Abstract
N-{4-[(2-amino-6-ethyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)thio]benzoyl}-L-glutamic acid 2 and thirteen nonclassical analogues 2a–2m were synthesized as potential dual thymidylate synthase (TS) and dihydrofolate reductase (DHFR) inhibitors and as antitumor agents. The key intermediate in the synthesis was 2-amino-6-ethyl-5-iodothieno[2,3-d]pyrimidin-4(3H)-one, 7, to which various aryl thiols were attached at the 5-position. Coupling 8 with L-glutamic acid diethyl ester and saponification afforded 2. X-ray crystal structure of 2 and 1 (the 6-methyl analogue of 2), DHFR and NADPH showed for the first time that the thieno[2,3-d]pyrimidine ring binds in a “folate” mode. Compound 2 was an excellent dual inhibitor of human TS (IC50 = 54 nM) and human DHFR (IC50 = 19 nM), and afforded nanomolar GI50 values against tumor cells in culture. The 6-ethyl substitution in 2 increases both the potency (by two- to three-orders of magnitude) as well as the spectrum of tumor inhibition in vitro compared to the 6-methyl analogue 1. Some of the nonclassical analogues were potent and selective inhibitors of DHFR from Toxoplasma gondii.
Introduction
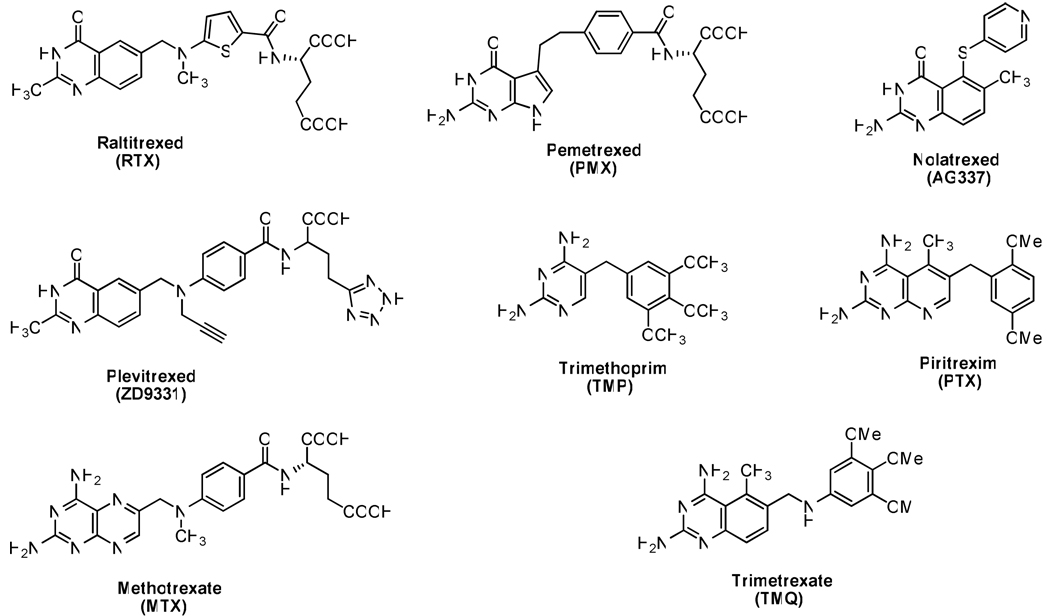
Folate metabolism has long been recognized as important and an attractive target for chemotherapy due to its crucial role in the biosynthesis of nucleic acid precursors.1,2 Inhibitors of folate-dependent enzymes have found clinical utility as antitumor, antimicrobial, and antiprotozoal agents 2–5 (Figure 1). Thymidylate synthase (TSa), which catalyzes the reductive methylation of deoxyuridylate (dUMP) to thymidylate (dTMP), has been of particular interest.6,7 This reaction affords 7,8-dihydrofolate (7,8-DHF), which is reduced by dihydrofolate reductase (DHFR) to tetrahydrofolate (THF).3, 5–7 Thus, TS and DHFR are crucial for the synthesis of dTMP in dividing cells. Several TS and DHFR inhibitors, as separate entities, have found clinical utility as antitumor agents.8–12 Usually a 2,4-diamino-substituted pyrimidine ring is considered important for potent DHFR inhibitory activity, while a 2-amino-4-oxopyrimidine or 2-methyl-4-oxopyrimidine ring is considered important for potent TS inhibitory activity.3,7,8 Examples of clinically used TS and DHFR inhibitors are raltitrexed,12 pemetrexed11 and methotrexate13 (MTX) illustrated in Figure 1. Raltitrexed is a quinazoline analogue that is transported into cells via the reduced folate carrier (RFC) and undergoes rapid polyglutamylation by the enzyme folylpolyglutamate synthetase (FPGS). Raltitrexed is approved as a first-line agent for advanced colorectal cancer in several European countries, Australia, Canada, and Japan. In pemetrexed a pyrrole ring replaces the pyrazine of folic acid and a methylene group replaces the N10-nitrogen in the bridge.11 Pemetrexed contains a 6-5 fused pyrrolo[2,3-d]pyrimidine scaffold and is designated a multitargeted antifolate (MTA). Pemetrexed and its polyglutamylated metabolites are reported to be inhibitors of several important folate-dependent enzymes including TS, DHFR, glycinamide ribonucleotide formyltransferase (GARFT), and aminoimidazole carboxamide ribonucleotide formyltransferase (AICARFT).11,16,17
Figure 1.
Representative antifolates.
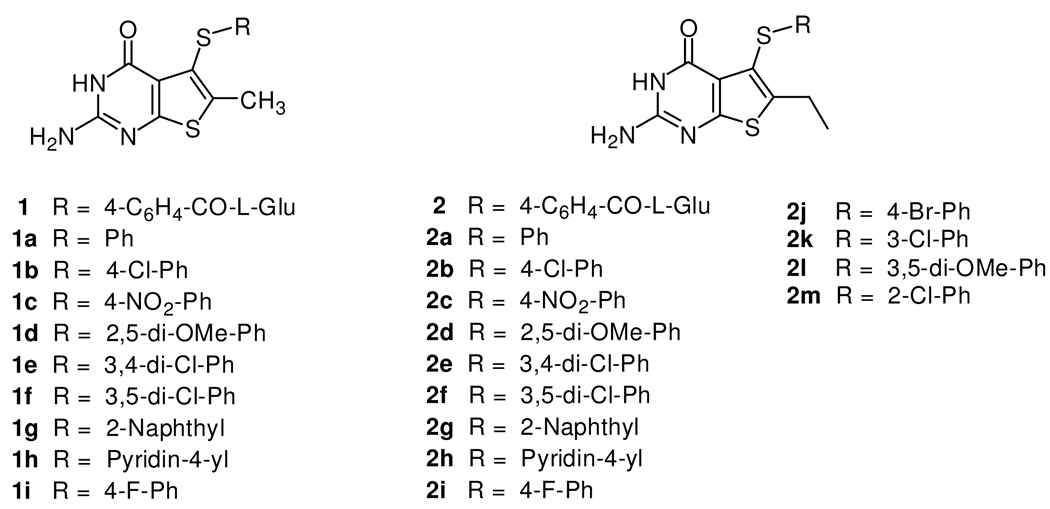
As part of a continuing effort to develop novel classical antifolates as antitumor agents, Gangjee et al.18 reported the synthesis of N-{4-[(2-amino-6-methyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)sulfanyl]benzoyl}-L-glutamic acid 1 (Figure 2) as a potent dual inhibitor of TS and DHFR with IC50 values in the 10−8 M range. Compound 1 also demonstrated moderate inhibitory activity against the growth of several human tumor cell lines in the National Cancer Institute (NCI)19 preclinical in vitro screen, with GI50 values of 10−5 to 10−6 M or lower. The dual TS/DHFR inhibitory potency and tumor cell inhibitory activities of 1 were, in part, attributed to its C6-methyl group. Molecular modeling using SYBYL 8.020 indicated that compound 1 could bind to human DHFR in the “flipped” mode compared to folic acid, in which the sulfur atom of thieno ring is superimposed on to the 4-oxo moiety of folate. Additionally, molecular modeling of 1 in human TS also suggested that homologation of the C6-methyl to an ethyl could further enhance the hydrophobic interaction with Trp109 and perhaps the antitumor activity as well.
Figure 2.
6-Methyl and Target 6-Ethyl–2-amino-4-oxo-5-substituted thieno[2,3-d]pyrimidines.
A disadvantage of classical antifolates as antitumor agents is that they require an active transport mechanism to enter cells, which, when impaired, causes tumor resistance.21,22 In addition, cells that lack these transport mechanisms, including many bacterial and protozoan cells, are not susceptible to the action of classical antifolates.23–26 In an attempt to overcome these potential drawbacks, nonclassical lipophilic antifolates have been developed as antitumor agents that do not require the folate transport system(s) but enter cells via diffusion. These lipophilic nonclassical antifolates such as nolatrexed (Figure 1) lack the polar glutamate moiety and hence do not depend on FPGS for their inhibitory activity.27,28 In addition, nonclassical antifolates do not require the reduced folate carrier (RFC) system for active uptake into the cell since they are lipophilic and are passively transported into cells.
An additional aspect of our interest in nonclassical dual TS-DHFR inhibitors lies in the treatment of opportunistic infections in immunocompromised patients such as those with acquired immunodeficiency syndrome (AIDS).29,30 The principal cause of death in patients with AIDS is opportunistic infections caused by Pneumocystis carinii (P. carinii)31 and Toxoplasma gondii (T. gondii).32 Current therapy includes the use of selective but weak inhibitors of protozoal DHFR such as trimethoprim (TMP) (Figure 1), in combination with sulfonamides to enhance potency. Toxicity of the sulfa drug component of these combinations is often a serious problem.32 In addition, the potent but toxic nonclassical antifolates trimetrexate (TMQ) and piritrexim (PTX) (Figure 1), co-administered with leucovorin for host rescue, are also used. Serious toxicities associated with the use of TMQ and PTX often force the cessation of treatment. Thus, it is of considerable interest to incorporate selectivity and potency into a single nonclassical antifolate that can be used alone to treat these infections.
