Abstract
Progranulin (PGRN) is a growth modulating factor released by a variety of cells. This molecule has gained the attention of the neuroscience community with recent discoveries of multifunctional roles of PGRN in normal brain and neurodegenerative disorders. We focus on novel roles of PGRN as a sex steroid-responsible gene in the developing and adult rodent brain. While the developing brain is feminine by default, hormone exposure, including androgen and estrogen, induces musculinization during the critical period. We have shown that PGRN is a sex steroid-responsible gene that may be involved in masculinization of the perinatal rat brain. We also found that in adult rats PGRN gene expression was up-regulated by estrogen in the hippocampus, suggesting that PGRN may mediate the mitogenic effects of estrogen in the active area of neurogenesis. Since it has been recently reported that mutations in PGRN gene are responsible for a type of frontotemporal lobar degeneration in humans, PGRN appears to be also involved in modulating neurodegeneration. Together, PGRN gene expression is induced by estrogen in both developing and adult brains, and it may play multifunctional roles in the organization of functional masculinization in the developing brain and the maintenance of adult brain function.
Keywords: brain, estrogen, neurogenesis, progranulin, sexual differentiation
Progranulin (PGRN) is a trophic factor that has gained attention with recent discoveries of its multifunctional roles in normal brain development and neurodegenerative disorders. In the last decade, we have demonstrated novel biological aspects of PGRN as a sex steroid-responsible gene in the developing and adult rodent brain. This review summarizes the recent results obtained in our and other laboratories concerning the potential roles of PGRN as a mediator of sex steroids in sexual differentiation of the developing brain and adult neurogenesis.
Background: What is PGRN?
PGRN, also called as granulin/epithelin precursor (GEP), proepithelin (PEPI), PC cells-derived growth factor (PCDGF) and acrogranin, is a glycosylated protein released by a varaiety of cells and is potentially mitogenic in cell culture [1]. It consists of 7 ½ sequentially arranged granulin (GRN) motifs in tandem. PGRN can be cleaved by extracellular proteases into several GRN peptides, which probably have separate functions. GRN peptides, also called epithelins [2], were initially identified as peptides of approximately 6-kDa, some of which can modulate the growth of cells in tissue culture [1]. They are rich in cystine and possess a unique structurally defined motif of six disulfide bonds. PGRN mRNA has been demonstrated in various tissues and organs including the reproductive organs, gastrointestinal tract, endocrinal organs, and neural tissues [3;4]. It is particularly prominent in epithelial and hematopoietic cells, and tends to be more highly expressed in tissues with high turnover rates such as gastric mucosa, lymphoid tissue, and tumor cell lines [4;5]. In contrast, most mitotically quiescent epthelia, such as those of the lung or renal tubules, express PGRN at relatively low levels [4]. PGRN is mitogenic for epithelial cells and several kinds of cancer cells and also promotes tumor cell invasiveness [6–12]. It has been suggested that PGRN is a steroid-regulated growth factor and mediates the mitogenic effect of estrogens in breast cancer tumorgenesis [13;14]. PGRN is expressed in the acrosome of the sperm [15] and oocytes [16] and modulates the development of early embryos in vitro [17].
PRGN has been also implicated in wound healing and inflammation [18–20]. During the wound repair response, PGRN is upregulated and stimulates neutrophil and macrophage infiltration and neovascularization of wound tissue [19]. PGRN and GRN peptides also regulate inflammation with opposing effects, and their production is regulated by novel interactions between PGRN, secretory leukocyte protease inhibitor (SLPI), and a serine protease elastase [19]. SLPI is a protein with protease inhibitor domains that has been implicated in regulating proteolysis. In the periphery the relative balance between the activities of elastase and SLPI influences the levels of the anti-inflammatory PGRN and the pro-inflammatory GRN proteins [19]. PGRN is also upregulated in response to hypoxia and acidotic stress in fibroblasts in culture [21], which indicates PGRN serves as a multiple-stress responsive factor.
