Abstract
It was shown that the TBDPS protecting group can serve as an efficient phenyl group donor for o-bromophenols via the Pd-catalyzed C—H arylation, followed by a routine TBAF deprotection of the forming silacycles. Employment of the newly designed Br-TBDPS protecting group in the same sequence allows for a facile introduction of a phenyl group in the ortho-position of phenols and anilines. Alternatively, switching desilylation to oxidation at the last step allows converting the forming silacycles into valuable ortho-biphenols.
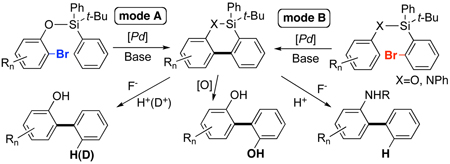
Aryl-aryl bond formation is an important process due to the unique pharmaceutical features and wide applications of biaryls in material science.1 Traditionally, the aryl-aryl bond is made via cross-coupling reactions between aryl halide (or equivalent) and aryl metal component.2 Recently, a number of excellent transition-metal catalyzed direct C-H arylation methodologies have emerged.3 However, these methods are not without boundaries. Thus, intermolecular versions often suffer from low reactivity and/or regioselectivity, which often could be circumvented by the introduction of a directing group,3k, 4 including a removable directing group. 5 Intramolecular arylations, although being much less problematic,3a,d,6 lead, by default, to cyclic biaryl products. To the best of our knowledge, there are no reports on the employment of easily removable tethers in the synthesis of biaryls via the C-H arylation motif.7 Herein, we wish to report that the common TBDPS protecting group can serve as an efficient aryl group donor for o-bromophenols via the Pd-catalyzed intramolecular arylation, followed by a deprotection step (mode A, eq 1). Moreover, it was found that the newly designed Br-TBDPS protecting group is even more efficient aryl group donor for simple phenols and anilines (mode B). Not only employment of this temporary silicon tether motif is beneficial due to the easiness of its deprotection, but also due to the fact that it provides easy access to deuteriated biaryls and biphenols (eq 1).
 |
(1) |
The temporary silicon connection method, coined by Stork,8 has been widely used for rendering intermolecular reactions intramolecular, which often resulted in superior regio- and stereoselectivities of the processes.9 Thus, we reasoned that employment of a suitable easily introducible and removable silicon tether may improve reactivity and regioselectivity of the Pd-catalyzed intermolecular C-H arylation reactions (vide supra). For these studies, we chose phenol, intermolecular arylation of which is a challenging task. 10 Initially, as a removable tether, we chose the tert-butyl-diphenylsilyl (TBDPS) protecting group, which, since its invention by Hanessian, 11 enjoyed extensive use in synthesis. 12 We hypothesized that if efficient conditions for intramolecular arylation of silicon-tethered phenyl groups are found, it can provide a convenient route to ortho-arylated phenols.10, 13 Accordingly, we examined the Pd-catalyzed intramolecular arylation of TBDPS-protected o-halophenols 1. After screening several standard conditions, it was found that employment of a modified Fagnou’s protocol3d,14 was the most efficient for arylation of o-bromophenol 1a.15,16
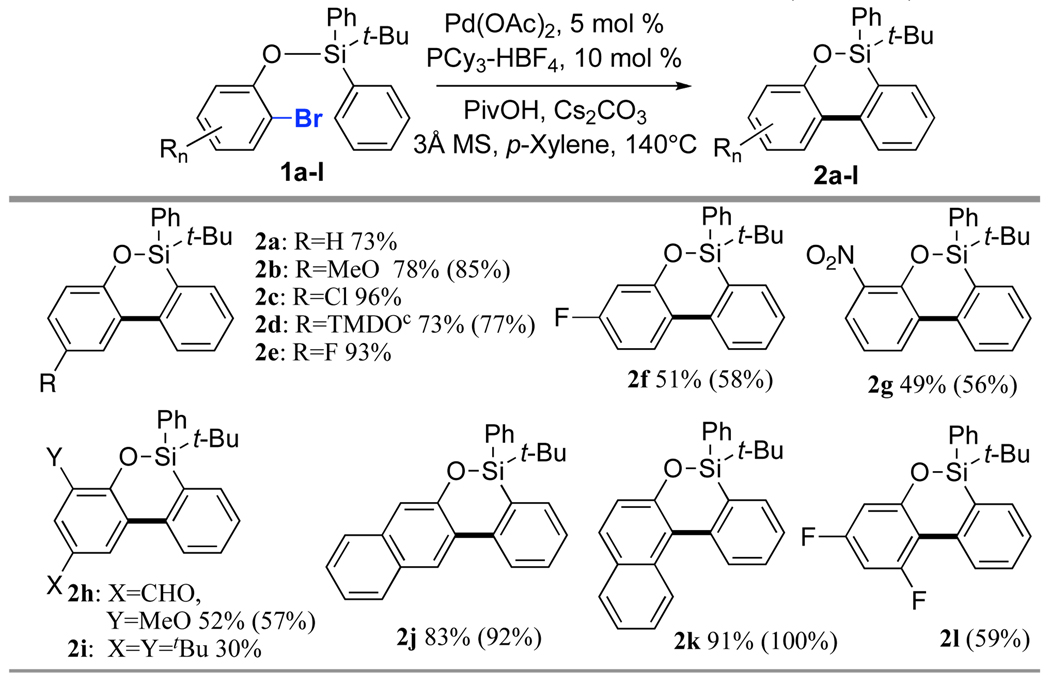
Next, the substrate scope was studied (Table 1). Gratifyingly, it was found that this method is quite general, as diverse substrates possessing MeO, Cl, F, NO2 and CHO groups, were perfectly tolerated under these reaction conditions to produce the corresponding oxasilacycles 2 in good to high yields. The yields were normally higher with electron-rich substrates and somewhat lower with electron deficient arenes. Expectedly, the highly sterically-congested 1i was less efficient in this reaction.