Gangjee et al.18 recently described the design and synthesis of several nonclassical 2-amino-4-oxo-5-arylthio-substituted-6-methylthieno[2,3-d]pyrimidine 1a–1i (Figure 2) as dual TS/DHFR inhibitors. All of the nonclassical analogues were reasonably potent inhibitors of human TS with IC50 values ranging from 0.11 to 4.6 µM. The electronic nature of the substitutent on the side chain phenyl was an important factor in determining inhibitory potency. Analogues with electron withdrawing substitutions on the phenyl ring were more potent than analogues with electron donating substitutions or the unsubstituted phenyl. Structure activity relationship (SAR) studies demonstrated that analogues with electron withdrawing groups at the 3- and/or 4-positions of the phenyl side chain provided optimum inhibitory potency against human TS. Nonclassical analogues such as 1b (IC50 = 0.26 µM), 1c (IC50 = 0.11 µM), 1e (IC50 = 0.11 µM), 1g (IC50 = 0.12 µM) or 1h (IC50 = 0.28 µM) were much more potent than the clinically used raltitrexed and pemetrexed against human TS, thus nonclassical analogues 2a–2m containing similar phenyl substituents were also synthesized. Interestingly, all the nonclassical compounds 1a–1i18were potent inhibitors of T. gondii DHFR with IC50 values ranging from 0.028 to 0.12 µM. The IC50 values of compounds 1b–1i against T. gondii DHFR were similar in potency to MTX, and were about 243-fold more potent than the clinically used TMP. In addition, all the nonclassical compounds showed good to excellent selectivity against T. gondii DHFR as compared to human DHFR. Analogue 1c (IC50 = 0.56 µM) was the most potent compound in this series against human DHFR, and it was 28-fold less potent against human DHFR than MTX but was more than 12-fold more potent than pemetrexed. Compound 1d with a 2,5-dimethoxy substitution on the phenyl ring was marginally active against human DHFR (IC50 = 22 µM), but very potent against T. gondii DHFR (IC50 = 56 nM) exhibiting 393-fold selectivity compared to human DHFR. As indicated above, molecular modeling (SYBYL 8.0) suggested that an extension of the 6-methyl group to an ethyl group might enhance the potency and selectivity against some pathogenic TS and DHFR. To determine the effect of 6-ethyl homologation on human TS and DHFR inhibitory activity in the classical and nonclassical analogues, compounds 2-2m (Figure 2) were synthesized. The synthesis and biological activities of analogues 2-2m are the subject of this report.
Chemistry
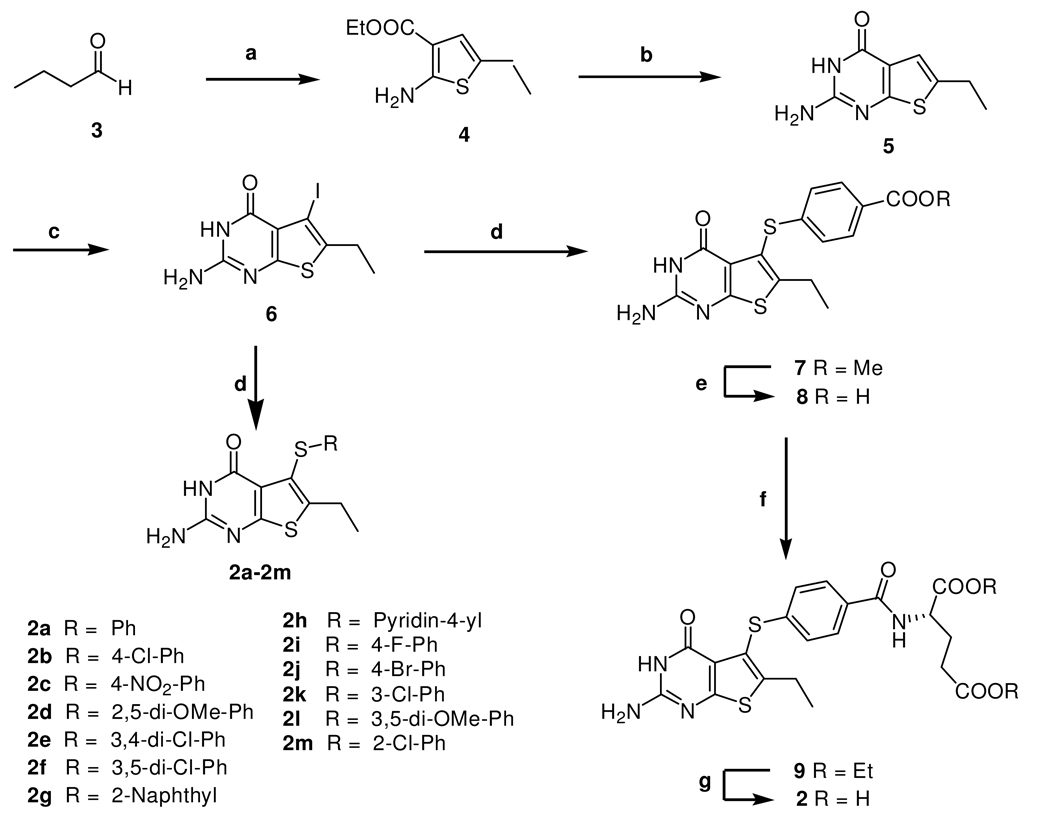
The synthetic strategy for target compounds 2-2m is shown in Scheme 1. The key intermediate in the synthesis was 2-amino-6-ethyl-5-iodothieno[2,3-d]pyrimidin-4(3H)-one, 6 (Scheme 1), which could undergo microwave assisted palladium catalyzed coupling reactions with appropriate aryl thiols to afford target compounds 2a–2m and intermediate 7 for the synthesis of classical analogue 2.
Scheme 1a.
a Reagents: (a) Ethylcyanoacetate, Et3N, Sulfur, DMF, 55 °C, 3h; (b) carbamimidic chloride hydrochloride, DMSO2, 120 °C, 1 h; (c) (1) Hg(AcO)2, AcOH, 100 °C, 3 h; (2) I2, CH2Cl2, rt, 5 h; (d) thiols, Pd2(dba)3, Xantphos, i-Pr2NEt, DMF, microwave 190 °C, 30 min; (e) 1 N NaOH, MeOH; (f) Lglutamate hydrochloride, 2-chloro-4,6-dimethoxy-1,3,5-triazine, N-methylmorpholine, DMF, rt, 5h; (g) 1 N NaOH, EtOH.
The required intermediate ethyl 2-amino-5-ethylthiophene-3-carboxylate, 4 was synthesized from commercially available butyraldehyde, 3 with ethylcyanoacetate, sulfur and triethylamine via reported methods of Gewald.33 With compound 4 in hand, we turned our attention to its conversion to the 2-amino-6-ethylthieno[2,3-d]pyrimidin-4-(3H)-one, 5. A literature search revealed that the synthesis of compound 5 was not reported. Gangjee et al.34 had previously reported that chlorformamidine hydrochloride on cyclization with ethyl 2-amino-5-methylthiophene-3-carboxylate, gave 2-amino-6-methylthieno[2,3-d]pyrimidin-4(3H)-one in reasonably good yield. Thus heating a mixture of 4 and chlorformamidine hydrochloride in DMSO2 under N2 for a period of 2 h at 120–125 °C gave 5 in good yields (86%) after column chromatography.
Recently, we reported a convenient C5-bromination for the 2-amino-4-oxo-6-methyl-thieno[2,3-d]pyrimidine template with Br2 under microwave irradiation.18 To our surprise, all efforts to perform this halogenation of 5 with Br2 or NBS using a variety of reaction conditions of time and temperature variations proved fruitless. Thus we had to consider an alternate halogenation method. A search of the literature revealed that there was no synthetic or other report for 6. However, Taylor et al.35 had reported mercuration methodology that could be adopted for the synthesis of the key intermediate 2-amino-6-ethyl-5-iodothieno[2,3-d]pyrimidin-4(3H)-one 6. Extending this methodology to the synthesis of 6 required the 5-chloromercuri derivative, which was obtained by mercuration of 5 with mercurate acetate in glacial acetic acid at 100 °C for 3 h, followed by treatment with NaCl solution. Without separation, this 5-chloromercuri derivative was treated with iodoine in CH2Cl2 at room temperature for 5 h to afford 6 in 42% yield (over two steps). With the key intermediate 6 in hand, attention was turned to its conversion to the target compounds 2a–2m and intermediate 7 (Scheme 1). Palladium-catalyzed cross-coupling reactions36 to form carbon-sulfur bonds with aryl bromides and aryl thiols appeared attractive for the synthesis of the target compounds 2a–2m and intermediate 7. Initial attempts to react the iodo derivative 6 with corresponding aryl thiols catalyzed by Pd2(dba)3 with variations of time (up to 8 h) and temperature (up to reflux) were unsuccessful.
Failure of the above attempts led us to explore an alternative strategy to perform this palladium-catalyzed cross-coupling reaction. Microwave irradiation has been widely applied in organic synthesis resulting in faster and cleaner reactions that sometimes exhibit different reactivities due to specific microwave absorption. It was therefore of interest to attempt this cross-coupling reaction under microwave irradiation. Thus heating a mixture of 6, the appropriate aryl thiols and i-Pr2NEt in DMF in the presence of Pd2(dba)3 and Xantphos under microwave irradiation at 190 °C for 1 h afforded the corresponding target compounds 2a–2m in the yields of 67–87%.
For the synthesis of the classical compound 2, the required intermediate, methyl 4-[(2-amino-6-ethyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)thio]benzoate, 7 (Scheme 1), was prepared using the same synthetic strategy as shown for 2a–2m. Methyl 4-mercaptobenzoate was used to afford 7 in a yield of 76%. Ester hydrolysis of 7 with 1 N NaOH at room temperature for 18 h afforded the corresponding free acid 8 in 96% yield. Coupling of the acid 8 (Scheme 1) with L-glutamic acid diethyl ester hydrochloride and 2-chloro-4,6-dimethoxy-l,3,5-triazine as the activating agent followed by column chromatographic purification afforded 9 in 70% yield. The 1H NMR of 9 revealed the newly formed peptide NH proton at 8.61 ppm as a doublet, which exchanged on addition of D2O. Compound 9 was characterized on the basis of NMR and MS. Hydrolysis of 9 with aqueous NaOH at room temperature, followed by acidification with 3 N HCl under ice cold conditions, afforded target compound 2 in 94% yield.