In the central nervous system (CNS), PGRN is widely expressed during early neural development but later on its expression becomes restricted to specific neuronal populations including cortical neurons in several layers, pyramidal cell layer and dentate gyrus of the hippocampus, ventromedial and arcuate nuclei of the hypothalamus, amygdale, and Purkinje cell layer in the cerebellum [4;22;23]. At the cellular level, PGRN immunoreactivity is found in neuronal perikarya, dendrites and axons. PGRN is also expressed in many non-neuronal cell types in CNS and particularly prominent in microglia [24], but also present in endothelial and smooth muscle cells and in the choroid plex and ependyma. There is no or little PGRN immunoreactivity in astrocytes or oligodendrocytes under normal conditions, but PGRN expression is observed in reactive astrocytes. While the exact function of PGRN in normal CNS is still unclear, recent results suggest that this molecule is involved in neurotrophic activity and neuroinflammation [23;25]. An in vitro study shows that PGRN enhances neuronal survival and neurite length in cultured cortical and motor neurons [26]. Interestingly, these effects were abolished by coadministration of SLPI, suggesiting that PGRN/GRN conversion plays a crucial role in their actions. In the CNS, SLPI has been known to be expressed in reactive astrocytes and upregulated with neuroprotective properties in ischemic stroke [27]. Furthermore, cultured microglial cells are known to produce elastase. Consequently, there may be interaction between PGRN, SLPI, elastase in the CNS neuronal repair process as in seen within wound healing in peripheral tissues [25].
Recently, much attention has been paid to the functional role of PGRN in the CNS, because PGRN plays a key role for disease progression in neurodegenerative diseases [23;24;28–33]. Mutations in PGRN gene were recently identified as the cause of some forms of autosomal dominant tau-negative frontotemporal lobar degeneration (FTLD) [24;28], which is represented by severe atrophy in the frontal lobe and temporal lobe of the brain and recognized as the common cause of dementia after Alzheimer’s disease (AD). The majority of FTLD-causing mutations in PGRN are predicted to cause functional null alleles by nonsense-mediated decay of the mutant mRNA [24;28]. Therefore, haploinsufficiency with reduced PGRN-induced neuronal survival is thought to cause neurodegeneration in FTLD. Apart from null-function mutations in FTLD, PGRN gene expression is increased in a number of neurodegenerative diseases in which microglial activation occurs, including lysosomal storage disorders, viral encephalitis, prion-related disease (Creutzfeldt-Jakob disease), AD, and Amyotrophic lateral Sclerosis (ALS) [23]. It is still unclear how PGRN contributes in the pathological progression of these inflammatory neurodegenerative disorders. In contrast to FTLD, mechanisms other than the lack of neurotrophic effects may play a role in PGRN-associated neurodegeneration as well. In a recent study, PGRN knockdown was found to induce caspase-dependent cleavage of TAR DNA binding protein-43 (TDP-43) with accumulation of its fragments, which is thought to work as the pathologic substrate of neuronal glial inclusions in FTLD and ALS [34].
PGRN as a steroid-inducible gene in the developing and adult rat brain
While the roles of PGRN in the normal brain and neurodegeneration remain to be determined, our recent observations propose novel biological aspects of this molecule. We have shown that PGRN is a sex steroid-responsible gene that may be involved in masculinization of the perinatal rat brain [22;35–38]. We also showed that PGRN may be involved in the mitogenic effects in the active area of neural generation (neurogenesis) of the adult rat brain [39].
PGRN in sexual differentiation of the developing brain
Some physiological and behavioral brain functions are gender-specific [40–42]. This is particularly evident, but not limited, in reproductive physiology such as patterning secretion of reproductive hormones and sexual behaviors. A variety of hormones and neurotransmitters show different degrees of variety between males and females, but sex differences have also been reported in non-sexual behaviors like spatial orientation and verbal fluency, or adaptive mechanisms of the adrenal axis to stress [43].
The developing brain is feminine by default, unless some specific stimuli drive it to a male phenotype. The mechanisms of sexual differentiation of the brain by sex steroids seem to be conserved throughout the mammalian species, although there may be some species differences. In rats, the critical period for sexual differentiation of the brain has been considered from embryonic 18 until around postnatal day 10 [41]. The organization of the brain during the critical period is followed by the activational effects of hormones on sexual behavior in adulthood, leading to the organizational/activational hypothesis of the brain and behavior [44]. While testicular androgen is a primary molecule to induce sexual differentiation in the male rat brain, administering large doses of estrogen can also induce mascualinization of females and appears to fully mimic the effects of endogenous androgen. The developing fetus has high levels of circulating α-fetoprotein, a protein that potently binds estrogen. Furthermore, brain nuclei that are sexually dimorphic express high levels of aromatase, the enzyme that converts testosterone to estrogen [45;46]. In the developing male rat, testosterone secreted from the testes, which is not bound to α–fetoprotein, can enter the brain, and is locally converted to estrogen by aromatase in specific nuclei. This hypothesis, named the aromatization hypothesis, has been supported by additional data that blocking of neuronal aromatase or absence of this key enzyme during development prevents normal sexual differentiation of the male rodent brain.
