Abstract
Objective
To test the hypothesis that emotional eaters show greater neural activation in response to food intake and anticipated food intake than nonemotional eaters and whether these differences are amplified during a negative versus neutral mood state.
Method
Female emotional eaters and nonemotional eaters (N = 21) underwent functional magnetic resonance imaging (fMRI) during receipt and anticipated receipt of chocolate milkshake and a tasteless control solution while in a negative and neutral mood.
Results
Emotional eaters showed greater activation in the parahippocampal gyrus and anterior cingulate (ACC) in response to anticipated receipt of milkshake and greater activation in the pallidum, thalamus, and ACC in response to receipt of milkshake during a negative relative to a neutral mood. In contrast, nonemotional eaters showed decreased activation in reward regions during a negative versus a neutral mood.
Discussion
Results suggest that emotional eating is related to increased anticipatory and consummatory food reward, but only during negative mood.
Keywords: emotional eating, fMRI, binge eating, negative effect, reward sensitivity
Introduction
Threshold and subthreshold bulimia nervosa and binge eating disorder are prevalent and associated with increased risk for future obesity, depression, substance abuse, and health problems.1,2 Thus, it is vital to examine risk factors for binge eating to inform the design of prevention and treatment interventions. The affect regulation model posits that some individuals may binge eat to decrease negative affect and increase positive affect.3 This is in contrast to the typical response to negative affect, which is a loss of appetite due to physiological effects of distress (including inhibition of gastric contractions and elevation of blood sugar4). Thus, binge eaters who eat in response to negative affect are doing so despite these normal physiological processes that decrease motivation to eat. We hypothesize that a heightened reward response to food intake and anticipated food intake in emotional eaters may explain their paradoxical eating behavior.
Prospective studies have found that negative affect increases risk for future onset of binge eating5,6 and bulimic pathology,7 as well as increases in bulimic symptoms8,9 in samples of adolescent girls. However, this relation typically has not emerged in prospective studies of adults,10,11 potentially because bulimic symptoms most often emerge during adolescence. One prospective study found that emotional eating increases the risk for future binge eating onset.6
Studies that have used ecological momentary assessment, wherein participants rate their moods immediately before, during, and after each binge episode or normal meal, have found that binge eaters have elevated negative affect prior to a binge episode and that negative affect decreases during the binge, but then increases after the binge.12,13 Additionally, individuals typically report elevated depression, anger, and guilt and less positive affect on days in which binge eating occurs relative to days in which binge eating does not occur.14
Experiments have found that negative affect induction results in increased caloric intake in lab settings among restrained eaters, but not unrestrained eaters.15-19 However, experimental negative mood inductions did not result in changes in caloric intake among individuals with bulimia nervosa and binge eating disorder.20,21
Thus, although prospective and ecological momentary assessment studies have provided support for the affect regulation model of binge eating, experiments have generated mixed support. The latter studies may have produced null findings because participants alter their behavior in a laboratory setting because they know they are being observed. Thus, it may be useful to utilize a functional magnetic resonance imaging (fMRI) paradigm, which provides an objective measure of neural activation in brain regions associated with reward and negative affect within an experiment that manipulated both food intake and affect.
Brain-imaging studies have found greater activity in the ventromedial prefrontal cortex (VMPFC) correlated with negative affective states.22,23 Additionally, greater activation in the medial and inferior prefrontal cortex, middle temporal cortex, and caudate was found during negative mood inducing films versus neutral mood films.24 Urry et al.22 found an increase in activation of the amygdala and a decrease in activation of the VMPFC when negative affect decreased. A recent meta-analysis25 reviewing brain response to mood inductions found that activation in the medial prefrontal cortex was present in response to general emotion processing, rather than during processing of any specific emotions. Fear was associated with increased amygdala activation, and sadness was associated with increased activation in the subcallosal cingulate. Finally, increased activation in the anterior cingulate and insula was associated with emotional imagery. If eating results in decreased negative affect among emotional eaters, we would expect greater activation in these prefrontal and limbic areas, but less activation in the VMPFC, in line with these previous studies of emotion response in the brain. Killgore and Yurgelun-Todd26 found that activation in areas associated with motivation to eat and reward (insula and orbitofrontal cortex) in response to high-calorie food images was positively correlated with self-report measures of negative affect, providing support for the notion that mood state influences the neural response to food.
We used an experimental paradigm that was designed to examine consummatory food reward (reward from food intake) and anticipatory food reward (anticipated reward from food intake). Because anticipatory food reward putatively plays a key role in overeating, an improved understanding of the neural substrates of this phenomenon may lead to improved treatment and preventive interventions for obesity, binge eating, and bulimic pathology.
Brain imaging studies with humans indicate that consumption of palatable foods, relative to control comparison conditions, results in greater activation of the caudal and medial orbitofrontal cortex, frontal operculum, Rolandic operculum, amygdala, superior and mid insula, striatum, midbrain, and anterior cingulate.27-30 Anticipation of food intake, relative to control conditions, results in greater activation in the caudal and medial orbitofrontal cortex, Rolandic operculum, dorsolateral prefrontal cortex, posterior amygdala, insula, anterior cingulate gyrus, striatum (caudate, putamen, and nucleus accumbens), midbrain, posterior cingulate, anterior hippocampus, parahippocampal gyrus, and fusiform gyrus.30-34 Many of these regions appear to respond to both consummatory and anticipatory food reward, which may be due to a learning effect, in which areas associated with consummatory reward begin to respond to anticipatory food reward as the association between cues and food delivery is strengthened. Although there is considerable overlap in brain response to these two phases of food reward, there is evidence that some regions respond preferentially to anticipatory food reward rather than consummatory food reward, and vice versa. O’Doherty et al.28 found that the receipt of a pleasant taste, relative to anticipation of a pleasant taste resulted in greater activation of the right anterior orbitofrontal cortex and that the anticipation of a pleasant taste, versus receipt of the taste, resulted in greater activation in the dopaminergic midbrain, nucleus accumbens of the ventral striatum, and the posterior right amygdala.