X-ray Crystal Structure
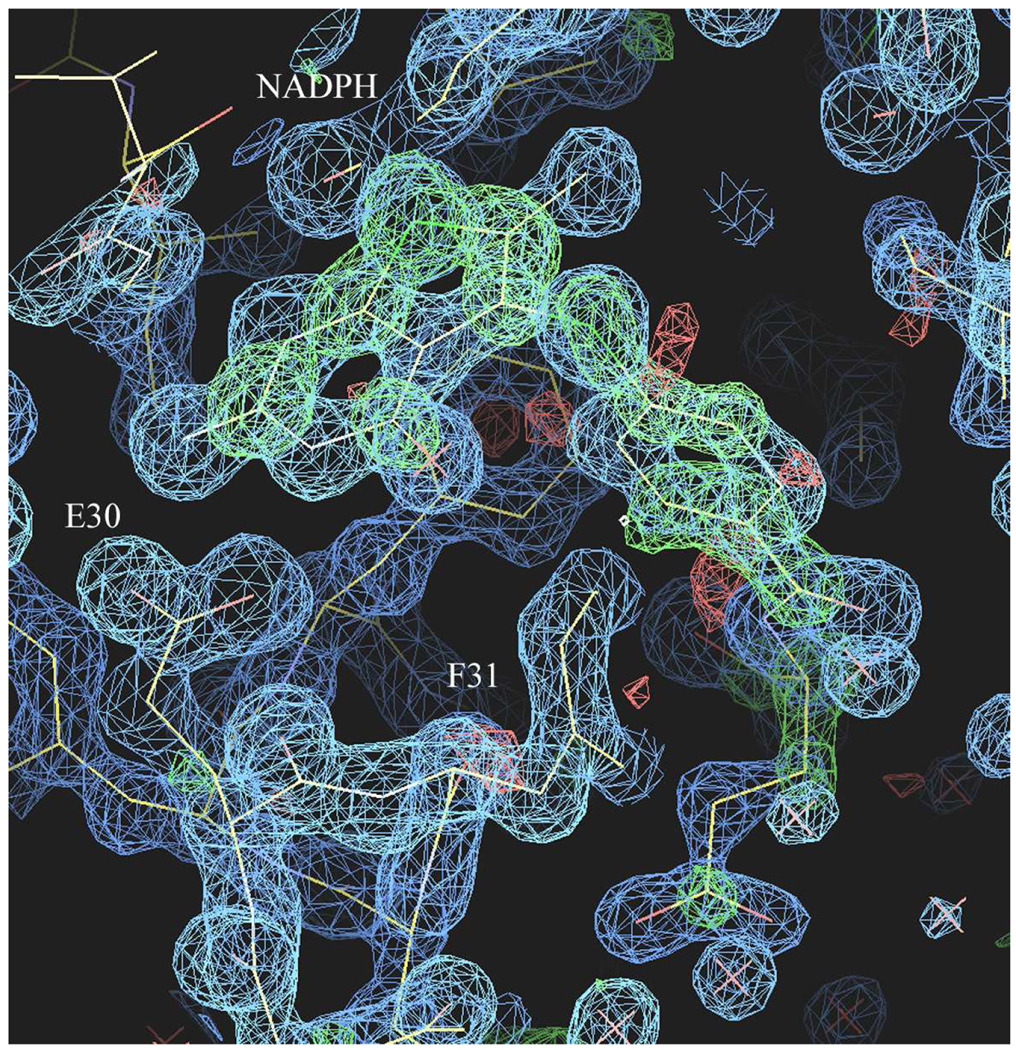
The X-ray crystal structures of the ternary complex of 2, NADPH, and the human DHFR double mutants (Q35K/N64F and Q35S/N64S) were determined, as well as complexes of 1 with the Q35K single mutant protein and wild type human DHFR. All DHFR ternary complex structures were refined to 1.3-1.5 Ǻ resolution. These results show, for the first time, that the thieno[2,3-d]pyrimidine antifolates 1 and 2 bind in a folate orientation such that the thieno sulfur occupies the N8 position observed in the binding of folic acid. Careful analysis of the difference electron density maps (Figure 3) was carried out to validate the binding orientation of 1 and 2. The difference electron density for all structures reveals that the thieno ring of 1 and 2 bind in the folate orientation.
Figure 3.
Difference electron density map (2Fo-Fc, 1σ blue, Fo-Fc 3σ green) for the ternary complex of NADPH and 1 in human DHFR
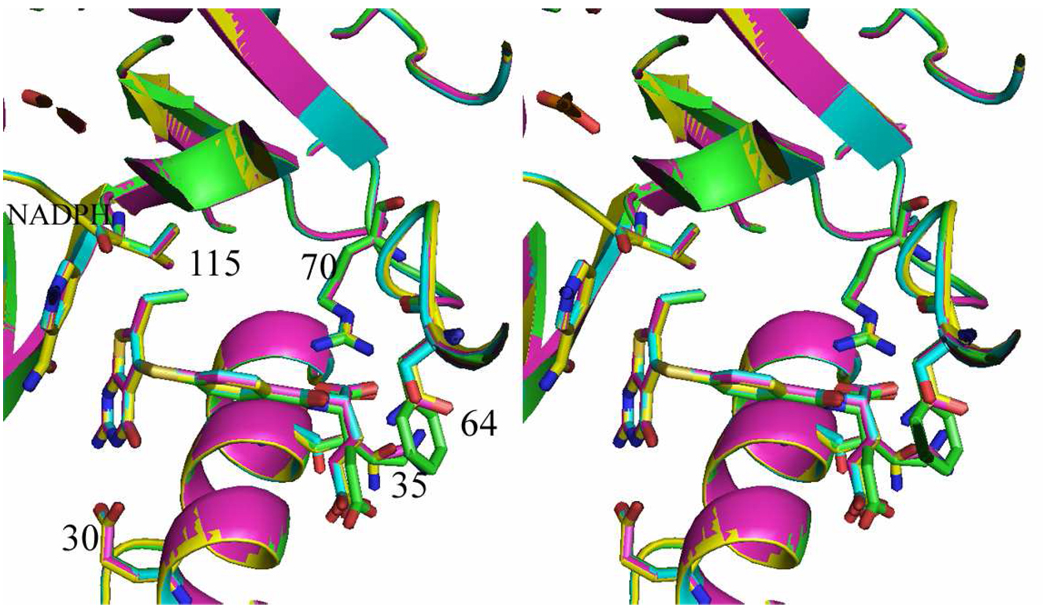
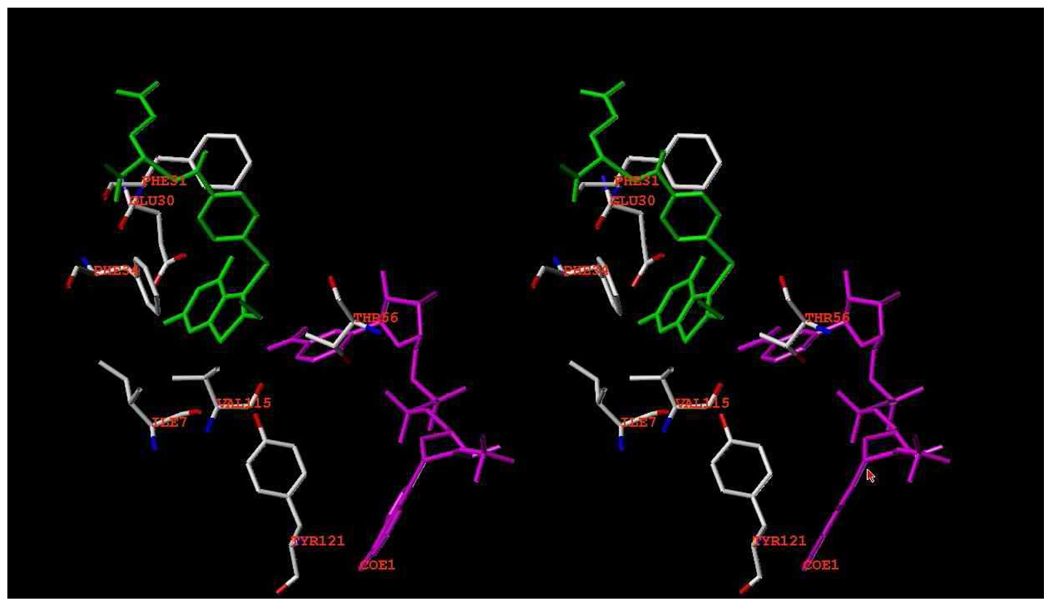
In these structures, the thieno ring has no hydrogen donor function. Nevertheless, the S7 of the thieno[2,3-d]pyrimidine ring makes intermolecular contacts with the carbonyl of Ile7 (3.6 Δ), Val115 (3.9 Δ), the hydroxyl of Tyr121 (3.8 Δ), and the carbonyl of the nicotinamide ring (3.4 Δ) (Figure 4). These values are longer than those observed for the 2,4-diamino pyrrolo[2,3-d]pyrimidine and furo[2,3-d]pyrimidine structures that showed a flipped orientation.29,37,38. Although molecular modeling studies carried out previously18 predicted that both a flipped as well as folate binding orientation was possible for 2-amino-4-oxo-6-methyl thieno[2,3-d]pyrimidine, the structural data for four examples of 1 and 2 bound to mutant human DHFR all show binding in the folate orientation.
Figure 4.
Stereoview of superposition of active site for human DHFR-Q35K/N64F double mutant ternary complex with the inhibitor 2 and NADPH (green), human DHFR-Q35S/N64S double mutant ternary complex with the inhibitor 2 and NADPH (cyan), human DHFR-Q35K single mutant ternary complex with 1 (violet) and human DHFR ternary complex with 1 (yellow). The figure was prepared by PyMol.
Biological Evaluation and Discussion
The classical analogue 2 and the nonclassical analogues 2a–2m were evaluated as inhibitors of human, Escherichia coli (E. coli), and T. gondii DHFR39 and TS.40 The inhibitory potencies (IC50) are listed in Table 1 and compared with pemetrexed, PDDF, MTX, and trimethoprim and the previously reported values for 1.
Table 1.
Inhibitory Concentrations (IC50 in µM) against TS and DHFR.a
| TS (µM) |
DHFR (µM) |
||||||
|---|---|---|---|---|---|---|---|
| compound | humanb | E. colib | T. gondiie | humanc | E. colid |
T. gondiie |
rh/tgf |
| 1g | 0.04 | 0.04 | 0.036 | 0.02 | 0.2 | 0.008 | 2.5 |
| 2 | 0.054 | 0.018 | 0.09 | 0.019 | 1.0 | 0.0021 | 9 |
| 2a | 5.6 | 28 | 8.4 | 2.6 | >33 (10)h | 0.033 | 79 |
| 2b | 0.75 | 2.5 | 2.2 | 0.3 | 42 | 0.009 | 33 |
| 2c | 0.22 | 1.7 | 1.0 | 0.26 | 39 | 0.0087 | 30 |
| 2d | 4.6 | >23 (11) | >23 (29) | 0.84 | >28 (0) | 0.014 | 60 |
| 2e | 0.23 | 2.3 | 1.0 | 0.29 | 22 | 0.0081 | 36 |
| 2f | 0.69 | 1.2 | 1.6 | 0.28 | >2.8 (0) | 0.014 | 20 |
| 2g | 2.3 | 1.4 | 1.1 | 2.2 | 28 | 0.0084 | 262 |
| 2h | 0.28 | 2.0 | 1.7 | 0.35 | >33 (17) | 0.017 | 21 |
| 2i | 1.3 | 2.9 | 2.6 | 0.62 | >31 (15) | 0.025 | 25 |
| 2j | 0.39 | 2.2 | 2.2 | 0.26 | 29 | 0.013 | 20 |
| 2k | 0.38 | 2.3 | 1.8 | 0.3 | >3.0 (0) | 0.012 | 25 |
| 2l | 1.2 | 1.8 | 2.3 | 0.54 | >27 (0) | 0.024 | 23 |
| 2m | 1.1 | 2.0 | 2.5 | 0.6 | >33 (0) | 0.027 | 22 |
| Pemetrexedi | 9.5 | 76 | 2.8 | 6.6 | 230 | 0.43 | 15.35 |
| PDDFj | 0.085 | 0.019 | 0.43 | 1.9 | 23 | 0.22 | 8.6 |
| MTX | nd | nd | nd | 0.02 | 0.0088 | 0.033 | 0.6 |
| Trimethoprim | nd | nd | nd | >340 (22) | 0.01 | 6.8 | >50 |
The percent inhibition was determined at a minimum of four inhibitor concentrations within 20% of the 50% point. The standard deviations for determination of 50% points were within ± 10% of the value given.
Kindly provided by Dr. Frank Maley, New York State Department of Health.
Kindly provided by Dr. Karen Anderson, Yale University, New Haven CT.
Kindly provided by Dr. J. H.Freisheim, Medical College of Ohio, Toledo, OH.
Kindly provided by Dr. R. L. Blakley, St. Jude Children’s hospital, Memphis TN.
rh/tg is Selectivity Ratio for T. gondii DHFR and is the IC50 against rhDHFR / IC50 against T. gondii DHFR.
