Abstract
In July of 2008, the National Heart, Lung, and Blood Institute convened experts in noninvasive cardiovascular imaging, outcomes research, statistics, and clinical trials to develop recommendations for future randomized controlled trials of the use of imaging in: 1) screening the asymptomatic patient for coronary artery disease; 2) assessment of patients with stable angina; 3) identification of acute coronary syndromes in the emergency room; and 4) assessment of heart failure patients with chronic coronary artery disease with reduced left ventricular ejection fraction. This study highlights several possible trial designs for each clinical situation.
Keywords: cardiovascular imaging, chest pain diagnosis, clinical trials
Cardiovascular imaging is a source of innovation and controversy for the health care community. Cardiologists and radiologists are now capable of obtaining high quality images that describe myocardial function and perfusion, define risk of major clinical events, and show coronary anatomy without need for invasive instrumentation (1). At the same time, there is concern that the rapid dissemination of cardiovascular imaging is a prime example of a costly technology that is enthusiastically embraced without appropriate supporting scientific evidence (2,3).
During the past 5 years, medical imaging has grown substantially, with Medicare Part B costs alone increasing from $6.89 billion in 2000 to $14.11 billion in 2005 (105%) of which an estimated one-third is cardiovascular (3,4). In addition, there is inconsistent use, with some areas of the country having utilization rates 10 times those of others (5). There is no clear explanation for the rapid growth; it cannot be ascribed entirely to aging of the population, changing disease rates, or improved outcomes (3,4). The “value” of imaging in terms of improved health outcomes or reduced cardiovascular events remains subjective, with limited evidence, often generated with flawed research methodology (6,7). There are also concerns that imaging can cause harm (8,9), that there are few rigorous regulatory controls, and that utilization is at least in part driven by self-referral (10) and, in some cases, even direct-to-consumer advertising (11).
A commonly cited model for efficacy in imaging describes 6 hierarchical tiers of evidence: 1) technical efficacy; 2) diagnostic accuracy; 3) diagnostic thinking; 4) therapeutic efficacy; 5) patient outcome; and 6) societal efficacy (12-14). A recently convened American College of Cardiology—Duke University think tank on imaging quality in cardiovascular medicine (15), noted that imaging research has primarily focused on diagnostic and prognostic accuracy, with little work directed at determining the direct impact of imaging on patient outcomes. As a result, among 745 recommendations for cardiovascular imaging in American College of Cardiology and American Heart Association guidelines, only 1% are based on Level of Evidence: A (16). In contrast, in cancer medicine, randomized trials have been completed or are under way for assessing the ability of imaging technologies to prevent major clinical events due to breast (17) or lung cancer (18).
Trial Design Considerations
Methodology
Though it may seem logical that diagnosing disease with “better” imaging tests will yield better outcomes, there are reasons why this may not be so. For example, some disease detected by sensitive technologies in fact reflects subclinical disease that if left alone would never become clinically manifest (19). This was discovered during large-scale studies of mass screening for neuroblastoma in children (20). Another unintended consequence of advanced imaging may be the detection of “nontarget” findings, such as noncalcified lung nodules, that may not have clinical relevance but require additional testing and/or procedures. Therefore, a number of scientists have argued that a preferred way to definitively determine whether or not any new diagnostic test improves outcomes is through properly designed randomized trials using clinical events as outcomes (21). However, there are a number of major methodological difficulties in designing and implementing randomized trials in which imaging tests themselves are the target of randomization (6). Effects, by definition, have to be indirect as tests do not directly affect clinical status. Instead we must presume that they lead clinicians and patients to modify behavior, which hopefully will lead to fewer clinical events.
Several issues represent important considerations when planning trials to determine if imaging can affect outcomes.
Comparison group
The initial consideration is whether one is testing a strategy of performing an imaging test versus not performing any imaging, or whether a comparison is desired between distinct imaging modalities. As an example of the latter design, 103 patients with chronic coronary artery disease (CAD) and left ventricular (LV) dysfunction being considered for revascularization (22) were randomized to either single-photon emission computed tomography (SPECT), myocardial perfusion imaging (MPI) or positron emission tomography (PET) for determination of viability. The imaging information was provided to clinicians for decision making blinded with regard to the imaging modality (with polar maps showing areas of ischemia, infarction, and the like) and patients were followed for 2- to 3-year outcomes. There was no difference in event-free survival between the 2 groups, suggesting that the use of either imaging modality to inform revascularization decisions results in similar outcomes. An ongoing study that represents the “imaging versus no imaging” approach is the WOMEN (What is the Optimal Method for Ischemia Evaluation in WomeN?) study, in which women with suspected CAD are randomized to an initial evaluation strategy of SPECT MPI versus an initial exercise electrocardiography (ECG) testing strategy, with the end point of 2-year negative predictive value for outcome events (23). These studies demonstrate that it is feasible to subject imaging modalities to the same rigorous comparisons that are standard for therapeutics.
End points
An area of substantial uncertainty in the evaluation of imaging outcomes is the appropriate end points for use in trials. Ideally, end points would involve important natural history outcomes such as death, cardiac death or composites of cardiac death, and nonfatal cardiovascular events including myocardial infarction (MI). However, the many decisions made “downstream” from the imaging results have a highly significant effect on outcomes, such that the imaging results themselves are only 1 of many influences on outcomes, and thus challenging to isolate. This has led to considerations of other end points occurring over a shorter time horizon, including such metrics as cost-to-diagnosis, cost-to-predict event, cost-to-prevent nonfatal events, and behavior change with risk factor modification.
