Abstract
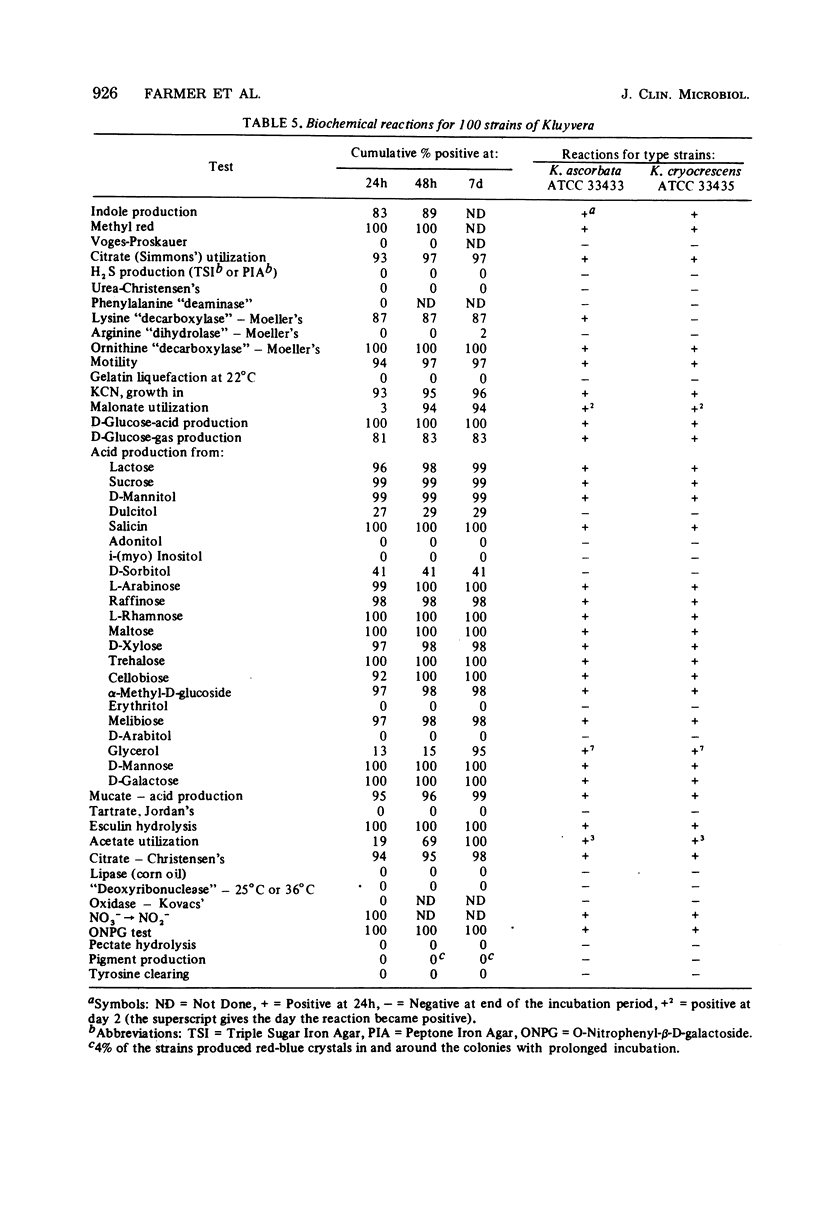
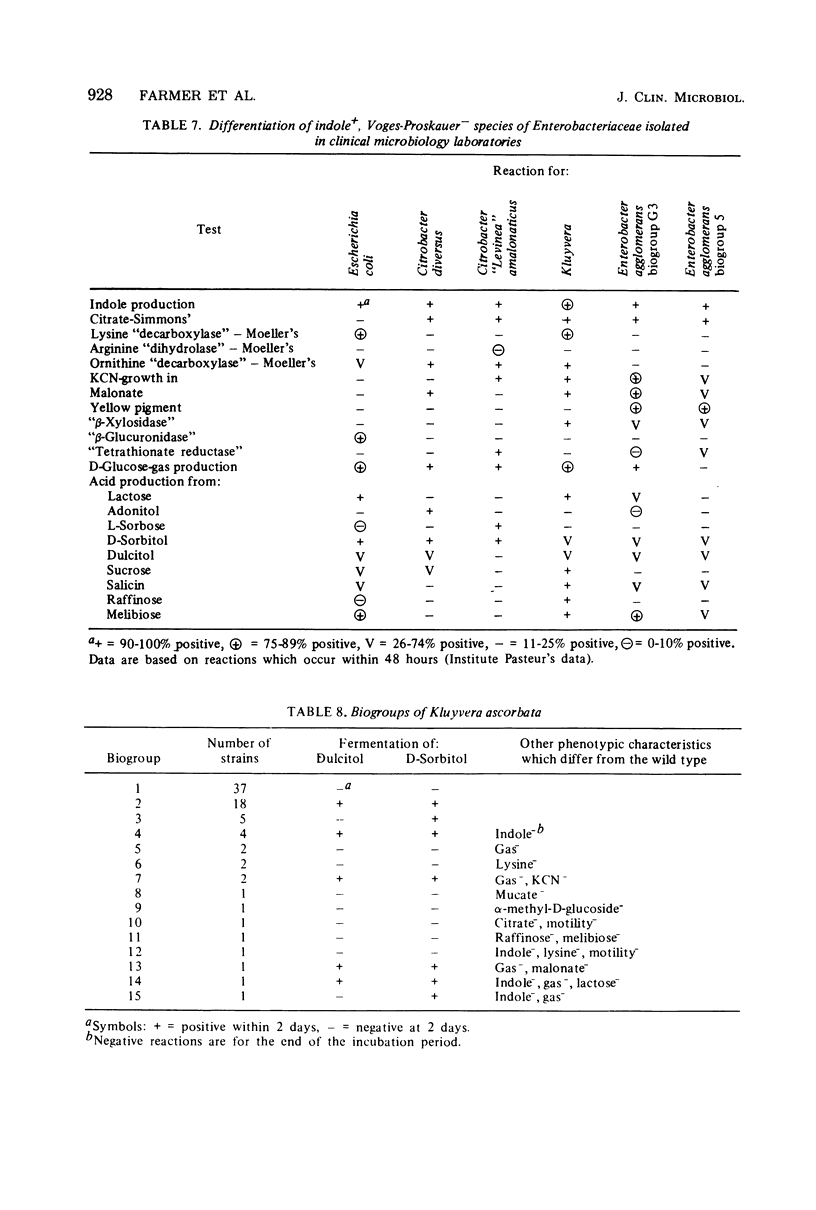
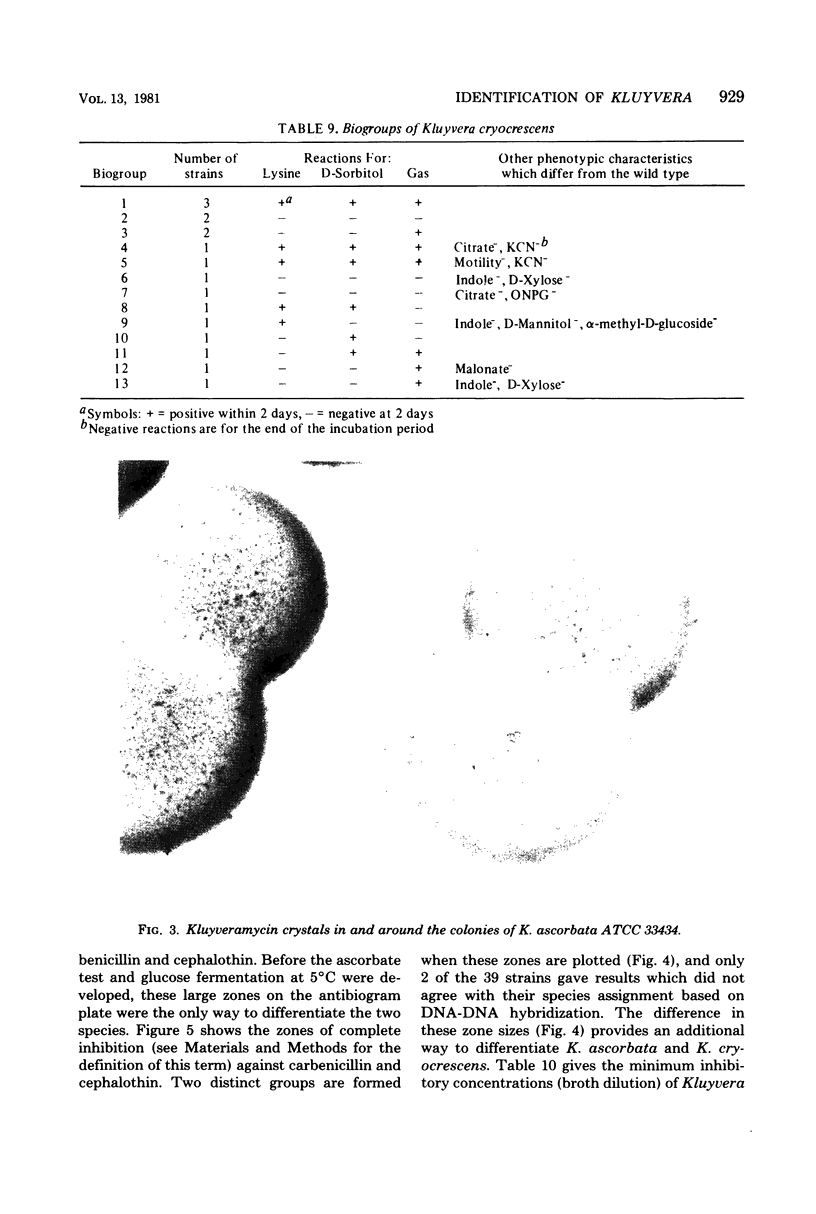
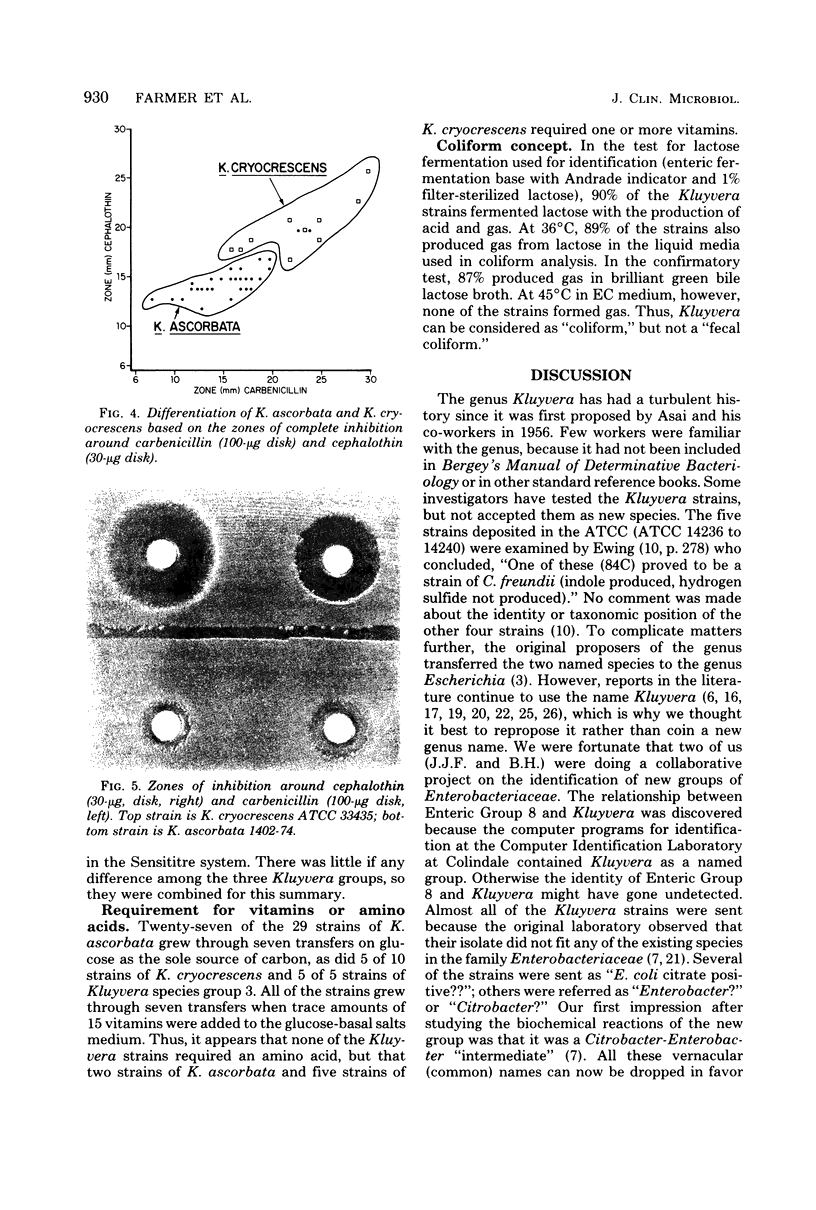
Kluyvera is proposed as a new genus for the group of organisms formerly known as Enteric Group 8 (synonym = API group 1). Strains of Kluyvera share the properties of most members of the family Enterobacteriaceae: they are gram-negative rods, motile with peritrichous flagella, catalase positive, and oxidase negative; they grow on MacConkey agar, ferment D-glucose with the production of acid and gas, and are susceptible to many antibiotics. Strains are usually indole positive, methyl red positive, Voges-Proskauer negative, citrate positive, H2S (triple sugar iron) negative, urea negative, phenylalanine deaminase negative, lysine decarboxylase positive, arginine dihydrolase negative, and ornithine decarboxylase positive. Kluyvera strains ferment many of the sugars and polyhydroxyl alcohols used in identification. By deoxyribonucleic acid-deoxyribonucleic acid hybridization, strains of Kluyvera were divided into three groups. Kluyvera