Summary
The skeletal muscle basement membrane fulfils several crucial functions during development and in the mature myotome and defects in its composition underlie certain forms of muscular dystrophy. A major component of this extracellular structure is the laminin polymer, which assembles into a resilient meshwork that protects the sarcolemma during contraction. Here we describe a zebrafish mutant, softy, which displays severe embryonic muscle degeneration as a result of initial basement membrane failure. The softy phenotype is caused by a mutation in the lamb2 gene, identifying laminin β2 as an essential component of this basement membrane. Uniquely, softy homozygotes are able to recover and survive to adulthood despite the loss of myofibre adhesion. We identify the formation of ectopic, stable basement membrane attachments as a novel means by which detached fibres are able to maintain viability. This demonstration of a muscular dystrophy model possessing innate fibre viability following muscle detachment suggests basement membrane augmentation as a therapeutic strategy to inhibit myofibre loss.
Keywords: Skeletal muscle, Zebrafish, Laminin β2, Basement membrane, Muscular dystrophy
INTRODUCTION
The basement membrane (BM) underlies the formation of most tissues and organs in the developing embryo, providing a resilient substratum of extracellular matrix that supports the overlying cell layers to which it adheres. One organ system in which BM formation and integrity is beginning to be well understood is the skeletal musculature. In skeletal muscle, the BM surrounds each myofibre and provides the elasticity that enables the sarcolemma to withstand the mechanical stress of repeated contraction (Sanes, 2003). A key component of all BMs is the glycoprotein laminin, which is secreted from the cell as a heterotrimer that self-assembles into a macromolecular lattice (McKee et al., 2007). The laminin subunit consists of α, β and γ chains, each of which are comprised of short and long arms at the N- and C-termini, respectively. The long arms form a coiled-coiled domain that binds the chains together, whereas the short arms are free, giving the trimer a cruciform structure (Yurchenco et al., 1992). The spontaneous polymerisation of laminin is facilitated by the interaction of the LN domains, globular domains of ∼250 amino acids at the N-terminal of each short arm (Odenthal et al., 2004; McKee et al., 2007).
In mammals, five α, three β and three γ laminin chains have been identified, combining in vivo to generate up to 15 different heterotrimers, many with tissue- or cell-type-specific expression (Aumailley et al., 2005). The laminin of the skeletal muscle BM, which consists of several spatially restricted isoforms (Patton, 2000), is involved in two major types of linkages to the sarcolemma. Firstly, it binds to the dystrophin glycoprotein complex (DGC) via a direct interaction with α-dystroglycan. Secondly, it forms an attachment via integrins to subsarcolemmal focal adhesion complexes. These two linkages represent the major connections between the actin cytoskeleton of the myofibre and the extracellular matrix, transmitting much of the force generated by muscle contraction across the sarcolemma (reviewed by Miner, 2008).
Recently, forward genetic strategies in the zebrafish have been instrumental in addressing the functional importance of these linkages in the context of muscle disease. Specifically, the characterisation of a small class of `dystrophic' mutants, which undergo rapid degeneration of the embryonic skeletal muscle, has established the zebrafish embryo as an informative model of human muscular dystrophy (Granato et al., 1996; Bassett and Currie, 2003). In the zebrafish embryonic myotome, laminin and the DGC are concentrated at the vertical myoseptum, where the muscle inserts into the connective tissue, a region referred to as the myotendinous junction (MTJ) (Parsons et al., 2002; Hall et al., 2007). The dystrophic mutant, sapje (sap) harbours a mutation in the zebrafish orthologue of the Duchenne muscular dystrophy gene (DMD, encoding dystrophin) and demonstrates that the dystrophin protein is essential for maintaining the integrity of the sarcolemma at the vertebrate MTJ (Bassett et al., 2003). A second mutant in this class, candyfloss (caf), has a mutation in the gene encoding the major α-laminin chain in skeletal muscle, laminin α2 (Lama2), and recapitulates the major features of muscle wasting in LAMA2-deficient congenital muscular dystrophy (MDC1A). The progressive, lethal degeneration seen in caf embryos results from the failure of the BM to maintain adhesion to the sarcolemma at fibre termini, leading to detachment of muscle fibres from the MTJ and subsequent fibre death (Hall et al., 2007).
Here, we describe another mutant in the dystrophic class, softy (sof). Like sap and caf, the damage seen in sof homozygous embryos results from the loss of muscle fibre adhesion at the MTJ. We demonstrate that in sof this failure occurs external to the sarcolemma, with many fibres detaching from the vertical myoseptum at the level of the BM. However, despite developing severe embryonic muscle pathology similar to the lethal dystrophy evident in other mutants of this class, the myotome is able to recover so effectively that a substantial proportion of sof homozygotes can survive to maturity. We attribute this recovery to the formation of ectopic fibre terminations (EFTs), novel attachment sites that preserve fibre viability by providing retracted fibre ends with a BM layer. The sof phenotype is caused by a missense mutation in lamb2, demonstrating a crucial role for laminin β2 (Lamb2) in MTJ stability and the maintenance of skeletal muscle attachment. Furthermore, the surprising ability of a subset of detached muscle fibres to remain viable in sof, despite defects in BM structure, has ramifications for the therapy of MDC1A and other diseases of BM failure.
MATERIALS AND METHODS
Zebrafish strains and maintenance
The softm272a, cafteg15a and sapta222a mutant alleles were obtained from the Tübingen Stock Collection. Attempts were made to revive the two other identified sof alleles, te234 and tz212 (Granato et al., 1996), by in vitro fertilisation, but heterozygotes could not be obtained. The tm272a allele was maintained in the AB* and TU strain backgrounds and crossed to the WIK strain for meiotic mapping, which was performed according to standard protocols (Geisler, 2002). The cafteg15a and sapta222a mutants have been described elsewhere (Bassett et al., 2003; Hall et al., 2007), as has the transgenic line Tg(9.7kb smhyc1:gfp)i104 (Elworthy et al., 2008). Zebrafish maintenance and embryo collection were carried out using established protocols (Westerfield, 1993), and all animal experimentation was approved by the Garvan/St Vincent's Animal Ethics Committee.
Histology and electron microscopy
For eye histology, embryos were fixed in 4% paraformaldehyde overnight at 4°C. Paraffin embedding and sectioning were carried out by standard methods (Nüsslein-Volhard and Dahm, 2002). Sections were cut at a thickness of 8 μm and stained with Hematoxylin and Eosin. For electron microscopy, embryos were collected at 72 hours post-fertilisation (hpf), fixed in 2.5% glutaraldehyde at 4°C and embedded in Spurr's resin. Ultrathin sections were imaged on a Philips EM410 transmission electron microscope.
Immunohistochemistry and in situ hybridisation
Whole-mount immunohistochemistry was performed by standard procedures (Guille, 1999). Primary antibodies were used at the following dilutions: anti-myosin heavy chain, slow isoform 1:10 (F59; DSHB, Iowa City, IA), anti-dystrophin 1:1000 (MANDRA1; Sigma, St Louis, MO), anti-β-dystroglycan 1:10 (MANDAG2; DSHB), anti-laminin 1:100 (L9393; Sigma). Alexa-fluor-488- and Alexa-fluor-568-conjugated fluorescent secondary antibodies (Invitrogen, Carlsbad, CA) were diluted 1:500. Whole-mount in situ hybridisation was carried out essentially as described previously (Oxtoby and Jowett, 1993). The lamb2 riboprobe was synthesised from a 347 bp DNA template (GenBank Accession Number FJ619350) encoding the last EGF-like domain of the short arm and the start of the long arm of zebrafish Lamb2. The lamb2l riboprobe template was an 859 bp partial cDNA (GenBank Accession Number FJ619351) encoding the last three EGF-like domains of the short arm and approximately 130 amino acids of the long arm of Lamb2l. The overlapping regions of the templates aligned with only 52% identity and did not result in significant overlap in expression pattern.