Efficacy versus effectiveness
Efficacy refers to the performance characteristics of a test under ideal conditions performed and interpreted by experts. Effectiveness refers to test performance under “real-life” conditions (24). An efficacious test does not necessarily translate into an effective test, and ideally imaging modalities would be subject to both types of analysis. Stowers et al. (25) reported SPECT imaging efficacy in a small study of 46 emergency department (ED) patients randomized to resting SPECT perfusion imaging or conventional clinical strategy. Length of stay and costs were lower in the imaging strategy arm. Effectiveness of rest perfusion imaging was studied in the ERASE Chest Pain (Emergency Room Assessment of Sestamibi for the Evaluation of Chest Pain) trial, in which over 2,500 patients were randomized to an initial ED evaluation strategy of resting SPECT perfusion imaging, in addition to standard testing, or to a nonimaging standard evaluation strategy (26). The results demonstrated a reduction in unnecessary hospital admissions associated with the imaging strategy, suggesting significant effectiveness of imaging in this situation.
The NHLBI Workshop on Imaging Outcomes Research
The National Heart, Lung, and Blood Institute (NHLBI) recently released its strategic plan for “Shaping the Future of Research” (27). The importance of optimizing diagnostic tests for improving outcomes is explicitly recognized in the plan, which states that “research is needed to evaluate the extent to which risk stratification and application of personalized approaches can improve effectiveness” (Challenge 3.1.a); that “studies are needed to reduce the inappropriate used of diagnostic tests and treatments” (Challenge 3.1.c); and that there is a need to “evaluate the risks, benefits, and costs of diagnostic tests and treatments in representative populations and settings” (Challenge 3.2.a).
Therefore, on July 21 and July 22, 2008, the NHLBI convened experts in noninvasive cardiovascular imaging, outcomes research, statistics, and clinical trials to develop a vision for imaging research that transcends current reliance on diagnostic and prognostic end points to a new paradigm that focuses on preventive and therapeutic value, where value implies an improved clinical outcome and/or reduced costs. The panel was specifically charged to develop a set of recommendations for future analyses and possible research funding by NHLBI, including sample trial designs for 4 pre-defined clinical scenarios commonly encountered in clinical practice. The 4 scenarios were: 1) screening the asymptomatic patient for CAD; 2) assessment of stable angina; 3) identification of acute coronary syndromes in the emergency room; and 4) assessment of heart failure patients with chronic CAD with reduced LV ejection fraction. The panel was asked to identify need, assess feasibility, and determine 1 to 2 examples of possible trial concepts for each scenario. Given the time limitations, it was recognized that these trial overviews would subsequently require substantial statistical and logistical analysis to become formal, detailed, and actionable trial designs.
Screening the Asymptomatic Patient for CAD
Forty years ago, the World Health Organization (28) first published principles around which screening programs can be justified (Table 1), and many of these principles also apply to vascular diseases such as CAD. Screening for abdominal aortic aneurysm is now an accepted practice for some patient groups based on multiple randomized controlled trials (29-31). However, there are also a number of unknowns that have blunted enthusiasm for screening for CAD (32,33). Controversy has arisen regarding whether imaging-based risk classification improves selection of patients for treatments and whether outcomes after screening are improved compared with traditional risk factor measurements and risk-factor based treatments (34-36). It is also unclear from existing data which patients to screen and how frequently to perform screening tests.
Table 1.
World Health Organization Criteria for Screening
|
Cohort studies using coronary calcium measurement have shown the ability of cardiac computed tomography (CT) to identify high-risk asymptomatic patients (37). For example, the MESA (Multi-Ethnic Study of Atherosclerosis) found that coronary calcium (CAC) scores were strongly and incrementally (compared with Framingham risk score [FRS]) associated with clinical vascular outcomes in 45-to 84-year-old subjects (38). Compared with CAC scores equal to 0, a CAC score of >300 was associated with a >6-fold higher odds of a major coronary event and a >9-fold higher odds of any CAD event. Consensus panels (37,39) have concluded that CAC scores are capable of stratifying patients into low-, intermediate-, and high-risk groupings. Similar predictive information has been published regarding carotid intima-media thickness measurements (40), although the MESA trial suggested that CAC is a stronger predictor of cardiovascular events (41). Thus, the use of CAC might be the preferred imaging strategy, independent of other considerations such as cost, availability, or impact of incidental scan findings, as a single imaging test for screening purposes.
Despite the demonstrated predictive value of CAC and intima-media thickness, enthusiasm among consensus panels for routine screening is limited (32-34), in part because of the absence of clinical trials data (34,42,43). The possibility that screening can cause harm in the form of radiation exposure (for CAC) and false reassurance for people with high risk factor scores but low levels of anatomic disease are often mentioned as reasons for caution in the adoption of screening using imaging tests (34,42,43). However, others have suggested that such data are absent for many other forms of screening and that trials of this sort are expensive and unlikely to be undertaken (35,36). In the absence of better outcomes data for use of an imaging strategy for screening and risk assessment, the controversy between screening advocates and screening detractors cannot be easily resolved.
Sample trial designs
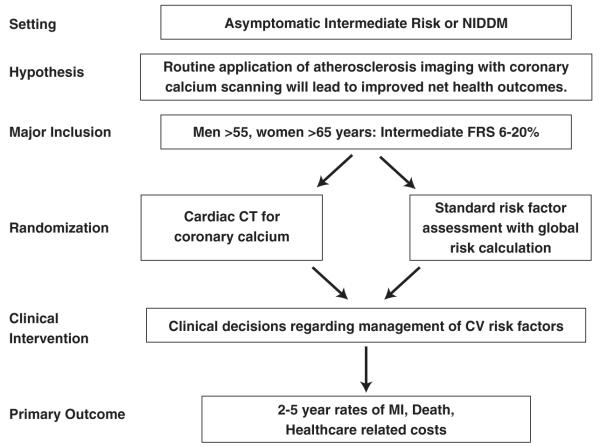
Workshop participants considered several sample study overviews designed to reach more definitive conclusions on the roles of imaging tests for cardiovascular screening. The first design was an effectiveness study of asymptomatic men and women with intermediate FRS (Fig. 1). The hypothesis was that CAC testing will improve risk stratification resulting in improved risk factor modification and leading to both reduced events and lower costs. Patients would be randomized to receive an invitation for coronary calcium testing versus no invitation for CAC testing. All patients would receive an individualized risk assessment and associated risk interpretation including FRS, which would be provided to all patients and to their doctors for subsequent treatment without specific guidance.
