine, and MK801 are widely used in adult animals as acute pharmacological models to study behavioral and neurochemical disruptions relevant to the disease (rev. in (Mouri et al., 2007)). Administration of PCP to rodents produces deficits in spatial working memory, in reversal learning, and in sustained attention (Jentsch and Roth, 1999; Stefani and Moghaddam, 2005b). Similarly, ketamine can impair performance on tasks testing executive function in humans (Krystal et al., 2005) and non-human primates (Stoet and Snyder, 2006), resembling executive functioning deficits that are associated with treatment-refractory aspects of schizophrenia (Kerns et al., 2008).
At the neurochemical level, acute exposure to NMDA-R antagonists in adulthood increases excitatory transmission in frontal and anterior cingulate cortex across species (Takahata and Moghaddam, 2003). This hyper-excitation has been related to an increase in thalamo-cortical glutamatergic excitation of downstream cortical regions such as the anterior cingulate and retrosplenial cortices (Tomitaka et al., 2000; Holcomb et al., 2005). PET scan studies have shown that schizophrenic subjects respond to ketamine with higher hypermetabolism than normal subjects. Even under resting conditions schizophrenia patients show altered activity in the prefrontal cortex (PFC) and parahippocampal regions (Garrity et al., 2007) and suffer from persistent symptoms and chronic deficiency in their cognitive ability.
Although acute exposures to NMDA-R antagonists can produce some symptoms of schizophrenia in healthy subjects, these are transient and disappear after washout of the drug. Repetitive NMDA-R antagonist treatment in animals produce more persistent effects on stereotypy and locomotor activity, as well as enduring cognitive deficits and neurochemical changes that resemble more accurately the alterations observed in schizophrenia (Jentsch and Roth, 1999; Morris et al., 2005; Mouri et al., 2007). For example, the initial hypermetabolism, observed after acute NMDA-R antagonist exposure, is followed by a decrease in metabolic activity in the PFC, as well as within structures of the auditory system, and the reticular nucleus of the thalamus (Cochran et al., 2003). Repetitive PCP decreases dopamine in the dorsolateral prefrontal cortex, prelimbic cortex, and cingulate cortex, but not in supplementary motor area (Jentsch and Roth, 1999). This regimen also elicits alterations in N-acetylaspartate (NAA) and N-acetylaspartylglutamate (NAAG) in temporal cortex and hippocampus (Reynolds et al., 2005), and decreases 5HT2A receptor binding in the PFC (Steward et al., 2004) in close similarity to schizophrenia pathology (Laruelle et al., 1993; Nudmamud et al., 2003). Furthermore, while the behavioral and neurochemical effects of acute exposures to NMDA-R antagonists are believed to occur upon disinhibition of cortical excitatory activity due to increased sensitivity of inhibitory systems to blockade of NMDA-R function (Olney et al., 1999; Homayoun and Moghaddam, 2007; Lisman et al., 2008; Middleton et al., 2008), they do not lead to the enduring changes in PV-interneurons observed in schizophrenia. Repetitive exposures to NMDA-R antagonists, on the other hand, produce a reduction in GAD67 expression in PV-interneurons of rodents (Behrens et al., 2007, 2008) as well as decreased expression of parvalbumin in rodents and non-human primates (Cochran et al., 2002; Keilhoff et al., 2004; Rujescu et al., 2006; Morrow et al., 2007).
The role of superoxide in the persistent effects of NMDA-R antagonists
Although exposure to one injection of the NMDA-R antagonist ketamine does not lead to the loss of GABAergic phenotype of PV-interneurons, injections of ketamine on two consecutive days is sufficient to produce this loss (Behrens et al., 2008). Similar exposures to ketamine in rats lead to an enduring decrease of inhibitory tone in prefrontal cortex (Zhang et al., 2008), supporting the idea that repetitive exposures to NMDA-R antagonists produce enduring effects resembling those observed in schizophrenia.
The repetitive, but not acute, exposure to NMDA-R antagonists disinhibited excitatory circuits and activated the superoxide producing enzyme NADPH oxidase-2 (Nox2) in three week old primary cortical neurons as well as in adult brain. Furthermore, the inhibition of Nox2 with apocynin or eliminating superoxide with a brain-penetrant SOD-mimetic prevented the loss of phenotype of PV-interneurons in vitro and in vivo, and the ketamine effects were absent in Nox2-deficient animals (Behrens et al., 2007, 2008). Exposure to PCP and more selective NMDA-R antagonists such as MK801 and CPP were shown to produce a rapid increase in reactive oxygen- and nitrogen-species (ROS) in vitro (Xia et al., 2002), and in vivo (Zuo et al., 2007; Fejgin et al., 2008) and repetitive exposures in vivo led to a substantial elevation of baseline levels of free radicals, suggesting that this treatment results in a persistent change in the oxidative state of the cortex (Zuo et al., 2007). Interestingly, recent results have shown that NMDA receptor activity is required for the expression of antioxidant enzymes (Papadia et al., 2008), further supporting the idea that prolonged blockade of NMDA receptors produces an increased oxidative state.