Iontophoresis and microinjection
Iontophoretic injection of Rhodamine Dextran into muscle fibres was performed as described (Hollway et al., 2007). Evans Blue dye injections were carried out according to Bassett et al. (Bassett et al., 2003). lamb2 morpholino antisense oligonucleotides (MOs) (Gene Tools, Philomath, OR) were diluted in distilled water and injected into one- to four-cell embryos. Two splice-site MOs (MO1 and MO2) and one translational blocking MO (MO3) were used. The sequences of the morpholinos used are as follows: MO1, 5′-CTGTGCGCTGACCGCTGACAGGACT-3′; MO2, 5′-GAGAACAGATGAGGACCTTACCTGC-3′; MO3, 5′-CTGTATGGTGATGAATCTTCAACTG-3′.
Production of the acta1:EosFPtd and acta1:Lamb2-EGFP transgenic constructs
The acta1:EosFPtd and acta1:Lamb2-EGFP constructs were created using the tol2kit according to Kwan et al. (Kwan et al., 2007). The acta1:EosFPtd vector was assembled from the entry clones p5E-acta1, pME-EosFPtd, p3E-polyA and the destination vector pDEST-tol2-pA2. The acta1:Lamb2-EGFP vector was assembled from the entry clones p5E-acta1, pME-Lamb2_noSTOP, p3E-EGFP and the destination vector pDEST-tol2-pA2. We generated p5E-acta1 by amplifying a 4 kb fragment from the upstream sequence of the alpha actin gene (Higashijima et al., 1997) and subcloning into pDONRp4-p1R. pME-EosFPtd was constructed by subcloning the coding sequence of EosFPtd (Wiedenmann et al., 2004) into pDONR 221. Similarly, pME-Lamb2_noSTOP was made by subcloning the rat Lamb2 coding sequence (Green et al., 1992) (kindly provided by Dr J. Miner, Washington University, St Louis, MO) into pDONR 221.
Genotyping of mutant alleles
Embryos produced from an incross of sof and caf compound heterozygotes were genotyped by restriction fragment length polymorphism analysis. lamb2 exons 3 and 4 were amplified from genomic DNA to produce a 780 bp fragment using the primers GTGGAAACTTGGCCTTTGC (forward) and TTCATTAACAGTTGGCAACCC (reverse). The softm272a mutation introduces a StyI site into exon 3, resulting in digestion products of 260 bp and 520 bp. The cafteg15a allele was genotyped using a derived cleaved amplified polymorphic sequences (dCAPS) assay (Neff et al., 2002), using the primers AGGTTGGTCATTGAGTTCGAGA (forward) and TGCCCAGGTCGAAGCTTAGGTGAGCCAGTC (reverse) to introduce a Tsp45I site into the mutant amplicon. PCR amplification with these primers generated a 136 bp fragment, which was cleaved by Tsp45I into products of 104 bp and 32 bp.
RESULTS
Muscle degeneration in sof embryos
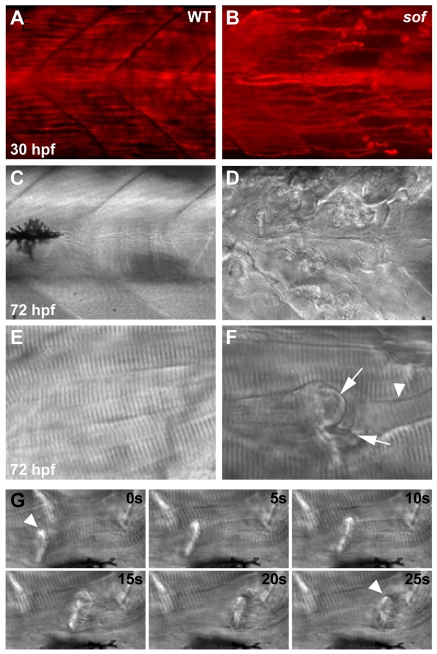
The softm272a mutant was isolated from a large-scale mutagenesis screen as one of several mutants displaying reduced motility with associated loss of muscle tissue (Granato et al., 1996). Although muscle development is normal in this class of mutant, the onset of spontaneous contractions is rapidly followed by degeneration of the axial myotome. Birefringence of polarised light through the parallel fibrillar arrays of the trunk muscle can be used to assay myofibre disruption (Granato et al., 1996). When assessed using this technique, the muscle degeneration in sof mutant embryos was similar in severity to that seen in the other dystrophic mutants caf and sap (Bassett et al., 2003; Hall et al., 2007) (Fig. 1).
Fig. 1.
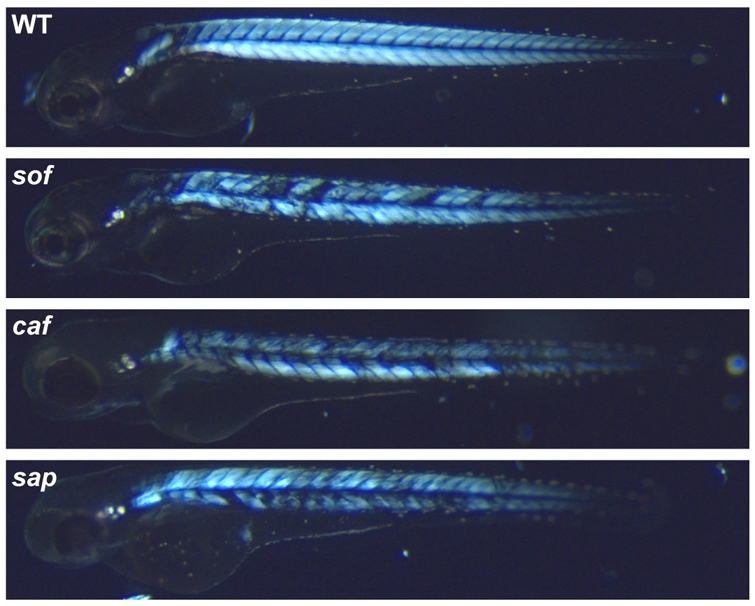
Muscle degeneration in the dystrophic mutants softm272a, cafteg15a and sapta222a. Birefringence images comparing the loss of striated muscle in mutant embryos at 72 hpf, viewed through a polarising filter as dark patches on the trunk. The distribution and severity of muscle lesions in sof are broadly similar to caf and sap, while wild-type embryos (WT) display no loss of birefringency.
In sof embryos, detachment of slow fibres from their insertion sites at the vertical myoseptum was readily seen by 30 hpf, using the F59 antibody, which specifically recognises slow muscle in zebrafish (Fig. 2A,B). This early detachment suggests that the muscle fibre termini in sof are prone to adhesion failure soon after mechanical load is applied by the onset of strong contraction (Kimmel et al., 1995). By 72 hpf, lesions had accumulated throughout the myotome, and multiple retracting fibres could be seen by differential interference contrast (DIC) microscopy, with some somites severely affected (Fig. 2D). Newly detached fibre ends possessed a faceted morphology (Fig. 2F, arrows) and retracted into the myotome leaving a clearly visible retraction groove (Fig. 2F, arrowhead). Fibre detachment could be observed in vivo using time-lapse photomicroscopy and the anaesthetic recovery technique described previously (Hall et al., 2007) (see Movie 1 in the supplementary material; Fig. 2G). Muscle damage was prevented by raising sof embryos in anaesthetic between 20 and 72 hpf, indicating that normal activity is sufficient to precipitate degeneration in the mutant and that muscle load is an essential component of the sof phenotype.