Table 1.
 |
See Supporting Information for the detailed procedure.
Isolated yield, NMR yield against CH2Br2 as internal standard in the parenthesis.
TMDO = 4,4,5,5-tetramethyl-1,3-dioxolan-2-yl.
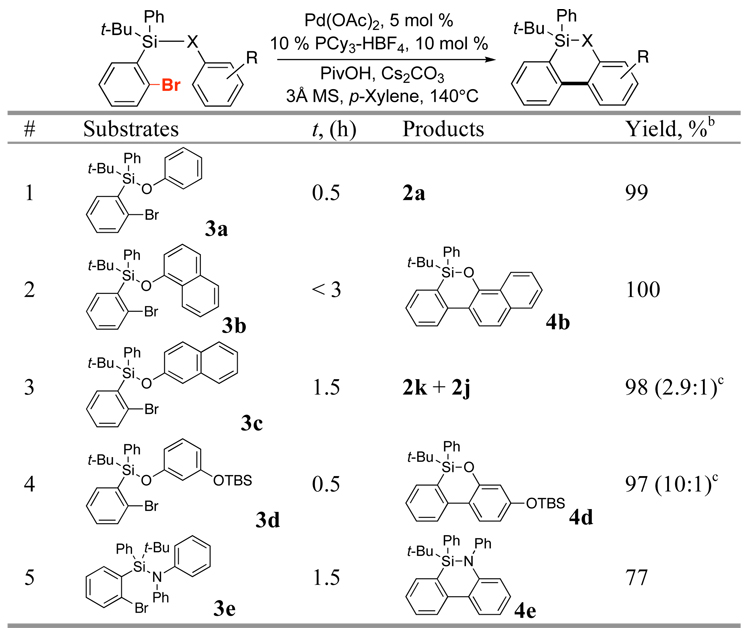
Inspired by a successful employment of TBDPS group as a tether and, at the same time, a donor of an aryl group for o-bromophenols, we aimed at the design of a silyl tether that will allow for arylation of simple phenols (mode B). To this end, we synthesized a Br-TBDPS group16 and tested it in the Pd-catalyzed arylation of phenols (Table 2). We were pleased to find that the O-Br-TBDPS-protected phenol (3a), under the same reaction conditions, underwent a nearly quantitative transformation into silacycle 2a (Table 2, entry 1). Likewise, O-Br-TBDPS-protected 1- and 2-naphthols were cleanly converted into the corresponding cyclization products. O-TBS-protected resorcinol reacted highly regioselectively to give oxasilacycle 4d in very high yield. Moreover, our initial experiments demonstrated that N-Br-TBDPS-protected anilines also can undergo the Pd-catalyzed intramolecular arylation to give azasilacycle 4e in good yield!17,18
Table 2.
Arylation of Br-TBDPS-Protected Phenols (Mode B)a
 |
Same conditions as those for mode A.
Isolated yield.
Major regioisomer shown.