ascorbata is proposed as the type species for the genus. Most strains of K. ascorbata have been isolated from clinical specimens. K. cryocrescens is proposed as the second species. It was occasionally isolated from clinical specimens, but it was isolated more commonly from the environment. Kluyvera species group 3 was heterogeneous, but was distinct from the two named species by deoxyribonucleic acid hybridization. This group was rare, so no species name will be proposed at this time. K. ascorbata can be differentiated from K. cryocrescens by its positive ascorbate test, inability to grow at 5 degrees C in a refrigerator, and smaller zones of inhibition around carbenicillin and cephalothin disks. The test normally used for identification does not clearly differentiate these two species. Kluyvera species are probably infrequent opportunistic pathogens. The most common source is sputum, where they are probably not clinically significant. Five strains have been from blood cultures. More information is needed about the incidence and clinical significance of the genus Kluyvera.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Wieczorek J., Reinecke W. Purification and properties of hamamelosekinase. Eur J Biochem. 1980 Jun;107(2):485–489. doi: 10.1111/j.1432-1033.1980.tb06054.x. [DOI] [PubMed] [Google Scholar]
- Braunstein H., Tomasulo M., Scott S., Chadwick M. P. A biotype of Enterobacteriaceae intermediate between Citrobacter and Enterobacter. Am J Clin Pathol. 1980 Jan;73(1):114–116. doi: 10.1093/ajcp/73.1.114. [DOI] [PubMed] [Google Scholar]
- Brisou B., Richard C., Lenriot A. Intérêt taxonomique de la recherche de la -xylosidase chez les Enterobacteriaceae. Ann Inst Pasteur (Paris) 1972 Sep;123(3):341–347. [PubMed] [Google Scholar]
- Hickman F. W., Farmer J. J., 3rd Salmonella typhi: identification, antibiograms, serology, and bacteriophage typing. Am J Med Technol. 1978 Dec;44(12):1149–1159. [PubMed] [Google Scholar]
- Kilian M., Bülow P. Rapid identification of Enterobacteriaceae. II. Use of a beta-glucuronidase detecting agar medium (PGUA agar) for the identification of E. coli in primary cultures of urine samples. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):271–276. [PubMed] [Google Scholar]