A major unveiled question was the underlying mechanism by which estrogen mediates sexual differentiation of the rat brain. Sex steroids exert profound and selective influences on brain development through the regulation of neuronal and glial proliferation and differentiation [40;45;47]. Most of these effects by estrogen occur through interactions with estrogen receptors, which serve as transcription factors for a wide variety of cellular target genes. Therefore, analysis of the specific gene expression or protein synthesis induced by sex steroids would provide an approach for understanding the underlying mechanism of sexual differentiation of the rat brain [48–50].
Under this hypothesis, we investigated genes differentially expressed between sexes or induced expression by steroid treatment in neonatal rat hypothalamus using cDNA subtraction [22], and the PGRN gene was identified as one of the sex steroid-inducible genes [22]. The cDNA subtraction method is a simple and efficient technique for isolating clones that are unequally distributed between two samples of cells or tissues. PGRN mRNA was strongly enriched in the hypothalamus of male and androgenized (testosterone-treated) female 5-day-old pups. Transcription of PGRN was also up-regulated by exogenous estrogen in the neonatal hypothalamus [35], indicating that in male rats androgen may induce PGRN gene expression after being converted to estrogen. The expression of PGRN in the hypothalamus of males is maintained at high levels throughout the critical period, while in females it gradually decreased and abruptly declines after birth [22]. In the brain of a 5-day-old male rat, PGRN mRNA was strongly enriched in the ventromedial hypothalamic nucleus (VMH) and the arcuate nucleus (ARC) of the hypothalamus [22]. Interestingly, a dense assembly of estrogen receptors has been shown in VMH and ARC, and sex steroids have been show to affect to the synaptic structures in these area. Furthermore, VMH is known to play essential roles in the dimorphism of sexual behavior in rats.
Different patterns of PGRN expression between the sexes led us to hypothesize that higher PGRN expression in the neonatal hypothalamus during the critical period is requisite for the process of masculinization of the brain. To test this possibility, we adapted the antisense oligo-deoxynucleotide (ODN) method [36]. This method has been applied to cell culture and animals to block translation of the selective mRNA into protein. We designed antisense ODN complementary to PGRN mRNA sequence and infused them into the third ventricle of male rats at 2 days of age. After maturation, the subject animals that were treated with the antisense ODN had compromised male sexual behaviors as adults [36].
As an alternative approach to block PGRN expression in the brain and to elucidate the physiological roles of PGRN in vivo, we recently generated a line of mice with targeted disruption of the PGRN gene, and investigated male sexual behavior, aggression and anxiety [38]. PGRN-deficient mice exhibited a decrease in ejaculation incidence, while the latency and frequency of both mount and intromission were unchanged. For the aggressive behavior test, the resident-intruder paradigm was used, and PGRN-deficient mice exhibited enhanced aggressiveness. In wild-type mice, males exhibited lower levels of anxiety than females by the open field test, while male PGRN-deficient mice exhibited an elevated level of anxiety and sex difference in anxiety was not observed. Interestingly, mRNA expression of the serotonergic receptor 5-HT1A, which could be related to the inhibition of aggression and anxiety, was significantly reduced in the hippocampus of PGRN-deficient mice after aggressive encounters. On the other hand, deficiency of the PGRN gene did not affect serum testosterone concentrations. These results suggest that PGRN gene plays a role in establishing sexual dimorphic behaviours at least partially by modulating the brain serotonergic system. We have further demonstrated that PGRN-deficient mice have a larger volume of the locus ceruleus (LC), which is known to participate in inducing anxiety-like behavior (Chiba et al., JRD under review). This suggests that PGRN plays a role in the organization of the LC, which eventually modulates anxiety in novel environments.