Data derived from ref18,nd = not determined.
Numbers in parentheses indicate the % inhibition at the stated concentration.
Kindly provided by Dr. Chuan Shih, Eli Lilly and Co.
Kindly provided by Dr. M. G. Nair, University of South Alabama.
The classical analogue 2 (Table 1) was an excellent dual inhibitor of human TS (IC50 = 54 nM) and human DHFR (IC50 = 19 nM). Against human TS, 2 was similar in potency to the previously reported compound 1 and about 2-fold more potent than PDDF and a remarkable 238-fold more potent than the clinically used pemetrexed.
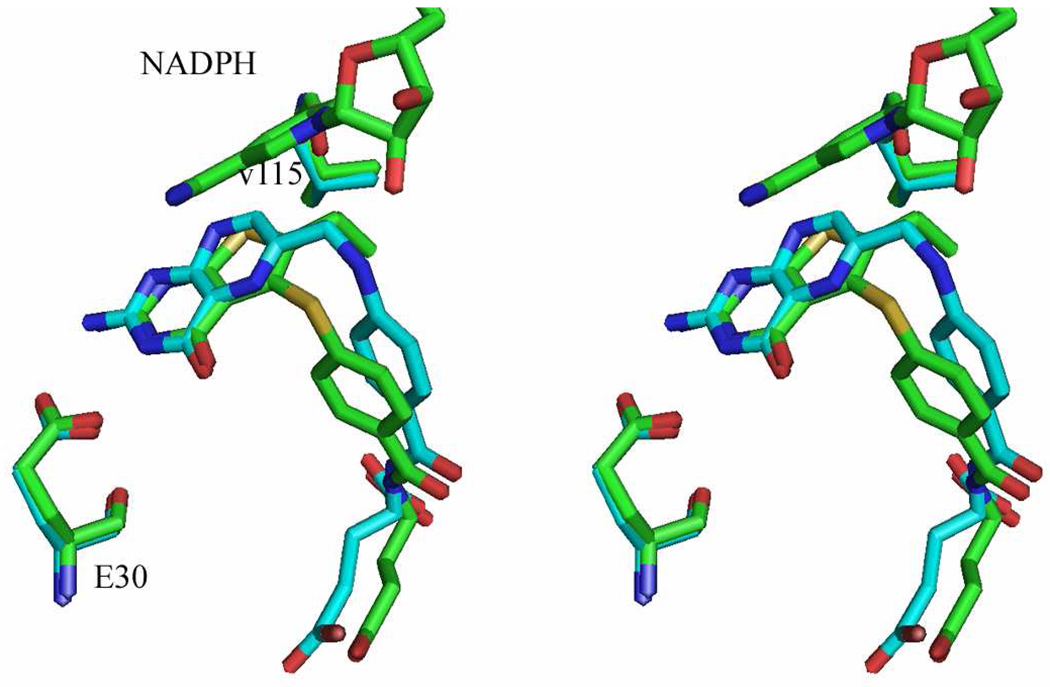
Against human DHFR (Table 1) 2 was similar in potency to 1 and the clinically used MTX (Table 1) and was 330-fold more potent than pemetrexed. Interestingly, compound 2 was 9-fold more potent against T. gondii DHFR than human DHFR, indicating a significant species difference. Compound 2 was somewhat more potent than 1 as an inhibitor of human DHFR. This increase in activity against human DHFR of 2 over 1 may be attributed to increased hydrophobic interaction of the 6-ethyl moiety of 2 and Val115 in human DHFR as predicted from molecular modeling and confirmed by the X-ray crystal structure (Figure 6). The biological data (IC50) of compounds 1 and 2 indicate that the methyl and ethyl groups at the C6-position respectively are both conducive for potent human TS and DHFR inhibition.
Figure 6.
Stereoview of active site for human DHFR-Q35S/N64S double mutant ternary complex with the inhibitor 2 and NADPH. The figure was prepared by SYBY 8.0.
The nonclassical analogues 2a–2m were also evaluated as inhibitors of TS and DHFR (Table 1). In the human TS assay, all of the nonclassical analogues were reasonably potent inhibitors with IC50 values ranging from 0.22 to 5.6 µM. The electronic nature of the substitutent on the side chain phenyl was an important factor in determining inhibitory potency. Analogues with electron withdrawing substitutions on the phenyl ring were more potent than analogues with electron donating substitutions or the unsubstituted phenyl. Electron withdrawing, 4-nitro, 3,4-dichloro, 3-chloro and 4-bromo substituents in analogues 2c, 2e, 2k and 2j, respectively, showed the most potent inhibition against isolated human TS. In addition, bulky substituents such as the 2-naphthyl (2g) showed marginal activity against human TS. These data are consistent with SAR studies previously reported for the C6-methyl analogues.18 The nonclassical analogues 1b, 1c, 1e, 1g and 1h of the 6-methyl series were potent human TS inhibitors.18 The corresponding 6-ethyl analogues 2b, 2c, 2e, 2g and 2h of this study were similar in potency except for 2g which was about 20-fold less potent than 1g. This difference in potency may reflect a steric intolerance of the larger 6-ethyl moiety with an adjacent naphthyl ring in 2g. Similar to the classical analogue 2, all of the nonclassical analogues were also more potent than pemetrexed as inhibitors of human TS. This result indicates that homologation of a 6-methyl to a 6-ethyl in thieno[2,3-d]pyrimidines maintains potent human TS inhibitory activity.
In the DHFR assay, the nonclassical analogues 2a–2m were also evaluated as inhibitors of human, E. coli and T. gondii DHFR. Against human DHFR, in general 2a–2m were moderately potent inhibitors with IC50 values ranging from 0.26 to 2.2 µM, and were more potent than the corresponding 6-methyl analogues.18 The most potent nonclassical analogues contained electron withdrawing groups such as 4-nitrophenyl 2c (IC50 = 0.26 µM) and 4-bromophenyl 2j (IC50 = 0.26 µM) in the 6-ethyl (2a–2m) series. Other substitutions such as an unsubstituted phenyl 2a (IC50 = 2.6 µM) and unsubstituted 2-naphthyl 2g (IC50 = 2.2 µM) cause a 10-fold drop in activity. In addition, compound 2c was the most potent compound in the nonclassical series, also demonstrating potent dual inhibitory activities against human TS (IC50 = 0.22 µM) and human DHFR (IC50 = 0.26 µM). The SAR among these analogues (2a–2m) suggests that potency for human DHFR is independent of the electronic nature of the substituent but mono para-electron withdrawing substitution is most favorable for human DHFR inhibition. Against T. gondii DHFR, in general, the nonclassical analogues 2a–2m were very potent inhibitors with IC50 values ranging from 0.27 to 0.0081 µM. The most potent single digit nanomolar nonclassical inhibitors contained electron withdrawing groups such as a 4-chlorophenyl 2b (IC50 = 0.009 µM), 4-nitrophenyl 2c (IC50 = 0.0087 µM), 3,4-dichlorophenyl 2e (IC50 = 0.0081 µM) and 2-naphthyl 2g (IC50 = 0.0084 µM). The IC50 values of compounds 2b, 2c, 2e and 2g against T. gondii DHFR were a remarkable 4-fold more potent than MTX, and were about 840-fold more potent than the clinically used trimethoprim (Table 1). To our knowledge these are some of the most potent nonclassical T. gondii DHFR inhibitors reported. Interestingly, a number of the nonclassical compounds in this series also showed good selectivity for T. gondii DHFR as compared to human DHFR. Compound 2g with a 2-naphthyl substitution was 262-fold more selective for T. gondii DHFR than human DHFR, which indicated a distinct species difference in DHFR from different sources. These results demonstrate that the nonclassical analogues 2a–2m in the 6-ethyl series follow similar trends of dual inhibition against human TS and DHFR as the 6-methyl analogues.
The classical analogue 2 was selected by the National Cancer Institute (NCI) for evaluation in its in vitro preclinical antitumor screening program. The full NCI panel of approximately 60 human cancer cell lines are grouped into disease subpanels including leukemia, nonsmall-cell lung, colon, central nervous system (CNS), melanoma, ovarian, renal, prostate, and breast tumors cell lines. The ability of compound 2 to inhibit the growth of tumor cell lines was measured as GI50 values, the concentration required to inhibit the growth of tumor cells in culture by 50% as compared to a control. In 8 of the 60 cell lines, compound 2 showed GI50 values of < 10−6 M (Table 2). It was also interesting to note that compound 2 was not a general cell poison but showed selectivity both within a type of tumor cell line as well as across different tumor cell lines with inhibitory values that in some instances differed by 1000-fold (data not shown). In addition, potency of tumor inhibition (GI50) was significantly increased for 2 over 1 (Table 2) by a hundred- to thousand-fold, indicating that homologation of the 6-methyl to the 6-ethyl was instrumental in increasing the potency as well as the spectrum of tumor inhibition in culture (Table 2). Thus the in vitro tumor cell inhibitory activity of 2 was much superior to that of 1. Possible explanation for this increased in vivo activity could be the increased lipophilicity of 2 compared to 1, which could facilitate passive diffusion and/or active transport of 2 into tumor cells. This is currently under investigation. Compound 2 is also currently under further evaluation by the NCI as an antitumor agent.
Table 2.
Cytotoxic Evaluation (GI50, M) of Compounds 1 and 2 against Selected Tumor Cell Lines.
| cell line | GI50 of 1 (M) | GI50 of 2 (M) |
|---|---|---|
| Leukemia | ||
| CCRF-CEM | 9.45 × 10−5 | 8.53 × 10−7 |
| HL-60 (TB) | 4.97 × 10−7 | |
| MOLT-4 | >1.00 × 10−4 | 4.78× 10−6 |
| Non-Small Cell Lung Cancer | ||
| NCI-H460 | 4.12 × 10−5 | 9.59 × 10−7 |
| NCI-H525 | >1.00 × 10−4 | 7.80 × 10−6 |
| NCI-H23 | >1.00 × 10−4 | 9.40 × 10−6 |
| Colon Cancer | ||
| HCC-2998 | >1.00 × 10−4 | 9.65 × 10−7 |
| HCT-116 | >1.00 × 10−4 | 2.84 × 10−7 |
| Melanoma | ||
| LOX IMVI | 6.56 × 10−5 | 9.09 × 10−8 |
| M14 | >1.00 × 10−4 | 4.32× 10−6 |
| Ovarian Cancer | ||
| OVCAR-8 | >1.00 × 10−4 | 5.79 × 10−7 |
| Renal Cancer | ||
| 786-0 | >1.00 × 10−4 | 8.17 × 10−7 |
| SN12C | >1.00 × 10−4 | 3.31 × 10−6 |
In summary, the 5-substituted 2-amino-4-oxo-6-ethyl-thieno[2,3-d]pyrimidine classical antifolate 2 and thirteen nonclassical analogues 2a–2m were designed and synthesized as potential dual TS-DHFR inhibitor. Compound 2 (TS IC50= 54 nM; DHFR IC50= 19 nM) maintained the potent human TS and DHFR inhibitory activity of 1. More importantly compound 2 significantly increased both the spectrum as well as the potency of the inhibition of the growth of tumor cells in culture compared with 1. This increase was clearly not attributable to enzyme inhibition difference because both 1 and 2 have about the same activity in isolated enzyme assays. Compound 2c was the most potent compound in the nonclassical series, also demonstrating potent dual inhibitory activities against human TS (IC50 = 0.22 µM) and human DHFR (IC50 = 0.26 µM). In addition, excellent potency and high selectivity for T. gondii DHFR compared to human DHFR was observed for all the analogues. This study indicated that the 5-substituted 2-amino-4-oxo-6-ethyl-thieno[2,3-d]pyrimidine scaffold is most conducive to dual human TS-DHFR inhibitory activity. The most remarkable finding was that elongation of the C6-methyl moiety of 2-amino-4-oxo-5-arylthio-substituted thieno[2,3-d]pyrimidine 1 to the 6-ethyl in 2 increases the inhibitory potency against the growth of several tumor cells in culture by two to three orders of magnitude and also increases the spectrum of tumor growth inhibition. These results are currently under investigation and may reflect transport and/or other differences between 1 and 2.
