Abstract
The proinflammatory cytokine IL-1β plays an important role in antifungal immunity; however the mechanisms by which fungal pathogens trigger IL-1β secretion are unclear. Here we show that infection with C. albicans is sensed by the Nlrp3 inflammasome resulting in the subsequent release of IL-1β. The ability of C. albicans to switch from a unicellular yeast form into a filamentous form is essential for activation of the Nlrp3 inflammasome as C. albicans mutants incapable of forming hyphae were defective in their ability to induce macrophage IL-1β secretion. Nlrp3-deficient mice also demonstrated increased susceptibility to infection with C. albicans consistent with a key role for Nlrp3 in innate immune responses to the pathogen C. albicans.
Introduction
C. albicans is a fungal pathogen that can cause severe opportunistic infections in immunocompromised patients. C. albicans can switch from a unicellular yeast form into two distinct filamentous multicellular forms, pseudohyphae and hyphae. The ability to switch between yeast and filamentous forms is believed to be important for Candida virulence (1). In addition, C. albicans dimorphism results in a differential interaction with dendritic cells skewing T helper cell responses both in vitro and in vivo (2).
Signaling through the IL-1 receptor has been shown to be important for host defenses against C. albicans (3). Consistent with this, IL-1α- and IL-1β-deficient mice have increased mortality rates and candidal burdens compared to wild-type mice challenged intravenously with C. albicans (4). Nlrp3 (also known as Nalp3 and cryopyrin), a member of the NLR (nucleotide-binding domain leucine-rich repeat containing) family, along with ASC and caspase-1, forms a multiprotein complex called the Nlrp3 inflammasome (5). The Nlrp3 inflammasome can activate caspase-1 in response to a number of diverse stimuli resulting in the processing and secretion of the proinflammatory cytokines IL-1β and IL-18 (5). Nlrp3 plays an important protective role against a number of pathogens including Listeria monocytogenes, Staphylococcus aureus and influenza virus (6–9); however, if and how fungi can activate the inflammasome remains unknown.
In this study we show that C. albicans can induce activation of caspase-1 and secretion of IL-1β through activation of the Nlrp3 inflammasome. We also demonstrate that the ability of C. albicans to switch from a unicellular yeast form into a filamentous form is essential for activation of the Nlrp3 inflammasome. Finally we show that the Nlrp3 inflammasome has a profound influence on the in vivo control of the fungal pathogen C. albicans.
Materials and Methods
Mice
The generation of Nlrp3-, ASC-, caspase-1-, and Nlrc4-deficient mice has been described previously (10, 11). All protocols used in this study were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
Candida strains and mutants
C. albicans clinical isolates FC20, FC16, FC10, FC5 (12), SC5314 wild-type and non-filamentous double mutant efg1Δ/Δcph1Δ/Δ (1), WO-1 (13), ATCC UC820, C. krusei clinical isolates P31, 932638 (14) and ATCC 6258, C. tropicalis clinical isolates T14, T362 and T5 (15) and C. glabrata clinical isolates 932474, 1480 and 932273 (16) were used in this study. For in vivo and in vitro experiments, 1 ml of a 24 h culture was diluted in 30 ml of YPD (1 % w/v yeast extract; 2% w/v peptone; 2% w/v dextrose) broth and grown for 4 h at 30°C. For hyphal induction, 1 ml of a 24 h culture was diluted in 30 ml of YPD supplement with 20% fetal calf serum and incubated at 37°C for 3 to 4 h. Heat-killed C. albicans were obtained by incubation for 90 min at 65°C. UV-killed C. albicans were obtained by exposure to 4 × 100 mjoules (UV Stratalinker 2400; Stratagene).