Fig. 2.
Muscle degeneration in sof mutants results from detachment of myofibres from the vertical myoseptum. Lateral views of wild-type muscle (A,C,E) show fibres spanning the somite between adjacent myosepta, whereas in sof mutants (B,D,F), lesions rapidly develop. (A,B) Whole-mount immunohistochemistry using an antibody against slow myosin demonstrates that, although slow fibres develop normally in sof embryos, by 30 hpf fibres have already detached from the myoseptum. (C-F) DIC images of axial muscle. By 72 hpf, the sof embryo appears severely damaged, with numerous detached fibres scarring the myotome. A magnified view of a single detached fibre (F) shows the characteristic invaginated membrane (arrows) and retraction groove (arrowhead). (G) Retraction of a single fibre (arrowhead), from left of frame to right, in a 72 hpf sof embryo. Still images taken from Movie 1 in the supplementary material, with elapsed time indicated in seconds.
Remarkably, despite a myopathology as severe as in the other, lethal dystrophic mutants, many sof larvae were able to recover completely and were viable. When expressed relative to wild-type sibling rates of survival, 64% (n=109) of homozygotes survived to maturity under standard rearing conditions and were fertile, swam normally and were indistinguishable from wild-type siblings. To test whether survival correlated with phenotypic severity, mildly and severely affected cohorts of sof mutant larvae were raised separately. Both groups survived at frequencies similar to that of the overall mutant sample (mild, 71%, n=21; severe, 66%, n=18), suggesting that viability of sof mutants was not restricted to mildly affected larvae.
The vertical myoseptum in sof mutants has an abnormal ultrastructure
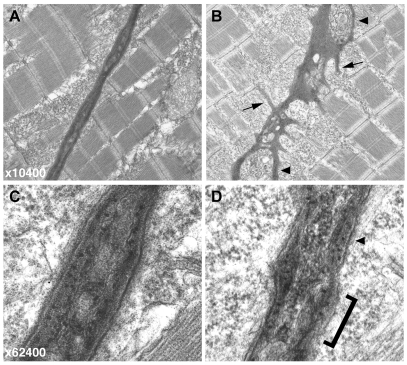
The muscle detachment apparent in sof mutants implied a failure of the anchorage of fibre ends to the embryonic MTJ at the vertical myoseptum. In order to look for direct evidence of a defective MTJ to explain this detachment, transmission electron microscopy was carried out on sections of 72 hpf sof mutant and sibling embryos (Fig. 3). The vertical myoseptum of wild-type siblings had a uniform, well-organised appearance with a layered structure visible at higher magnification (Fig. 3A,C). By contrast, even in regions where the fibres were intact, the mutant myoseptum appeared grossly distorted, with an irregular thickness and numerous blisters within the extracellular matrix (Fig. 3B, arrowheads). Multiple short processes branching off the myoseptum were also noticeable (Fig. 3B, arrows). A higher magnification view revealed that the surface of the mutant myoseptum was rough and disorganised compared with the wild type (Fig. 3C,D). The overall appearance of the vertical myoseptum suggested that the underlying defect in sof resided in the junctional extracellular matrix.
Fig. 3.
Electron micrographs of the vertical myoseptum reveal a highly irregular MTJ in sof embryos. (A,B) At lower magnification (×10,400), the wild-type myoseptum is compact and linear (A) whereas the sof myoseptum appears severely distorted, even in the absence of fibre detachment (B). Numerous processes extending into the myotome are clearly visible in the mutant (B, arrows), and blisters within the myoseptum are also indicated (arrowheads). (C,D) At higher magnification (×62,400), the smooth, layered structure of the BM at the wild-type MTJ is evident (C). By contrast, the sof BM lacks organisation, with a rough surface (bracketed) and only patches of layering (arrowhead) (D).
softm272a has a mutation in the zebrafish lamb2 gene
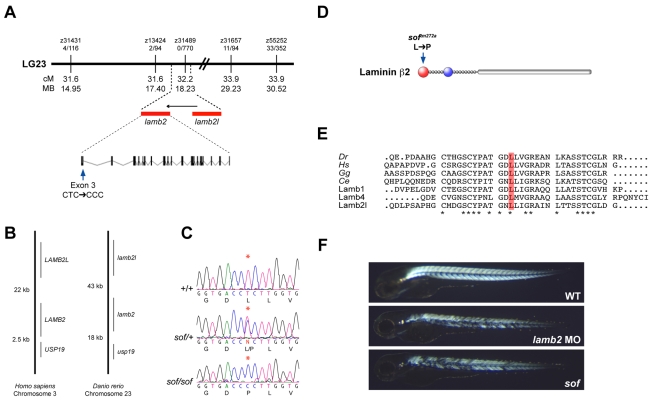
To investigate the molecular basis of the phenotype, meiotic mapping of the sof mutation was carried out using standard approaches (Geisler, 2002). Linkage analysis defined a region on Chromosome 23 between SSLP markers z13424 (31.6 cM; 2 recombinants out of 94 meioses) and z31657 (33.9 cM; 11 recombinants out of 94 meioses) and a non-recombinant marker was found (z31489, 32.2 cM; 0 out of 770 meioses), indicating tight linkage to the sof mutation (Fig. 4A). An inspection of the region surrounding z31489 in the Ensembl genome assembly (Zv7; http://www.ensembl.org) revealed duplicate genes that were strong candidates for the mutation. One gene, lamb2 (ENSDARG00000002084), encoded a predicted protein with significant similarity to human laminin β2 (LAMB2; 59% identity). The other candidate was the predicted gene ENSDARG00000033950, which encoded a protein 49% identical to lamb2. This second gene, lamb2l, was situated in the same relative location as human LAMB2-like, a pseudogene, which is adjacent and upstream of LAMB2 (Durkin et al., 1999). The gene order in this region is conserved between human and zebrafish (Fig. 4B). This implies an ancestral tandem duplication and subsequent inactivation of LAMB2-like in the mammalian lineage.
Fig. 4.