Although it appears that no previous research has investigated activation of brain areas related to affective processes or food reward among emotional eaters compared with nonemotional eaters, there is evidence that abnormalities in neural circuitry is related to obesity. Specifically, one fMRI study found that obese, relative to lean, adolescent girls showed greater activation in the anterior and mid insula, frontal operculum, parietal operculum, and Rolandic operculum in response to anticipatory and consummatory food reward, but decreased activation in the caudate nucleus in response to consummatory food reward.35 Another study found increased activation in the right parietal and temporal cortices after exposure to pictured food in obese but not lean women and that this activation correlated positively with hunger ratings.36 Rothemund et al.37 found greater dorsal striatum response to pictures of high-calorie foods in obese verse lean adults and that BMI correlated positively with response in insula, claustrum, cingulate, postcentral gyrus (somatosensory cortex), and lateral OFC.
This study attempts to elucidate differences in brain activation between emotional eaters and nonemotional eaters in response to consuming chocolate milkshake and anticipating consumption of chocolate milkshake in a neutral and negative mood state. We consider the brain activation in response to a cue signaling the impending delivery of a chocolate milkshake (versus tasteless solution) to reflect anticipatory food reward, and we consider the brain activation in response to actually receiving a taste of milkshake (versus tasteless solution) to reflect consummatory food reward. We hypothesized that emotional eaters would show greater evidence of anticipatory and consummatory food reward based on the activation in regions associated with these reward processes while in a negative mood state versus a neutral mood state, whereas the opposite pattern would emerge for nonemotional eaters. We predicted this opposite pattern for nonemotional eaters in accordance with the normal response of decreased appetite during negative mood.4 Additionally, we hypothesized activation in the limbic system, specifically the amygdala and insula, reflecting difference in reward response as well as emotional response to food stimuli. We expected to see decreased activation in the VMPFC in response to anticipation and receipt of chocolate milkshake among emotional eaters in a negative mood, as this would imply a decrease in negative affect.22 To our knowledge, no prior fMRI study has examined activations in regions associated with food reward and emotional processing that compared emotional eaters to nonemotional eaters in different mood states. We attempted to improve upon prior research by experimentally manipulating mood, rather than relying on self-reported mood, experimentally manipulating exposure to food cues and food intake, and examining activation in brain regions thought to encode food reward and emotional experience using objective fMRI techniques. We conducted this study on college women to remain consistent with prior research on the affect regulation model, predominantly studied in women because most individuals with eating disorders are women.1
Method
Participants
Participants were 21 female college students recruited from introductory psychology courses, aged 17 to 24 (M = 20.1, SD = 2.0). The sample of women was 4% Hispanic, 84% Caucasian, 4% Asian, and 8% American Indian or Alaska Native. Participants had a mean body mass index (BMI) of 24.4 (SD = 4.5).
Procedure
Students taking introductory psychology courses completed the emotional eating subscale of the Dutch Eating Behavior Questionnaire (n = 709; 64% female)38 and female students scoring in the top and bottom quartiles were invited to participate in a brain imaging study of mood and taste. Qualifying scores were greater than 3.6 for the emotional eating group and less than 1.6 on a 1- to 5-point scale for the nonemotional eating group. Of the 42 women contacted via email, 27 replied, and 21 agreed to participate. Thus, the sample comprised 10 participants scoring in the top quartile and 11 participants in the bottom quartile of the emotional eating scale.
Participants were asked to consume a usual meal and then refrain from eating and drinking caffeinated beverages for 4-6 h before the imaging session for standardization purposes. All scans were conducted in the afternoon on weekdays (between noon and 3 pm). Participants completed questionnaires and were familiarized with the fMRI procedure.
The experimental paradigm was conducted in a negative mood state and in a neutral mood state for each participant. This resulted in a between- and within-subjects experimental design, such that mood was compared within each subject, and emotional eating status was compared between the subjects. A musical mood induction was used to induce the negative and neutral moods. Participants listened to 3 min of Prokofiev’s Russia Under the Mongolian Yoke played at half speed (negative mood) and Adams’s Common Tones in Simple Time (neutral mood). During presentation of each musical piece, participants were instructed to imagine a situation in which they recently felt that mood. This procedure has been used successfully in previous mood induction studies.39,40 After the mood induction (lasting 3 min), participants rated their mood on a scale of 0-100 for how sad, anxious, or stressed they were. A manipulation check based on these ratings is reported in the results section.
For the fMRI paradigm, the stimuli consisted of three black shapes (cues) that signaled the delivery of 0.5 ml of either a chocolate milkshake, a tasteless (neutral) solution, or no solution. The milkshake consisted of 1 cup of Häagen Daz vanilla ice cream, 2 tablespoons of 2% milk, and 2 tablespoons of Hershey’s chocolate syrup. The tasteless solution, which was designed to mimic the natural taste of saliva, consisted of 25 mM KCl and 2.5 mM NaHCO3. Stimuli were presented using MATLAB run from a Windows computer. Images were presented with a digital projector/reverse screen display system to a screen at the back end of the MRI scanner bore and were visible via a mirror mounted on the birdcage head coil. The taste delivery occurred 4-11 s after onset of the cue. Tastes were delivered using two programmable syringe pumps (Braintree Scientific BS-8000) controlled by MATLAB to ensure consistent volume, rate, and timing of taste delivery (0.5 ml over 5 s). This rate and amount of liquid is consistent with prior research in the sensory neuroscience literature and has resulted in clear brain activation in expected regions in these studies (e.g., Ref. 41). Sixty-milliliter syringes filled with the chocolate milkshake and the tasteless solution were connected via Tygon tubing through a wave guide to a manifold, developed by Pierce Laboratories, CT, which was attached to the birdcage head coil in the MRI scanner (see Fig. 1). The manifold fit into the participants’ mouths and delivered the taste to a consistent segment of the tongue. The taste cue remained on the screen for 8.5 s after the taste was delivered, and participants were instructed to swallow when the shape went off. The next cue appeared 1-5 s after the prior cue disappeared.
FIGURE 1.