PGRN and endocrine disruptors
Endocrine disruptors (or environmental endocrine disrupting chemicals, EDCs) are exogenous substances that act like hormones in the endocrine system and disrupt the physiologic function of endogenous hormones [51]. Studies have linked endocrine disruptors to adverse biological effects in animals, giving rise to concerns that low-level exposure might cause similar effects in human beings. Due to the plasticity and the high responsiveness of the fetal brain, its exposure to compounds able to interfere with these mechanisms during the critical period of brain sexual differentiation might cause detrimental effects on reproductive physiology [52;53]. More precise methods are necessary to define the impact of EDCs on the sexual differentiation of the brain. According to our observations of the steroid-dependent induction and sexually different expressing patterns, PGRN gene may be a good parameter for assessing sex steroid properties of EDCs in the neonatal brain. We recently assessed the effects of perinatal exposure of some phthalate/adipate esters, which are suspected to interfere with the endocrine system as EDCs, on PGRN gene expression in the neonatal hypothalamus and sexual behaviors after maturation [54]. PGRN expression was affected in the brains of male and female neonatal rats by perinatal exposure to these chemicals, while these treatments decreased sexual behaviors after maturation in other cohorts of rats. Perinatal exposure to these compounds may lead inappropriate expression of PGRN gene in the hypothami of neonatal rats, followed by permanent effects on the hypothalamus to alter the exhibition of sexual behaviors after maturation.
PGRN in adult neurogenesis
Recent studies have shown the presence of active neurogenesis even in specific areas of the adult brain, and it has been suggested that estrogen and various growth factors influence the processes of adult neurogenesis. In the hippocampus, neural precursor cells are located in the subgranular zone, which is the border region between the granule cell layer and the hilus in the dentate gyrus, and these precursor cells proliferate and produce daughter cells that are capable of differentiation into mature granule neurons [55]. Adult neurogenesis in the dentate gyrus has been recognized to be involved in learning and also the behavioral effects of antidepressants. Among the factors regulating neurogenesis, more attention has been paid to estrogen since it is known to enhance cell proliferation and increase the number of immature neurons in the adult dentate gyrus [56–59]. Enhanced neurogenesis is thought to be one of the routes through which estrogen exerts its effect on cognitive functions [60].
Given increasing evidence that PGRN is potentially mitogenic in culture and its expression is up-regulated by sex steroids in the developing CNS, we hypothesized that PGRN is also involved in the mitogenic effects of sex steroids in the active areas of neurogenesis in the adult brain. To answer this possibility, we recently assessed cell proliferation in the dentate gyrus and the mRNA expression levels of PGRN in the hippocampus 4 hours after treatment with estrogen in young adult (3-month old) and aged (12-month old) ovariectomized rats [39]. In young adult rats, PGRN gene expression and cell proliferation were increased by estrogen. However, neither PGRN gene expression nor cell proliferation in the dentate gyrus was affected by estrogen in aged females. Additionally, estrogen enhanced the proliferation of neural progenitor cells derived from hippocampal tissue of 3-month-old female rats in vitro; this was inhibited by neutralization of PGRN with specific antibodies. Together, these results suggest that PGRN may be involved in the mitogenic effects of estrogen in active areas of neurogenesis in the adult brain and that the product of this gene is involved in the mitotic effects of estrogen in the dentate gyrus, although the responses to estrogen decline with age.