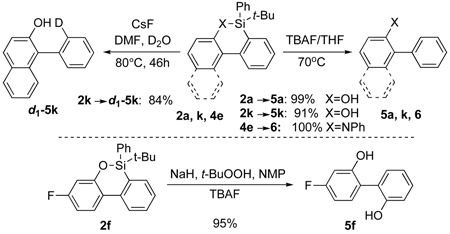
Naturally, after development of efficient intramolecular C-H arylations of silicon-tethered o-Br-phenols, phenols, and aniline, we performed deprotection of the silicon tether. Thus, standard TBAF deprotection protocol proved successful on oxasilacycles 2 and azasilacycle 4e to produce arylated phenols 5a, 5k, and aniline 6 in very high to quantitative yields (eq 2). Moreover, treatment of 2k with anhydrous CsF in DMF/D2O lead to d1-5k in 84 % yield. Furthermore, employment of a modified Woerpel’s oxidation conditions16,19 for 2f produced unsymmetrical biphenol 5f in excellent yield!
 |
(2) |
In summary, two methods employing a removable silicontether for the Pd-catalyzed arylation of phenols and anilines have been developed. It was shown that the TBDPS protecting group could serve as a convenient aryl group donor for ortho-bromophenols via an intramolecular arylation/deprotection sequence. It was also shown that the newly designed Br-TBDPS group could serve as an even more efficient aryl group donor for simple phenols and anilines. Remarkably, switching desilylation to oxidation at the last step allows converting the forming silacycles into valuable orthobiphenols.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (Grant GM-64444).
Footnotes
Supporting Information Available: Experimental data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Hassan J, Sévignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359. doi: 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]
- 2.For a general review, see for example: De Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Weinheim: Wiley-VCH; 2004.
- 3.For reviews, see: Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174. doi: 10.1021/cr0509760. Seregin IV, Gevorgyan V. Chem. Soc. Rev. 2007;36:1173. doi: 10.1039/b606984n. Campeau L-C, Stuart DR, Fagnou K. Aldrich Chim. Acta. 2007;40:35. For representative works on biaryl formation, see: Campeau L-C, Parisien M, Fagnou K. J. Am. Chem. Soc. 2004;126:9186. doi: 10.1021/ja049017y. Lafrance M, Rowley CN, Woo TK, Fagnou K. J. Am. Chem. Soc. 2006;128:8754. doi: 10.1021/ja062509l. Park C-H, Ryabova V, Seregin IV, Sromek AW, Gevorgyan V. Org. Lett. 2004;6:1159. doi: 10.1021/ol049866q. Lane BS, Brown MA, Sames D. J. Am. Chem. Soc. 2005;127:8050. doi: 10.1021/ja043273t. Daugulis O, Zaitsev VG. Angew. Chem. Int. Ed. 2005;44:4046. doi: 10.1002/anie.200500589. Wang C, Piel I, Glorius F. J. Am. Chem. Soc. 2009;131:4194. doi: 10.1021/ja8100598. Chiong HA, Pham Q-N, Daugulis O. J. Am. Chem. Soc. 2007;129:9879. doi: 10.1021/ja071845e. Do H-Q, Kashif Khan RM, Daugulis O. J. Am. Chem. Soc. 2008;130:15185. doi: 10.1021/ja805688p. Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975. Stuart DR, Fagnou K. Science. 2007;316:1172. doi: 10.1126/science.1141956. Deprez NR, Kalyani D, Krause A, Sanford MS. J. Am. Chem. Soc. 2006;128:4972. doi: 10.1021/ja060809x. Berman AM, Lewis JC, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:14926. doi: 10.1021/ja8059396. Do H-Q, Daugulis O. J. Am. Chem. Soc. 2007;129:12404. doi: 10.1021/ja075802+. Brasche G, García-Fortanet J, Buchwald SL. Org. Lett. 2008;10:2207. doi: 10.1021/ol800619c. Ackermann L, Althammer A, Fenner S. Angew. Chem. Int. Ed. 2009;48:201. doi: 10.1002/anie.200804517. Miyasaka M, Fukushima A, Satoh T, Hirano K, Miura M. Chem. Eur. J. 2009;15:3674. doi: 10.1002/chem.200900098. Voutchkova A, Coplin A, Leadbeater NE, Crabtree RH. Chem. Commun. 2008:6312. doi: 10.1039/b813998a.
- 4.For directed intermolecular arylation, see: Wang D-H, Mei T-S, Yu J-Q. J. Am. Chem. Soc. 2008;130:17676. doi: 10.1021/ja806681z. Yang S, Li B, Wan X, Shi Z. J. Am. Chem. Soc. 2007;129:6066. doi: 10.1021/ja070767s. Özdemir I, Demir S, Çetinkaya B, Gourlaouen C, Maseras F, Bruneau C, Dixneuf PH. J. Am. Chem. Soc. 2008;130:1156. doi: 10.1021/ja710276x. Ackermann L. Top. Organomet. Chem. 2007;24:35. Norinder J, Matsumoto A, Yoshikai N, Nakamura E. J. Am. Chem. Soc. 2008;130:5858. doi: 10.1021/ja800818b. Lazareva A, Daugulis O. Org. Lett. 2006;8:5211. doi: 10.1021/ol061919b. Kametani Y, Satoh T, Miura M, Nomura M. Tetrahedron Lett. 2000;40:2655. Ackermann L, Vicente R, Althammer A. Org. Lett. 2008;10:2299. doi: 10.1021/ol800773x.