Figure 1. Sample Trial Design for Imaging in Asymptomatic Patients.
CT = computed tomography; CV = cardiovascular; FRS = Framingham risk score; MI = myocardial infarction; NIDDM = non insulin-dependent diabetes mellitus.
Inclusion criteria would be asymptomatic individuals with intermediate FRS (>6% but <20%) and without known CAD, cardiovascular disease, peripheral artery disease, or renal disease. The primary outcome would be a combined end point consisting of major clinical events (MI, stroke, congenital heart disease [CHD] death). Major secondary end points would include total health care costs estimated from hospitalization and doctor and ED visits, medications, additional tests, quality of life measurements, behavior changes after testing, cardiovascular drug use, risk factor changes, clinically indicated coronary revascularization, and other CHD events. The workshop discussants proposed a 10% to 20% reduction in major cardiovascular disease end points as study design goals. Similar trials in different populations, such as in asymptomatic type 2 diabetes mellitus, commonly considered a CHD risk equivalent, were also considered.
To address concerns that reliance on usual physician care may increase the likelihood of a negative result, a fully managed trial testing the efficacy of a guideline-based treatment approach versus a CAC plus risk factor-based approach to individualized therapy of cardiovascular risk was proposed. The trial would have similar inclusion and exclusion criteria and end points as already discussed.
Assessment of Stable Angina
Evaluation of imaging modalities used in stable angina patients
Imaging modalities for use in patients with suspected or known CAD have generally been evaluated on the basis of accuracy for the detection of angiographic CAD. All of the contemporary imaging modalities—SPECT or PET MPI, stress echocardiography, cardiac computed tomographic angiography (CCTA), and cardiac magnetic resonance (CMR)—perform to a clinically acceptable standard. The next tier of evaluation focusing on prognostic or risk-stratification studies has generally demonstrated that greater abnormalities on SPECT MPI and stress echo imaging are associated with a higher risk of an incident cardiovascular event during follow-up (44,45), documenting the “incremental value” of the imaging data over previously available and less expensive to obtain clinical or stress ECG information (46-48). Only a very few imaging randomized controlled studies have been performed to date of the kind that might be considered to constitute “higher level evidence” from the prism of therapeutic trials (26); however, these demonstrate that it is feasible to subject imaging modalities to the same rigorous analysis that is standard for therapeutics.
Sample trial designs
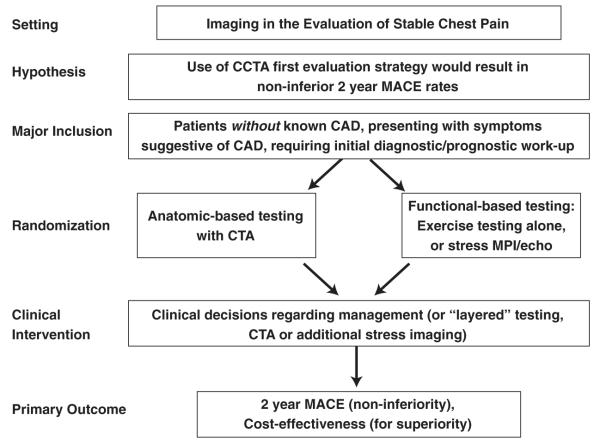
In patients without known CAD who present with symptoms suggestive of CAD and requiring initial diagnostic/prognostic work-up, workshop participants proposed a trial design randomizing patients to an initial CCTA strategy as compared with an initial functional-based testing strategy (Fig. 2). The primary hypotheses were that CCTA would result in non-inferior 12-month major adverse cardiovascular event rates and would be cost-efficient. Secondary end points could include rates of invasive angiography, effective biological radiation dosages received by patients, cost-effectiveness in the low likelihood group (hypothesizing that CCTA is more cost-effective), and cost-effectiveness in the high likelihood group (hypothesizing that functional imaging is more cost-effective).
Figure 2. Sample Trial Design for Imaging in the Evaluation of Patients With Stable Chest Pain.
CAD = coronary artery disease; CCTA = cardiac computed tomographic angiography; MACE = major adverse cardiac events; MPI = myocardial perfusion imaging.
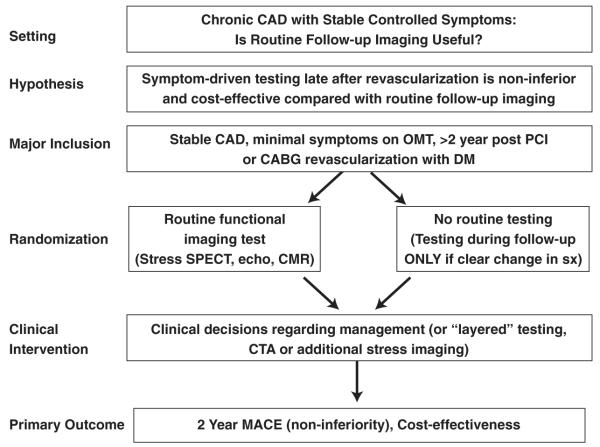
To address the impact of imaging in clinically stable patients with known CAD and previous myocardial revascularization, workshop participants proposed randomizing clinically stable patients >2 years after revascularization to either routine late “screening” for recurrent ischemia with stress imaging versus symptom-driven testing (Fig. 3). The hypothesis would be that periodic imaging after revascularization is non-inferior for major adverse cardiac events and cost-effective. For patients randomized to the initial imaging strategy group (including any functional imaging test such as SPECT MPI, PET MPI, stress echo, stress CMR), the results would be provided to their physicians to act on as they see fit. Secondary end points could evaluate the “yield” of routine late post-revascularization stress functional imaging, the clinical predictors of a positive test (to potentially enhance the yield of imaging), and the influence of time from revascularization.