In vitro stimulation of macrophages
Bone marrow-derived macrophages (BMM) were generated as previously described (14). BMM that were either unstimulated or primed with 50 ng/ml LPS for 3–4 h were infected with Candida at a multiplicity of infection (MOI) of 1 macrophage (Mϕ) to 10 yeast for 6 h, or at the indicated time and concentration. Antibody pairs for the IL-1β ELISA were from R&D Systems. Western blotting was performed as previously described (14). Cytochalasin D, cytochalasin B, cathepsin B inhibitor CA-074-Me and the cathepsin L inhibitor V were obtained from Calbiochem and added 10 min (for cytochalasin) and 30 min (for cathepsin inhibitors) prior to the addition of C. albicans.
In vivo infection with C. albicans
Nlrp3-deficient mice (n=4) and WT mice (n=4) were infected i.v. with 5 × 105 colony forming units (CFU) of C. albicans strain FC20. To assess organ colonization kidney, liver and spleen were harvested 6 days post infection, and dilutions of homogenized organs were plated onto YPD plates and counted.
Results and Discussion
C. albicans induces macrophage IL-1β secretion in vitro
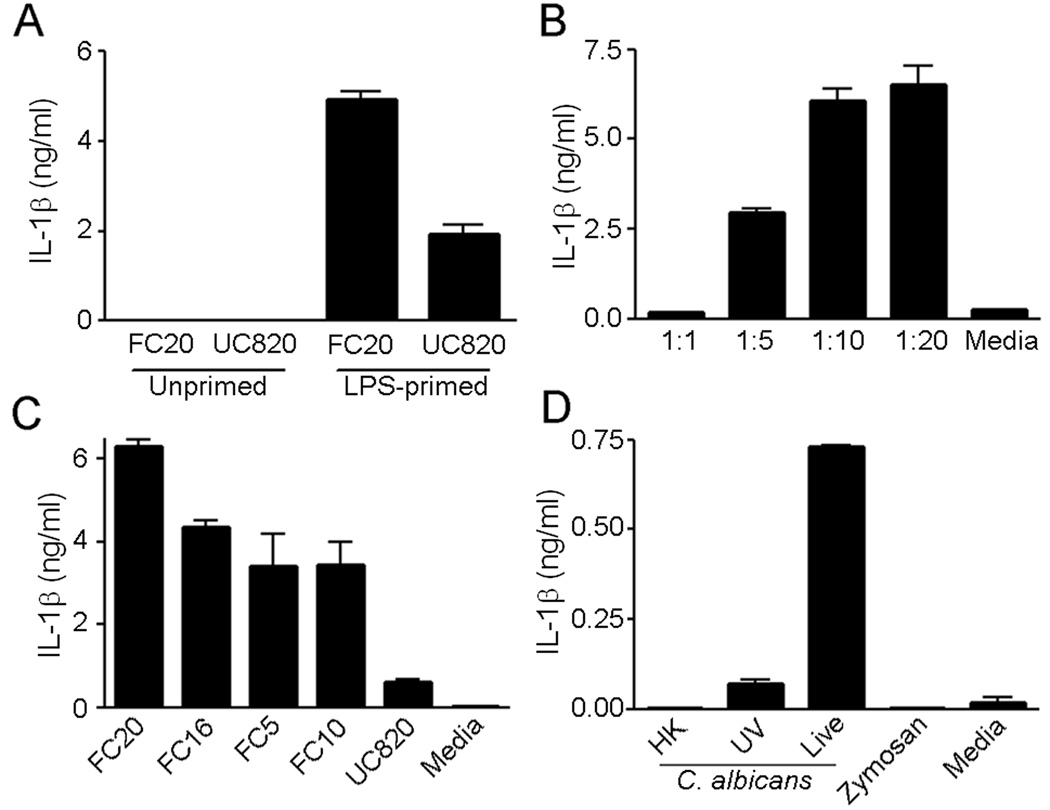
To determine if C. albicans could directly induce the secretion of IL-1β we infected WT Mϕ with two different strains of C. albicans (Fig. 1A). Infection of unprimed Mϕ with C. albicans failed to induce the secretion of IL-1β. However, infection of LPS-primed Mϕ resulted in a dose dependent secretion of IL-1β (Fig. 1A and B). Maximal IL-1β secretion was reached 4 h after infection (Supplemental Fig. 1A). To ensure that the ability of C. albicans to induce IL-1β secretion from LPS-primed Mϕ was not strain specific we tested multiple clinical isolates of C. albicans from different genetic clades (12). All C. albicans strains tested were capable of inducing IL-1β secretion from LPS-primed WT Mϕ (Fig. 1C) although the UC820 strain was less potent compared to the clinical isolates at the same MOI. Heat-killed or UV inactivated C. albicans and zymosan failed to induce IL-1β secretion from LPS-primed Mϕ (Fig. 1D) suggesting that C. albicans-mediated activation of Mϕ for IL-1β secretion is an active process on the part of Candida and is independent of the sole stimulation of major surface receptors for C. albicans such as Dectin-1 or TLR2.