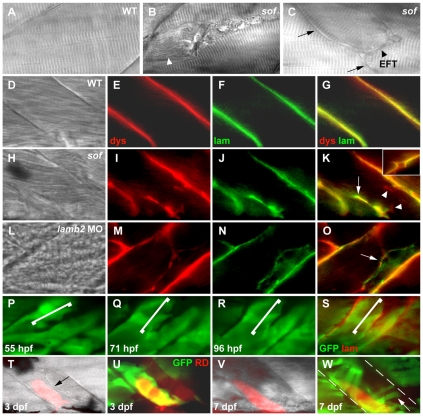
softm272a is an allele of lamb2. (A) softm272a was meiotically mapped to a 2.3 cM region on chromosome 23, delineated by the SSLP markers z13424 and z31657. The non-recombinant marker z31489 was found to reside in an intron of lamb2l. The markers used have been placed on a physical map of chromosome 23, with recombination frequencies shown (number of recombinants/number of meioses). The numbers below the line represent the genetic (top) and physical (bottom) location of markers (Zv7; http://www.ensembl.org). The arrow above the genes indicates transcriptional orientation. (B) The zebrafish lamb2 and lamb2l genes maintain a syntenic relationship with USP19 in human. LAMB2 regions of human chromosome 3 and zebrafish chromosome 23 are displayed, with distances between genes shown (not to scale). Distances were taken from the Ensembl database and Durkin et al. (Durkin et al., 1999). (C) Genomic sequence electropherograms of the mutated region in wild-type, heterozygote and homozygote sof embryos. A single T to C transition was found in exon 3 of lamb2 (asterisk), resulting in the substitution of a proline for a leucine at amino acid 38 of the unprocessed form of Lamb2. The translation is shown below each electropherogram. (D) Domains of Lamb2. The softm272a mutation is in the LN domain, shown in red. Other domains represented are LF domain (blue), EGF-like domain (grey circles) and coiled-coiled domain (grey bar) (Aumailley et al., 2005). (E) Amino acid sequence alignment of the N-terminal region of laminin β2 from zebrafish (Dr), human (Hs), chicken (Gg) and the sole Caenorhabditis elegans β-laminin, LAM-1 (Ce), as well as zebrafish laminin β1, β4 and β2-like. The mutation affects a completely conserved leucine, 21 amino acids from the predicted secreted N-terminal of zebrafish Lamb2. Asterisks denote universally conserved residues. The signal peptide cleavage sites of each sequence were predicted using SignalP3.0 (Bendtsen et al., 2004). (F) Injection of a lamb2 antisense MO precisely phenocopies the loss of birefringency seen in sof homozygous mutants. Top, uninjected wild-type embryo; middle, lamb2 MO3-injected embryo; bottom, sof mutant embryo.
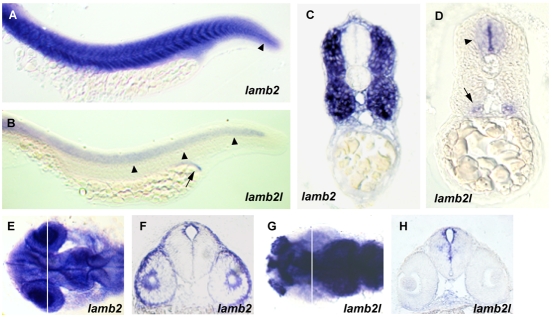
As well as being genetically linked to the sof mutation, lamb2 and lamb2l are candidates from a functional perspective because mammalian LAMB2 is abundant at the MTJ, where it co-localises with LAMA2 in a laminin α2β2γ1 complex (Pedrosa-Domellof et al., 2000). As mentioned, the zebrafish lama2 mutant, caf, also displays a muscle detachment phenotype (Hall et al., 2007). In addition, Lamb2 knockout mice have defects at the MTJ of the diaphragm (Miner et al., 2006). The expression of the duplicate genes was, therefore, examined by whole-mount in situ hybridisation (Fig. 5). At 24 hpf, the highest level of lamb2 expression was seen in the axial muscle (Fig. 5A,C). Expression was also seen in the eye and brain (Fig. 5E,F). Expression of lamb2l was detected in the neural tube and the pronephric duct (Fig. 5B,D), with strong expression in the brain at 24 hpf but in a pattern distinct from the neural expression of lamb2 (Fig. 5G,H). No expression of lamb2l was detected in the skeletal muscle of wild-type embryos, nor was it upregulated in sof mutant embryos (not shown). These patterns of expression, which, when combined, broadly encompass the expression pattern of mammalian Lamb2, were consistent with a functional role for zebrafish Lamb2, but not Lamb2l, in skeletal muscle attachment and so we proceeded to sequence lamb2 in sof mutants.
Fig. 5.
Embryonic expression of the lamb2 and lamb2l genes. (A) Lateral view of lamb2 expression in the trunk and tail at 24 hpf. Strong expression is seen in the skeletal muscle. Expression is also seen in the notochord of the tail (arrowhead). (B) Lateral view of lamb2l expression in the trunk and tail at 24 hpf, showing staining in the neural tube (arrowheads) and pronephric duct (arrow). (C,D) Transverse sections of (C) lamb2- and (D) lamb2l-stained embryos, showing distinct expression domains. Neural tube (arrowhead) and pronephric duct (arrow) expression of lamb2l is indicated in D. (E) Dorsal view of head expression of lamb2 at 24 hpf. (F) In a transverse section through the head, at the position indicated by the line in E, lamb2 expression is visible in the cornea, lens and diencephalon. (G) Dorsal view of head expression of lamb2l at 24 hpf. Staining is prominent in the olfactory placode, telencephalon and midbrain. (H) Transverse section of the head of a lamb2l-stained embryo, at the position indicated by the line in G. Expression is seen around the diencephalic ventricle but not in the eye.
Sequencing of lamb2 exons from genomic DNA of sof mutant embryos identified a single base change within the open reading frame that altered the amino acid sequence. This was a T to C transition in exon 3, resulting in a Leu38→Pro substitution in the LN domain of the predicted Lamb2 protein (Fig. 4C,D). This leucine residue is completely conserved in all known Lamb2 sequences as well as in other β-laminin chains (Fig. 4E). Prolines are known to induce kinks in amino acid chains, so this substitution is likely to affect the function of the globular LN domain, which participates in the polymerisation of the laminin lattice (Colognato et al., 1999; McKee et al., 2007). Sequencing of exon 3 in 14 affected and 14 unaffected embryos confirmed segregation of the homozygous mutation with the sof phenotype.
To demonstrate that a loss of Lamb2 function was responsible for the sof phenotype, antisense MOs were injected into wild-type embryos at the one- to four-cell stage. Initially, two splice-site MOs (MO1 and MO2), complementary to the splice donor and acceptor sequences, respectively, of exon 3 of lamb2 were used. Both in combination and singly, these MOs were able to induce early fibre detachment similar to that seen in the mutant (MO1+MO2 100 μM each, 49% of injected embryos had fibre detachment, n=47; MO1 200 μM, 39%; MO2 200 μM, 50%, n=32) but did not produce as severe a muscle loss as evident in sof homozygotes at later stages. A third, translational blocking lamb2 MO (MO3) was, however, highly effective in inducing muscle detachment up to and beyond 30 hpf and completely phenocopied sof mutants. Thus, at 72 hpf, the MO3-injected embryos displayed the same stochastic loss of birefringency as sof mutants (Fig. 4F), with fibre detachment seen in 53% of injected embryos (200 μm MO, n=270). All morphants survived beyond 7 days post-fertilisation (dpf), indicating that transient knockdown of Lamb2 protein is not lethal. Moreover, when larvae were raised to 26 dpf, lamb2 morphant and sof mutant rates of survival were similar (sof 68% survival, n=50; lamb2 MO3 71% survival, n=49). Taken together, these results strongly suggested that the sof phenotype was caused by loss of normal Lamb2 function in muscle.
Transgenic expression of Lamb2 in sof does not prevent fibre detachment
The nature of the mutation in sof led us to question whether the muscle detachment could be prevented by cell-autonomous expression of wild-type Lamb2. To that end, we constructed a rat Lamb2-GFP fusion transgene under the control of the skeletal muscle α-actin promoter and injected it into sof embryos to generate mosaic expression of Lamb2 in sof muscle fibres (see Fig. S1 in the supplementary material). The full-length Lamb2 cDNA used in this construct has been demonstrated to be functional in rescuing defects in the Lamb2-/- mouse (Miner et al., 2006). As a control, we injected an EosFPtd construct (Wiedenmann et al., 2004) under the same promoter, in order to mosaically label muscle fibres in sof mutants for a baseline reading of the proportion of detached fluorescent fibres under `non-rescue' conditions. Transgenic fibres were quantitated at 72 hpf.