Figure 3. Sample Trial Design for Imaging in the Evaluation of Patients With Stable CAD.
CABG = coronary artery bypass graft; CMR = cardiac magnetic resonance; DM = diabetes mellitus; OMT = optical medical therapy; SPECT = single-photon emission computed tomography; sx = symptoms; other abbreviations as in Figure 2.
Diagnosis of Chest Pain in the Emergency Room
Emergency department visits in the U.S. for suspected acute coronary syndrome patients exceed 10 million individuals (8%), 6.24 million of whom undergo a fairly extensive evaluation. Of the latter, 50% are ultimately determined to have a noncardiac diagnosis. Unfortunately, current technology is often inadequate to differentiate the roughly 85% of patients with noncardiac problems from the small minority with an acute cardiovascular disease presentation (49). Risk factors (50), risk scores (e.g., Thrombolysis In Myocardial Infarction [TIMI]) (51), the physical exam, chest radiography, and even the arrival ECG are nondiagnostic in 98% of patients (49), and even interpretation of the patient’s symptoms is constrained by language barriers, recall quality, and the fact that as many as one-third of confirmed MI patients do not have chest pain (52).
Given far greater risk associated with an inappropriate discharge as compared with additional diagnostic testing or hospitalization, test sensitivity is critical. Highly specific testing, though valuable when positive, may be inadequate for safe discharge. Unfortunately, currently available biomarker tests have high specificity but sensitivity as low as 10% (53), although a “chest pain center” strategy of serial markers and selective stress testing decreases mortality and increases discharges by 37% and 36%, respectively, compared with usual care (54). Thus, use of this model has sky rocketed (55-59), despite tremendous cost, average length of hospitalization of 17 h, and great inconvenience to the patient. Adding the use of imaging technology to usual care may improve the system, but prospective data are sparse. This limitation has resulted in vague guideline statements that suggest “the potential benefit of noninvasive coronary angiography is likely to be greatest in symptomatic patients who are at intermediate risk for coronary artery disease after initial risk stratification” (61). Future research is required before general use of ED imaging can be adopted.
Sample trial design
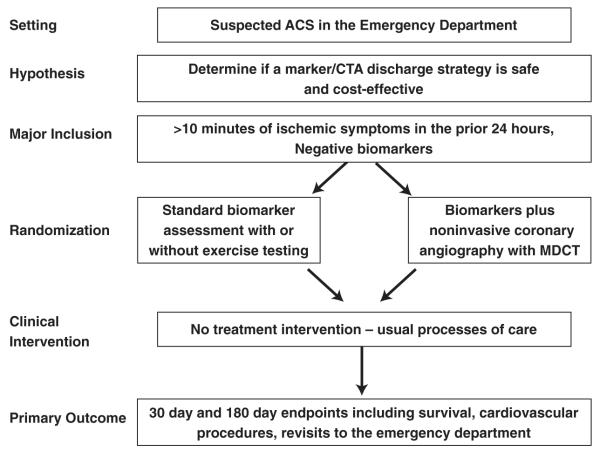
In patients presenting with symptoms suggestive of acute coronary syndromes, workshop participants proposed randomizing patients to an initial cardiac marker and CCTA strategy as compared with usual care (Fig. 4). The primary hypothesis would be that use of a biomarker plus CTA discharge strategy is safe and effective compared with the present standard of care. Eligible patients would be those presenting to the ED with ischemic symptoms and negative cardiac marker determinations. Major exclusion criteria would include cardiac catheterization indications (diagnostic ECG changes or local positive troponin) and CCTA exclusions (reduced renal function, known CAD, or significant arrhythmia). The primary end point would be a combination (major adverse cardiac events) of death, coronary revascularization intervention, or of heart failure. Secondary end points could include additional clinical outcomes and resource implications of a marker/CTA strategy such as ED process time, time to accurate diagnosis, rates of noncardiac diagnoses, and percutaneous coronary interventions (PCI) performed as a consequence of strategy used, dye load and complications, radiation exposure, patient satisfaction, and revisits.
Figure 4. Sample Trial Design for Imaging in the Evaluation of Patients With Acute Chest Pain in the Emergency Department.
ACS = acute coronary syndromes; other abbreviations as in Figure 2.
Assessment of Heart Failure Patients With Chronic CAD With Reduced Ejection Fraction
Imaging plays several important roles in the current management of patients with LV systolic dysfunction including: to assess its severity, to identify those with underlying CAD, to determine the extent and severity of myocardial ischemia, and to identify the magnitude of dysfunctional but viable myocardium. Only LV ejection fraction has been studied in prospective randomized clinical trials. Among the many candidate clinical trials in patients with ischemic LV dysfunction discussed at this workshop, the assessment of myocardial viability and the role of imaging in ischemic mitral regurgitation (MR) were selected for consideration, as these 2 topics have both clinical need and potential public health impact.
Myocardial viability
Numerous studies have demonstrated the potential of PET, SPECT, dobutamine echo, and contrast-enhanced CMR to identify viable myocardium in patients with CAD and LV dysfunction, and to predict recovery of LV function following percutaneous (PCI) or surgical (coronary artery bypass graft [CABG]) revascularization (61-63), as well as improved survival and symptomatic status compared with the results of medical therapy (62-64). However, these studies were all retrospective in nature, often with treatment biases based on the results of the imaging tests, and the medically treated patients often did not receive aggressive evidence-based medical management.