Several studies have reported increased oxidative and nitrosative state, as well as a diminished antioxidant capacity in schizophrenia patients (reviewed in Do et al., 2009). Glutathione (GSH), responsible for detoxification of reactive oxygen and other radical species, is consistently decreased in cerebrospinal fluid of drug-naïve schizophrenia patients (Do et al., 2000), as well as in postmortem tissue (Yao et al., 2006). Polymorphisms in genes coding for enzymes that participate in GSH synthesis have been linked to schizophrenia risk (Tosic et al., 2006; Gysin et al., 2007), and acute frontal-brain GSH depletion in adult rodents was recently shown to produce disruptions in short-term memory, supporting the link between depletion of brain GSH levels and cognitive impairments that occur in schizophrenia (Pileblad et al., 1989; Jacobsen et al., 2005; Dean et al., 2009). Moreover, acute GSH depletion potentiates the release of dopamine produced by amphetamine in striatum and potentiates the behavioral effects of NMDA-R antagonists as a well as those of amphetamine (Jacobsen et al., 2005). Cysteine, the limiting substrate in the synthesis GSH in neurons, is transported from the extracellular space by the main glutamate transporter present in neurons, EAAC1 (Aoyama et al., 2006). EAAC1 as well as EAAC2 have been shown to be highly sensitive to oxidative conditions: reducing agents activate, and oxidation inactivates glutamate transport (Trotti et al., 1997). Thus, the increased superoxide production caused by activation of the IL-6/Nox2 pathway after exposure to NMDA-R antagonists would be expected to produce the redox inactivation of EAAC1 and a decrease in cysteine transport, leading to diminished GSH content in brain. If such activation of the IL-6/Nox2 pathway occurs in situations of genetically diminished brain-GSH levels, such as those described in some schizophrenia cohorts, it will lead to further decrease in GSH levels, NMDA-R hypofunction and increased oxidative damage to macromolecules and lipids (Do et al., 2009). Recent results showing that treatment with N-acetyl-cysteine, a precursor of GSH, improves negative symptoms, and corrects mismatch negativity in schizophrenia patients further support the idea of a redox imbalance in schizophrenia (Berk et al., 2008; Lavoie et al., 2008).
Mechanism of activation of Nox2 in neurons
One of the most consistent findings in schizophrenia patients is an imbalance in plasma and cerebrospinal fluid levels of cytokines (Muller et al., 2000). In particular, elevated plasma levels of IL-6 have been consistently reported in patients and first-degree relatives with mood disorders (Ganguli et al., 1994; Naudin et al., 1996; Nunes et al., 2005), and correlate with exacerbation of psychotic episodes (Ganguli et al., 1994; Naudin et al., 1996; Lin et al., 1998; Zhang et al., 2002; Kudoh et al., 2003; Nunes et al., 2005). Furthermore, treatment with atypical antipsychotics can alter circulating cytokines (Pollmacher et al., 2000). The increased levels of cytokines in schizophrenia patients, together with the important role played by Nox2-dependent NADPH oxidase in inflammatory processes outside the CNS suggests the possible involvement of proinflammatory molecules in the effects of NMDA-R antagonists.
Prolonged exposure to ketamine in vitro or repetitive exposures in vivo increased the levels of IL-6 mRNA, without affecting the levels of IL-1β or TNFα mRNAs (Behrens et al., 2008). Furthermore, brain IL-6 production is necessary and sufficient to produce the induction and activation of Nox2, and the consequent loss of the GABAergic phenotype of PV interneurons (Behrens et al., 2008). Interestingly, increased activity of Nox2 was shown in neutrophils isolated from schizophrenia patients, which correlated with negative symptoms (Sirota et al., 2003), suggesting that the increased IL-6 levels in schizophrenia patients may also lead to induction of the peripheral enzyme.
However, although the effects of repetitive exposures to NMDA-R antagonist resemble more accurately the dysfunction of PV-interneurons observed in schizophrenia patients, these effects are slowly reversible in both the in vitro and in vivo models, requiring 48 h in culture (Kinney et al., 2006) or several days in the absence of drug in vivo (Behrens et al., 2008). Thus, although causing persistent effects, repetitive adult exposures to NMDA-R antagonists do not produce the irreversible changes in inhibitory circuitry observed in schizophrenia.
Neurodevelopmental origins of schizophrenia: Activation of the IL-6/Nox2 pathway may alter the development of PV-interneurons
Mild developmental impairments caused either by a genetic predisposition or by immune activation during development are believed to contribute to the appearance of schizophrenic symptoms in early adulthood (Rapoport et al., 2005). There is a strong correlation between infections in mid-gestation and the incidence of schizophrenia in the offspring (reviewed in (Brown, 2006; Patterson, 2008). Cytokine induction due to abnormal immune activation, or inflammation, derails normal brain development leading to alterations in cognition in adulthood (Gilmore and Jarskog, 1997; Nawa et al., 2000; Smith et al., 2007). Recently, using a rodent maternal infection model Smith and collaborators found that maternal IL-6 induction during infection is responsible for the delayed schizophrenia-like behavior observed in the adult offspring (Smith et al., 2007). A lasting imbalance in cytokine levels was observed in the offspring throughout the first month of age. Since activation of the IL-6/Nox2 pathway and consequent increase in superoxide production in brain is required for the reversible loss of phenotype of PV-interneurons in adulthood (Behrens et al., 2008), activation of this pathway may be responsible for the delayed effects observed in the maternal-infection model of schizophrenia. Furthermore, decreased antioxidant capacity during early postnatal period induce cognitive derangements relevant to schizophrenia, as well as a decreased number of PV-interneurons in adulthood (Cabungcal et al., 2007).