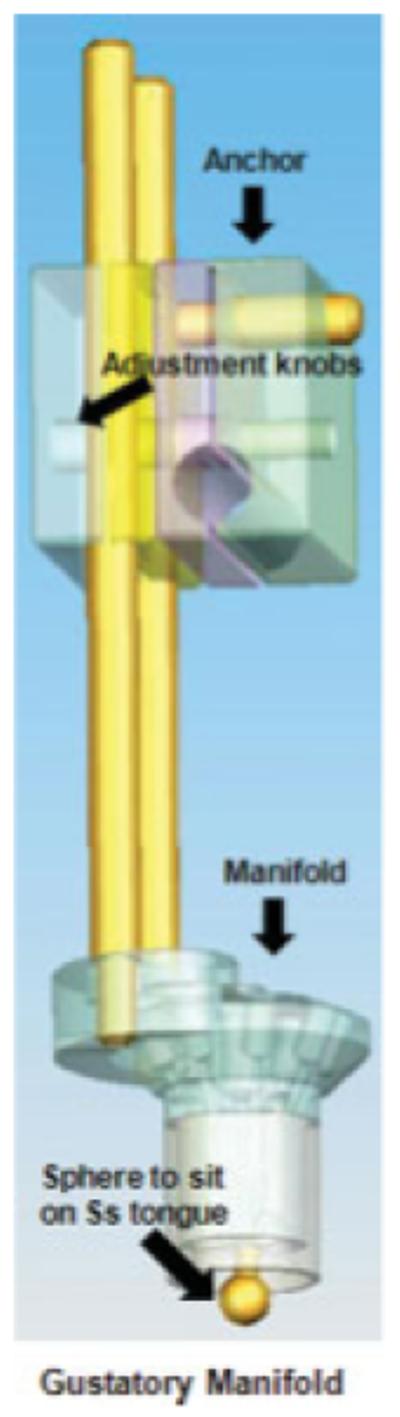
The gustatory manifold is anchored to the headcoil. New tubing and syringes are used for each subject and the mouthpiece is cleaned and sterilized between uses. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Each shape corresponded with a particular taste (or no taste). However, on 50% of the chocolate and tasteless solution trials, the taste was not delivered as expected to permit investigation of activation in response to anticipation that was not confounded by activation in response to actual milkshake receipt. Thus, there were five conditions: chocolate valid (chocolate cue followed by chocolate taste), chocolate invalid (chocolate cue followed by no taste), tasteless solution valid (tasteless solution cue followed by tasteless solution), tasteless solution invalid (tasteless solution cue followed by no taste), and no taste valid (no taste cue followed by no taste). Each block of the experiment included four trials of each condition and lasted 5 min. Four blocks were run in each mood condition for every participant, for a total of 16 trials per condition per mood condition. Mood conditions and shape/taste combination were counter-balanced between participants, (e.g., for some participants, a circle corresponded with chocolate, and they had a negative mood induction first, and some participants had a neutral mood first and a circle corresponded with tasteless solution).
Experiments were performed on a Siemens Allegra 3.0 Tesla head-only MRI scanner. A standard birdcage coil was used to acquire data from the entire brain. A thermofoam vacuum pillow and additional padding was used to restrict head motion. High-resolution T1-weighted structural images were acquired using the 3D MP-RAGE pulse sequence. Blood oxygen level-dependent, echo-planar images (BOLD-EPI) were acquired using T2*-weighted gradient echo sequence, 64 × 64 voxel matrix, TE = 30 ms, TR = 2 s, Flip angle = 90°, 32 interleaved (no skip) slices with 4 mm thickness.
Data were analyzed using general linear model in SPM5 (Wellcome Department of Cognitive Neurology, London, UK). The functional images were time-acquisition corrected to the slice obtained at 50% of the TR. All functional images were then realigned to the mean. The images (anatomical and functional) were normalized to the T1 nii SPM5 template with an average of 152 brains. Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for structural images. Functional images were smoothed with a 6-mm FWHM isotropic Gaussian kernel. For time series analysis of all participants, a high-pass filter was included in the filtering matrix (according to convention in SPM5) to remove low-frequency noise and slow drifts in the signal, which could bias the estimates of the error.
Condition-specific effects at each voxel were estimated using the general linear model. The responses to events (i.e., indicated by stimulus onsets) were modeled by a canonical hemodynamic response function (HRF), consisting of a mixture of 2 gamma functions that emulate the early peak at 5 s and the subsequent undershoot.
Within- and between-group comparisons were performed using a random effects model to account for intersubject variability. Parameter estimate images from contrasts were entered into a second-level 2 × 2 ANOVA (high vs. low emotional eaters) by (negative vs. neutral mood). SPM assigns significance t-fields from all analyses using the theory of Gaussian Random Fields.42,43 Activations are considered significant at p < .05 after correction at the cluster level using random field theory (RFT) for multiple comparisons either across all voxels in the volume or within regions of interest, including the limbic lobe, frontal cortex, and somatosensory cortex. All regions discussed in the Results section met these conservative criteria for significance, although uncorrected p-values are reported.
Measures
Handedness was assessed with the Edinburgh Handedness Questionnaire,44 and both the laterality quotient and the laterality scale were calculated.45 Scores above zero indicate more dominant use of the right hand, and below zero indicate more dominant use of the left hand. Someone scoring exactly zero would have no preference of one hand over the other. Mean score for the sample was 0.80, reflecting predominantly right-handed participants. None of the participants were left-handed.
Mood ratings were made after each mood induction by taking an average of three ratings (sadness, anxiety, and stress). Ratings were measured by asking the following for each mood: “Please rate your current level of sadness from 0 to 100, where 0 is no sadness and 100 is extremely sad.”
Emotional eating was assessed with the Dutch Eating Behavior Questionnaire—Emotional Eating subscale.38 The scale consists of 10 questions asking participants the frequency of particular eating habits, on a 5-point scale. The scale has demonstrated internal consistency (α = 0.86 to 0.9738) and shows predictive validity for binge eating onset.6
Depressive symptoms were assessed with the Beck Depression Inventory (BDI46). The BDI has acceptable internal consistency (α = 0.73 to 0.95), test-retest reliability (r = .60 to .90), and convergent validity with clinician ratings of depressive symptoms (M r = 0.7546).