Experimental Section
Analytical samples were dried in vacuo (0.2 mm Hg) in a CHEM-DRY drying apparatus over P2O5 at 80 °C. Melting points were determined on a MEL-TEMP II melting point apparatus with FLUKE 51 K/J electronic thermometer and are uncorrected. Nuclear magnetic resonance spectra for proton (1H NMR) were recorded on a Bruker WH-400 (400 MHz) spectrometer. The chemical shift values are expressed in ppm (parts per million) relative to tetramethylsilane as an internal standard: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad singlet. Mass spectra were recorded on a VG-7070 double-focusing mass spectrometer or in a LKB-9000 instrument in the electron ionization (EI) mode. Chemical names follow IUPAC nomenclature. Thin-layer chromatography (TLC) was performed on Whatman Sil G/UV254 silica gel plates with a fluorescent indicator, and the spots were visualized under 254 and 366 nm illumination. Proportions of solvents used for TLC are by volume. Column chromatography was performed on a 230–400 mesh silica gel (Fisher, Somerville, NJ) column. Elemental analyses were performed by Atlantic Microlab, Inc., Norcross, GA. Element compositions are within 0.4% of the calculated values. Fractional moles of water or organic solvents frequently found in some analytical samples of antifolates could not be prevented in spite of 24–48 h of drying in vacuo and were confirmed where possible by their presence in the 1H NMR spectra. Microwave-assisted synthesis was performed utilizing an Emrys Liberator microwave synthesizer (Biotage) utilizing capped reaction vials. All microwave reactions were performed with temperature control. All solvents and chemicals were purchased from Aldrich Chemical Co. or Fisher Scientific and were used as received.
Ethyl 2-amino-5-ethylthiophene-3-carboxylate (4)
Et3N (4.7 g, 0.05 mol) was added to a stirred suspension of ethylcyanoacetate (9.74 g, 90 mmol) and sulfur (2.76 g, 90 mol) in 50 mL DMF under a N2 atmosphere. The resulting mixture was stirred at 55 °C (temperature of bath) for 1 h. Butyraldehyde 3 (6.5 g, 90 mmol) was then added into this suspension dropwise while maintaining the temperature at 55° C. After addition, the reaction was allowed to cool to room temperature and stirred for 2 h. The mixture was transferred to a separating funnel containing ethyl acetate (80 mL) and H2O (30 mL). The organic layer was separated, washed with brine (4 × 20 mL), and concentrated under reduced pressure to afford yellow oil. The residue was loaded on a column packed with silica gel and eluted with 5% ethyl acetate in hexanes and fractions containing the desired product (TLC) were pooled and evaporated to afford 8.9 g (56%) of 4 as a yellow solid: mp 70–72° C; Rf 0.45 (ethyl acetate/n-hexane, 1:3); 1H NMR (DMSO-d6) δ 1.08 (t, 3 H, J = 7.2 Hz), 1.19 (t, 3 H, J = 7.2 Hz), 2.48 (q, 2 H, J = 7.2 Hz), 4.11 (q, 2 H, J = 7.2 Hz), 6.45 (s, 1H), 7.07 (s, 2 H).
2-Amino-6-ethylthieno[2,3-d]pyrimidin-4-(3H)-one (5)
Ethyl 2-amino-5-ethylthiophene-3-carboxylate 4 (9.5 g, 47.8 mmol), carbamimidic chloride hydrochloride (5.5 g, 48.3 mmol) and 25 g DMSO2 was placed in a 250 mL flask. The reaction mixture was heated to 120–125 °C under N2 for 2 h. Then H2O (50 mL) was added to the reaction mixture right away to quench the reaction. The resulting solution was cooled in an ice bath, and the pH was adjusted to 8 with dropwise addition of concentrated NH4OH with stirring. This suspension was left at 5° C for 3 h and filtered. The residue was washed well with small amounts of acetone and water, purified by flash chromatography on silica gel (gradient: 2% MeOH/CHCl3 to 5% MeOH/CHCl3) to afford 8.1 g (86%) of 5 as a yellow solid: mp 126–128° C; Rf 0.63 (MeOH/CHCl3, 1:10); 1H NMR (DMSO-d6) δ 1.20 (t, 3 H, J = 7.6 Hz), 2.71 (q, 2 H, J = 7.6 Hz), 6.45 (s, 2 H), 6.79 (s, 1 H), 10.82 (s, 1 H). Anal. (C8H9N3OS•0.09CH3COCH3) C, H, N, S.
2-Amino-6-ethyl-5-iodothieno[2,3-d]pyrimidin-4(3H)-one (6)
To a suspension of 5 (3.5 g, 17.9 mmol) in 50 mL of glacial acetic acid at room temperature was added mercuric acetate (8.5 g, 26.8 mmol). The resulting solution was stirred at 100 °C for 3 h, then poured into a saturated NaCl (50 mL), and stirred for 20 min. The solid was collected by filtration, washed with water (20 mL), hexane (20 mL) and dried to give a dark solid (5-chloromercury derivative), which was directly used for iodination reaction without further purification. This dark material was dissolved in CH2Cl2 (30 mL) containing I2 (6.8 g, 26.8 mmol), stirred for 5 h at room temperature. The solvent was evaporated, the residue was washed with 2 N NaS2O3 (35 mL) and dried in vacuo. The crude product was purified by column chromatography on silica gel with 3% MeOH/CHCl3 as the eluent to afford 2.4 g (42%) of 6 as a white solid: mp 185–187° C; Rf 0.45 (MeOH/CHCl3, 1:5); 1H NMR (DMSO-d6) δ 1.13 (t, 3 H, J = 7.6 Hz), 2.67 (q, 2 H, J = 7.6 Hz), 6.57 (s, 2 H), 10.93 (s, 1 H). Anal. (C8H8IN3OS•0.34CH2Cl2) C, H, N, I, S.
Methyl 4-[(2-amino-6-ethyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)thio]benzoate (7)
The microwave reaction vial was charged with 6 (1.05 g, 3.3 mmol), i-Pr2NEt (1.2 mL, 6.6 mmol) and 15 mL dry DMF. The mixture was evacuated and backfilled with nitrogen (3 cycles). Catalyst Pd2dba3 (76 mg, 0.08 mmol), Xantphos (94 mg, 0.16 mmol) and methyl 4-mercaptobenzoate (1.1 g, 6.6 mmol) were added and then the reaction mixture was degassed twice. The reaction mixture was irradiated in a microwave apparatus at 190° C, 1 h. After the reaction mixture was cooled to ambient temperature, the product was filtered, the filtrate was concentrated, and the crude mixture was purified by silica gel column chromatography using 2% MeOH in CHCl3 as the eluent. Fractions containing the product (TLC) were combined and evaporated to afford 0.9 g (76%) of 7 as a white solid: Rf = 0.47 (MeOH/CHCl3, 1:5); mp 156–158° C; 1H NMR (DMSO-d6) δ 1.10 (t, 3 H, J = 7.2 Hz), 2.81 (q, 2 H, J = 7.2 Hz), 6.61 (s, 2 H), 7.05 (d, 2 H, J = 8.4 Hz), 7.77 (d, 2 H, J = 8.4 Hz), 10.79 (s, 1 H). HRMS (EI) calcd for C16H15N3O3S2 m/z = 361.0554, found m/z = 361.0556.
2-Amino-6-ethyl-5-(phenylsulfanyl)thieno[2,3-d]pyrimidin-4(3H)-one (2a)
Compound 2a (synthesized as described for 7): yield 87%; mp 215–220° C; TLC Rf = 0.48 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.10 (t, 3 H, J = 7.6 Hz), 2.82 (q, 2 H, J = 7.6 Hz), 6.57 (s, 2 H), 6.97–7.24 (m, 5 H), 10.76 (s, 1 H). HRMS (EI) calcd for C14H13N3OS2 m/z = 303.0500, found m/z = 303.0503.
2-Amino-5-[(4-chlorophenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2b)
Compound 2b (synthesized as described for 7): yield 81%; mp 174–175° C; TLC Rf = 0.47 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.11 (t, 3 H, J = 7.6 Hz), 2.82 (q, 2 H, J = 7.6 Hz), 6.59 (s, 2 H), 6.98 (d, 2 H, J = 8.4 Hz), 7.27 (d, 2 H, J = 8.4 Hz), 10.76 (s, 1 H). HRMS (EI) calcd for C14H12N3OClS2 m/z = 337.0110, found m/z = 337.0093.
2-Amino-6-ethyl-5-[(4-nitrophenyl)sulfanyl]thieno[2,3-d]pyrimidin-4(3H)-one (2c)
Compound 2c (synthesized as described for 7): yield 74%; mp 162–163° C; TLC Rf = 0.48 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.12 (t, 3 H, J = 7.6 Hz), 2.82 (q, 2 H, J = 7.6 Hz), 6.64 (s, 2 H), 7.16 (d, 2 H, J = 9.2 Hz), 8.07 (d, 2 H, J = 9.2 Hz), 10.83 (s, 1 H). HRMS (EI) calcd for C14H13N4O3S2 m/z = 349.0429, found m/z = 349.0403.
2-Amino-5-[(2,5-dimethoxyphenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2d)
Compound 2d (synthesized as described for 7): yield 82%; mp 178–180° C; TLC Rf = 0.50 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.08 (t, 3 H, J = 7.2 Hz), 2.77 (q, 2 H, J = 7.2 Hz), 3.52 (s, 3 H), 3.79 (s, 3 H), 5.92 (s, 2 H), 6.58–6.88 (m, 3 H), 10.78 (s, 1 H). HRMS (EI) calcd for C16H17N3O3S2 m/z = 363.0711, found m/z = 363.0706.
2-Amino-5-[(3,4-dichlorophenyl)thio]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2e)
Compound 2e (synthesized as described for 7): yield 72%; mp 154-116° C; TLC Rf = 0.48 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.12 (t, 3 H, J = 7.6 Hz), 2.86 (q, 2 H, J = 7.6 Hz), 6.62 (s, 2 H), 6.90 (dd, 1 H, J = 2.4 Hz, J = 8.4 Hz), 7.3 (d, 2 H, J = 2.4 Hz), 7.46 (d, 1 H, J = 8.4 Hz), 10.80 (s, 1 H). HRMS (EI) calcd for C14H11N3OS2Cl2 m/z = 370.9715, found m/z = 370.9720.