Several neurodevelopmental models of schizophrenia converge on a sustained dysfunction of the PV-interneuronal system occurring during late prenatal/early postnatal development. Studies in the maternal infection model, the DISC1 model, and prenatal exposure to methylazoxymethanol acetate (MAM), have shown that alterations of brain development during specific periods of pre or postnatal development produce discrete disruptions that lead to behavioral and neurochemical effects that include a decreased number of PV-interneurons (Hikida et al., 2007; Lodge and Grace, 2007; Meyer et al., 2007; Lodge et al., 2009). In the MAM model, where the mitotoxin is applied during interneuronal proliferation/migration stage, the number of PV-interneurons decreased in specific brain regions that correlated with alterations in oscillatory activity and decreased lateral inhibition in adulthood (Lodge et al., 2009).
In rodents and primates, maturation of the PV-interneuronal system occurs postnatally (reviewed in (Lewis et al., 2004; Huang, 2009)). Studies performed in rodents show that migration from the medial ganglionic eminence to the cortical plate is complete by around embryonic day 15, but the neurons remain silent until the beginning of the second postnatal week when their maturation begins. Before the first week of age, parvalbumin expression is absent in these neurons, but appears when volleys of excitatory activity arrive from thalamo-cortical projections during the second postnatal week. Further maturation of PV-interneuron synaptic contacts develops independently of thalamic inputs and reaches adult levels by the end of the first month of age (Di Cristo et al., 2004). A recent study in mouse cortex showed pronounced transcriptional and electrophysiological changes in PV-interneurons occurring during the first month of postnatal development (Okaty et al., 2009). Profound changes in the ion- channel repertoire expressed at different time-points during the first postnatal month shape the electrophysiological maturation required to attain the characteristic fast-spiking non-accommodating current patterns observed in adulthood. Oscillatory activity in the gamma range also begins during this second postnatal week, and matures throughout the following weeks to reach adult levels by the end of the adolescent period (Doischer et al., 2008). In non-human primates, the appearance of parvalbumin expression and development of PV-synaptic contacts begins at around 3 months of age, and develops profusely throughout childhood and adolescence (Reynolds and Beasley 2001; Cruz et al., 2003). During adolescence, however, the restructuring and pruning of synaptic contacts reduces the levels to those found in adulthood (Cruz et al., 2003). Cortical neural synchrony and cognitive performance also show a protracted maturation in humans, reaching mature levels only in early adulthood after a period of decreased cognitive performance and synchrony during adolescence (Uhlhaas et al., 2009). The profound reorganization of synaptic activity that occurs during adolescence could be responsible for the significant reductions in phase synchronization observed. The emergence of psychotic symptoms in schizophrenia during late adolescence/early adulthood could be a consequence of this reorganization process in compromised inhibitory networks (Feinberg 1982).
The pro-psychotic effects of NMDA-R antagonists appear only in early adulthood and exposure of four week old rats to ketamine on two consecutive days did not reduce GAD67 immunoreactivity (Zhang et al., 2008). However, 24 h continuous exposure of three week old cultured neurons to ketamine produces a substantial reduction in GAD67 immunoreactivity (Kinney et al, 2006). These results suggest that PV-interneurons are more resistant to the effects of NMDA-R antagonists during the rodent equivalent to the adolescence period in humans. The overwhelming proliferation of PV-synaptic contacts observed before adolescence (Cruz et al., 2003), as well as the slow functional maturation of PV-inhibitory network (Doischer et al., 2008; Okaty et al., 2009) may explain the lack of pro-psychotic effects of NMDA-R antagonists before early adulthood. However, hormonal influences or changes in receptor-expression patterns (Okaty et al,. 2009) and changes in response to modulatory neurotransmitters (Tseng and O'Donnell, 2007) that occur during adolescence could also explain the lack of pro-psychotic effects of NMDA-R antagonists.
In contrast to the reversible effects observed in adult animals (Behrens et al., 2008), embryonic and repetitive exposures during the second postnatal week to NMDA-R antagonists can produce loss of PV-interneurons and persistent behavioral and neurochemical deficits that appear only in adulthood (Stefani and Moghaddam, 2005a; Mouri et al., 2007; Wang et al., 2008). Mice expressing 10% of the normal NMDA-R activity develop cognitive disruptions resembling those found in schizophrenia (reviewed in (Gainetdinov et al., 2001)). Ablation of NR1 subunits in GABAergic neurons during early postnatal development leads to a loss of parvalbumin expression in PV-interneurons in adulthood and to behavioral disruptions reminiscent to those found in schizophrenia (Belforte et al., 2008). Taken together, these results support the idea of a critical role for NMDA-R function in the postnatal maturation of PV-interneurons, and raise a note of caution in the use of anesthetics with demonstrated NMDA-R antagonist activity in children.