Negative and positive affect was assessed with the Positive and Negative Affect Schedule (PANAS47). Participants rate how much 20 adjectives describe their current mood on a 5-point scale. This scale has shown internal consistency (α = 0.95), 3-week test-retest reliability (r = .78), convergent validity, and predictive validity.48,49
Eating disorder symptoms were assessed with the Eating Disorder Diagnostic Scale (EDDS50). The EDDS assesses DSM-IV diagnostic criteria for anorexia nervosa, bulimia nervosa, and binge eating disorder and yields a continuous symptom composite that was used in the study. The EDDS has shown high agreement (κ = 0.78-0.83) with eating disorder diagnoses made with the Eating Disorder Examination (EDE51), internal consistency (α = 0.89), 1-week test-retest reliability (r = .87), sensitivity to detecting intervention effects, and predictive validity for future onset of eating pathology and depression.50,52
Restrained eating was measured with the Dutch Eating Behavior Questionnaire—Restraint Scale.38 The DRES has shown internal consistency (α = 0.95), 2-week test-retest reliability (r = .82), convergent validity with self-reported caloric intake (though it shows weaker relations to objectively measured intake), and predictive validity for bulimic symptom onset.38,52
Expectations of food’s consequences were assessed with the Eating Expectancy Inventory (EEI53). The factors of this scale are as follows: eating helps manage negative affect, eating is pleasurable and useful as a reward, eating leads to feeling out of control, eating enhances cognitive competence, and eating alleviates boredom. The EEI has shown internal consistency (α = 0.90) and association with eating disorder symptoms among adolescents.54,55
Hunger was assessed with the Three Factor Eating Questionnaire—Hunger Scale (TFEQ-H56). The scale consists of 10 true/false statements and three questions asking how often the participant is hungry. This scale has shown internal consistency and test-retest reliability.57
Results
Descriptive and Group Differences
We performed independent t-tests to test whether emotional eaters differed from nonemotional eaters on all survey measures, to characterize the sample. These results are displayed in Table 1. As would be expected, in comparison to nonemotional eaters, emotional eaters reported more negative affect and depressive symptoms. Also as would be expected, relative to nonemotional eaters, emotional eaters were significantly more likely to hold the expectation that eating helps manage negative affect and that eating alleviated boredom. Additionally, compared with nonemotional eaters, emotional eaters were significantly more likely to hold the expectation that eating leads to feeling out of control. No other significant differences were found between emotional eaters and nonemotional eaters on the variables included in the study. It is important to note that one outlier was removed from group comparisons on BMI and depressive symptoms. Analyses of fMRI data were conducted with and without this outlier, and results remained the same. Thus, the results in this report are from the entire sample.
TABLE 1. Means, standard deviations, and independent sample t-tests on self-report measures.
| Group Means |
|||||
|---|---|---|---|---|---|
| Emot. Eat. | Non-Emot. | ||||
| Variable | t(19) | p | η2 | Mean (SD) | |
| Dietary restraint | -0.29 | .77 | 0.004 | 3.04 (0.72) | 2.93 (1.04) |
| Hunger | 1.28 | .22 | 0.08 | 21.5 (1.84) | 22.45 (1.57) |
| Positive affect | 1.34 | .20 | 0.09 | 2.98 (0.82) | 3.39 (0.57) |
| Negative affect | -2.30 | .033 | 0.22 | 2.24 (0.61) | 1.69 (0.49) |
| Depressive symptoms [with outlier removed; t(18)] | -3.75 | .001 | 0.44 | 13.56 (7.14) | 3.91 (4.25) |
| Body mass index [with outlier removed; t(18)] | -1.60 | .13 | 0.14 | 24.51 (2.62) | 22.68 (2.23) |
| Eating disorder symptoms | -1.57 | .14 | 0.13 | 19.0 (11.52) | 11.6 (9.06) |
| Expectation that eating manages negative affect | -5.12 | <.001 | 0.58 | 3.32 (0.55) | 2.16 (0.48) |
| Expectation that eating alleviates boredom | -2.19 | .041 | 0.20 | 3.32 (0.55) | 2.82 (0.25) |
| Expectation that eating is rewarding | 0.92 | .85 | 0.04 | 3.02 (0.17) | 3.13 (0.38) |
| Expectation that eating enhances cognitive comp | -0.77 | .45 | 0.03 | 3.55 (0.80) | 3.23 (1.08) |
| Expectation that eating leads to feeling out of control | -3.99 | .001 | 0.46 | 3.00 (0.49) | 2.30 (0.31) |
A manipulation check confirmed that the mood induction was successful. A paired sample t-test revealed significant differences between participants’ negative affect ratings in the negative (M = 44.02, SD = 19.23) and neutral conditions (M = 27.14, SD = 18.53, t[20] = 5.02, p < .001, η2 = 0.56). Although this effect was present for both emotional eaters and nonemotional eaters, there was a significant interaction between emotional eating status and the effect of the mood induction on negative affect ratings (F[1,19] = 8.68, p = .008, η2 = 0.31), such that emotional eaters rated the negative condition (M = 49.83, SD = 16.74) more negatively than nonemotional eaters (M = 38.73, SD = 20.56) and the neutral condition (M = 24.13, SD = 18.38) less negatively than nonemotional eaters (M = 29.88, SD = 19.12). These results suggest that emotional eaters may be more emotionally reactive than nonemotional eaters.
Effects of Mood Condition and Emotional Eating on Anticipatory Food Reward
A 2 × 2 ANOVA indicated that there were no significant main effects of emotional eating status on neural activation in response to anticipating receipt of chocolate milkshake versus receipt of the tasteless control solution (the anticipatory food reward contrast). This contrast utilized the incongruent trials (e.g., when the cue signaled chocolate, but no taste was delivered). This allowed us to separate the effect of anticipation from the effect of taste delivery. There was a significant main effect of mood condition for this contrast. This comparison revealed a greater activation in the left ventral anterior cingulate during the negative mood condition than in the neutral mood condition for the anticipatory reward contrast, averaging across emotional and nonemotional eaters. (See Table 2 for a summary of brain areas and contrasts.) The opposite pattern emerged in the neutral mood condition, in which there was greater activation in this region in response to milkshake cue, tasteless solution cue. Additionally, we found a greater activation in the left thalamus during the neutral mood condition than in the negative mood condition in the anticipatory food reward contrast, averaging across emotional and nonemotional eaters. Again, the opposite pattern emerged in the negative mood, with greater activation in the left thalamus in response to anticipated receipt of the control solution versus milkshake.