2-Amino-5-[(3,5-dichlorophenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2f)
Compound 2f (synthesized as described for 7): yield 74%; mp 176–178° C; TLC Rf = 0.45 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.12 (t, 3 H, J = 7.6 Hz), 2.84 (q, 2 H, J = 7.6 Hz), 6.58 (s, 2 H), 6.98 (s, 1 H), 7.05 (s, 1 H), 7.20 (s, 1 H), 10.76 (s, 1 H). HRMS (EI) calcd for C14H11N3OS2Cl2 m/z = 370.9708, found m/z = 370.9720.
2-Amino-6-ethyl-5-(2-naphthylthio)thieno[2,3-d]pyrimidin-4(3H)-one (2g)
Compound 2g (synthesized as described for 7): yield 87%; mp 154–156° C; TLC Rf = 0.51 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.12 (t, 3 H, J = 7.2 Hz), 2.86 (q, 2 H, J = 7.2 Hz), 6.59 (s, 2 H), 7.15–7.82 (m, 7 H), 10.74 (s, 1 H). HRMS (EI) calcd for C18H15N3OS2 m/z = 353.0656, found m/z = 353.0653.
2-Amino-6-ethyl-5-(pyridin-4-ylsulfanyl)thieno[2,3-d]pyrimidin-4(3H)-one (2h)
Compound 2h (synthesized as described for 7): yield 67%; mp 185–186° C; TLC Rf = 0.50 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.11 (t, 3 H, J = 7.2 Hz), 2.80 (q, 2 H, J = 7.2 Hz), 6.63 (s, 2 H), 6.92 (d, 2 H, J = 4.8 Hz), 8.28 (d, 2 H, J = 4.8 Hz), 10.83 (s, 1 H). HRMS (EI) calcd for C13H13N4OS2 m/z = 305.0531, found m/z = 305.0528.
2-Amino-6-ethyl-5-[(4-fluorophenyl)sulfanyl]thieno[2,3-d]pyrimidin-4(3H)-one (2i)
Compound 2i (synthesized as described for 7): yield 75%; mp 194–196° C; TLC Rf = 0.50 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.12 (t, 3 H, J = 7.2 Hz), 2.84 (q, 2 H, J = 7.2 Hz), 6.59 (s, 2 H), 7.07–7.09 (m, 4 H), 10.77 (s, 1 H). HRMS (EI) calcd for C14H12N3OFS2 m/z = 321.0405, found m/z = 321.0404.
2-Amino-5-[(4-bromophenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2j)
Compound 2j (synthesized as described for 7): yield 74%; mp 186–187° C; TLC Rf = 0.51 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.11 (t, 3 H, J = 7.2 Hz), 2.82 (q, 2 H, J = 7.2 Hz), 6.60 (s, 2 H), 6.92 (d, 2 H, J = 6.8 Hz), 7.39 (d, 2 H, J = 6.8 Hz), 10.76 (s, 1 H). HRMS (EI) calcd for C14H12N3OBrS2 m/z = 380.9611, found m/z = 380.9605.
2-Amino-5-[(3-chlorophenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2k)
Compound 2k (synthesized as described for 7): yield 72%; mp 186–187° C; TLC Rf = 0.53 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.14 (t, 3 H, J = 7.2 Hz), 2.87 (q, 2 H, J = 7.2 Hz), 6.62 (s, 2 H), 6.90 (m, 3 H), 7.24 (s, 1H), 10.80 (s, 1 H). HRMS (EI) calcd for C14H12ClN3OS2 m/z = 337.0119, found m/z = 337.0110.
2-Amino-5-[(3,5-dimethoxyphenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2l)
Compound 2l (synthesized as described for 7): yield 83%; mp 151–154° C; TLC Rf = 0.45 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.14 (t, 3 H, J = 7.6 Hz), 2.89 (q, 2 H, J = 7.6 Hz), 3.67 (s, 3 H), 6.55 (s, 2 H), 6.56 (dd, 1 H, J = 2.4 Hz, J = 8.4 Hz), 6.83 (d, 2 H, J = 2.4 Hz), 6.87 (d, 1 H, J = 8.4 Hz), 10.78 (s, 1 H). HRMS (EI) calcd for C16H17N3O3S2 m/z = 363.0721, found m/z = 363.0711.
2-Amino-5-[(2-chlorophenyl)sulfanyl]-6-ethylthieno[2,3-d]pyrimidin-4(3H)-one (2m)
Compound 2m (synthesized as described for 7): yield 72%; mp 221–224° C; TLC Rf = 0.53 (CHCl3/MeOH, 5:1); 1H NMR (DMSO-d6) δ 1.14 (t, 3 H, J = 7.2 Hz), 2.85 (q, 2 H, J = 7.2 Hz), 6.63 (s, 2 H), 6.90 (m, 4 H), 10.80 (s, 1 H). HRMS (EI) calcd for C14H12ClN3OS2 m/z = 337.0113, found m/z = 337.0110.
4-[(2-Amino-6-ethyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)thio]benzoic acid (8)
To a solution of 7 (0.72 g, 1.6 mmol) in MeOH (10 mL) was added 1 N NaOH (32 mL) and the reaction mixture stirred at room temperature for 18 h until TLC showed that the reaction was complete. The reaction mixture was evaporated to dryness under reduced pressure. The residue was dilute with H2O (5 mL). The resulting solution was adjusted to pH 4 with 3 N aq HCl then stored at 0° C for 24 h. The precipitated solid was collected and dried in vacuo using P2O5 to afford 0.53 g (96%) of 8 as a white powder: mp 216–218° C; Rf 0.34 (MeOH/CHCl3, 1:4); 1H NMR (DMSO-d6) δ 1.13 (t, 3 H, J = 7.6 Hz), 2.83 (q, 2 H, J = 7.6 Hz), 6.62 (s, 2 H), 7.03 (d, 2 H, J = 8.4 Hz), 7.76 (d, 2 H, J = 8.4 Hz), 10.79 (s, 1 H). Anal. (C15H13N3O3S2•1.94CH3COCH3) C, H, N, S.
Diethyl N-{4-[(2-amino-6-ethyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)thio]-benzoyl}-L-glutamate (9)
To a suspension of benzoic acid 8 (0.4 g, 1.1 mmol) in DMF (10 mL) at 25 °C was added N-methylmorpholine (0.12 mL, 1.21 mmol) followed by 2-chloro-4,6-dimethoxy-1,3,5-triazine (0.21 g, 1.1 mmol), and the resulting solution was stirred at 25° C for 2 h. At this point, N-methylmorpholine (0.12 mL, 1.21 mmol) was added to this solution followed by L-glutamic acid diethyl ester hydrochloride (0.31 g, 1.32 mmol), and the resulting mixture was stirred at 25° C for another 4 h until the starting material 8 disappeared (TLC). The reaction solution was evaporated in vacuo to dryness, and the residue was purified by column chromatography on silica gel with 5% MeOH in CHCl3 as the eluent. Fractions containing the product (TLC) were combined and evaporated to afford 0.41 g (70%) of 9 as a white solid: mp 150–152° C; Rf 0.57 (MeOH/CHCl3, 1:5); 1H NMR (DMSO-d6) δ 1.12–1.17 (m, 6 H), 1.14 (t, 3 H, J = 7.6 Hz), 1.93–2.10 (m, 2 H), 2.39 (m, 2 H), 2.86 (q, 2 H, J = 7.6 Hz), 4.05 (m, 4 H), 4.37 (m, 1 H), 6.61 (s, 1 H), 7.02 (d, 2 H, J = 8.4 Hz), 7.69 (d, 2 H, J = 8.4 Hz), 8.61 (d, 1 H, J = 7.6 Hz), 10.79 (br s, 1 H). Anal. (C24H28N4O6S2·0.59CH3COCH3) C, H, N, S. HRMS (EI) calcd for C24H28N4O6S2 m/z = 532.1450, found m/z = 532.1444.
N-{4-[(2-amino-6-ethyl-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-5-yl)thio]benzoyl }-L-glutamic acid (2)
To a solution of 9 (0.35 g, 0.65 mmol) in ethanol (10 mL) was added 1 N NaOH (10 mL), and the mixture was stirred at room temperature for 24 h until the starting material 9 disappeared (TLC). The reaction mixture was evaporated to dryness under reduced pressure. The residue was dissolved in water (3 mL), the resulting solution was cooled in an ice bath, and the pH was adjusted to 3–4 with dropwise addition of 3 N HCl. This suspension was left at 5° C for 24 h. The precipitated solid was collected by filtration, washed with brine, and dried in vacuo to afford 3 (0.31 g, 94%) of as an offwhite solid: mp 202–204° C; Rf 0.35 (MeOH/CHCl3, 1:4); 6.61 (s, 1 H), 7.02 (d, 2 H, J = 8.0 Hz), 7.69 (d, 2 H, J = 8.0 Hz), 8.61 (d, 1 H, J = 7.6 Hz), 10.79 (br s, 1 H). Anal. (C20H20N4O6S2•0.31CH3COCH3 . 0.79HCl) C, H, N, S.
Construction and Expression of Mutant Human DHFR
Mutations were introduced into the cDNA of human DHFR and verified by sequencing (Roswell Park Cancer Center, Buffalo, NY). DNA oligonucleotides were obtained from Integrated DNA Technologies (Coralville, IA) and used without further purification.
mutants in pDS5
Plasmid DNA was purified using the plasmid mini kit (Qiagen). Mutagenesis was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). All primers were PAGE purified and were synthesized by Alpha DNA (Montreal, Quebec) or IDT (Coralville, IA). Primers were designed according to manufacturer’s recommendations. PCR reactions were performed according to manufacturer’s recommendations with adjustments made for Tm of corresponding primers.
Primers (5’ to 3’)
| N65F for: | GGTTCTCCATTCCTGAGAAGTTTCGACCTTTAAAGGGTAG |
| N65F rev: | CTACCCTTTAAAGGTCGAAACTTCTCAGGAATGGAGAACC |
| N65S for: | GGTTCTCCATTCCTGAGAAGAGTCGACCTTTAAAGGGTAG |
| N65S rev: | CTACCCTTTAAAGGTCGACTCTTCTCAGGAATGGAGAACC |
| Q36K for: | CAGATATTTCAAGAGAATGACCACAACC |
| Q36K rev: | GGTTGTGGTCATTCTCTTGAAATATCTG |
| Q36S for: | CAGATATTTCTCGAGAATGACCACAACC |
| Q36S rev: | GGTTGTGGTCATTCTCGAGAAATATCTG |
The original wt human DHFR (pDS5 vector) was used for PCR and all subsequent mutagenesis experiments. Four single mutants were created (N65F, N65S, Q36K, Q36S) with the QuikChange Site Directed Mutagenesis Kit following the manufacturer’s recommended conditions.
PCR: (50ng of dsDNA template, 100ng of each primer, 5mM dNTPs, 2.5U/µL Taq) 1 cycle of 95EC for 30sec, 16 cycles of 95EC for 30sec, 55EC for 1min, 68EC for 3min The two double mutants (Q35K/N64F and Q35S/N64S) were created by using parental template DNA having one confirmed single residue mutation and using primers for the second desired mutation during PCR.