Ketamine, although not approved for use in humans less than 17 years of age, is commonly used as an anesthetic in children (Mellon et al., 2007). Ketamine and other NMDA-R antagonists cause neurodegeneration in the developing brain in rodents and primates (Olney, 2002; Wang and Slikker, 2008). Although the doses used in these experiments are higher relative to those used in humans, repetitive subanesthetic doses, by activating the IL-6/Nox2 pathway in brain, may halt the maturation process of PV-interneurons and lead to a permanent dysfunction of cognitive processes. This is supported by the finding that antioxidants applied during perinatal exposure to NMDA-R antagonists prevent the development of behavioral disruptions (Wang et al., 2003), and that Nox2-deficient mice are protected from the decrease in PV-interneurons observed in animals that were exposed to NMDA-R antagonists during the second postnatal week (Shehktman and Behrens, unpublished).
Glutamate receptors and the GABAergic phenotype of PV-Interneurons
In common with other glutamatergic synapses, glutamate preferentially activates AMPA-type glutamate receptors in mature PV-interneurons. However, unlike receptors on pyramidal neurons, AMPA receptors on PV-interneurons do not express GluR2 subunits, making them highly Ca2+ permeable (Goldberg et al., 2003). Regarding group 1 metabotropic glutamate receptors, cortical and hippocampal PV-interneurons preferentially express mGluR5 (Cauli et al., 1997; van Hooft et al., 2000). On the other hand, NMDA-Rs in PV-interneurons have a subunit composition that differs from neighboring pyramidal neurons, with NR2A and NR2C subunits being highly expressed (Kinney et al., 2006; Xi et al., 2009). NMDA-Rs exert a tight control of the basal excitability in PV-interneurons, and are highly sensitive to NMDA-R antagonists (Grunze et al., 1996; Li et al., 2002; Middleton et al., 2008). Given this composition of glutamate receptors, it is expected that glutamatergic transmission in PV-interneurons will be substantially different than transmission in pyramidal neurons.
Blockade of NMDA-Rs during the third week of development in culture leads to the loss of the GABAergic phenotype of PV-interneurons, and this loss can be prevented by strategies that increase intracellular calcium levels (Kinney et al., 2006). As noted above, PV-interneurons express GluR2-less AMPA receptors and are permeable to Ca2+. Since the initial disinhibition caused by the NMDA-R antagonists lead to an increase in excitatory transmission, it would be expected that AMPA receptors in PV-interneurons would permeate enough Ca2+ to prevent the deleterious effects of blockade of NMDA-Rs. Furthermore, the increase in excitatory transmission caused by disinhibition should activate group I metabotropic glutamate receptors present in PV-interneurons and also lead to increases in intracellular Ca2+, now through release from intracellular stores. However, co-exposure to a calcium-channel opener or an activator of group I metabotropic receptors was needed to preserve the GABAergic phenotype of PV-interneurons in the presence of NMDA-R antagonists (Kinney et al., 2006). This lack of response of AMPA and mGluR5 receptors to the increased excitatory transmission after disinhibition led to the hypothesis that prolonged blockade of NMDA-Rs in PV-interneurons results in an enduring change in AMPA and mGluR5-mediated responses to glutamate (Behrens et al., 2007).
What is the difference between PV-interneurons and pyramidal neurons that makes the former so sensitive to NMDA-R antagonists? Perhaps the specific subunit composition of the glutamate receptor in PV-interneurons is responsible. NMDA-Rs containing NR2C subunits have a reduced Mg2+ block, and consequently may be highly sensitive to ambient glutamate concentrations. Under normal physiological conditions Mg2+ concentrations are high enough to block NR2A/B-containing receptors, whereas NR2C and NR2D containing receptors will not show such blockade. Recent evidence shows that at physiological Mg2+ concentrations NMDA-R antagonists such as ketamine or memantine have negligible inhibitory effects on NR2A or NR2B containing receptors, whereas their inhibitory effects on NR2C or NR2D containing receptors remain unaltered (Kotermanski and Johnson, 2009). Thus, since NMDA-Rs in PV-interneurons contain higher levels of NR2A and NR2C receptors, antagonists such as ketamine would have stronger effects in these interneurons than on pyramidal neurons. In the presence of NMDA-R antagonists, lack of function of NR2A/NR2C containing receptors in PV-interneurons may then lead to profound changes in the function of this inhibitory system. Indeed, repetitive exposure to MK801 in vivo was recently shown to decrease the expression of NMDA-R subunits in PV-interneurons in the prefrontal cortex of rats (Xi et al., 2009). Interestingly, the NR2C subunit decreased most (~87 fold) after treatment.