Conclusion
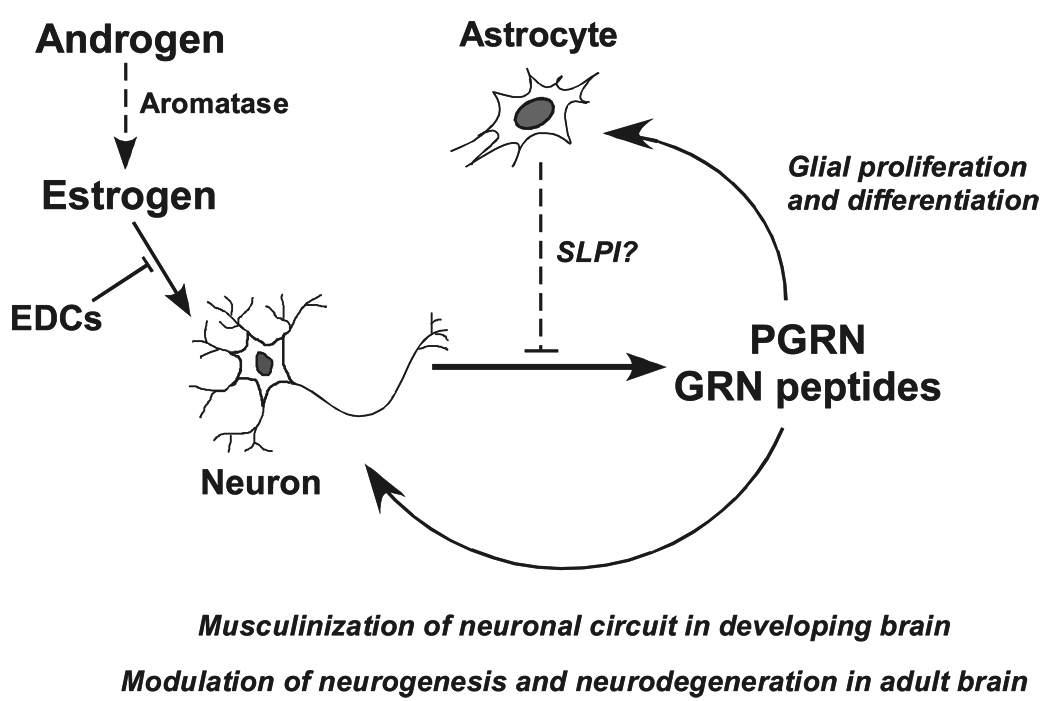
We have shown that sex-steroid exposure during the perinatal period increased PGRN expression in the rat hypothalamus. The reduction or depletion of PGRN expression influenced the exhibition of sexual dimorphic behaviors in the antisense ODN-treated rats or PGRN-deficient mice. Furthermore, PGRN works as a good parameter for assessing the estrogenic or anti-estrogenic actions of EDCs on the neonatal brain. These observations support our hypothesis that PGRN is involved in the organization of the male brain during the critical period. PGRN during the critical period might play a role in modulating the proliferation and differentiation of neurons and/or glial cells in an autocrine or paracrine manner, and consequently may masculinize the neuronal circuit necessary for exhibiting sexual dimorphic behaviors [37]. PGRN is a complex protein that has distinct functional properties as a precursor protein and its cleaved peptides. Although secretory and processing mechanisms of PGRN in the CNS still need to be resolved, SLPI may contribute to PGRN processing in the CNS since this protein is known to be generated in astrocytes. Additionally, the steroid-dependent induction of PGRN gene was also observed in the dentate gyrus of adult rat hippocampus, suggesting PGRN may play a key role to modulate the mitotic effects of estrogen in active areas of neurogenesis. Our findings indicate that PGRN may be involved in both sexual differentiation of the brain during perinatal period and neurogenesis in the hippocampus in adulthood in a sex-steroid dependent manner. This suggests that there is a common molecular mechanism between the developmental and neurotrophic effects of sex-steroids in the brain. Since haploinsufficiency of PGRN has been shown to cause FTLD, PGRN appears to play multifunctional roles in the organization of functional masculinization in developing brain and the maintenance of adult brain functions by modulating neurogenesis and neurodegeneration (Fig. 1).
Fig. 1.
Hypothesis of the roles of PGRN in the developing and adult brain. Estrogen converted from androgen by aromatase increases PGRN expression in neurons. The secreted PGRN or cleaved GRN peptides modulates the proliferation and differentiation of neurons and/or glial cells in an autocrine and/or paracrine manner, and consequently masculinizes the neuronal circuit in developing brain and modulates neurogenesis and neurodegeneration in adult and aged brain. Exposure to endocrine disruptors (EDCs) may lead inappropriate expression of PGRN gene. Astrocytes may influence protease activity by secretory leukocyte protease inhibitor (SLPI) and control the levels of PGRN and GRN peptides as in seen within wound healing in peripheral tissues.
It is important to understand how estrogen modulates PGRN expression and its trophic effects in the developing and adult CNS. Additional studies using in vitro systems and PGRN-deficient mice might be helpful to answer these questions. Further investigations will lead us to understand the biological roles of PGRN in the normal CNS and neurodegenerative diseases.
Acknowledgements
Research in the authors’ laboratory was funded in part by the grant from NIH/NINDS R21-NS06104, the Les Turner ALS foundation (to M.S.) and Grants-in-Aid for Scientific Research (19040004 and 20248030) from the Japan Society for the Promotion Science (to N.M.). We are extremely grateful to Ms. Jacalyn McHugh for helpful comments on the manuscript.