FIGURE 1.
C. albicans induces IL-1β secretion from LPS-primed Mϕ. A, BMM from WT mice were either primed with 50 ng/ml LPS or left untreated. Mϕ were infected with the C. albicans clinical isolate FC20 or ATCC strain UC820 and culture supernatants collected 6 h later; IL-1β was measured by ELISA. B, LPS-primed Mϕ were infected with C. albicans strain FC20 at the indicated MOI for 6 h; culture supernatants were collected and IL-1β release measured by ELISA. LPS-primed Mϕ were infected with the indicated C. albicans strains (C), and live, heat killed (HK) or UV killed FC20 and Zymosan (100 µg/ml) (D). IL-1β release into culture supernatants 6 h after challenge was measured by ELISA. Determinations were performed in triplicate and presented as the mean ± SEM. Results are representative of two (C, D) and three (A, B) separate experiments.
C. albicans induces IL-1β secretion in a Nlrp3 dependent manner
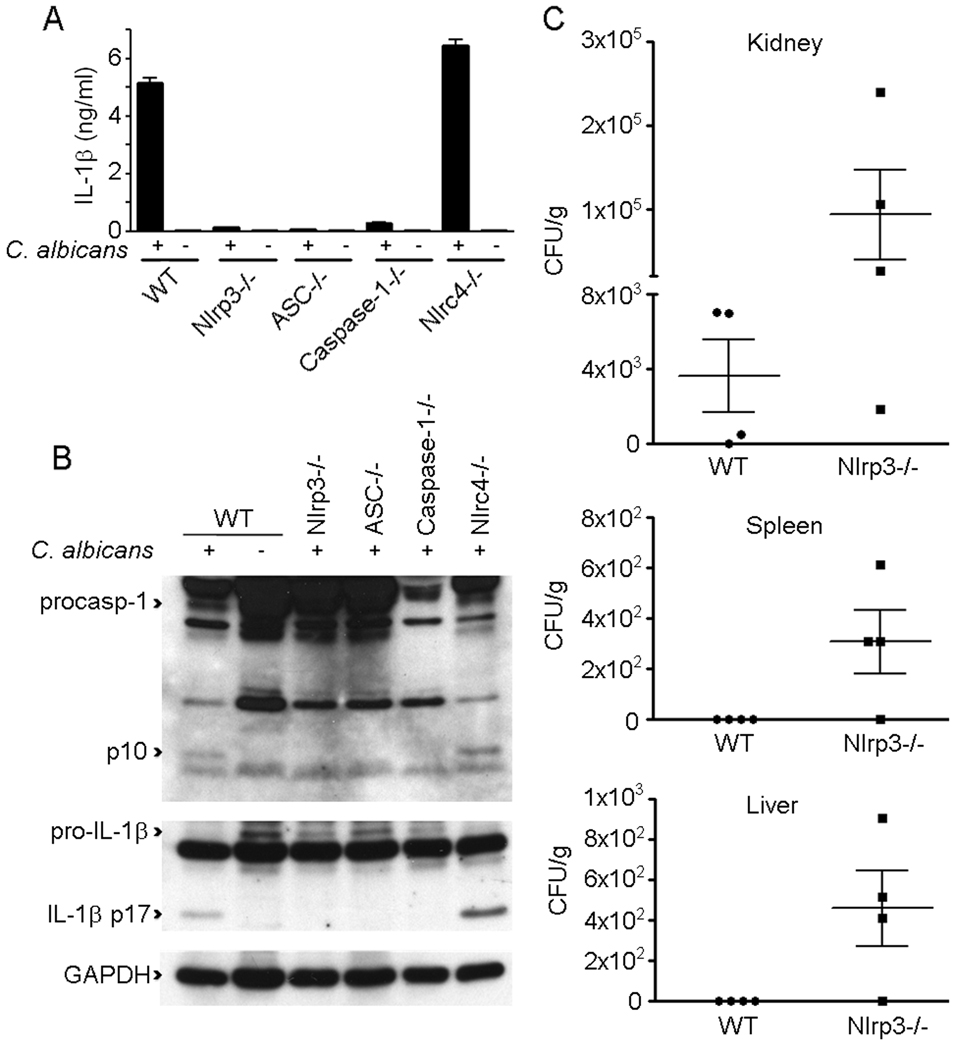
We used Mϕ deficient in specific components of the Nlrp3 inflammasome to test if they were required for C. albicans-induced IL-1β secretion. LPS-primed Nlrp3-, ASC- and caspase-1-deficient Mϕ displayed a marked defect in their ability to secrete IL-1β in response to C. albicans (Fig. 2A and B). In contrast, Mϕ deficient in Nlrc4, which is important for Mϕ caspase-1 activation in response to infection with type III and type IV secretion system carrying bacteria (5), had an intact response to infection with C. albicans and were capable of secreting IL-1β (Fig. 2A). C. albicans infection of LPS-primed WT Mϕ resulted in the activation of caspase-1 as detected by Western blot with the appearance of the p10 cleavage product (Fig. 2B). We did not observe caspase-1 activation in response