Expression and purification of human DHFR pDS5/mutant pDS5
Expression of mutant human DHFR in pDS5 vector in E. coli BL21 (DE3) carried out with 200 mL of LB medium containing 100 ug/mL ampicillin and inoculated with glycerol stock of human DHFR at 37EC with shaking at 300 rpm overnight. One liter of fresh LB/AMP was inoculated with the 200 mL overnight culture. The culture was grown at 37E C at 300 rpm until the O.D.600 0.8–0.9. Cells were then induced with 2 mM IPTG overnight at 16E C (16–18 hr) and harvested by centrifugation at 13,000 × g. Cells were resuspended in 100 mL of ice-cold M9 salt solution (12.8 g Na2HPO4-7H20, 3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl in a volume of 1 L).
Cells were lysed in 100 mL of ice-cold buffer containing 6.8 g KH2PO4, 3.7 g KCl, dissolved in 900 mL of H20, 1.0 mL of 1.0 M EDTA is added and the pH adjusted to 7.0 with KOH before bringing volume to 1 L. Cells were disrupted by passing through a microfluidizer at 18,000 psi (Microfluidics, Inc). The resulting lysate was subjected to centrifugation for 30 min. at 7,000 × g. Ammonium sulfate was added to supernatant over a period of 60 min. to a final saturation of 85% at 0E C. Precipitated protein was centrifuged for 30 min. at 7,000 × g and the pellet resuspended in 50 mL of methotrexate column binding buffer (100 mM KCl, 50 mM KPO4, pH 7.0). The resulting sample was passed over a 25 mL methotrexate affinity column. The column was extensively washed (> 5 column volumes of buffer) to remove unbound protein. DHFR was subsequently eluted in 5 mL fractions by passing a solution of 4 mM folic acid, 50 mM KPO4, pH 8.0 over the column. SDS-PAGE was performed on fractions to determine which contain DHFR. The corresponding fractions were then pooled and dialyzed extensively against DEAE column buffer (50 mM KPO4, pH 7.5) to remove folic acid from solution. On the following day the sample was applied to a 120 mL DEAE ion exchange column (GE Healthcare). The unbound protein fractions (containing DHFR) were collected. Remaining bound proteins and residual folic acid were eluted from the column by a linear gradient of DEAE affinity buffer supplemented with 500 mM NaCl. The column was stripped and regenerated with 1 column volume of 3 M NaCl. All fractions were analyzed by SDS-PAGE. Fractions containing highly pure (>95%) DHFR were pooled, concentrated to 1 mg/mL, flash-frozen in liquid nitrogen, and stored at −80E C.
Structure Determination and Refinement
The recombinant proteins were washed in a centricon-10 with 10 mM HEPES buffer, pH 7.4 and concentrated to 33.2 mg/mL. The protein was incubated with NADPH and an excess of the inhibitor for one hour over ice prior to crystallization using the hanging drop vapor diffusion method. The reservoir solution contained 100 mM KPO4, pH 6.9, 60% saturated NH4SO4, 3% v/v ethanol. Protein droplets contained 100 mM KPO4, pH 6.9, 30% saturated NH4SO4. Crystals of both mutant and wild type DHFR complexes grew over several days time at 14E C and are trigonal, space group H3 and diffracted to 1.3 Ǻ resolution. Data were collected at liquid N2 temperatures on an Rigaku RaxisIV imaging plate system and then later on beamlines 11-1 and 9-2 at the Stanford Synchrotron Research Laboratory (SSRL) imaging plate system and the data processed with using Mosflm41. The Rmerge for all data was 0.063 with a 3-fold multiplicity. The overall completeness of the data was 94.8 and 92.8 for data in the shell between 2.05 and 2.15 Ǻ. The data for the ternary complex refined to 17.9% for all data and 25.9% for the test data (5%) (Table 3).
Table 3.
Data collection and refinement statistics for human DHFR-NADPH-2 ternary complexes.
| Data collection | Q35S/N64S NADPH-2 |
Q35K/N64F NADPH-2 |
Wild type NADPH-1 |
Q35K NADPH-1 |
|---|---|---|---|---|
| PDB accession # | 3 ghc | 3ghv | 3ghw | 3gi2 |
| Space group | H3 | H3 | H3 | H3 |
| Cell dimensions (Ǻ) | 84.36 77.77 | 84.21 78.03 | 84.56 78.05 | 84.57 77.94 |
| Beamline | SSRL 11-1 | SSRL 11-1 | SSRL 9-2 | SSRL 9-2 |
| Resolution (Ǻ) | 1.01 | 1.20 | 1.20 | 1.50 |
| Wavelength (Ǻ) | 1.00 | 1.00 | 1.00 | 1.00 |
| Rsym (%) a,b | 0.050 (0.162) | 0.058 (0.121) | 0.036(2.3) | 0.046 (0.29) |
| Rmerge | 0.045 (0.14) | 0.044 (0.10) | 0.114(4.2) | 0.037 (0.25) |
| Completeness (%) a | 99.8 (95.3) | 100.0 (99.8) | 100.0 (94.0) | 98.9 (98.9) |
| Observed reflections | 269,146 | 388,944 | 839,426 | 64,773 |
| Unique reflections | 50,745 | 50,446 | 85,159 | 32,939 |
| I/σ(I) | 23.0 (10.3) | 26.3 (11.2) | 15.1 (0.3) | 11.6 (2.5) |
| Multiplicity a | 5.3 (4.3) | 5.3 (4.3) | 9.9 (4.1) | 2.0 (1.9) |
| Refinement and model quality | ||||
| Resolution range (Ǻ) | 26.0 – 1.30 | 53.3 – 1.30 | 22.59-1.24 | 42.30-1.53 |
| No. of reflections | 48155 | 48184 | 56,537 | 29,450 |
| R-factor c | 17.0 | 15.9 | 18.0 | 15.7 |
| Rfree-factor d | 19.8 | 18.3 | 20.0 | 20.1 |
| Total protein atoms | 1942 | 2030 | 1933 | 1939 |
| Total water atoms | 354 | 384 | 347 | 358 |
| Average B-factor (Ǻ2) | 13.8 | 14.0 | 18.2 | 18.3 |
| Rms deviation from ideal | ||||
| Bond lengths (Ǻ) | 0.009 | 0.029 | 0.009 | 0.010 |
| Bond angles (°) | 1.858 | 1.943 | 1.76 | 1.73 |
| Luzzati | 0.131 | 0.127 | 0.144 | 0.143 |
| Ramachandran plot | ||||
| Most favored regions (%) | 97.8 | 98.9 | 98.9 | 97.8 |
| Additional allowed regions (%) | 2.2 | 1.1 | 1.1 | 2.2 |
| Generously allowed regions (%) | 0.0 | 0.0 | 0.0 | 0.0 |
| Disallowed regions (%) | 0.0 | 0.0 | 0.0 | 0.0 |
The values in parentheses refer to data in the highest resolution shell.
Rsym = ∑h∑i|Ih,i − <Ih>| / ∑h∑i|Ih,i|, where <Ih> is the mean intensity of a set of equivalent reflections.
R-factor = ∑|Fobs − Fcalc| / ∑Fobs, where Fobs and Fcalc are observed and calculated structure factor amplitudes.
Rfree-factor was calculated for R-factor for a random 5% subset of all reflections.
The structure was solved by molecular replacement methods using the coordinates for human DHFR (u072) in the program Molref41. Inspection of the resulting difference electron density maps were made using the program COOT42 running on a Mac G5 workstation revealed density for a ternary complex. The final cycles of refinement were carried out using the program Refmac5 in the CCP4 suite of programs. The Ramachandran conformational parameters from the last cycle of refinement generated by PROCHECK43 showed that more than 91% of the residues have the most favored conformation and none are in the disallowed regions. Coordinates for these structures have been deposited with the Protein Data Bank (PDB code ???).
Supplementary Material
Figure 5.
Stereoview of the superposition of folate (cyan) from hDHFR (PDB1drf) on the structure of the hDHFR NADPH double mutant protein Q35K/N64F with 2 (green).
Acknowledgement
This work was supported, in part, by a grant from the National Institutes of Health and National Institute of Allergy and Infectious Diseases AI069966 (AG) and the National Institute of General medical Sciences GM51670 (VC).
Footnotes
Abbreviations: TS, thymidylate synthase; dUMP, deoxyuridylate; dTMP, deoxythymidylate; 7,8-DHF, 7,8-dihydrofolate; DHFR, dihydrofolate reductase; MTX, methotrexate; RFC, reduced folate carrier; FPGS, folylpolyglutamate synthetase; GARFT, glycinamide ribonucleotide formyltransferase; AICARFT, aminoimidazole carboxamide ribonucleotide formyltransferase; AIDS, acquired immunodeficiency syndrome; P. carinii, Pneumocystis carinii; T. gondii, Toxoplasma gondii; E. coli, Escherichia coli; PTX, piritrexim; NCI, National Cancer Institute.
Supporting Information Available:
Results from elemental analysis and high resolution mass spectrometry. This material is available free of charge via the Internet at http://pubs.acs.org.