- 5.(a) Boebel TA, Hartwig JF. J. Am. Chem. Soc. 2008;130:7534. doi: 10.1021/ja8015878. [DOI] [PubMed] [Google Scholar]; (b) Pastine SJ, Gribkov DV, Sames D. J. Am. Chem. Soc. 2006;128:14220. doi: 10.1021/ja064481j. [DOI] [PubMed] [Google Scholar]; (c) Maehara A, Tsurugi H, Satoh T, Miura M. Org. Lett. 2008;10:1159. doi: 10.1021/ol8000602. [DOI] [PubMed] [Google Scholar]; (d) Giri R, Wasa M, Breazzano SP, Yu J-Q. Org. Lett. 2006;8:5685. doi: 10.1021/ol0618858. [DOI] [PubMed] [Google Scholar]; (e) Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; (f) Giri R, Chen X, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884. [DOI] [PubMed] [Google Scholar]
- 6.For intramolecular arylations toward biaryls, see: Lafrance M, Lapointe D, Fagnou K. Tetrahedron. 2008;64:6015. García-Cuadrado D, de Mendoza P, Braga AAC, Maseras F, Echavarren AM. J. Am. Chem. Soc. 2007;129:6880. doi: 10.1021/ja071034a. García-Cuadrado D, Braga AAC, Maseras F, Echavarren AM. J. Am. Chem. Soc. 2006;128:1066. doi: 10.1021/ja056165v. Pascual S, de Mendoza P, Braga AAC, Maseras F, Echavarren AM. Tetrahedron. 2008;64:6021.
- 7.For cleavage of the cyclic product of intramolecular arylation, see: Campeau L-C, Parisien M, Jean A, Fagnou J. J. Am. Chem. Soc. 2006;128:581. doi: 10.1021/ja055819x.. For arylations proceeding via norbornene-tethered intermediates, see Catellani M, Motti E, Della C' N. Acc. Chem. Res. 2008;41:1512. doi: 10.1021/ar800040u.
- 8.Stork G, Suh HS, Kim G. J. Am. Chem. Soc. 1991;113:7054. [Google Scholar]
- 9.For a review, see for example: Fensterbank L, Malacria M, Sieburth SMcN. Synthesis. 1997;813
- 10.Efficient intermolecular arylation of phenols reported only on substrates possessing a bulky o-substituent. See: Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. Angew. Chem. Int. Ed. 2003;42:112. doi: 10.1002/anie.200390037. Bedford RB, Limmert ME. J. Org. Chem. 2003;68:8669. doi: 10.1021/jo030157k. Bedford RB, Betham M, Caffyn AJM, Charmant JPH, Lewis-Alleyne LC, Long PD, Polo-Cerón D, Prashar S. Chem. Commun. 2008:990. doi: 10.1039/b718128k.
- 11.Hanessian S, Lavallee P. Can. J. Chem. 1975;53:2975. [Google Scholar]
- 12.(a) Kocienski PJ. Protecting Groups. Stuttgart, Germany: Thieme; 1994. [Google Scholar]; (b) Greene TW, Wuts PGM. Protective Groups in Organic Synthesis. 3rd ed. New York: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 13.(a) Oi S, Watanabe S-i, Fukita S, Inoue Y. Tetrahedron Lett. 2003;44:8665. [Google Scholar]; (b) Hennings DD, Iwasa S, Rawal VH. J. Org. Chem. 1997;62:2. doi: 10.1021/jo961876k. [DOI] [PubMed] [Google Scholar]; (c) Bajracharya GB, Daugulis O. Org. Lett. 2008;10:4625. doi: 10.1021/ol801897m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kawamura Y, Satoh T, Miura M, Nomura M. Chem. Lett. 1999;961 [Google Scholar]
- 14.Lafrance M, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2007;129:14570. doi: 10.1021/ja076588s. [DOI] [PubMed] [Google Scholar]
- 15.Iodo- and chlorophenols were less efficient, whereas the corresponding triflates were not stable under these conditions at all.
- 16.See Supporting Information for details.
- 17.For a direct intermolecular C-H arylation of anilides, see ref. 3h,3q.
- 18.In all cases, no arylation of a silyl-bound phenyl group of Br-TBDPS, leading to an alternative five-membered silacycle was observed.
- 19.Smitrovich JH, Woerpel KA. J. Org. Chem. 1996;61:6044. doi: 10.1021/jo991312r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