to C. albicans in either Nlrp3- or ASC-deficient LPS-primed Mϕ (Fig. 2B). Despite the marked defect in caspase-1 activation and IL-1β secretion observed in Nlrp3-deficient Mϕ, Nlrp3-deficiency did not alter the cytotoxic effects of C. albicans on the Mϕ (Supplemental Fig. 1A and B). These data identify the Nlrp3 inflammasome as required for caspase-1-mediated IL-1β secretion in response to infection with C. albicans.
FIGURE 2.
IL-1β secretion induced by C. albicans is dependent on the Nlrp3 inflammasome. A, LPS-primed BMM from WT, Nlrp3-, ASC-, caspase-1-, or Nlrc4-deficient mice were stimulated for 6 h with or without C. albicans strain FC20 at an MOI of 1:10. Culture supernatants were collected and IL-1β secretion quantified by ELISA. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of two separate experiments. B, Lysates from LPS- primed Mϕ from WT, Nlrp3-, ASC-, caspase-1-, or Nlrc4-deficient mice were collected after 6h exposure to C. albicans strain FC20 and immunoblotted with antibodies against the p10 subunit of caspase-1, IL-1β and GAPDH. Results are representative of two separate experiments. C, WT (n=4) and Nlrp3−/− (n=4) mice were infected i.v. with 5 × 105 CFU of C. albicans strain FC20. After 6 days C. albicans colonization was assessed in the left kidney, spleen and liver. Data are expressed as the mean ± SEM.
To assess the relevance of these findings in vivo, fungal burdens in organs of WT and Nlrp3−/− mice after i.v. infection with C. albicans was determined (Fig. 2C). Fungal burdens in the kidney, spleen and liver were higher (10–100 fold) in Nlrp3-deficient mice compared with WT. Hence, Nlrp3 is important in innate immune defenses required to control the replication of C. albicans in vivo.