The control EosFPtd construct, when injected into wild-type siblings, elicited 0% fibre detachment (n=113 fibres; ten embryos). When this construct was injected into sof embryos, detachment was seen in 17% of fluorescent fibres (n=206; eight embryos). The Lamb2-GFP fusion construct did not induce detachment in wild-type siblings (0%, n=105; five embryos), ruling out the possibility of a dominant-negative detrimental effect of overexpression of the cDNA. Expression of Lamb2-GFP in sof mutants resulted in detachment in 20% of transgenic fibres (n=93; five embryos), clearly showing that the transgene failed to prevent muscle detachment (see Fig. S1 in the supplementary material). This outcome suggests that, as the sof phenotype cannot be rescued by secretion of wild-type protein, it does not arise from the cell-autonomous failure of laminin-mediated adhesion but, rather, from a structural weakness within the laminin matrix at the MTJ caused by a lack of functional Lamb2.
Ocular development is unaffected in sof mutants
As lamb2 is expressed in the zebrafish lens and cornea and mutations in the human orthologue can cause malformation of these tissues (Zenker et al., 2004), we decided to look for ocular defects in sof mutant embryos and lamb2 MO-injected embryos. By visual inspection and dark-field microscopy, the eyes appeared normal at all developmental stages in vivo (see Fig. S2A in the supplementary material). Histological analyses of sof mutants at 72 hpf (not shown) and 7 dpf (see Fig. S2B,C in the supplementary material) revealed that eye development was overtly normal. However, we cannot rule out the presence of subtle abnormalities or synaptic defects, such as those observed in the Lamb2 knockout mouse (Libby et al., 1999).
Two distinct modes of fibre detachment in sof mutants
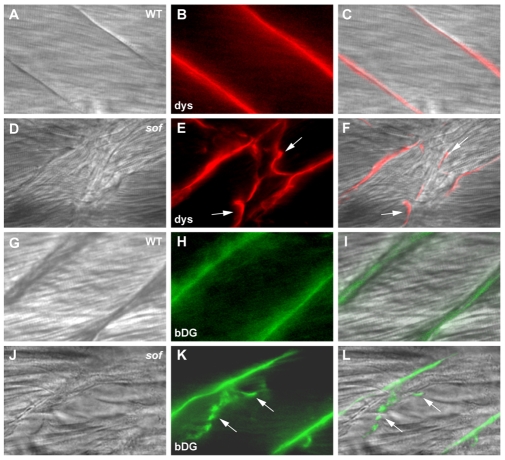
Next, the pathobiology of the muscle detachment in sof was explored by whole-mount fluorescence immunohistochemistry. This revealed that components of the DGC, such as the intracellular protein, dystrophin and the integral membrane protein, β-dystroglycan, were present at the fibre ends of wild-type embryos at 72 hpf (Fig. 6A-C,G-I). In sof embryos, however, these proteins remained associated with the sarcolemma of detached fibres upon retraction (Fig. 6D-F,J-L, arrows), consistent with attachment failure occurring external to the sarcolemma. Upon closer inspection, two types of lesions could be seen in the myotome of sof mutants and lamb2 morphants from 72 hpf (Fig. 7A-C; and data not shown). Firstly, as described earlier, individual fibres that had detached at one end and retracted from their insertion point at the myoseptum were observed (Fig. 7B). These fibres were distinguished by highly condensed myofibrils within the retracted end. A second type of detachment was associated with fissures that appeared similar to the vertical myosepta and were often seen to branch off these myosepta into the myotome to span several fibre widths (Fig. 7C). In other cases, fissures were found in the middle of the somite (e.g. Fig. 7P-S). We have termed these novel structures ectopic fibre terminations (EFTs), defined as ectopic, myoseptum-like structures within the mutant myotome, clearly visible by DIC microscopy, to which fibres are anchored. The fibres attached to these EFTs maintained normal myofibrillar striations but often had an orientation oblique to that of the remainder of the myotome.
Fig. 6.
Analysis of detached fibres indicates that muscle attachment failure occurs external to the sarcolemma. Whole-mount immunohistochemistry of mutant embryos at 72 hpf reveals the presence of dystrophin and β-dystroglycan at the ends of detached fibres. (A-F) Wild-type (A-C) and sof (D-F) embryos stained with anti-dystrophin. (G-L) Wild-type (G-I) and sof (J-L) embryos stained with anti-β-dystroglycan. (A,D,G,J) DIC images, (B,E,H,K) fluorescence images, (C,F,I,L) merged images. Arrows indicate fibre detachments. All panels show lateral views with anterior to the left. dys, anti-dystrophin; bDG, anti-β-dystroglycan.
Fig. 7.
Two modes of fibre detachment occur in sof muscle. (A-C) In vivo observation of intact fibres in the wild type (A) in comparison with a retracted fibre (B) and an EFT (arrowhead, C) in sof embryos at 120 hpf. Condensed myofibrils can be seen in the retracted fibre (arrowhead, B), whereas fibres attached to the EFT maintain a normal striated appearance. The vertical myoseptum is indicated by arrows in C. (D-K) Antibody co-staining with anti-dystrophin (E,I) and anti-laminin (F,J) reveals the presence of sarcolemma-BM linkages at EFTs in sof embryos (arrow in K), whereas single detached fibres retain sarcolemma only (arrowheads in K). BM detachment can be seen at the junction of the vertical myoseptum and an EFT (inset in K). (L-O) EFTs seen in lamb2 morphants appear identical to those in sof embryos (arrow in O). (P-R) Time points from the monitoring of a single EFT (bracketed) on the Tg(9.7kb smhyc1:gfp)i104 background, with panels labelled according to the stages at which images were captured. The last 16 hours of this period are depicted in Movie 2 in the supplementary material. (S) Anti-laminin staining of the same somite, in red, overlaid onto the GFP fluorescence image, confirming lesion as an EFT. (T-W) Labelling of fibres adjacent to an EFT (arrow in T) with Rhodamine Dextran illustrates viability of detached fibres over 4 days under load-bearing conditions. New fibres occasionally elongate over the EFT (arrow in W), extending to the myoseptum (dashed lines). Panels are labelled according to the stages at which images were captured. (A-C,D,H,L) DIC images, (E,F,I,J,M,N,P-R) fluorescence images, (G,K,O,S,U,W) merge of fluorescence images, (T,V) merge of DIC and fluorescence images. All panels show lateral views with anterior to the left. dys, anti-dystrophin; lam, anti-laminin.
In addition to their distinct appearances in vivo, the two types of lesions described above could be differentiated immunohistochemically. Double antibody staining was carried out on 120 hpf sof mutant embryos using antibodies against dystrophin and laminin to label the terminal sarcolemma and BM, respectively. These proteins localise to the MTJ of wild-type embryos (Fig. 7D-G). Within the myotome of sof embryos, the ends of single detached fibres were immunoreactive for dystrophin, whereas the EFTs were immunoreactive for both dystrophin and laminin, indicative of an MTJ-like linkage (Fig. 7H-K). Interestingly, lamb2 morphants formed EFTs very similar in appearance and quantity to those seen in sof embryos (sof 4.4±0.8 EFTs/embryo, n=10 embryos; lamb2 MO3 5.3±1.2, n=10). This suggests that the EFTs are not a distinct consequence of the sof missense mutation but are more generally linked to the loss of Lamb2 function (Fig. 7L-O). As the maintenance of a functional sarcolemma-BM attachment at these sites may permit the survival of the detached fibres, the EFTs were investigated further.