TABLE 2. Significant brain regions (Talairach coordinates of cluster centers), cluster Z-score, p-value, and effect size for each contrast.
| Contrast | x | y | z | Max Z | p | η2 | Brain Region |
|---|---|---|---|---|---|---|---|
| Anticipatory reward: Main effect of mood condition | |||||||
| -3 | 27 | -3 | 3.48 | <.001 | 0.53 | Left ventral anterior cingulate | |
| -15 | -33 | 6 | 2.76 | .003 | 0.33 | Left thalamus | |
| Anticipatory reward: Interaction between emotional eating and mood condition | |||||||
| -30 | -39 | -6 | 3.48 | <.001 | 0.53 | Left parahippocampal gyrus | |
| 12 | 57 | 15 | 3.36 | <.001 | 0.50 | Right anterior cingulate | |
| -6 | 42 | 18 | 3.29 | <.001 | 0.48 | Left anterior cingulate | |
| 24 | -39 | -6 | 3.09 | .001 | 0.32 | Right parahippocampal gyrus | |
| 6 | 27 | 15 | 2.85 | .002 | 0.30 | Right anterior cingulate | |
| Consummatory reward: Main effect of emotional eating status | |||||||
| -12 | 9 | 6 | 3.18 | .001 | 0.32 | Left caudate nucleus, left pallidum | |
| Consummatory reward: Main effect of mood condition | |||||||
| 3 | 36 | 12 | 3.43 | <.001 | 0.61 | Right anterior cingulate | |
| -3 | 36 | 24 | 2.90 | .002 | 0.39 | Left anterior cingulate | |
| Consummatory reward: Interaction between emotional eating and mood condition | |||||||
| 18 | -3 | 0 | 3.75 | <.001 | 0.50 | Right pallidum | |
| -18 | -21 | 9 | 3.22 | .001 | 0.47 | Left thalamus | |
| -24 | -12 | 3 | 3.18 | .001 | 0.37 | Left pallidum | |
| -15 | -9 | 21 | 2.95 | .002 | 0.51 | Left anterior thalamus | |
| 3 | -12 | 6 | 2.82 | .002 | 0.44 | Right thalamus | |
| 6 | 36 | 21 | 2.76 | .003 | 0.60 | Right anterior cingulate | |
More importantly, the ANOVA model revealed a significant interaction between emotional eating status and mood condition for brain activation in the anticipatory food reward contrast. This interaction revealed greater activation in the left parahippocampal gyrus in this contrast for emotional eaters in the negative mood state. Interestingly, the opposite was found for nonemotional eaters in the negative mood state, with greater activation in the left parahippocampal gyrus in response to tasteless solution cue—milkshake cue. Furthermore, effects were reversed in the neutral mood state, such that greater activation in this region was found in milkshake cue—tasteless solution cue for nonemotional eaters and in response to tasteless solution cue—milkshake cue for emotional eaters. Additionally, this same pattern of effects was seen bilaterally in the anterior cingulate and in the right hippocampal gyrus. Coordinates and statistics for these effects are shown in Table 2, and Figure 2 shows activation location for this comparison.
FIGURE 2.
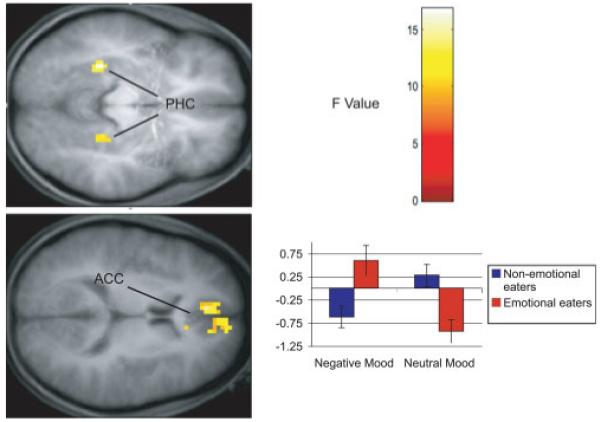
Results from ANOVA models of anticipatory food reward. The color bar represents the F values representative for both figures. Axial sections of increased activation bilaterally in the anterior cingulate cortex (ACC) and parahippocampal gyrus (PHC) in response to anticipated receipt of chocolate versus tasteless control solution in emotional eaters during a negative mood state compared with nonemotional eaters in the negative mood and emotional eaters during a neutral mood. The bar graph represents relative activation in the left parahippocampal gyrus [-30, -39, -3] in response to anticipatory reward. Results from other regions followed the same overall pattern of activation.
Effects of Mood Condition and Emotional Eating on Consummatory Food Reward
A 2 × 2 ANOVA model revealed that emotional eaters showed greater activation in the left caudate nucleus and left pallidum in response to milkshake receipt—tasteless solution receipt (the consummatory food reward contrast). Results also revealed a significant main effect of mood condition on this contrast such that greater activation was found bilaterally in the anterior cingulate in the neutral mood condition in response to milkshake receipt—tasteless solution receipt compared with the negative mood.
Furthermore, there was a significant interaction between emotional eating status and mood state on the consummatory food reward contrast. This interaction revealed greater activation in the right pallidum in this contrast for emotional eaters in the negative mood state. The opposite was found for nonemotional eaters, with greater activation during negative mood in the right pallidum in response to receipt of the tasteless control solution than in response to the receipt of the milkshake. Furthermore, effects were reversed in the neutral mood state, such that greater activation in this region was found in response to milkshake receipt—tasteless solution for nonemotional eaters and in response to tasteless solution receipt—milkshake for the emotional eaters in the neutral mood state. This same pattern emerged in bilateral thalamus, left pallidum, and right anterior cingulate. Figure 3 shows activation locations for this comparison.
FIGURE 3.
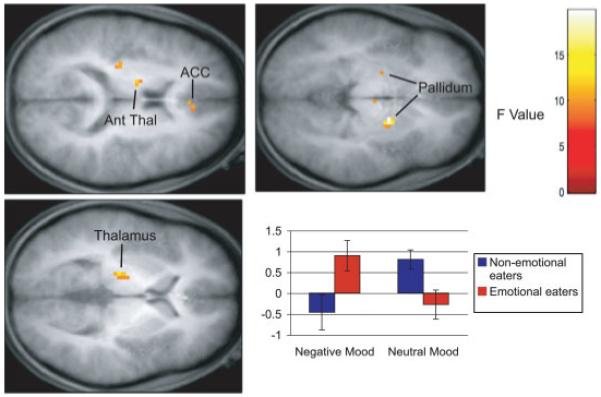
Results from ANOVA models of consummatory food reward. The color bar represents the F values representative for all figures. Axial sections of increased activation bilaterally in the pallidum, in the left thalamus, anterior thalamus (Ant Thal), and right anterior cingulate cortex (ACC) in response to receipt of chocolate versus tasteless control solution in emotional eaters during a negative mood state compared with nonemotional eaters in the negative mood and emotional eaters during a neutral mood. The bar graph represents relative activation in the right pallidum [18, -3, 0] in response to consummatory reward. Results from other regions followed the same overall pattern of activation.