C. albicans induced IL-1β secretion requires internalization and cathepsin B activity
In order to understand how C. albicans might activate the Nlrp3 inflammasome, we tested if the endocytic ability of Mϕ was required for C. albicans-induced IL-1β production. Inhibiting actin polymerization with cytochalasin D or B inhibited IL-1β production from Mϕ infected with C. albicans (Supplemental Fig. 2A), suggesting that phagocytosis of C. albicans by Mϕ is needed to activate the Nlrp3 inflammasome for the resultant processing and secretion of IL-1β. Neither cytochalasin D or B reduced IL-1β production in response to stimulation with ATP, which uses the P2X7 receptor to activate the Nlrp3 inflammasome, confirming that macrophages were still viable and capable of secreting Nlrp3-dependent IL-1β (Supplemental Fig. 2A).
Using the cathepsin B inhibitor CA-074-Me, but not the cathepsin L inhibitor V, we observed an inhibition in the secretion of IL-1β following infection with C. albicans (Supplemental Fig. 2B). This result suggests that similar to the activation of the inflammasome by silica, amyloid-β and influenza, (5, 8, 17), C. albicans activation of the Nlrp3 inflammasome may be linked to lysosomal damage. A recent study by Gross and colleagues (18) showed that cathepsin B was dispensable for C. albicans induced IL-1β secretion in dendritic cells. Further studies to examine the difference in the requirement for cathepsin B between dendritic cells and Mϕ are needed. C. albicans induced secretion of IL-1β was however independent of endogenous ATP release as Mϕ deficient in P2X7R were still capable of secreting IL-1β (Supplemental Fig. 2C). Taken together these data suggest that C. albicans-induced activation of the Nlrp3 inflammasome shares similar pathways used by other danger associated molecular patterns and pathogens that are capable of activating the Nlrp3 inflammasome.
The ability for C. albicans to form hyphae is essential for their efficient activation of the Nlrp3 inflammasome
As the switch to a filamentous form is crucial for C. albicans to avoid killing by providing a means to evade the phagosome (1), we hypothesized that membrane disruption by hyphae or pseudohyphae may be necessary for inflammasome activation. Interestingly, IL-1β secretion by LPS-primed Mϕ exposed to hyphae was markedly lower than LPS-primed Mϕ challenged with the yeast form (Fig. 3A). Studies examining the interaction between C. albicans and Mϕ have shown that 1 h following ingestion of yeast by the Mϕ they start to form germ tubes that eventually develop into hyphae (1). We therefore tested if the transition to a hyphal form was important for triggering IL-1β secretion as opposed to the Mϕ interaction with the hyphae themselves. We studied the ability of the C. albicans efg1Δ/Δcph1Δ/Δ double mutant, locked in the yeast phase and therefore deficient in both pseudohyphae and hyphae (1), to induce IL-1β secretion by LPS-primed Mϕ. The C. albicans efg1Δ/Δcph1Δ/Δ double mutant failed to induce IL-1β secretion compared to its parental wild-type strain even at high MOIs (Fig. 3B). Infection of LPS-primed Mϕ with the wild-type parental strain resulted in the activation of caspase-1 as detected by the appearance of p10 by Western blotting at MOIs of 1:10 and 1:20 (Fig. 3C) whereas no caspase-1 activation was observed in response to the efg1Δ/Δcph1Δ/Δ double mutant at similar MOIs (Fig. 3C). Consistent with previous findings (1), we did not observe differences in the ability of Mϕ to bind and phagocytose the efg1Δ/Δcph1Δ/Δ double mutant compared to the wild-type strain (data not shown). Infection of WT mice with the efg1Δ/Δcph1Δ/Δ double mutant in vivo also resulted in significantly less serum IL-1β compared to mice infected with wild-type C. albicans (Fig. 3D). To further examine the role of the C. albicans mycelial form in Nlrp3 inflammasome activation we studied the ability of phenotypic switching to modulate IL-1β secretion. Phenotypic switching is a spontaneous and reversible mechanism affecting cellular morphology, colony shape and cell physiology of most C. albicans strains. The WO-1 strain switching system consists of a reversible transition between a white domed-shaped colony “white phase”, and a larger flat grey colony “opaque phase” (13). More importantly for our study, the WO-1 opaque phenotype multiplies solely as a unicellular cell (yeast form) while the white phenotype retains the ability to form hyphae and pseudohyphae (13). Consistent with our findings that the efg1Δ/Δcph1Δ/Δ double mutant fails to induce Nlrp3 inflammasome activation, the WO-1 opaque phenotype, which does not produce hyphae and pseudohyphae, had a markedly diminished ability to induce the secretion of Mϕ IL-1β (Fig. 3E).