Even the ongoing NHLBI-funded STICH (Surgical Treatment for Ischemic Heart Failure) trial (65) will leave unresolved a number of important questions as patients were randomized to revascularization versus aggressive medical management in patients with ischemic LV dysfunction independent of imaging results. Further studies are needed to address whether an imaging strategy is useful in actually guiding management decisions in patients with ischemic LV dysfunction.
Sample trial design
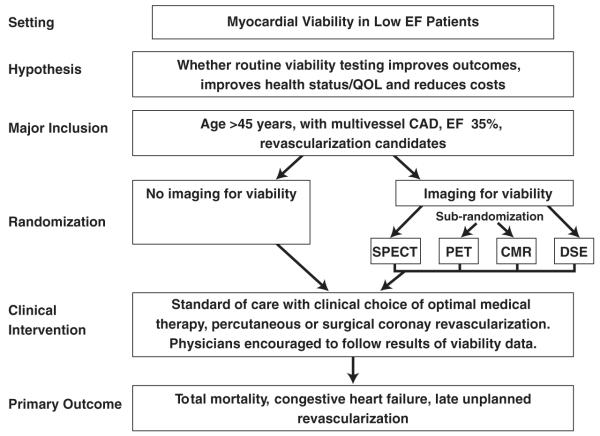
A possible trial in this area could test the effectiveness of routine viability imaging versus nonimaging strategy in the management of patients with symptomatic heart failure and multivessel CAD by improving outcomes, health status, and/or quality of life and reducing cost. Following angiography, patients with 2- or 3-vessel CAD and ejection fraction 35% or less would be randomized to standard of care therapy (optimal medical therapy or PCI or CABG) without imaging or to a strategy of imaging followed by standard of care (Fig. 5). Ideally, a second randomization would be performed within the imaging arm, in which patients would be randomized to one of 4 imaging strategies—PET, SPECT, dobutamine stress echo, or CMR—to determine the relative effect of each of these tests. Patient care would be determined at the discretion of the treating physician, but physicians are encouraged to follow the results of the viability data in patients randomized to imaging. The clinical end points could include cardiovascular mortality and cardiac readmissions for MI, unstable angina, heart failure, and late revascularization (excluding planned PCI or CABG based on initial testing).
Figure 5. Sample Trial Design for Imaging in the Evaluation of Patients With Low EF.
EF = ejection fraction; PET = positron emission tomography; QOL = quality of life; other abbreviations as in Figures 2 and 3.
Ischemic MR
Patients with ischemic cardiomyopathy who have MR have a worse outcome in terms of mortality, development of heart failure, and hospitalization than patients without MR (66,67). In this situation, MR develops secondary to LV dysfunction with dilation and displacement of the papillary muscles, mitral annular dilation and tethering of the mitral valve leaflets (68). It is unclear whether the resulting “functional” MR is merely a marker of a greater degree of LV dysfunction or whether it contributes actively to progression of LV dysfunction. It is also unclear whether surgery to repair or replace the mitral valve leads to a better outcome (69,70), or whether mild to moderate MR should be repaired at the time of CABG.
Possible trial design
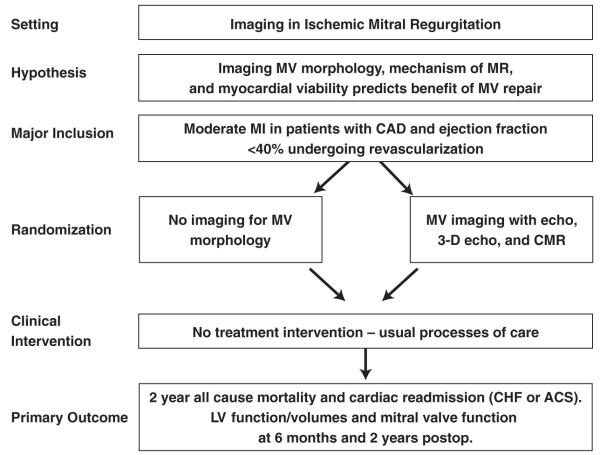
This clinical trial proposal involves using the infrastructure developed by the NHLBI Cardiothoracic Surgery Clinical Research Network to assess whether mild to moderate MR should be repaired at the time of CABG in patients with LV dysfunction and could include 3-dimensional echocardiography and CMR as part of the prospective evaluation of patients enrolled in such studies. This study would examine the importance of imaging in identifying which patients with ischemic MR benefit from mitral valve repair at the time of CABG through follow-up echocardiography and CMR at 6 months and 2 years after surgery (Fig. 6). The study would also examine whether and how imaging influences operative decision making and outcomes of all-cause mortality and hospital readmissions, including whether imaging is helpful in determining which patients will benefit.
Figure 6. Sample Trial Design for Imaging of Patients With Ischemic MR.
CHF = congestive heart failure; LV = left ventricular; MR = mitral regurgitation; MV = mitral valve; other abbreviations as in Figures 1 and 2.
Common Themes and Concerns
The sample trials considered by the workshop share some common themes and limitations that, taken together, provide a practical lesson in how to think about outcomes research in imaging. Most trial designs focused on real-world populations and were large practical trials intended to assess effectiveness and not efficacy. All but 1 specified the use of “usual care” in which decisions regarding further testing or therapeutic intervention were left to the patients’ care team following randomization. In other words, the trials did not specify how physicians were to act upon imaging results. Some were based on randomization to the use of imaging or not and others randomized patients between imaging strategies.