Post Hoc Analyses
Because certain variables may influence brain response, we ran analyses with these variables as covariates. When depression was included as a covariate, 14 of the 16 significant activation clusters remained significant. When BMI was included as a covariate, 11 of the 16 significant activation clusters remained significant. All activation clusters remained significant when we included menstrual phase as a covariate. Menstrual phase was included due to the heightened reward response found during the midfollicular phase (4-8 days after onset of menses).58 Phase was calculated individually for each participant based on the start date of their last menstrual period and the number of days in their cycle. Only two participants were in the midfollicular phase during the scan, one in each group.
To ensure that learning effects could not account for null findings for anticipatory reward, we conducted analyses testing brain activation for anticipatory reward for trials at the beginning of the scan session to trials at the end of the scan session. In response to the cue signaling, chocolate milkshake delivery versus the cue signaling tasteless solution delivery, greater activation was found during early trials in the left posterior insula. During later trials, greater activation was found for the same contrast in the right and left parahippocampal gyrus. Thus, there does not appear to be a clear learning effect, as we would have expected greater activation in reward circuitry during later trials if the association between the cue and the chocolate taste had strengthened due to learning. There was no evidence of change in activation across the rest of the brain regions listed in Table 2, which also suggests limited learning effects.
Discussion
According to the affect regulation model of binge eating, greater activation should be found in brain regions responding to anticipatory and consummatory food reward and affect among emotional eaters compared with nonemotional eaters. Unexpectedly, these main effects did not emerge for anticipatory reward. Results did reveal a significant difference between emotional eaters and nonemotional eaters in the left caudate nucleus and pallidum in response to receipt of the chocolate milkshake versus tasteless control solution, suggesting greater food reward sensitivity among emotional eaters than nonemotional eaters across mood states. These findings provide support for the hypothesis that some individuals may experience hyperactivation of food reward circuitry in response to food intake, which may increase risk for overeating and binge eating.59 Interestingly, these findings are in contrast to the decreased activation of the caudate found among obese relative to lean women in response to chocolate milkshake receipt.35 These latter findings are more consistent with the hypothesis that some individuals may experience hypoactivation of food reward circuitry in response to food intake, which could cause them to overeat in a compensatory fashion.60 Yet, it is possible that the reduced activation in the striatum is a product of reduced dopamine receptor density that results from chronic overeating.59 The negative mood condition was associated with greater activation in the left ventral anterior cingulate than the neutral mood condition averaged across emotional and nonemotional eaters in response to anticipating receipt of chocolate versus control solution, although inconsistent findings emerged in the left thalamus for anticipatory reward. However, because there were significant interaction effects between emotional eating status and mood, we focus primarily on the interpretation of the interaction effects rather than these main effects.
There was a significant interaction between emotional eating status and mood condition bilaterally in the parahippocampal gyrus and anterior cingulate in response to anticipatory food reward. As noted previously, the parahippocampal gyrus is one of the few limbic regions implicated in anticipated reward from food intake.30,32 The parahippocampal gyrus may respond specifically to anticipatory reward, as prior studies of consummatory reward have not found effects in this area.27-30 Additionally, the anterior cingulate has been associated with reward and food palatability.61 These effects were in the hypothesized direction, with greater activation in these regions among emotional eaters during a negative mood state compared with the neutral mood and the nonemotional eaters in the negative mood. Further, we found an interaction between emotional eating status and mood condition bilaterally in the pallidum and thalamus, the left anterior thalamus, and right anterior cingulate in consummatory food reward. This converges with prior research that has implicated the pallidum and other limbic areas in hedonic reward response to taste, specifically consummatory food reward.62,63 In each of these interactions, emotional eaters showed heightened neural response in these brain regions to anticipatory and consummatory reward for the milkshake versus tasteless control in the negative mood condition, which supports our hypothesis that these individuals show heightened brain response to food during a negative mood. Additionally, the finding that nonemotional eaters had less of a neural response to anticipatory and consummatory food intake of milkshake in the negative mood accords with past findings indicating that people show a diminished interest in food while in a negative mood (e.g., Ref. 4). Furthermore, these findings provide support for the DEBQ-Emotional Eating scale, as it appears to predict important individual differences in reward response during negative moods.
It is noteworthy that different brain regions emerged for anticipatory and consummatory food reward. This supports prior evidence that these two phases of food reward involve somewhat different circuitry.28 Additionally, it is striking that activations in brain regions associated with negative affect, such as the VMPFC and amygdala, were not significantly different in emotional and nonemotional eaters in the negative or neutral mood conditions. Thus, it appears that emotional eaters differ from nonemotional eaters not in their emotional response to food but rather in their activation of neural circuitry in response to food and food cues. Although these individuals have the expectation that eating will reduce negative affect, it may be that food is actually more rewarding or pleasurable for them in negative mood states. This has important implications for the affect model of binge eating, which suggests that women utilize eating to decrease or alleviate negative affect. Although their behavior may be due to an incorrect expectation, it appears that the brain does not encode any changes in affect in response to tastes of chocolate milkshake or to anticipation of milkshake. The heightened neural response to food in a negative mood state for emotional eaters may be due to repeated episodes of eating during negative moods, which lead to a conditioned association between food reward and negative mood. However, it is possible that if participants had consumed more food, typical of a regular eating episode, it may have reduced activation indicative of negative affect and/or increased activation indicative of positive affect.
Indeed, one limitation of this study is the inability to relate these findings to clinically meaningful emotional eating, as these findings reflect brain responses to a taste of chocolate milkshake. Future research should investigate neural activation in response to intake of larger quantities of palatable foods in the scanner for emotional and nonemotional eaters. Second, it is unclear whether differences in neural activation between groups are a product of emotional eating or result from depressive symptoms or negative affect, as emotional eaters showed significantly greater levels of both depression and negative affect. Although most effects remained when depression was included as a covariate, this remains a limitation shared by all cross-sectional brain imaging studies. Because of the correlational nature of fMRI studies, it is impossible to rule out or control for all possible third variables that may account for differences in brain activation. Third, the present paradigm might not optimally separate anticipatory from consummatory food reward, as participants always anticipated receipt of the milkshake taste when they received it in our paradigm (there was no un-cued delivery of milkshake). Fourth, our paradigm could have resulted in learning that produces differential brain response to cues over time as participants learn to associate the cue with the taste. This would impact results for anticipatory food reward, as later trials may produce stronger effects than earlier trials, although this does not appear to be the case in this study. Fifth, this study included a complex design, testing the effects of emotional eating, mood, and two tastes on brain activation. We may have had low power to detect small effectsa. This could explain why effects were not found in all brain regions associated with reward circuitry. Finally, the predominantly white college sample may not be representative of the population of people developing binge-eating behaviors, although this sample does represent a population at risk for these symptoms. Future research should test these relations among more diverse samples to further elucidate the brain differences in anticipatory and consummatory reward between emotional and nonemotional eaters in different mood states.