Reference
- 1.Chan DCM, Anderson AC. Towards Species-specific Antifolates. Curr. Med. Chem. 2006;13:377–398. doi: 10.2174/092986706775527938. [DOI] [PubMed] [Google Scholar]
- 2.Hawser S, Lociuro S, Islam K. Dihydrofolate Reductase Inhibitors as Antibacterial Agents. Biochem. Pharmacol. 2006;71:941–948. doi: 10.1016/j.bcp.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 3.Gmeiner HW. Novel Chemical Strategies for Thymidylate Synthase Inhibition. Curr. Med.Chem. 2005;12:191–202. doi: 10.2174/0929867053363432. [DOI] [PubMed] [Google Scholar]
- 4.Gangjee A, Kurup S, Namjoshi O. Dihydrofolate Reductase as a Target for Chemotherapy in Parasites. Curr. Pharm. Des. 2007;13:609–639. doi: 10.2174/138161207780162827. [DOI] [PubMed] [Google Scholar]
- 5.Berman EM, Werbel LM. The Renewed Potential for Folate Antagonists in Contemporary Cancer Chemotherapy. J. Med. Chem. 1991;34:479–485. doi: 10.1021/jm00106a001. [DOI] [PubMed] [Google Scholar]
- 6.Blakley RL. Dihydrofolate Reductase. In: Blakley RL, Benkovic SJ, editors. Folate and Pterins. Vol. I. New York: Wiley-Interscience; 1984. pp. 191–253. [Google Scholar]
- 7.MacKenzie RE. Biogenesis and Interconversion of Substituted Tetrahydrofolates. In: Blakley RL, Benkovic SJ, editors. Folates and Pterins Chemistry and Biochemistry. Vol. I. New York: Wiley; 1984. pp. 255–306. [Google Scholar]
- 8.Petero GJ, Kohne CH. Fluoropyrimidines as Antifolate Drugs. Antifolate Drugs Cancer Ther. 1999:101–145. [Google Scholar]
- 9.Rosowsky A. Chemistry and Biological Activity of Antifolates. In: Ellis GP, West GB, editors. Progress in Medicinal Chemistry. Amsterdam: Elsevier Science Publishers; 1989. pp. 1–252. [DOI] [PubMed] [Google Scholar]
- 10.Gangjee A, Elzein E, Kothare M, Vasudevan A. Classical and Nonclassical Antifolates as Potential Antitumor, Antipneumocystis and Antitoxoplasma Agents. Curr. Pharm. Des. 1996;2:263–280. [Google Scholar]
- 11.Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, Jannatipour M, Moran RA. Dideazatetrahydrofolate Analogue Lacking a Chiral Center at C-6, N-[4-[2-(2-Amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-y1)ethylbenzoyl]-L glutamic Acid, Is an Inhibitor of Thymidylate Synthase. J. Med. Chem. 1992;35:4450–4454. doi: 10.1021/jm00101a023. [DOI] [PubMed] [Google Scholar]
- 12.Jackman AL, Taylor GA, Gibson W, Kimbell R, Brown M, Calvert AH, Judson IR, Hughes LR. ICI D1694, a Quinazoline Antifolate Thymidylate Synthase Inhibitor That Is a Potent Inhibitor of L1210 Tumour Cell Growth in Vitro and in Vivo: A New Agent for Clinical Study. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- 13.Bertino JR, Kamen B, Romanini A. Folate Antagonists. In: Holland JF, Frei E, Bast RC, Kufe DW, Morton DL, Weichselbaum RR, editors. Cancer Medicine. Vol. 1. Baltimore, MD: Williams and Wilkins; 1997. pp. 907–921. [Google Scholar]
- 14.Gibson W, Bisset GMF, Marsham PR, Kelland LR, Judson IR, Jackman AL. The Measurement of Polyglutamate Metabolites of the Thymidylate Synthase Inhibitor, ICI D1694,in Mouse and Human Cultured Cells. Biochem. Pharmacol. 1993;45:863–869. doi: 10.1016/0006-2952(93)90170-2. [DOI] [PubMed] [Google Scholar]
- 15.Sikora E, Jackman AL, Newell DF, Calvert AH. Formation and Retention and Biological Activity of N10-Propargyl-5,8-dideazafolic Acid (CB3717) Polyglutamates in L1210 Cells in Vitro. Biochem. Pharmacol. 1988;37:4047–4054. doi: 10.1016/0006-2952(88)90094-9. [DOI] [PubMed] [Google Scholar]
- 16.Jackman AL, Newell DR, Gibson W, Jodrell DI, Taylor GA, Bishop JA, Hughes LR, Calvert AH. The Biochemical Pharmacology of the Thymidylate Synthase Inhibitor 2-Desamino-2-methyl-N10-propargyl-5,8-dideazafolic Acid (IC1 198583) Biochem. Pharmacol. 1991;41:1885–1895. doi: 10.1016/0006-2952(91)90586-t. [DOI] [PubMed] [Google Scholar]
- 17.Nair MG, Abraham A, McGuire JJ, Kisliuk RL, Galivan J. Polyglutamylation as a Determinant of Cytotoxicity of Classical Folate Analogue Inhibitors of Thymidylate Synthase and Glycinamide Ribonucleotide Formyltransferase. Cell. Pharmacol. 1994;1:245–249. [Google Scholar]
- 18.Gangjee A, Qiu Y, Li W, Kisliuk RL. Potent Dual Thymidylate Synthase and Dihydrofolate Reductase Inhibitors: Classical and Nonclassical 2-Amino-4-oxo-5-arylthio-substituted-6-methylthieno[2,3-d]pyrimidine Antifolates. J. Med. Chem. 2008;51:5789–5797. doi: 10.1021/jm8006933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.We thank the Developmental Therapeutics Program of the National Cancer Institute for performing the in vitro anticancer evaluation.
- 20.Tripos Inc., 1699 South Handley Rd, Suite 303, St. Louis, MO 63144
- 21.Assaraf YG. Molecular Basis of Antifolate Resistance. Cancer Metastasis Rev. 2007;26:153–181. doi: 10.1007/s10555-007-9049-z. [DOI] [PubMed] [Google Scholar]
- 22.Matherly LH, Hou Z. Structure and Function of the Reduced Folate Carrier a Paradigm of a Major Facilitator Superfamily Mammalian Nutrient Transporter. Vitam. Horm. 2008;79:145–184. doi: 10.1016/S0083-6729(08)00405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao W, Matherly LH. Structural Determinants of Folate and Antifolate Membrane Transport by the Reduced Folate Carrier. In: Lash LH, editor. Drug Metabolism and Transport. Totowa: Humana Press; 2005. pp. 291–318. [Google Scholar]
- 24.Fry DW, Jackson RC. Membrane Transport Alterations as a Mechanism of Resistance to Anticancer Agents. Cancer SurV. 1986;5:47–49. [PubMed] [Google Scholar]
- 25.Schornagel JH, Pinard MF, Westerhof GR, Kathmann I, Molthoff CFM, Jolivet J, Jansen G. Functional Aspects of Membrane Folate Receptors Expressed in Human Breast Cancer Lines with Inherent and Acquired Transport-Related Resistance to Methotrexate. Proc.Am. Assoc. Cancer Res. 1994;35:302. [Google Scholar]
- 26.Wong SC, Zhang L, Witt TL, Proefke SA, Bhushan A, Matherly LH. Impaired Membrane Transport in Methotrexate-Resistant CCRF-CEM Cells Involves Early Translation Termination and Increased Turnover of a Mutant Reduced Folate Carrier. J. Biol. Chem. 1999;274:10388–10394. doi: 10.1074/jbc.274.15.10388. [DOI] [PubMed] [Google Scholar]
- 27.Webber SE, Bleckman TM, Attard J, Deal JG, Katherdekar V, Welsh KM, Webber S, Janson CA, Matthews DA, Smith WW, Freer ST, Jordan SR, Bacquet RJ, Howland EF, Booth CJL, Ward RW, Hermann SM, White J, Morse CA, Hilliard JA, Bartlett CA. Design of Thymidylate Synthase Inhibitors Using Protein Crystal Structures: The Synthesis and Biological Evaluation of a Novel Class of 5-Substituted Quinazolines. J. Med. Chem. 1993;36:733–746. doi: 10.1021/jm00058a010. [DOI] [PubMed] [Google Scholar]
- 28.Hughes A, Calvert AH. Preclinical and Clinical Studies with the Novel Thymidylate Synthase Inhibitor Nolatrexed Dihydrochloride (Thymitaq, AG337) In: Jackman AL, editor. Antifolate Drugs in Cancer Therapy. Totowa: Humana Press; 1999. pp. 229–241. [Google Scholar]
- 29.Gangjee A, Guo X, Queener SF, Cody V, Galitsky N, Luft JR, Pangborn W. Selective Pneumocystis carinii Dihydrofolate Reductase Inhibitors: Design, Synthesis, and Biological Evaluation of New 2,4-Diamino-5-substituted-furo[2,3-d]pyrimidines. J. Med. Chem. 1998;41:1263–1271. doi: 10.1021/jm970537w. [DOI] [PubMed] [Google Scholar]
- 30.Klon EA, Heroux A, Ross LJ, Pathak V, Johnson AC, Piper JR, Borhani WD. Atomic Structures of Human Dihydrofolate Reductase Complexed with NADPH and two Lipophilic Antifolates at 1.09 Å and 1.05 Å Resolution. J. Mol. Biol. 2002;320:677–693. doi: 10.1016/s0022-2836(02)00469-2. [DOI] [PubMed] [Google Scholar]
- 31.Kovacs JA, Hiemenz JW, Macher AM, Stover D, Murray HW, Shelhamer J, Lane HC, Urmacher U, Honig C, Longo DL, Parker MM, Nataneon JE, Parrillo JE, Fauci AS, Pizzo PA, Maeur H. Pneumocystis carinii pneumonia: a Comparison between Patients with the Acquired Immunodeficiency Syndrome and Patients with other Immunodeficiencies. Ann. Intern. Med. 1984;100:68–71. doi: 10.7326/0003-4819-100-5-663. [DOI] [PubMed] [Google Scholar]
- 32.Klepser ME, Klepser TB. Drug Treatment of HIV-related Opportunistic Infections. Drugs. 1997;53:40–73. doi: 10.2165/00003495-199753010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Gewald K. Heterocyclen aus CH-aciden nitrilen. VII. 2-Aminothiophene aus α-oxo-Mercaptanen und Methylenaktiven Nitrilen. Chem. Ber. 1966;98:3571–3577. [Google Scholar]
- 34.Gangjee A, Qiu Y, Kisliuk RL. Synthesis of Classical and Nonclassical 2-Amino-4-oxo-6-benzyl-thieno[2,3-d]pyrimidines as Potential Thymidylate Synthase Inhibitors. J. Heterocycl. Chem. 2004;41:941–946. [Google Scholar]
- 35.Taylor EC, Young WB, Chaudhari R, Patel M. Synthesis of a Regioisomer of N-{4-[2-(2-amino-4(3H)-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic Acid ( LY231514), an Active Thymidylate Synthase Inhibitor and Antitumor Agent. Heterocycles. 1993;36:1897–1908. [Google Scholar]
- 36.Itoh T, Mase T. A General Palladium-Catalyzed Coupling of Aryl Bromides/Triflates and Thiols. Org. Lett. 2004;24:4587–4590. doi: 10.1021/ol047996t. [DOI] [PubMed] [Google Scholar]
- 37.Gangjee A, Yu J, McGuire JJ, Cody V, Galitsky N, Kisliuk RL, Queener SF. Design, Synthesis, and X-ray Crystal Structure of a Potent Dual Inhibitor of Thymidylate Synthase and Dihyhdrofolate Reductase as an Antitumor Agent. J. Med. Chem. 2003;43:3837–3851. doi: 10.1021/jm000200l. [DOI] [PubMed] [Google Scholar]
- 38.Cody V, Galitsky N, Luft JR, Pangborn W, Gangjee A, Devraj R, Queener SF, Blakely RL. Comparison of Ternary Complexes of Pneumocystis carinii and Wild Type Human Dihydrofolate Reductase With a Novel Classical Antitumor Furo[2,3-d]pyrimidine Antifolate. Acta Cryst. 1997;D53:638–649. doi: 10.1107/S090744499700509X. [DOI] [PubMed] [Google Scholar]
- 39.Kisliuk RL, Strumpf D, Gaumont Y, Leary RP, Plante L. Diastereoisomers of 5,10-Methylene-5,6,7,8-tetrahydropteroyl-D glutamic Acid. J. Med. Chem. 1977;20:1531–1533. doi: 10.1021/jm00221a038. [DOI] [PubMed] [Google Scholar]
- 40.Wahba AJ, Friedkin M. The Enzymatic Synthesis of Thymidylate. Early Steps in the Purification of Thymidylate Synthetase of Escherichia coli. J. Biol. Chem. 1962;237:3794–3801. [PubMed] [Google Scholar]
- 41.Collaborative Computational Project, Number 4, The CCP4 Suite: Programs for Protein Crystallography. Acta Crystallogr. 1994;D50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 42.Emsley P, Cowtan K. Coot: Model-Building Tools for Molecular Graphics. Acta Crystallogr. 2004;D60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 43.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: A Prorgram to Check the Stereochemical Quality of Protein Structures. J. Applied Crystal. 1993;26:283–291. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.