References
- 1.Bateman A, Bennett HP. Granulins: the structure and function of an emerging family of growth factors. J Endocrinol. 1998;158:145–151. doi: 10.1677/joe.0.1580145. [DOI] [PubMed] [Google Scholar]
- 2.Shoyab M, McDonald VL, Byles C, Todaro GJ, Plowman GD. Epithelins 1 and 2: isolation and characterization of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci U S A. 1990;87:7912–7916. doi: 10.1073/pnas.87.20.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhandari V, Giaid A, Bateman A. The complementary deoxyribonucleic acid sequence, tissue distribution, and cellular localization of the rat granulin precursor. Endocrinology. 1993;133:2682–2689. doi: 10.1210/endo.133.6.8243292. [DOI] [PubMed] [Google Scholar]
- 4.Daniel R, He Z, Carmichael KP, Halper J, Bateman A. Cellular localization of gene expression for progranulin. J Histochem Cytochem. 2000;48:999–1009. doi: 10.1177/002215540004800713. [DOI] [PubMed] [Google Scholar]
- 5.Serrero G, Ioffe OB. Expression of PC-cell-derived growth factor in benign and malignant human breast epithelium. Hum Pathol. 2003;34:1148–1154. doi: 10.1016/s0046-8177(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 6.He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res. 2002;62:5590–5596. [PubMed] [Google Scholar]
- 7.Tangkeangsirisin W, Serrero G. PC cell-derived growth factor (PCDGF/GP88, progranulin) stimulates migration, invasiveness and VEGF expression in breast cancer cells. Carcinogenesis. 2004;25:1587–1592. doi: 10.1093/carcin/bgh171. [DOI] [PubMed] [Google Scholar]
- 8.He Z, Bateman A. Progranulin (granulin-epithelin precursor, PC-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med. 2003;81:600–612. doi: 10.1007/s00109-003-0474-3. [DOI] [PubMed] [Google Scholar]
- 9.Davidson B, Alejandro E, Florenes VA, Goderstad JM, Risberg B, Kristensen GB, Trope CG, Kohn EC. Granulin-epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer. 2004;100:2139–2147. doi: 10.1002/cncr.20219. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura N, Mandai M, Miyanishi M, Fukuhara K, Baba T, Higuchi T, Kariya M, Takakura K, Fujii S. Oncogenic property of acrogranin in human uterine leiomyosarcoma: direct evidence of genetic contribution in in vivo tumorigenesis. Clin Cancer Res. 2006;12:1402–1411. doi: 10.1158/1078-0432.CCR-05-2003. [DOI] [PubMed] [Google Scholar]
- 11.Monami G, Gonzalez EM, Hellman M, Gomella LG, Baffa R, Iozzo RV, Morrione A. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103–7110. doi: 10.1158/0008-5472.CAN-06-0633. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Hayashi J, Serrero G. PC cell-derived growth factor confers resistance to dexamethasone and promotes tumorigenesis in human multiple myeloma. Clin Cancer Res. 2006;12:49–56. doi: 10.1158/1078-0432.CCR-05-0929. [DOI] [PubMed] [Google Scholar]
- 13.Lu R, Serrero G. Mediation of estrogen mitogenic effect in human breast cancer MCF-7 cells by PC-cell-derived growth factor (PCDGF/granulin precursor) Proc Natl Acad Sci U S A. 2001;98:142–147. doi: 10.1073/pnas.011525198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MB, Houwink AP, Freeman BK, Greenwood TM, Lafky JM, Lingle WL, Berchuck A, Maxwell GL, Podratz KC, Maihle NJ. The granulin-epithelin precursor is a steroid-regulated growth factor in endometrial cancer. J Soc Gynecol Investig. 2006;13:304–311. doi: 10.1016/j.jsgi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Baba T, Hoff HB, III, Nemoto H, Lee H, Orth J, Arai Y, Gerton GL. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34:233–243. doi: 10.1002/mrd.1080340302. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M, Matsumuro M, Hirabayashi K, Ogawara M, Takahashi M, Nishihara M. Oocyte-specific expression of granulin precursor (Acrogranin) in rat ovary. Journal of Reproduction and Development. 2000;46:271–277. [Google Scholar]
- 17.Qin J, az-Cueto L, Schwarze JE, Takahashi Y, Imai M, Isuzugawa K, Yamamoto S, Chang KT, Gerton GL, Imakawa K. Effects of progranulin on blastocyst hatching and subsequent adhesion and outgrowth in the mouse. Biol Reprod. 2005;73:434–442. doi: 10.1095/biolreprod.105.040030. [DOI] [PubMed] [Google Scholar]