Redox dysregulation of NMDA-R mediated transmission in PV-interneurons
Inactivation of synaptic proteins through oxidation is a well described phenomenon, and considered to be behind many of the derangements of the nervous system observed in disease states (Rowan et al., 2005; Butterfield, 2006; Satoh and Lipton, 2007). Regulatory redox sites have been found in many proteins that are key to glutamatergic neurotransmission including, serine-racemase that is responsible for the synthesis of the endogenous modulator of the glycine site in NMDA receptors (Mustafa et al., 2007); glutamine synthase that is responsible for glutamate synthesis (Pinteaux et al., 1996); as well as the excitatory amino acid transporters that together with glutamine synthase are involved in the regulation of extraneuronal levels of glutamate (Volterra et al., 1994). Last, but not least, the NMDA receptor itself is highly sensitive to redox modulation through a redox-sensitive site (Herin and Aizenman, 2004), and decreases in the main antioxidant in brain, GSH, or reduced activity of GSH-peroxidase lead to oxidized hypofunctional NMDA-Rs (Jiang et al., 2000; Steullet et al., 2006). In particular, receptors composed of NR1:NR2A subunits were shown to have a highly reversible and rapid current potentiation by sulfhydryl redox agents, including GSH, acting on a specific redox site in NR2A (Kohr et al., 1994). The oxidation status of this redox site can affect the regulation of these receptors by spermine and protons, as well as the inhibition mediated by the high-affinity Zn2+ site (Lipton et al., 2002). On the other hand, oxidation of receptors containing NR2C is slowly reversible (Kohr et al., 1994). Given this heightened response to oxidative conditions, NMDA-R function in PV-interneurons may remain blocked when the IL-6/Nox2 pathway is activated, and this could lead to the enduring changes we observed in the phenotype of PV-interneurons in adult animals (Behrens et al., 2007, 2008). The less oxidizing conditions produced by inactivation of the IL-6/Nox2 pathway upon drug washout would then slowly reverse these effects.
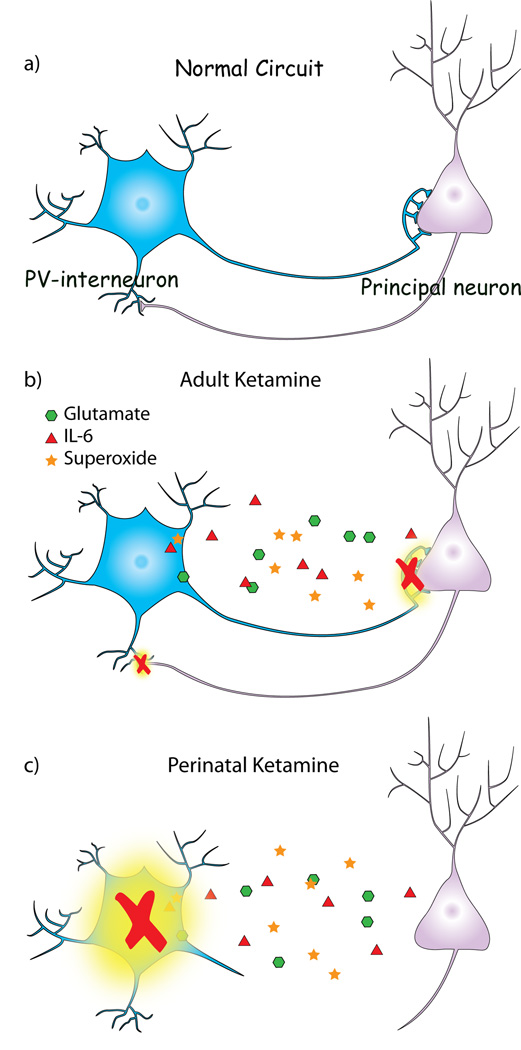
Under normal physiological conditions the brain maintains a physiological range of superoxide production that is required for normal nervous system function. Perturbations of superoxide levels in either direction are associated with impaired LTP, altered glutamatergic neurotransmission, and poorer cognitive performance (Kishida and Klann, 2007). Thus, it could be assumed that the effects of transient activation of the IL-6/Nox2 pathway may be a consequence of normal regulatory mechanisms in brain, where brief activation of the pathway does not lead to enduring effects on inhibitory circuits. Indeed, this is what is observed 24 h after one injection of NMDA-R antagonists (Figure 1a and Zuo et al., 2007; Behrens et al., 2008). Repetitive exposures in adulthood, however, through disinhibition-induced activation of the IL-6/Nox2 pathway induce an enduring, albeit reversible, dysfunction of the PV-interneuronal system (Figure 1b).
Figure 1. Schematic representation of the effects of activation of the IL-6/Nox2 pathway on the PV-interneuronal system in the adult and perinatal brain.
a) PV-interneuronal networks, through feedforward inhibition, control cortical output and generate oscillatory synchrony in the adult brain. b) In the adult brain, the initial disinhibition (increased glutamate) caused by repetitive exposures to sub-anesthetic concentrations of NMDA-R antagonists (i.e. ketamine) leads to the sustained activation of the IL-6/Nox2 pathway. The superoxide thus produced tips the redox balance in brain, leading to the reversible loss of GABAergic phenotype of PV-interneurons. c) If the activation of the IL-6/Nox2 pathway is triggered by repetitive exposure to NMDA-R antagonists during the maturation of PV-interneuronal networks, it produces irreversible loss of PV-interneurons and permanent dysfunction of the PV-interneuronal system in adulthood.