EFTs support the viability of detached fibres in sof mutants
We attempted to determine the stability of the EFTs, as well as the fibres attached to them, by two methods: time-lapse photomicroscopy and iontophoretic labelling of single cells. Firstly, we crossed the sof allele onto the Tg(9.7kb smhyc1:gfp)i104 transgenic line, which expresses GFP in the single layer of slow muscle fibres at the lateral extremity of the myotome (Elworthy et al., 2008). This enabled us to detect and follow EFTs and their associated slow fibres by fluorescence microscopy in vivo. When a single EFT in an anaesthetised sof embryo was monitored for 48 hours, neither its position nor its width changed significantly and cell turnover was not in evidence, illustrating the stability of these structures over a period of days (Fig. 7P-R; see Movie 2 in the supplementary material). The length of the EFT appeared to decrease over this period, perhaps as a consequence of some repair occurring at the tip of the lesion. Immunostaining of the embryo at the conclusion of this period confirmed that the lesion that had been monitored was, indeed, a true EFT, as laminin was detected at the ends of the fibres (Fig. 7S).
Next, we undertook iontophoretic injection of fibres adjacent to EFTs to determine their fate under load-bearing conditions. Rhodamine Dextran was injected into detached slow fibres of sof mutants at prominent EFTs on the aforementioned transgenic background at 3 dpf and the embryos were allowed to develop and swim normally (Fig. 7T,U, n=9). Embryos were observed again at 7 dpf (Fig. 7V,W). Using this approach, we were able to visualise fibres attached to EFTs that remained viable for at least 4 days post-injection. In some instances, new fibres were even able to elongate across the EFT to attach to the MTJ (Fig. 7W, arrow). These observations suggest that formation of EFTs in sof mutants can enhance overall myotomal stability and contribute to the viability of this mutant.
The different modes of fibre detachment in sof have opposing effects on sarcolemmal integrity
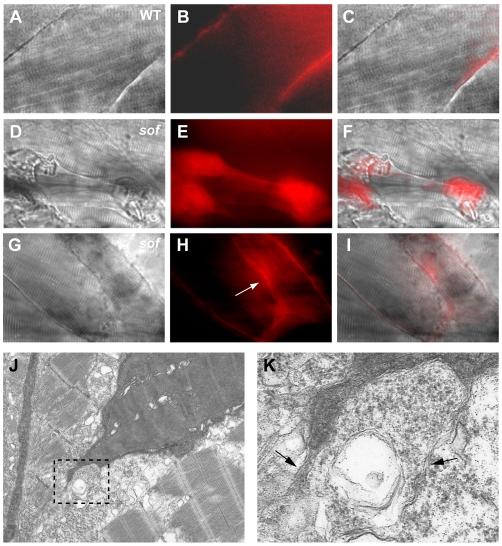
To determine if sarcolemmal damage was occurring in sof, the fluorescent dye, Evans Blue, was injected into sof and wild-type sibling embryos at 72 hpf. As expected, the dye was excluded from the myotome of wild-type embryos (Fig. 8A-C). However, uptake did occur in a subset of retracting fibres in sof embryos, and this correlated with the mode of fibre detachment. Thus, while the dye permeated the EFTs in a similar manner to the myoseptum, the fibres attached to them remained impervious to it. By contrast, staining was seen in single fibres, often before complete sarcolemmal detachment from the myoseptum (Fig. 8D-I). Transmission electron microscopy of a single detached fibre in a sof embryo appeared to show filaments of trailing sarcolemma, providing ultrastructural evidence of membrane damage (Fig. 8J,K). Permeation of the dye into the EFTs can be explained by the observation that Evans Blue binds to connective tissue (Straub et al., 1997). These results led to the conclusion that sarcolemmal detachment from the BM in sof was accompanied by rupture of the sarcolemma but that detachment of the BM itself, and EFT formation, did not damage the sarcolemma. This provides a further demonstration of: (1) the distinct nature of the two types of fibre detachment; and (2) the importance of the EFTs to the viability of the damaged myotome in sof mutants.
Fig. 8.
Sarcolemmal rupture correlates with the mode of fibre detachment in sof muscle. (A-C) Evans Blue is excluded from the myotome of wild-type embryos. (D-F) The dye infiltrates a subset of damaged fibres in sof embryos before complete detachment. The fibre depicted still spans the somite, but the contractile cytoskeleton has collapsed in the centre. (G-I) Evans Blue permeates an EFT (arrow in H) but does not enter the fibres attached to it. (J) Electron micrograph of a retracting fibre in a sof embryo at 72 hpf. The vertical myoseptum is to the left of the fibre. (K) A magnified view of the boxed area in J showing trailing sarcolemma at the newly detached tip of the fibre (arrows). (A,D,G) DIC images, (B,E,H) fluorescence images, (C,F,I) merge of DIC and fluorescence images. All panels show lateral views with anterior to the left.
Sarcolemmal detachment affects membrane integrity differently in sof and the lama2 mutant caf
It has previously been shown that the sarcolemma of detached fibres in lama2-null caf embryos remains intact and impermeable to Evans Blue dye (Hall et al., 2007). As Lama2 and Lamb2 associate in the same laminin trimer at the MTJ, the uptake of Evans Blue by a subset of detaching fibres in sof mutants was a surprising finding. In order to verify this difference in phenotype between sof and caf, Evans Blue was injected into embryos of both mutants in parallel at 72 hpf (see Fig. S3 in the supplementary material). This demonstrated a clear difference in sarcolemmal integrity between the two mutants. Although the dye again entered detached fibres in the sof myotome, there was no uptake by detaching fibres in the caf myotome. This experiment also reinforced the difference in the morphology of the detached ends, with the sof fibres often appearing invaginated, compared with the more angular fibre ends in the caf mutant (compare Fig. S3A with S3D in the supplementary material). This may indicate that caf fibres can maintain their sarcolemmal shape and tension upon detachment whereas the terminal sarcolemma of sof fibres collapses following tearing and detachment from the BM.
Analysis of softy;candyfloss double mutant embryos
As Lama2 and Lamb2 are co-localised at the MTJ, we sought to determine the epistatic relationship between the two laminin chains. Embryos produced from an incross of sof/+;caf/+ compound heterozygotes were assayed by birefringence at 120 hpf. As no additive phenotype was evident, those embryos with the greatest loss of birefringency were genotyped for the presence of the mutant alleles. This revealed that 59% (n=22) of the selected embryos were homozygous for the caf allele whereas 41% were homozygous for both caf and sof alleles (i.e. double mutants). As no sof mutants alone were selected in this assay, we concluded that, by 120 hpf, the muscle degeneration in caf has progressed more than in sof. Furthermore, the presence of one mutant lama2 allele on a homozygous sof background did not increase the severity of the sof mutant phenotype, as assayed by loss of birefringence (n=12). These results demonstrate that caf is epistatic to sof, such that the presence of a mutant Lamb2 chain does not exacerbate the phenotype caused by loss of Lama2 (see Fig. S4 in the supplementary material).