- 18.Zanocco-Marani T, Bateman A, Romano G, Valentinis B, He ZH, Baserga R. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res. 1999;59:5331–5340. [PubMed] [Google Scholar]
- 19.Zhu J, Nathan C, Jin W, Sim D, Ashcroft GS, Wahl SM, Lacomis L, Erdjument-Bromage H, Tempst P, Wright CD, Ding A. Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell. 2002;111:867–878. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Ong CH, Halper J, Bateman A. Progranulin is a mediator of the wound response. Nat Med. 2003;9:225–229. doi: 10.1038/nm816. [DOI] [PubMed] [Google Scholar]
- 21.Guerra RR, Kriazhev L, Hernandez-Blazquez FJ, Bateman A. Progranulin is a stress-response factor in fibroblasts subjected to hypoxia and acidosis. Growth Factors. 2007;25:280–285. doi: 10.1080/08977190701781222. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki M, Yoshida S, Nishihara M, Takahashi M. Identification of a sex steroid-inducible gene in the neonatal rat hypothalamus. Neurosci Lett. 1998;242:127–130. doi: 10.1016/s0304-3940(98)00008-1. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed Z, Mackenzie IR, Hutton ML, Dickson DW. Progranulin in frontotemporal lobar degeneration and neuroinflammation. J Neuroinflammation. 2007;4:7. doi: 10.1186/1742-2094-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 25.Eriksen JL, Mackenzie IR. Progranulin: normal function and role in neurodegeneration. J Neurochem. 2008;104:287–297. doi: 10.1111/j.1471-4159.2007.04968.x. [DOI] [PubMed] [Google Scholar]
- 26.Van DP, Van HA, Lambrechts D, Vanacker P, Bogaert E, van SJ, Carmeliet P, Van Den BL, Robberecht W. Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Biol. 2008;181:37–41. doi: 10.1083/jcb.200712039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilzecka J, Stelmasiak Z. Increased serum levels of endogenous protectant secretory leukocyte protease inhibitor in acute ischemic stroke patients. Cerebrovasc Dis. 2002;13:38–42. doi: 10.1159/000047744. [DOI] [PubMed] [Google Scholar]
- 28.Cruts M, Gijselinck I, van der ZJ, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin JJ, van DC, Peeters K, Sciot R, Santens P, De PT, Mattheijssens M, Van den BM, Cuijt I, Vennekens K, De Deyn PP, Kumar-Singh S, Van BC. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–924. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 29.Brouwers N, Sleegers K, Engelborghs S, Maurer-Stroh S, Gijselinck I, van der ZJ, Pickut BA, Van den BM, Mattheijssens M, Peeters K, Schymkowitz J, Rousseau F, Martin JJ, Cruts M, De Deyn PP, Van BC. Genetic variability in progranulin contributes to risk for clinically diagnosed Alzheimer disease. Neurology. 2008;71:656–664. doi: 10.1212/01.wnl.0000319688.89790.7a. [DOI] [PubMed] [Google Scholar]
- 30.Cruts M, Van BC. Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet. 2008;24:186–194. doi: 10.1016/j.tig.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Sleegers K, Brouwers N, Maurer-Stroh S, van Es MA, Van DP, van Vught PW, van der ZJ, Serneels S, De PT, Van den BM, Cruts M, Schymkowitz J, De JP, Rousseau F, van den Berg LH, Robberecht W, Van BC. Progranulin genetic variability contributes to amyotrophic lateral sclerosis. Neurology. 2008;71:253–259. doi: 10.1212/01.wnl.0000289191.54852.75. [DOI] [PubMed] [Google Scholar]
- 32.van Swieten JC, Heutink P. Mutations in progranulin (GRN) within the spectrum of clinical and pathological phenotypes of frontotemporal dementia. Lancet Neurol. 2008;7:965–974. doi: 10.1016/S1474-4422(08)70194-7. [DOI] [PubMed] [Google Scholar]
- 33.Irwin D, Lippa CF, Rosso A. Progranulin (PGRN) expression in ALS: An immunohistochemical study. J Neurol Sci. 2009;276:9–13. doi: 10.1016/j.jns.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson D, Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki M, Yonezawa T, Fujioka H, Matuamuro M, Nishihara M. Induction of granulin precursor gene expression by estrogen treatment in neonatal rat hypothalamus. Neurosci Lett. 2001;297:199–202. doi: 10.1016/s0304-3940(00)01699-2. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki M, Bannai M, Matsumuro M, Furuhata Y, Ikemura R, Kuranaga E, Kaneda Y, Nishihara M, Takahashi M. Suppression of copulatory behavior by intracerebroventricular infusion of antisense oligodeoxynucleotide of granulin in neonatal male rats. Physiol Behav. 2000;68:707–713. doi: 10.1016/s0031-9384(99)00241-3. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki M, Nishiahara M. Granulin precursor gene: a sex steroid-inducible gene involved in sexual differentiation of the rat brain. Mol Genet Metab. 2002;75:31–37. doi: 10.1006/mgme.2001.3274. [DOI] [PubMed] [Google Scholar]