Although the effects of activation of the IL-6/Nox2 pathway appear to be reversible in the adult brain, similar exposures during the second postnatal week produce an irreversible loss of PV-interneurons (Wang et al., 2008 and our unpublished results). GAD67 expression is required for the correct development of PV-synaptic contacts during postnatal development (Chattopadhyaya et al., 2007) and NMDA-R antagonists decrease the expression of this enzyme in PV-interneurons (Kinney at el., 2006, Behrens et al., 2007, 2008). Thus, it is possible that the decreased expression of GAD67 caused by NMDA-R antagonists during the second week of postnatal development could halt the maturational of PV-interneurons, profoundly affecting the development of this critical inhibitory system (Figure 1c). This would impair the development of cortical networks involved in gamma frequency generation and synchrony, and could eventually lead to the cognitive dysfunctions observed in schizophrenia. These effects should be more pronounced in at risk individuals that show diminished antioxidant defenses, as suggested for schizophrenia patients carrying specific single nucleotide polymorphisms in genes coding for the enzymes involved in GSH synthesis (reviewed in Do et al., 2009).
Summary
The evidence reviewed here points toward a precipitating oxidative period early in the development of PV-interneurons that, after a cascade of compensatory changes to other neurons, may leave the cortex in a highly vulnerable state. This may account for the long delay before the symptoms of schizophrenia appear in early adulthood, when synaptic reorganization, hormonal changes and environmental stresses could tip the balance toward the dysregulation of cortical circuits (Figure 1). Only a few of the molecular mechanisms that might be responsible for this long chain of events have been uncovered so far. In particular the activation of Nox2 by IL-6 may trigger the oxidation of the NMDA-R on PV interneurons during the critical period of their maturation, leading to the permanent downregulation of GABAergic transmission by these interneurons. Critical experiments to test this hypothesis in vivo are underway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers
References
- Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utilizing reversible cryogenic depression. Brain Res. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Ba A, Seri BV. Psychomotor functions in developing rats: ontogenetic approach to structure-function relationships. Neurosci Biobehav Rev. 1995;19:413–425. doi: 10.1016/0149-7634(94)00042-y. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M. Maturation of medial temporal lobe memory functions in rodents, monkeys, and humans. Hippocampus. 1993;3:191–201. [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–355. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal ablation of NMDA receptors in corticolimbic interneurons leads to schizophrenia-like behavior. Soc. Neurosci. Abs. 2008 doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA. Oxidative stress in neurodegenerative disorders. Antioxid Redox Signal. 2006;8:1971–1973. doi: 10.1089/ars.2006.8.1971. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Preissmann D, Delseth C, Cuenod M, Do KQ, Schenk F. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol Dis. 2007;26:634–645. doi: 10.1016/j.nbd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009 doi: 10.1038/nature08002. 2009 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54(6):889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002;46:206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28:265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Lewis DA. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J Comp Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Dean O, Bush AI, Berk M, Copolov DL, van den Buuse M. Glutathione depletion in the brain disrupts short-term spatial memory in the Y-maze in rats and mice. Behav Brain Res. 2009;198:258–262. doi: 10.1016/j.bbr.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment and schizophrenia. Curr Op Neurobiol. 2009 doi: 10.1016/j.conb.2009.05.001. in press. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17(4):319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- Fejgin K, Palsson E, Wass C, Svensson L, Klamer D. Nitric oxide signaling in the medial prefrontal cortex is involved in the biochemical and behavioral effects of phencyclidine. Neuropsychopharmacology. 2008;33:1874–1883. doi: 10.1038/sj.npp.1301587. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Caron MG. Genetic animal models: focus on schizophrenia. Trends Neurosci. 2001;24:527–533. doi: 10.1016/s0166-2236(00)01886-5. [DOI] [PubMed] [Google Scholar]
- Ganguli R, Yang Z, Shurin G, Chengappa KN, Brar JS, Gubbi AV, Rabin BS. Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Res. 1994;51:1–10. doi: 10.1016/0165-1781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF. Exposure to infection and brain development: cytokines in the pathogenesis of schizophrenia. Schizophr Res. 1997;24:365–367. doi: 10.1016/s0920-9964(96)00123-5. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable AMPA and NMDA receptors to subthreshold Ca2+dynamics. J Physiol. 2003;551:67–78. doi: 10.1113/jphysiol.2003.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuenod M, Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herin GA, Aizenman E. Amino terminal domain regulation of NMDA receptor function. Eur J Pharmacol. 2004;500:101–111. doi: 10.1016/j.ejphar.