FIGURE 3.
C. albicans bud-hyphae transition is essential for Nlrp3 inflammasome activation. A, IL-1β release from LPS-primed WT Mϕ was assessed by ELISA after 6 h exposure (MOI of 1:10) to C. albicans strain FC20 yeast and hyphae. B, LPS-primed WT Mϕ were infected with C. albicans strain SC5314 (wild-type) or the efg1Δ/Δcph1Δ/Δ̣ mutant at the indicated MOI. IL-1β release into culture supernatants 6 h after challenge was measured by ELISA. C, Lysates from LPS- primed WT Mϕ after a 6h infection with C. albicans strain SC5314 (wild-type) or the efg1Δ/Δcph1Δ/Δ̣ mutant at the indicated MOI were immunoblotted with antibodies against the p10 subunit of caspase-1 and GAPDH. D, WT mice were infected i.v. with 5 × 106 CFU of C. albicans strain SC5314 (wild-type) or the efg1Δ/Δcph1Δ/Δ̣ mutant; 4 h post infection serum IL-1β was determined by ELISA. * p = 0.0159 by two-tailed Mann Whitney test. E, LPS-primed WT Mϕ were infected with C. albicans strains FC20 and WO-1 white and opaque phenotypes at an MOI of 1:10 for 6 h and IL-1β release was measured by ELISA. F, LPS-primed WT Mϕ were infected with three strains each of C. krusei (C.k., 1: ATCC6258, 2: P31, 3: 932638), C. tropicalis (C.t., 1: T4, 2: T362, 3: T5), and C. glabrata (C.g, 1: 932273, 2: 1480, 3: 932474) and C. albicans (C.a.) FC20 at an MOI of 1:10 for 6 h and IL-1β was measured by ELISA. Determinations were performed in triplicate and presented as the mean ± SEM (A, B, E). Determinations were performed in duplicate and presented as the mean (F). Results are representative of two (B, C, E, F) and three (A) separate experiments.
Because hyphae and pseudohyphae are two different filamentous forms with distinct properties, we next evaluated whether inflammasome activation was strictly dependent on the bud-hyphae transition or if pseudohyphae could also mediate this pathway. We tested the ability of C. tropicalis and C. krusei (which form pseudohyphae but not hyphae) and C. glabrata (which produces rare pseudohyphae under specific conditions (19) but not hyphae) to induce IL-1β secretion from LPS-primed Mϕ. Infection of LPS-primed Mϕ with C. tropicalis and C. krusei, but not C. glabrata, resulted in IL-1β secretion (Fig. 3F). Although C. glabrata was still capable of inducing the secretion of TNFα from LPS-primed Mϕ (data not shown), there may be additional species differences, other than hyphae production, between C. glabrata and C. albicans that may account for the lack of induction of IL-1β secretion. Together, these data demonstrate that candidal activation of the inflammasome is dependent upon Candida’s ability to form filaments, although not necessarily the filaments themselves. This further underscores the role of phenotypic plasticity in C. albicans modulation of the host innate immune response.