A particularly important direction for future research will be to determine whether the differences in neural activation in response to food intake are due to emotional eating versus greater depression or temperamental emotionality. Emotional eaters had higher levels of negative affect and depressive symptoms and also had a strong reported response to the mood inductions, which could explain the difference in brain response. Future studies should include subjective and objective measures of emotionality of the participants to explore this question. Further research is also necessary to determine whether these brain differences reflect a risk for binge eating or bulimic pathology. It is possible that differences in brain activation in response to receipt of food may only emerge under conditions of moderate hunger, thus it would be useful for future research to test these questions before and after participants eat a healthy meal. Additionally, it is important to match the change in brain activation with change in mood ratings to see how these findings compare to findings from ecological momentary assessment studies.
In sum, these findings provide support for the affect regulation model of eating based on differential activation of brain regions that encode anticipatory and consummatory food reward, but not in brain regions that encode emotional experience. Emotional eaters appear to have increased neural response to anticipatory and consummatory food reward when compared with nonemotional eaters, but this effect was only found in the negative mood state. It may be that emotional eaters have a reward sensitivity during negative mood states that may explain a tendency to binge eat or overeat in this mood, but not that food results in decreased negative affect. Additionally, the relation between negative mood and eating may be a learned association, with emotional eaters learning to anticipate reward from food when encountering negative mood after associating negative affect with eating episodes. Addressing abnormalities in food reward may be important in preventing binge eating and bulimic pathology.
Footnotes
Because of the number of conditions and groups included in these analyses, we were concerned about power to detect effects. All effect sizes for activation clusters in the study were large. However, we may not have had adequate power to detect small or moderate effects. Friston et al.42 have suggested that groups with 20 participants provide adequate power for fMRI studies, thus additional effects may have gone undetected in this study, with 10 participants per group.
References
- 1.Wilson GT, Becker CB, Heffernan K. Eating disorders. In: Barkley RA, Mash EJ, editors. Child Psychopathology. 2nd ed. The Guilford Press; New York: 2003. pp. 687–715. [Google Scholar]
- 2.Stice E, Bulik C. Eating disorders. In: Beauchaine TP, Hinshaw SP, editors. Child and Adolescent Psychopathology. 2008. pp. 643–669. [Google Scholar]
- 3.McCarthy M. The thin ideal, depression, and eating disorders in women. Behav Res Ther. 1990;28:205–218. doi: 10.1016/0005-7967(90)90003-2. [DOI] [PubMed] [Google Scholar]
- 4.Schachter S, Goldman R, Gordon A. Effect of fear, food deprivation, and obesity on eating. J Pers Soc Psychol. 1968;10:91–97. doi: 10.1037/h0026284. [DOI] [PubMed] [Google Scholar]
- 5.Stice E, Agras WS. Predicting onset and cessation of bulimic behaviors during adolescence: A longitudinal grouping analysis. Behav Ther. 1998;29:257–276. [Google Scholar]
- 6.Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: A 2-year prospective investigation. Health Psychol. 2002;21:131–138. [PubMed] [Google Scholar]
- 7.Killen JD, Taylor CB, Hayward C, Haydel KF, Wilson DM, Hammer L, et al. Weight concerns influence the development of eating disorders: A 4-year prospective study. J Consult Clin Psychol. 1996;64:936–940. doi: 10.1037//0022-006x.64.5.936. [DOI] [PubMed] [Google Scholar]
- 8.Cooley E, Toray T. Body image and personality predictors of eating disorder symptoms during the college years. Int J Eat Disord. 2001;30:28–36. doi: 10.1002/eat.1051. [DOI] [PubMed] [Google Scholar]
- 9.Stice E. A prospective test of the dual-pathway model of bulimic pathology: Mediating effects of dieting and negative affect. J Abnorm Psychol. 2001;110:124–135. doi: 10.1037//0021-843x.110.1.124. [DOI] [PubMed] [Google Scholar]
- 10.Vogeltanz-Holm ND, Wonderlich SA, Lewis BA, Wilsnack SC, Harris TF, Wilsnack RW, et al. Longitudinal predictors of binge eating, intense dieting, and weight concerns in a national sample of women. Behav Ther. 2000;31:221–235. [Google Scholar]
- 11.Vohs KD, Bardone AM, Joiner TE, Abramson LY. Perfectionism, perceived weight status, and self-esteem interact to predict bulimic symptoms: A model of bulimic symptom development. J Abnorm Psychol. 1999;108:695–700. doi: 10.1037//0021-843x.108.4.695. [DOI] [PubMed] [Google Scholar]
- 12.Davis R, Freeman RJ, Garner DM. A naturalistic investigation of eating behavior in bulimia nervosa. J Consult Clin Psychol. 1988;56:273–279. doi: 10.1037//0022-006x.56.2.273. [DOI] [PubMed] [Google Scholar]
- 13.Lingswiler VM, Crowther JH, Stephens MA. Affective and cognitive antecedents to eating episodes in bulimia and binge eating. Int J Eat Disord. 1989;8:533–539. [Google Scholar]
- 14.Wegner KE, Smyth JM, Crosby RD, Wittrock D, Wonderlich SA, Mitchell JE. An evaluation of the relationship between mood and binge eating in the natural environment using ecological momentary assessment. Int J Eat Disord. 2002;32:352–361. doi: 10.1002/eat.10086. [DOI] [PubMed] [Google Scholar]
- 15.Baucom DH, Aiken PA. Effect of depressed mood in eating among obese and nonobese dieting and nondieting persons. J Pers Soc Psychol. 1981;41:577–585. doi: 10.1037//0022-3514.41.3.577. [DOI] [PubMed] [Google Scholar]
- 16.Cools J, Schotte DE, McNally RJ. Emotional arousal and overeating in restrained eaters. J Abnorm Psychol. 1992;101:348–351. doi: 10.1037//0021-843x.101.2.348. [DOI] [PubMed] [Google Scholar]
- 17.Polivy J, Herman CP, McFarlane T. Effects of anxiety on eating: Does palatability moderate distress-induced overeating in dieters? J Abnorm Psychol. 1994;103:505–510. doi: 10.1037//0021-843x.103.3.505. [DOI] [PubMed] [Google Scholar]
- 18.Ruderman AJ. Dysphoric mod and overeating: A test of restraint theory’s disinhibition hypothesis. J Abnorm Psychol. 1985;94:78–95. doi: 10.1037//0021-843x.94.1.78. [DOI] [PubMed] [Google Scholar]
- 19.Schotte DE, Cools J, McNally RJ. Film-induced negative affect triggers overeating in restrained eaters. J Abnorm Psychol. 1990;99:317–320. doi: 10.1037//0021-843x.99.3.317. [DOI] [PubMed] [Google Scholar]