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb HH, Lahti AC, Medoff DR, Cullen T, Tamminga CA. Effects of noncompetitive NMDA receptor blockade on anterior cingulate cerebral blood flow in volunteers with schizophrenia. Neuropsychopharmacology. 2005;30:2275–2282. doi: 10.1038/sj.npp.1300824. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JP, Rodriguiz RM, Mork A, Wetsel WC. Monoaminergic dysregulation in glutathione-deficient mice: possible relevance to schizophrenia? Neuroscience. 2005;132:1055–1072. doi: 10.1016/j.neuroscience.2005.01.059. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jiang D, Akopian G, Ho YS, Walsh JP, Andersen JK. Chronic brain oxidation in a glutathione peroxidase knockout mouse model results in increased resistance to induced epileptic seizures. Exp Neurol. 2000;164:257–268. doi: 10.1006/exnr.2000.7431. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Correlation of physiological subgroupings of nonpyramidal cells with parvalbumin- and calbindinD28k-immunoreactive neurons in layer V of rat frontal cortex. J Neurophysiol. 1993;70:387–396. doi: 10.1152/jn.1993.70.1.387. [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Nuechterlein KH, Braver TS, Barch DM. Executive functioning component mechanisms and schizophrenia. Biol Psychiatry. 2008;64:26–33. doi: 10.1016/j.biopsych.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer's drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Jr, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, Macdougall L, Abi-Saab W, D'Souza DC. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Takase H, Takahira Y, Katagai H, Takazawa T. Postoperative confusion in schizophrenic patients is affected by interleukin-6. J Clin Anesth. 2003;15:455–462. doi: 10.1016/j.jclinane.2003.03.008. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Casanova MF, Toti R, Weinberger DR, Kleinman JE. Selective abnormalities of prefrontal serotonergic receptors in schizophrenia. A postmortem study. Arch Gen Psychiatry. 1993;50:810–818. doi: 10.1001/archpsyc.1993.01820220066007. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuenod M, Buclin T, Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulate and retrosplenial cortices: a potential mechanism of neurotoxicity. J Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Kenis G, Bignotti S, Tura GJ, De Jong R, Bosmans E, Pioli R, Altamura C, Scharpe S, Maes M. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Takahashi H, Zhang D, Li W, Godzik A, Bankston LA. Cysteine regulation of protein function--as exemplified by NMDA-receptor modulation. Trends Neurosci. 2002;25:474–480. doi: 10.1016/s0166-2236(02)02245-2. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27:11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Mellon RD, Simone AF, Rappaport BA. Use of anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509–520. doi: 10.1213/01.ane.0000255729.96438.b0. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2007;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Middleton S, Jalics J, Kispersky T, Lebeau FE, Roopun AK, Kopell NJ, Whittington MA, Cunningham MO. NMDA receptor-dependent switching between different gamma rhythm-generating microcircuits in entorhinal cortex. Proc Natl Acad Sci U S A. 2008;105:18572–18577. doi: 10.1073/pnas.0809302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Morrow BA, Elsworth JD, Roth RH. Repeated phencyclidine in monkeys results in loss of parvalbumin-containing axo-axonic projections in the prefrontal cortex. Psychopharmacology (Berl) 2007;192:283–290. doi: 10.1007/s00213-007-0708-0. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Gruber R, Ackenheil M, Schwarz MJ. The immune system and schizophrenia. An integrative view. Ann N Y Acad Sci. 2000;917:456–467. doi: 10.1111/j.1749-6632.2000.tb05410.x. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Kumar M, Selvakumar B, Ho GP, Ehmsen JT, Barrow RK, Amzel LM, Snyder SH. Nitric oxide S-nitrosylates serine racemase, mediating feedback inhibition of D-serine formation. Proc Natl Acad Sci U S A. 2007;104:2950–2955. doi: 10.1073/pnas.0611620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudin J, Mege JL, Azorin JM, Dassa D. Elevated circulating levels of IL-6 in schizophrenia. Schizophr Res. 1996;20:269–273. doi: 10.1016/0920-9964(96)00014-x. [DOI] [PubMed] [Google Scholar]
- Nawa H, Takahashi M, Patterson PH. Cytokine and growth factor involvement in schizophrenia--support for the developmental model. Mol Psychiatry. 2000;5:594–603. doi: 10.1038/sj.mp.4000730. [DOI] [PubMed] [Google Scholar]
- Nudmamud S, Reynolds LM, Reynolds GP. N-acetylaspartate and N-Acetylaspartylglutamate deficits in superior temporal cortex in schizophrenia and bipolar disorder: a postmortem study. Biol Psychiatry. 2003;53:1138–1141. doi: 10.1016/s0006-3223(02)01742-0. [DOI] [PubMed] [Google Scholar]