In conclusion, we demonstrated a key role for the Nlrp3 inflammasome in host defense against C. albicans. A recent study by Gross and colleagues is consistent with our finding that Nlrp3 is important in anti-candidal host defense (18). Their findings and our results highlight the difference in the mechanisms of inflammasome activation in different myeloid cells. In this study, we show that C. albicans activation of the Nlrp3 inflammasome in Mϕ requires a two step activation process with the first signal consisting of a TLR ligand. In contrast, dendritic cells appear to obtain both signal 1 and 2 from C. albicans, with signaling through syk/CARD9 acting as signal 1 (18). Importantly, we showed that the ability of C. albicans to transition from the yeast to the filamentous phase is a crucial component for signal 2 in Nlrp3 inflammasome activation. Consistent with this difference in the requirement of a separate signal 1 by Mϕ compared to dendritic cells, Goodridge et al. demonstrated that Dectin-1-CARD9 failed to activate NF-κB and trigger TNF-α production in resting Mϕ (20). Taken together these findings have intriguing implications for the role of the Nlrp3 inflammasome in the pathogenesis of fungal diseases and stimulation of the Nlrp3 inflammasome may provide a possible therapeutic adjunct for the treatment of candidal infections.
Supplementary Material
Acknowledgments
This work was supported by grants K08 AI065517 (F.S.S.) and K08 AI067736 (S.L.C.) from the National Institutes of Health.
We thank Richard Flavell, Anthony Coyle, Ethan Grant, and John Bertin for providing mutant mice and Gerald Fink for providing the C. albicans efg1Δ/Δcph1Δ/Δ mutant.
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Lo HJ, Köhler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 2.d'Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L. Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J. Exp. Med. 2000;191:1661–1674. doi: 10.1084/jem.191.10.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L. The contribution of the Toll-like/IL-1 receptor superfamily to innate andadaptive immunity to fungal pathogens in vivo. J Immunol. 2004;172:3059–3069. doi: 10.4049/jimmunol.172.5.3059. [DOI] [PubMed] [Google Scholar]
- 4.Vonk AG, Netea MG, van Krieken JH, Iwakura Y, van der Meer JW, Kullberg BJ. Endogenous interleukin (IL)-1α and IL-1β are crucial for host defense against disseminated candidiasis. J Infect Dis. 2006;193:1419–1426. doi: 10.1086/503363. [DOI] [PubMed] [Google Scholar]
- 5.Pedra JH, Cassel SL SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21:10–16. doi: 10.1016/j.coi.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 7.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza a virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Lara-Tejero M, Sutterwala FS, Ogura Y, Grant EP, Bertin J, Coyle AJ, Flavell RA, Galán JE. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pujol C, Joly S, Lockhart SR, Noel S, Tibayrenc M, Soll DR. Parity among the randomly amplified polymorphic DNA method, multilocus enzyme electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans. J. Clin. Microbiol. 1997;35:2348–2358. doi: 10.1128/jcm.35.9.2348-2358.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. "White-opaque transition": a second high-frequency switching system in Candida albicans. J. Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joly S, Pujol C, Schroppel K, Soll DR. Development of two species-specific fingerprinting probes for broad computer-assisted epidemiological studies of Candida tropicalis. J. Clin. Microbiol. 1996;34:3063–3071. doi: 10.1128/jcm.34.12.3063-3071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joly S, Maze CM, McCray PB, Jr, Guthmiller JM. Human β-defensins demonstrate strain-selective activity against oral microorganisms. J. Clin. Micro. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lockhart SR, Joly S, Pujol C, Sobel J, Pfaller M, Soll DR. Development and verification of fingerprinting probes for Candida glabrata. Microbiology. 1997;143:3733–3746. doi: 10.1099/00221287-143-12-3733. [DOI] [PubMed] [Google Scholar]
- 17.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, Mocsai A, Tschopp J, Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 19.Lachke SA, Joly S, Daniels K, Soll DR. Phenotypic switching and filamentation in Candida glabrata. Microbiology. 2002;148:2661–2674. doi: 10.1099/00221287-148-9-2661. [DOI] [PubMed] [Google Scholar]
- 20.Goodridge HS, Shimada T, Wolf AJ, Hsu YS, Becker CA, Lin X, Underhill DM. Differential use of CARD9 by dectin-1 in macrophages and dendritic cells. J. Immunol. 2009;182:1146–1154. doi: 10.4049/jimmunol.182.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.