In general, the sample trial designs advocated for use of “hard end points” such as death or myocardial infarction over at least a 1-year period for primary end points, rather than relying on softer outcomes such as clinical worsening or use of medications. Most also included a broad variety of secondary end points such as radiation exposure, assessment of quality of life, behavior change with risk factor modification, and economic analyses, including such metrics as cost-to-diagnosis, cost-to-predict event, and cost-to-prevent MI. Motivation for these additional metrics included the wish to incorporate end points that may be less challenging to develop over a shorter time horizon, as well as interest in the variables themselves. They also reflect the broad range of concerns around imaging use.
Many limitations common to the sample trials are noted. The trial designs were not subject to rigorous evaluation of feasibility, in part due to acknowledged time constraints during the workshop and lack of analytic expertise needed to construct detailed clinical protocols, but also by intent so as not to limit creativity. These challenges also extend beyond the time constraints of the workshop format as it is difficult to properly estimate a sample size in the absence of reliable community-based data on prevalence of disease, test performance, end point occurrence, effect size, and cross over rates, among other concerns. It is possible that initial pilot studies or simulations may be helpful in more detailed planning.
Another concern was the duration of time required to perform such studies, especially related to the rapid pace of technologic change, and whether the results would still be relevant at the time of trial completion. Finally, all of the sample trials would be “large” and “expensive” trials and that the cost of even 1 such trial would be quite high, perhaps even prohibitive, an especially important consideration for NHLBI, as the convener of the conference and for any future Request For Applications (RFA) that might arise as a result of the workshop deliberations. Several alternative solutions in addition to conventional federal funding were discussed including pooling resources from the private sector (industry, payers) with National Institutes of Health funds, using only clinically indicated (and therefore “covered”) testing or creating other incentives for enrollment that might mitigate this concern. Other strategies proposed to minimize costs included combining the emergency room and stable angina trials, with identical end points to allow pooling of data, and administrative approaches to achieve economies of scale such as using a single coordinating center and using common sites and/or data collection forms for several trials.
Because such practical considerations will be critical going forward, and many of these would require additional thought by multiple stakeholders, an Imaging Outcomes Consortium was proposed to facilitate further, indepth exploration of these strategies. Such a consortium could also be used to further review trial proposals developed at the workshop, engage key stakeholders, conduct large or smaller trials sub-studies or registries, and provide ongoing oversight and support to the emerging outcomes research standard for imaging.
Summary
Given that Medicare spends over $14 billion per year on Part B imaging alone, about one-third of which is cardiovascular imaging (4), it is imperative that a robust effort be made to understand the scientific basis for the use of imaging and its contribution to the nation’s health. Fortunately the research paradigm regarding imaging is changing, with growing recognition that there is both urgent need and great opportunity in this area (6,15). Future imaging trials must address actual patient outcomes, instead of sensitivity/specificity and prognostic value. The workshop deliberations, as summarized in this proceedings document, amply demonstrate both a commitment on the part of multiple stakeholders to this goal and a shared belief that this is feasible and timely. There is much work remaining to be done, from creating more detailed and practical trial designs to determining sources of funding. It is hoped that, in the near future, clinicians ordering cardiovascular imaging tests will have a clear idea of their value in improving the health of their patients.
Supplementary Material
Footnotes
APPENDIX For a list of workshop participants, please see the online version of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Di Carli M, Hachamovitch R. New technology for noninvasive evaluation of coronary artery disease. Circulation. 2007;115:1464–80. doi: 10.1161/CIRCULATIONAHA.106.629808. [DOI] [PubMed] [Google Scholar]
- 2.Demaria AN. The growth of diagnostic imaging services. J Am Coll Cardiol. 2005;45:2093–4. doi: 10.1016/j.jacc.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Lucas FL, DeLorenzo MA, Siewers AE, Wennberg DE. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113:374–9. doi: 10.1161/CIRCULATIONAHA.105.560433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Government Accountability Office [Accessed August 16, 2008];Medicare Part B Imaging Services: Rapid Spending Growth and Shift to Physician Offices Indicate Need for CMS to Consider Additional Management Practices. Available at: http://www/gao.gov/news.items/d08452.pdf.
- 5.Wennberg DE, Kellett MA, Dickens JD, Malenka DJ, Keilson LM, Keller RB. The association between local diagnostic testing intensity and invasive cardiac procedures. JAMA. 1996;275:1161–4. [PubMed] [Google Scholar]
- 6.Shah BR, Patel MR, Peterson ED, Douglas PS. Defining optimal research study design for cardiovascular imaging using computed tomography angiography as a model. Am J Cardiol. 2008;102:943–8. doi: 10.1016/j.amjcard.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 7.Redberg RF. Computed tomographic angiography: more than just a pretty picture? J Am Coll Cardiol. 2007;49:1827–9. doi: 10.1016/j.jacc.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007;298:317–23. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 9.Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 10.Levin DC, Rao VM. Turf wars in radiology: updated evidence on the relationship between self-referral and the overutilization of imaging. J Am Coll Radiol. 2008;5:806–10. doi: 10.1016/j.jacr.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Lee TH, Brennan TA. Direct-to-consumer marketing of high-technology screening tests. N Engl J Med. 2002;346:529–31. doi: 10.1056/NEJM200202143460715. [DOI] [PubMed] [Google Scholar]