- Nunes SO, Borelli SD, Matsuo T, Watanabe MA, Itano EN. The association of the HLA in patients with schizophrenia, schizoaffective disorder, and in their biological relatives. Schizophr Res. 2005;76:195–198. doi: 10.1016/j.schres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW. New insights and new issues in developmental neurotoxicology. Neurotoxicology. 2002;23:659–668. doi: 10.1016/S0161-813X(01)00092-4. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Papadia S, Soriano FX, Leveille F, Martel MA, Dakin KA, Hansen HH, Kaindl A, Sifringer M, Fowler J, Stefovska V, McKenzie G, Craigon M, Corriveau R, Ghazal P, Horsburgh K, Yankner BA, Wyllie DJ, Ikonomidou C, Hardingham GE. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat Neurosci. 2008;11:476–487. doi: 10.1038/nn2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.12.016. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Pileblad E, Magnus T, Fornstedt B. Reduction of brain glutathione by L-buthionine sulfoximine potentiates the dopamine-depleting action of 6-hydroxydopamine in rat striatum. J Neurochem. 1989;52:978–980. doi: 10.1111/j.1471-4159.1989.tb02550.x. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Copin JC, Ledig M, Tholey G. Modulation of oxygen-radical-scavenging enzymes by oxidative stress in primary cultures of rat astroglial cells. Dev Neurosci. 18:397–404. doi: 10.1159/000111433. 2996. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Haack M, Schuld A, Kraus T, Hinze-Selch D. Effects of antipsychotic drugs on cytokine networks. J Psychiatr Res. 2000;34:369–382. doi: 10.1016/s0022-3956(00)00032-7. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex--development and deficits in schizophrenia. J Chem Neuroanat. 2001;22:95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Cochran SM, Morris BJ, Pratt JA, Reynolds GP. Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartate and N-acetylaspartylglutamate in rat brain. Schizophr Res. 2005;73:147–152. doi: 10.1016/j.schres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MJ, Klyubin I, Wang Q, Anwyl R. Synaptic plasticity disruption by amyloid beta protein: modulation by potential Alzheimer's disease modifying therapies. Biochem Soc Trans. 2005;33:563–567. doi: 10.1042/BST0330563. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Moller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Lipton SA. Redox regulation of neuronal survival mediated by electrophilic compounds. Trends Neurosci. 2007;30:37–45. doi: 10.1016/j.tins.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Sirota P, Gavrieli R, Wolach B. Overproduction of neutrophil radical oxygen species correlates with negative symptoms in schizophrenic patients: parallel studies on neutrophil chemotaxis, superoxide production and bactericidal activity. Psychiatry Res. 2003;121:123–132. doi: 10.1016/s0165-1781(03)00222-1. [DOI] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009 doi: 10.1038/nature07991. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005a;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Systemic and prefrontal cortical NMDA receptor blockade differentially affect discrimination learning and set-shift ability in rats. Behav Neurosci. 2005b;119:420–428. doi: 10.1037/0735-7044.119.2.420. [DOI] [PubMed] [Google Scholar]
- Steullet P, Neijt HC, Cuenod M, Do KQ. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience. 2006;137:807–819. doi: 10.1016/j.neuroscience.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Steward LJ, Kennedy MD, Morris BJ, Pratt JA. The atypical antipsychotic drug clozapine enhances chronic PCP-induced regulation of prefrontal cortex 5-HT2A receptors. Neuropharmacology. 2004;47:527–537. doi: 10.1016/j.neuropharm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Stoet G, Snyder LH. Effects of the NMDA antagonist ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology. 2006;31:1675–1681. doi: 10.1038/sj.npp.1300930. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK-801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci. 2000;12:1420–1430. doi: 10.1046/j.1460-9568.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, Matthey ML, Parnas J, Preisig M, Saraga M, Solida A, Timm S, Wang AG, Werge T, Cuenod M, Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet. 2006;79:586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti D, Rizzini BL, Rossi D, Haugeto O, Racagni G, Danbolt NC, Volterra A. Neuronal and glial glutamate transporters possess an SH-based redox regulatory mechanism. Eur J Neurosci. 1997;9:1236–1243. doi: 10.1111/j.1460-9568.1997.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine modulation of prefrontal cortical interneruons changes during adolescence. Cereb Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0900390106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A, Trotti D, Floridi S, Racagni G. Reactive oxygen species inhibit high-affinity glutamate uptake: molecular mechanism and neuropathological implications. Ann N Y Acad Sci. 1994;738:153–162. doi: 10.1111/j.1749-6632.1994.tb21800.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg. 2008;106:1643–1658. doi: 10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, West JB, Bao J, Anastasio N, Guidry JA, Ye Y, Salvemini D, Johnson KM. Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J Pharmacol Exp Ther. 2003;304:266–271. doi: 10.1124/jpet.102.041798. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci U S A. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: Application of laser microdissection technique. J Neurosci Methods. 2009;176:172–181. doi: 10.1016/j.jneumeth.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Cai ZY, Thi LL, Kim-Han JS, Dugan LL, Covey DF, Rothman SM. The estrogen receptor is not essential for all estrogen neuroprotection: New evidence from a new analog. Neurobiol Dis. 2002;9:282–293. doi: 10.1006/nbdi.2002.0478. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YC. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res. 2002;57:247–258. doi: 10.1016/s0920-9964(01)00296-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo DY, Wu YL, Yao WX, Cao Y, Wu CF, Tanaka M. Effect of MK-801 and ketamine on hydroxyl radical generation in the posterior cingulate and retrosplenial cortex of free-moving mice, as determined by in vivo microdialysis. Pharmacol Biochem Behav. 2007;86:1–7. doi: 10.1016/j.pbb.2006.05.010. [DOI] [PubMed] [Google Scholar]