- 20.Agras WS, Telch CF. The effects of caloric deprivation and negative affect on binge eating in obese binge-eating disordered women. Behav Ther. 1998;29:491–503. [Google Scholar]
- 21.Telch CF, Agras WS. Do emotional states influence binge eating in the obese? Int J Eat Disord. 1996;20:271–279. doi: 10.1002/(SICI)1098-108X(199611)20:3<271::AID-EAT6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 22.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA. 2002;99:2450–2454. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauregard M, Leroux J, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, et al. The functional neuroanatomy of major depression: An fMRI study using an emotional activation paradigm. Neuroreport. 1998;9:3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- 25.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 26.Killgore WDS, Yurgelun-Todd DA. Affect modulates appetite-related brain activity to images of food. Int J Eat Disord. 2006;39:357–363. doi: 10.1002/eat.20240. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Gao JH, Liu HL, Fox PT. The temporal response of the brain after eating revealed by functional MRI. Nature. 2000;405:1058–1062. doi: 10.1038/35016590. [DOI] [PubMed] [Google Scholar]
- 28.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–826. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- 29.O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 30.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: From pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 31.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 32.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 33.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: Food-craving activation during fMRI. Neuroimage. 2004;23:815–826. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food related stimuli: Effects of fasting and gender. Behav Brain Res. 2006;169:111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J Abnorm Psychol. doi: 10.1037/a0013600. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain. 1997;120:1675–1684. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- 37.Rothemund Y, Preuschof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37:410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 38.van Strien T, Frijters JER, Bergers GPA, Defares PB. The Dutch eating behavior questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5:295–315. [Google Scholar]
- 39.Clark DM. On the induction of depressed mood in the laboratory: Evaluation and comparison of the Velten and musical procedures. Adv Behav Res Ther. 1983;5:27–49. [Google Scholar]
- 40.Heatherton TF, Striepe M, Wittenberg L. Emotional distress and disinhibited eating: The role of self. Pers Soc Psychol Bull. 1998;24:301–313. [Google Scholar]
- 41.Veldhuizen M, Bender G, Constable RT, Small DM. Tasting in the absence of taste: Modulation of early gustatory cortex by attention to taste. Chem Senses. 2007;32:569–581. doi: 10.1093/chemse/bjm025. [DOI] [PubMed] [Google Scholar]
- 42.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- 43.Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited-again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 44.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Schacter SC. Studies of handedness and anomalous dominance. In: Galaburda AM, editor. Dyslexia and Development. Harvard University Press; Cambridge, MA: 1993. pp. 269–296. [Google Scholar]
- 46.Beck AT, Steer RA, Garbin M. Psychometric properties of the beck depression inventory: 25 years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- 47.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 48.Stice E, Trost A, Chase A. Healthy weight control and dissonance-based eating disorder prevention programs: Results from a controlled trial. Int J Eat Disord. 2003;33:10–21. doi: 10.1002/eat.10109. [DOI] [PubMed] [Google Scholar]
- 49.Watson D, Clark LA. On traits and temperament: General and specific factors of emotional experience and their relation to the five-factors model. J Pers. 1992;60:441–476. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 50.Stice E, Telch CF, Rizvi SL. Development and validation of the eating disorder diagnostic scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2000;12:123–131. doi: 10.1037//1040-3590.12.2.123. [DOI] [PubMed] [Google Scholar]
- 51.Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, Assessment, and Treatment. 12th ed. The Guilford Press; New York: 1993. pp. 317–331. [Google Scholar]
- 52.Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: Additional evidence of reliability and validity. Psychol Assess. 2004;16:60–71. doi: 10.1037/1040-3590.16.1.60. [DOI] [PubMed] [Google Scholar]
- 53.Hohlstein LA, Smith GT, Atlas JG. An application of expectancy theory to eating disorder: Development and validation of measures of eating and dieting expectancies. Psychol Assess. 1998;10:49–58. [Google Scholar]
- 54.MacBrayer EK, Smith GT, McCarthy DM, Demos S, Simmons J. The role of family of origin food-related experiences in bulimic symptomatology. Int J Eat Disord. 2001;30:149–160. doi: 10.1002/eat.1067. [DOI] [PubMed] [Google Scholar]
- 55.Simmons JR, Smith GT, Hill KK. Validation of eating and dieting expectancy measures in two adolescent samples. Int J Eat Disord. 2002;31:461–473. doi: 10.1002/eat.10034. [DOI] [PubMed] [Google Scholar]
- 56.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 57.Bond MJ, McDowell AJ, Wilkinson JY. The measurement of dietary restraint, disinhibition and hunger: An examination of the factor structure of the three factor eating questionnaire (TFEQ) Int J Obes. 2001;25:900–906. doi: 10.1038/sj.ijo.0801611. [DOI] [PubMed] [Google Scholar]
- 58.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci USA. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis C, Strachan S, Berkson M. Sensitivity to reward: Implications for overeating and obesity. Appetite. 2004;42:131–138. doi: 10.1016/j.appet.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: Implications for obesity. Expert Opin Ther Targets. 2002;6:601–609. doi: 10.1517/14728222.6.5.601. [DOI] [PubMed] [Google Scholar]
- 61.DeAraujo IET, Rolls ET. Representation in the human brain of food texture and oral fat. J Neurosci. 2004;24:3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tindell AJ, Smith KS, Peciña S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: When a bad taste turns good. J Neuropshysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]