- 12.Gazelle GS, McMahon PM, Siebert U, Beinfeld MT. Cost-effectiveness analysis in the assessment of diagnostic imaging technologies. Radiology. 2005;235:361–70. doi: 10.1148/radiol.2352040330. [DOI] [PubMed] [Google Scholar]
- 13.Soman P, Udelson JE. The price for probability: comparing the costs of diagnostic testing strategies. J Nucl Cardiol. 2007;14:142–4. doi: 10.1016/j.nuclcard.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 14.Fryback DG, Thornbury JR. The efficacy of diagnostic imaging. Med Decis Making. 1991;11:88–94. doi: 10.1177/0272989X9101100203. [DOI] [PubMed] [Google Scholar]
- 15.Douglas PS, Iskandrian AE, Krumholz HM, et al. Achieving quality in cardiovascular imaging: proceedings from the American College of Cardiology-Duke University Medical Center Think Tank on Quality in Cardiovascular Imaging. J Am Coll Cardiol. 2006;48:2141–51. doi: 10.1016/j.jacc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 16.Douglas PS. Improving imaging: our professional imperative. J Am Coll Cardiol. 2006:2152–3. doi: 10.1016/j.jacc.2006.04.107. [DOI] [PubMed] [Google Scholar]
- 17.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359:909–19. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 18.Marshall E. Medicine. A bruising battle over lung scans. Science. 2008;320:600–3. doi: 10.1126/science.320.5876.600. [DOI] [PubMed] [Google Scholar]
- 19.Patz EF, Jr., Goodman PC, Bepler G. Screening for lung cancer. N Engl J Med. 2000;343:1627–33. doi: 10.1056/NEJM200011303432208. [DOI] [PubMed] [Google Scholar]
- 20.Schilling FH, Spix C, Berthold F, et al. Neuroblastoma screening at one year of age. N Engl J Med. 2002;346:1047–53. doi: 10.1056/NEJMoa012277. [DOI] [PubMed] [Google Scholar]
- 21.Lord SJ, Irwig L, Simes RJ. When is measuring sensitivity and specificity sufficient to evaluate a diagnostic test, and when do we need randomized trials? Ann Intern Med. 2006;144:850–5. doi: 10.7326/0003-4819-144-11-200606060-00011. [DOI] [PubMed] [Google Scholar]
- 22.Siebelink HM, Blanksma PK, Crijns HJ, et al. No difference in cardiac event-free survival between positron emission tomography-guided and single-photon emission computed tomography-guided patient management: a prospective, randomized comparison of patients with suspicion of jeopardized myocardium. J Am Coll Cardiol. 2001;37:81–8. doi: 10.1016/s0735-1097(00)01087-1. [DOI] [PubMed] [Google Scholar]
- 23.The WOMEN Study [Accessed August 24, 2008];What is the Optimal Method for Ischemia Evaluation in WomeN [TCT00282711] Available at: www.clinicaltrials.gov.
- 24.Nallamothu BK, Hayward RA, Bates ER. Beyond the randomized clinical trial: the role of effectiveness studies in evaluating cardiovascular therapies. Circulation. 2008;118:1294–303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 25.Stowers SA, Eisenstein EL, Wackers FJ Th, et al. An economic analysis of an aggressive diagnostic strategy with single photon emission computed tomography myocardial perfusion imaging and early exercise stress testing in emergency department patients who present with chest pain but nondiagnostic electrocardiograms: results from a randomized trial. Ann Emerg Med. 2000;35:17–25. doi: 10.1016/S0196-0644(00)70100-4. [DOI] [PubMed] [Google Scholar]
- 26.Udelson JE, Beshansky JR, Ballin DS, et al. Myocardial perfusion imaging for evaluation and triage of patients with suspected acute cardiac ischemia: a randomized controlled trial. JAMA. 2002;288:2693–700. doi: 10.1001/jama.288.21.2693. [DOI] [PubMed] [Google Scholar]
- 27.National Heart, Lung, and Blood Institute [Accessed May 11, 2009];Shaping the future of research. Available at: http://apps.nhlbi.nih.gov/strategicplan/StrategicPlan_Appendix.pdf.
- 28.Wilson JM. The evaluation of the worth of early disease detection. J R Coll Gen Pract. 1968;16(Suppl 2):48–57. [PMC free article] [PubMed] [Google Scholar]
- 29.Multicentre Aneurysm Screening Study Group The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet. 2002;360:1531–9. doi: 10.1016/s0140-6736(02)11522-4. [DOI] [PubMed] [Google Scholar]
- 30.Scott R, Ashton H, Kay D. Routine ultrasound screening in management of abdominal aortic aneurysms. Br Med J. 1988;296:1709–10. doi: 10.1136/bmj.296.6638.1709-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott R, Bridgewater S, Ashton H. Randomized clinical trial of screening for abdominal aortic aneurysm in women. Br J Surg. 2002;89:283–5. doi: 10.1046/j.0007-1323.2001.02014.x. [DOI] [PubMed] [Google Scholar]
- 32.Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–501. doi: 10.1161/CIRCULATIONAHA.107.181546. [DOI] [PubMed] [Google Scholar]
- 33.Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G. The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review. Health Technol Assess. 2006;10:iii–iv. ix–x, 1–41. doi: 10.3310/hta10390. [DOI] [PubMed] [Google Scholar]
- 34.Greenland P, Lloyd-Jones D. Defining a rational approach to screening for cardiovascular risk in asymptomatic patients. J Am Coll Cardiol. 2008;52:330–2. doi: 10.1016/j.jacc.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Cohn JN, Duprez DA. Time to foster a rational approach to preventing cardiovascular morbid events. J Am Coll Cardiol. 2008;52:327–9. doi: 10.1016/j.jacc.2008.02.085. [DOI] [PubMed] [Google Scholar]
- 36.Naghavi M, Falk E, Hecht HS, et al. From vulnerable plaque to vulnerable patient part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Greenland P, Bonow RO, Brundage BH, et al. American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) Society of Atherosclerosis Imaging and Prevention. Society of Cardiovascular Computed Tomography ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. on behalf of. [DOI] [PubMed] [Google Scholar]
- 38.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 39.Budoff MJ, Achenbach S, Blumenthal RS, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–91. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 40.Stein JH, Korcarz CE, Hurst RT, et al. American Society of Echocardiography Carotid Intima-Media Thickness Task Force Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. on behalf of. [DOI] [PubMed] [Google Scholar]