Double mutant embryos were also immunostained for dystrophin and laminin to ascertain whether Lama2 is required for the formation of EFTs (see Fig. S5 in the supplementary material). When compared with sof and caf mutant embryos, it was evident that the double mutants were most similar to caf. EFTs were readily seen in sof mutants, whereas intramyotomal co-localisation of dystrophin and laminin in caf and sof;caf mutants was restricted to condensed fragments of membrane at the ends of retracted fibres. This clearly indicates that the formation of EFTs is dependent on the availability of Lama2 in the extracellular matrix.
DISCUSSION
Previous work has demonstrated that loss of crucial components of the zebrafish MTJ, either intracellularly (dystrophin, integrin-linked kinase) or extracellularly (Lama2), results in an embryonic myofibre detachment phenotype (Bassett et al., 2003; Hall et al., 2007; Postel et al., 2008). In this study we extend our knowledge of the role of another extracellular component, Lamb2, by showing that a disorganised MTJ, detachment of muscle fibres and sarcolemmal damage all result from a missense mutation in the zebrafish lamb2 gene in the softm272a mutant. Whereas secondary changes in levels of LAMB2 immunoreactivity have been seen in congenital muscular dystrophy, this laminin chain has not been implicated as a direct cause of inherited muscle disease (Cohn et al., 1997). Rather, mutations in LAMB2 cause Pierson syndrome, an autosomal recessive, oculorenal disease with neurological involvement (Zenker et al., 2004). No dystrophy has been reported in Pierson syndrome; however, deletion of Lamb2 in the mouse results in MTJ defects in addition to renal and motor endplate dysfunction (Miner et al., 2006). As we have shown, the specificity of the sof phenotype, compared with mammalian alleles, can be explained by gene duplication and subdivision of expression domains of the duplicate genes, lamb2 and lamb2l. These findings reinforce the importance of Lamb2 in maintaining the structural integrity of the skeletal muscle BM and the stability of myofibre attachment.
The initial severity of the sof phenotype may be explained by the Leu38→Pro substitution being located in the LN domain of Lamb2. This domain participates in assembly of the laminin polymer via non-covalent tripartite interactions with the LN domains of adjacent β and γ chains (Colognato et al., 1999; McKee et al., 2007). A compromised polymerisation interface will have an impact on the integrity of the laminin network as a whole, thereby affecting the strength of extracellular matrix adhesion to the sarcolemma at the MTJ. Although the short stretches of layered BM seen in electron micrographs of sof MTJ imply that the mutant protein can still be assembled into a macromolecular lattice, the onset of muscle activity, imposing physical strain on the MTJ, will expose any weakness in the polymer, triggering the muscle attachment failure seen in sof. The inability of exogenous Lamb2 to prevent detachment also supports a model of overall instability of the laminin lattice in sof mutants that cannot be overcome by localised, cell-autonomous secretion of wild-type protein into the extracellular matrix.
In terms of aetiology, sof is most similar to the zebrafish lama2 mutant, caf, a model for MDC1A (Hall et al., 2007). caf is also epistatic to sof, with no increased severity seen in sof;caf double homozygotes. This implies that the major function of Lamb2 in stabilising muscle attachments is mediated through its complex with Lama2, rather than another α chain. However, a number of intriguing phenotypic differences exists between the two mutants. These include different MTJ pathologies, differences in the permeability of detached single fibres to Evans Blue dye and a contrasting capacity for recovery and survival. In addition, although the nature of the respective mutations is different - truncation of Lama2 in caf, amino acid substitution in Lamb2 in sof - the precise recapitulation of the sof phenotype by knockdown of Lamb2 in morphant embryos strongly suggests that the sof mutation is also a loss-of-function mutation. Furthermore, the presence of normal levels of immunoreactive laminin at fibre ends in lamb2 morphants indicates that an alternate laminin β chain may be present. The laminin complex containing this alternate β chain, the identity of which is under investigation, may be sufficient for initial attachment but, when Lamb2 levels are reduced, may not provide the necessary resilience to prevent fibre detachment in the morphants. Together, these findings suggest a complexity of interaction between multiple laminin isoforms in the developing skeletal muscle BM that is only beginning to be explored (Snow et al., 2008).
A major, unexpected finding of this report is that, despite a severe muscle pathology comparable to that seen in other dystrophic zebrafish mutants, sof is viable. This contrasts with the other dystrophic mutants, which have an average life span of three weeks or less (Bassett et al., 2003; Hall et al., 2007). In attempting to elucidate the basis of this capacity for recovery, we uncovered ectopic fibre terminations associated with a subset of retracted fibres as a phenotypic element unique to sof. The fact that these were also seen in lamb2 morphant embryos indicates that the loss of functional Lamb2 protein predisposes to EFT formation. Furthermore, our evidence suggests that the EFTs act as a stable substrate, providing a mechanical support and/or survival factor(s) to the associated fibres, despite having detached from the myoseptum. The retracted fibres may even continue to secrete BM components, increasing the strength of the EFT. Overall, the viability afforded by attachment to EFTs may act to slow down the cascade of dystrophic muscle degeneration and allow regenerative processes to take effect in the damaged myotome. EFT formation may thus be a pivotal factor contributing to the recovery seen in sof mutants.
The inherited muscular dystrophies are a large group of diseases characterised by skeletal muscle wasting and loss of muscle function (Emery, 2002). None of these diseases are currently curable, and many experimental therapies are being investigated. A viable zebrafish muscular dystrophy model has the potential to make a significant contribution to research in this field (Bassett and Currie, 2003; Guyon et al., 2007). Uncovering the mechanisms that enable the sof mutant to recover from severe, early muscle degeneration and thrive as adults may ultimately have important ramifications. Although our understanding of EFT formation is incomplete, their contribution to stabilising a damaged myotome offers promise for a novel therapeutic approach to the muscular dystrophies. If the BM of diseased muscle fibres can be augmented in a way that mimics the structures described here, then the severity of dystrophic muscle damage may be alleviated.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/19/3367/DC1
Supplementary Material
We are grateful to Dr H.-G. Frohnhöfer and Prof. P. Ingham for fish strains. We thank J. Cocks, C. Jenkin and P. Yudhyantara for expert fish care. We also thank Dr M. Wouters and Dr I. Martin for useful discussion and the Fatkin Lab for technical help. The F59 and MANDAG2 antibodies (developed by F. Stockdale and G. Morris, respectively) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA. This work was funded by the NHMRC, Australia, the MDA, USA and the Human Frontiers Science Program. E.B.-N. and D.L.S. were supported by the Wellcome Trust (WT 077037/Z/05/Z, WT 077047/Z/05/Z). Deposited in PMC for release after 6 months.