- 38.Kayasuga Y, Chiba S, Suzuki M, Kikusui T, Matsuwaki T, Yamanouchi K, Kotaki H, Horai R, Iwakura Y, Nishihara M. Alteration of behavioural phenotype in mice by targeted disruption of the progranulin gene. Behav Brain Res. 2007;185:110–118. doi: 10.1016/j.bbr.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Chiba S, Suzuki M, Yamanouchi K, Nishihara M. Involvement of granulin in estrogen-induced neurogenesis in the adult rat hippocampus. J Reprod Dev. 2007;53:297–307. doi: 10.1262/jrd.18108. [DOI] [PubMed] [Google Scholar]
- 40.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Negri-Cesi P, Colciago A, Pravettoni A, Casati L, Conti L, Celotti F. Sexual differentiation of the rodent hypothalamus: hormonal and environmental influences. J Steroid Biochem Mol Biol. 2008;109:294–299. doi: 10.1016/j.jsbmb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 43.McCarthy MM, Konkle AT. When is a sex difference not a sex difference? Front Neuroendocrinol. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 45.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 46.MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 1987;50:459–474. doi: 10.1016/0039-128x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- 47.MacLusky NJ, Naftolin F. Sexual differentiation of the central nervous system. Science. 1981;211:1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- 48.Yonehara K, Suzuki M, Yamanouchi K, Nishihara M. Expression analyses of sex steroid-regulated genes in neonatal rat hypothalamus. J Reprod Dev. 2003;49:547–552. doi: 10.1262/jrd.49.547. [DOI] [PubMed] [Google Scholar]
- 49.Yonehara K, Suzuki M, Yamanouchi K, Nishihara M. Androgen induces p130 mRNA expression in the neonatal rat hypothalamus. Neurosci Lett. 2002;334:107–110. doi: 10.1016/s0304-3940(02)01114-x. [DOI] [PubMed] [Google Scholar]
- 50.Yonehara K, Suzuki M, Nishihara M. Sex-related differences in gene expression in neonatal rat hypothalamus assessed by cDNA microarray analysis. Endocr J. 2002;49:131–137. doi: 10.1507/endocrj.49.131. [DOI] [PubMed] [Google Scholar]
- 51.Waldron AC, Naber EC. Importance of feed as an unavoidable source of pesticide contamination in poultry meat and eggs. 2. Residues in eggs and tissues. Poult Sci. 1974;53:1428–1435. doi: 10.3382/ps.0531428. [DOI] [PubMed] [Google Scholar]
- 52.Dohler KD. Influence of hormones and hormone antagonists on sexual differentiation of the brain. Arch Toxicol Suppl. 1998;20:131–141. doi: 10.1007/978-3-642-46856-8_12. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki M, Lee HC, Chiba S, Yonezawa T, Nishihara M. Effects of methoxychlor exposure during perinatal period on reproductive function after maturation in rats. Journal of Reproduction and Development. 2004;50:455–461. doi: 10.1262/jrd.50.455. [DOI] [PubMed] [Google Scholar]
- 54.Lee HC, Yamanouchi K, Nishihara M. Effects of perinatal exposure to phthalate/adipate esters on hypothalamic gene expression and sexual behavior in rats. J Reprod Dev. 2006;52:343–352. doi: 10.1262/jrd.17096. [DOI] [PubMed] [Google Scholar]
- 55.Gould E, Tanapat P, Rydel T, Hastings N. Regulation of hippocampal neurogenesis in adulthood. Biol Psychiatry. 2000;48:715–720. doi: 10.1016/s0006-3223(00)01021-0. [DOI] [PubMed] [Google Scholar]
- 56.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 58.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 59.Brannvall K, Korhonen L, Lindholm D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol Cell Neurosci. 2002;21:512–520. doi: 10.1006/mcne.2002.1194. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]