- 41.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168:1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lauer MS. Screening for coronary heart disease: has the time for universal imaging arrived? Cleve Clin J Med. 2007;74:645–56. doi: 10.3949/ccjm.74.9.645. [DOI] [PubMed] [Google Scholar]
- 43.Wald NJ. Screening: a step too far. A matter of concern. J Med Screen. 2007;14:163–4. doi: 10.1258/096914107782912040. [DOI] [PubMed] [Google Scholar]
- 44.Klocke FJ, Baird MG, Lorell BH, et al. American College of Cardiology. American Heart Association Task Force on Practice Guidelines. American Society for Nuclear Cardiology ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging— executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging) J Am Coll Cardiolg. 2003;42:1318–33. doi: 10.1016/j.jacc.2003.08.011. on behalf of. [DOI] [PubMed] [Google Scholar]
- 45.Cheitlin MD, Alpert JS, Armstrong WF, et al. ACC/AHA guidelines for the clinical application of echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. J Am Coll Cardiol. 1997;29:862–79. doi: 10.1016/s0735-1097(96)90000-5. [DOI] [PubMed] [Google Scholar]
- 46.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–14. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 47.Gibbons RJ, Hodge DO, Berman DS, et al. Long-term outcome of patients with intermediate-risk exercise electrocardiograms who do not have myocardial perfusion defects on radionuclide imaging. Circulation. 1999;100:2140–5. doi: 10.1161/01.cir.100.21.2140. [DOI] [PubMed] [Google Scholar]
- 48.Bangalore S, Gopinath D, Yao SS, Chaudhry FA. Risk stratification using stress echocardiography: incremental prognostic value over historic, clinical, and stress electrocardiographic variables across a wide spectrum of Bayesian pretest probabilities for coronary artery disease. J Am Soc Echocardiogr. 2007;20:244–52. doi: 10.1016/j.echo.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342:1163–70. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 50.Han JH, Lindsell CJ, Storrow AB, et al. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med. 2007;49:145–52. 152, e1. doi: 10.1016/j.annemergmed.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 51.Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 52.Canto JG, Shlipak MG, Rogers WJ, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283:3223–9. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 53.Peacock WI, Emerman CL, McErlean ES, et al. Prediction of short- and long-term outcomes by troponin T levels in low-risk patients evaluated for acute coronary syndromes. Ann Emerg Med. 2000;35:213–20. [PubMed] [Google Scholar]
- 54.Kugelmass AD, Anderson AL, Brown PP. Does having a chest pain center impact the treatment and survival of acute myocardial infarction patients (abstr)? Circulation. 2004;110:III409. [Google Scholar]
- 55.Mitchell AM, Garvey JL, Chandra A, Diercks D, Pollack CV, Kline JA. Prospective multicenter study of quantitative pretest probability assessment to exclude acute coronary syndrome for patients evaluated in emergency department chest pain units. Ann Emerg Med. 2006;47:447. doi: 10.1016/j.annemergmed.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 56.Reilly BM, Evans AT, Schaider JJ, Wang Y. Triage of patients with chest pain in the emergency department: a comparative study of physicians’ decisions. Am J Med. 2002;112:95–103. doi: 10.1016/s0002-9343(01)01054-3. [DOI] [PubMed] [Google Scholar]
- 57.Freas GC. Medicolegal aspects of acute myocardial infarction. Emerg Med Clin North Am. 2001;19:511–21. doi: 10.1016/s0733-8627(05)70198-x. [DOI] [PubMed] [Google Scholar]
- 58.Pilote L, Granger C, Armstrong PW, Mark DB, Hlatky MA. Differences in the treatment of myocardial infarction between the United States and Canada. A survey of physicians in the GUSTO trial. Med Care. 1995;33:598–610. doi: 10.1097/00005650-199506000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Graff LG, Dallara J, Ross MA, et al. Impact on the care of the emergency department chest pain patient from the chest pain evaluation registry (CHEPER) study. Am J Cardiol. 1997;80:563–8. doi: 10.1016/s0002-9149(97)00422-0. [DOI] [PubMed] [Google Scholar]
- 60.Bluemke DA, Achenbach S, Budoff M, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention, and the Councils on Clinical Cardiology and Cardiovascular Disease in the Young. Circulation. 2008;118:586–606. doi: 10.1161/CIRCULATIONAHA.108.189695. [DOI] [PubMed] [Google Scholar]
- 61.Bonow RO. Identification of viable myocardium. Circulation. 1996;94:2674–80. doi: 10.1161/01.cir.94.11.2674. [DOI] [PubMed] [Google Scholar]
- 62.Schinkel AF, Bax JJ, Poldermans D, Elhendy A, Ferrari R, Rahimtoola SH. Hibernating myocardium: diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32:375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 63.Camici PG, Prasad SK, Rimoldi OE. Stunning, hibernation, and assessment of myocardial viability. Circulation. 2008;117:103–14. doi: 10.1161/CIRCULATIONAHA.107.702993. [DOI] [PubMed] [Google Scholar]
- 64.Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151–8. doi: 10.1016/s0735-1097(02)01726-6. [DOI] [PubMed] [Google Scholar]
- 65.Velazquez EJ, Lee KL, O’Connor CM, et al. STICH Investigators The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg. 2007;134:1540–7. doi: 10.1016/j.jtcvs.2007.05.069. on behalf of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bursi F, Enriquez-Sarano M, Nkomo VT, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;111:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 67.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 68.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–58. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 69.Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol. 2005;45:381–7. doi: 10.1016/j.jacc.2004.09.073. [DOI] [PubMed] [Google Scholar]
- 70.Mehra MR, Gheorghiade M, Bonow RO. Mitral regurgitation in chronic heart failure: more questions than answers? Curr Cardiol Rep. 2004;6:96–9. doi: 10.1007/s11886-004-0005-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.