References
- Aumailley, M., Bruckner-Tuderman, L., Carter, W. G., Deutzmann, R., Edgar, D., Ekblom, P., Engel, J., Engvall, E., Hohenester, E., Jones, J. C. et al. (2005). A simplified laminin nomenclature. Matrix Biol. 24, 326-332. [DOI] [PubMed] [Google Scholar]
- Bassett, D. I. and Currie, P. D. (2003). The zebrafish as a model for muscular dystrophy and congenital myopathy. Hum. Mol. Genet. 12 Spec No 2, R265-R270. [DOI] [PubMed] [Google Scholar]
- Bassett, D. I., Bryson-Richardson, R. J., Daggett, D. F., Gautier, P., Keenan, D. G. and Currie, P. D. (2003). Dystrophin is required for the formation of stable muscle attachments in the zebrafish embryo. Development 130, 5851-5860. [DOI] [PubMed] [Google Scholar]
- Bendtsen, J. D., Nielsen, H., von Heijne, G. and Brunak, S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783-795. [DOI] [PubMed] [Google Scholar]
- Cohn, R. D., Herrmann, R., Wewer, U. M. and Voit, T. (1997). Changes of laminin beta 2 chain expression in congenital muscular dystrophy. Neuromuscul. Disord. 7, 373-378. [DOI] [PubMed] [Google Scholar]
- Colognato, H., Winkelmann, D. A. and Yurchenco, P. D. (1999). Laminin polymerization induces a receptor-cytoskeleton network. J. Cell Biol. 145, 619-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin, M. E., Jager, A. C., Khurana, T. S., Nielsen, F. C., Albrechtsen, R. and Wewer, U. M. (1999). Characterization of the human laminin beta2 chain locus (LAMB2): linkage to a gene containing a nonprocessed, transcribed LAMB2-like pseudogene (LAMB2L) and to the gene encoding glutaminyl tRNA synthetase (QARS). Cytogenet. Cell Genet. 84, 173-178. [DOI] [PubMed] [Google Scholar]
- Elworthy, S., Hargrave, M., Knight, R., Mebus, K. and Ingham, P. W. (2008). Expression of multiple slow myosin heavy chain genes reveals a diversity of zebrafish slow twitch muscle fibres with differing requirements for Hedgehog and Prdm1 activity. Development 135, 2115-2126. [DOI] [PubMed] [Google Scholar]
- Emery, A. E. (2002). The muscular dystrophies. Lancet 359, 687-695. [DOI] [PubMed] [Google Scholar]
- Geisler, R. (2002). Mapping and cloning. In Zebrafish (ed. C. Nüsslein-Volhard and R. Dahm), pp. 175-212. Oxford: Oxford University Press.
- Granato, M., van Eeden, F. J., Schach, U., Trowe, T., Brand, M., Furutani-Seiki, M., Haffter, P., Hammerschmidt, M., Heisenberg, C. P., Jiang, Y. J. et al. (1996). Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development 123, 399-413. [DOI] [PubMed] [Google Scholar]
- Green, T. L., Hunter, D. D., Chan, W., Merlie, J. P. and Sanes, J. R. (1992). Synthesis and assembly of the synaptic cleft protein S-laminin by cultured cells. J. Biol. Chem. 267, 2014-2022. [PubMed] [Google Scholar]
- Guille, M. (1999). Molecular Methods in Developmental Biology: Xenopus and Zebrafish. New York: Humana Press.
- Guyon, J. R., Steffen, L. S., Howell, M. H., Pusack, T. J., Lawrence, C. and Kunkel, L. M. (2007). Modeling human muscle disease in zebrafish. Biochim. Biophys. Acta 1772, 205-215. [DOI] [PubMed] [Google Scholar]
- Hall, T. E., Bryson-Richardson, R. J., Berger, S., Jacoby, A. S., Cole, N. J., Hollway, G. E., Berger, J. and Currie, P. D. (2007). The zebrafish candyfloss mutant implicates extracellular matrix adhesion failure in laminin alpha2-deficient congenital muscular dystrophy. Proc. Natl. Acad. Sci. USA 104, 7092-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima, S., Okamoto, H., Ueno, N., Hotta, Y. and Eguchi, G. (1997). High-frequency generation of transgenic zebrafish which reliably express GFP in whole muscles or the whole body by using promoters of zebrafish origin. Dev. Biol. 192, 289-299. [DOI] [PubMed] [Google Scholar]
- Hollway, G. E., Bryson-Richardson, R. J., Berger, S., Cole, N. J., Hall, T. E. and Currie, P. D. (2007). Whole-somite rotation generates muscle progenitor cell compartments in the developing zebrafish embryo. Dev. Cell 12, 207-219. [DOI] [PubMed] [Google Scholar]
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. and Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253-310. [DOI] [PubMed] [Google Scholar]
- Kwan, K. M., Fujimoto, E., Grabher, C., Mangum, B. D., Hardy, M. E., Campbell, D. S., Parant, J. M., Yost, H. J., Kanki, J. P. and Chien, C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088-3099. [DOI] [PubMed] [Google Scholar]
- Libby, R. T., Lavallee, C. R., Balkema, G. W., Brunken, W. J. and Hunter, D. D. (1999). Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J. Neurosci. 19, 9399-9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee, K. K., Harrison, D., Capizzi, S. and Yurchenco, P. D. (2007). Role of laminin terminal globular domains in basement membrane assembly. J. Biol. Chem. 282, 21437-21447. [DOI] [PubMed] [Google Scholar]
- Miner, J. H. (2008). Laminins and their roles in mammals. Microsc. Res. Tech. 71, 349-356. [DOI] [PubMed] [Google Scholar]
- Miner, J. H., Go, G., Cunningham, J., Patton, B. L. and Jarad, G. (2006). Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: implications for Pierson syndrome. Development 133, 967-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff, M. M., Turk, E. and Kalishman, M. (2002). Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 18, 613-615. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard, C. and Dahm, R. (2002). Zebrafish: A Practical Approach. Oxford: Oxford University Press.
- Odenthal, U., Haehn, S., Tunggal, P., Merkl, B., Schomburg, D., Frie, C., Paulsson, M. and Smyth, N. (2004). Molecular analysis of laminin N-terminal domains mediating self-interactions. J. Biol. Chem. 279, 44504-44512. [DOI] [PubMed] [Google Scholar]
- Oxtoby, E. and Jowett, T. (1993). Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 21, 1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, M. J., Pollard, S. M., Saude, L., Feldman, B., Coutinho, P., Hirst, E. M. and Stemple, D. L. (2002). Zebrafish mutants identify an essential role for laminins in notochord formation. Development 129, 3137-3146. [DOI] [PubMed] [Google Scholar]
- Patton, B. L. (2000). Laminins of the neuromuscular system. Microsc. Res. Tech. 51, 247-261. [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellof, F., Tiger, C. F., Virtanen, I., Thornell, L. E. and Gullberg, D. (2000). Laminin chains in developing and adult human myotendinous junctions. J. Histochem. Cytochem. 48, 201-210. [DOI] [PubMed] [Google Scholar]
- Postel, R., Vakeel, P., Topczewski, J., Knoll, R. and Bakkers, J. (2008). Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev. Biol. 318, 92-101. [DOI] [PubMed] [Google Scholar]
- Sanes, J. R. (2003). The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 278, 12601-12604. [DOI] [PubMed] [Google Scholar]
- Snow, C. J., Goody, M., Kelly, M. W., Oster, E. C., Jones, R., Khalil, A. and Henry, C. A. (2008). Time-lapse analysis and mathematical characterization elucidate novel mechanisms underlying muscle morphogenesis. PLoS Genet. 4, e1000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, V., Rafael, J. A., Chamberlain, J. S. and Campbell, K. P. (1997). Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield, M. (1993). The Zebrafish Book. Eugene, OR: University of Oregon Press.
- Wiedenmann, J., Ivanchenko, S., Oswald, F., Schmitt, F., Rocker, C., Salih, A., Spindler, K. D. and Nienhaus, G. U. (2004). EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA 101, 15905-15910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco, P. D., Cheng, Y. S. and Colognato, H. (1992). Laminin forms an independent network in basement membranes. J. Cell Biol. 117, 1119-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenker, M., Aigner, T., Wendler, O., Tralau, T., Muntefering, H., Fenski, R., Pitz, S., Schumacher, V., Royer-Pokora, B., Wuhl, E. et al. (2004). Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13, 2625-2632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.