- Nara T., Okachi R., Misawa M. Enzymatic synthesis of D(-)-alpha-aminobenzylpenicillin by Kluyvera citrophila. J Antibiot (Tokyo) 1971 May;24(5):321–323. doi: 10.7164/antibiotics.24.321. [DOI] [PubMed] [Google Scholar]
- Serizawa N., Nakagawa K., Kamimura S., Miyadera T., Arai M. Stereo-specific synthesis of 3-trifluoromethylcephalosporin derivative by microbial acylase. J Antibiot (Tokyo) 1979 Oct;32(10):1016–1018. doi: 10.7164/antibiotics.32.1016. [DOI] [PubMed] [Google Scholar]
- Steigerwalt A. G., Fanning G. R., Fife-Asbury M. A., Brenner D. J. DNA relatedness among species of Enterobacter and Serratia. Can J Microbiol. 1976 Feb;22(2):121–137. doi: 10.1139/m76-018. [DOI] [PubMed] [Google Scholar]
- Thanbichler A., Beck E. Catabolism of hamamelose. The anaerobic dissimilation of D-hamamelose by Kluyvera citrophila 627. Eur J Biochem. 1974 Dec 16;50(1):191–196. doi: 10.1111/j.1432-1033.1974.tb03887.x. [DOI] [PubMed] [Google Scholar]
- Thurner K., Busse M. Numerisch taxonomische Untersuchungen an Enterobakterien aus Oberflächenwasser. Zentralbl Bakteriol B. 1978 Sep;167(3):262–271. [PubMed] [Google Scholar]