Abstract
CSRP3 or Muscle LIM protein (MLP) is a nucleocytoplasmic shuttling protein and a mechanosensor in cardiac myocytes. MLP regulation and function was studied in cultured neonatal rat myocytes treated with pharmacological or mechanical stimuli. Either verapamil or BDM decreased nuclear MLP while phenylephrine and cyclic strain increased it. These results suggest that myocyte contractility regulates MLP subcellular localization. When RNA polymerase II was inhibited with α-amanitin, nuclear MLP was reduced by 30%. However, when both RNA polymerase I & II were inhibited with actinomycin D, there was a 90% decrease in nuclear MLP suggesting that its nuclear translocation is regulated by both nuclear and nucleolar transcriptional activity. Using cell permeable synthetic peptides containing the putative nuclear localization signal (NLS) of MLP, nuclear import of the protein in cultured rat neonatal myocytes was inhibited. The NLS of MLP also localizes to the nucleolus. Inhibition of nuclear translocation prevented the increased protein accumulation in response to phenylephrine. Furthermore, cyclic strain of myocytes after prior NLS treatment to remove nuclear MLP resulted in disarrayed sarcomeres. Increased protein synthesis and brain natriuretic peptide expression were also prevented suggesting that MLP is required for remodeling of the myofilaments and gene expression. These findings suggest that nucleocytoplasmic shuttling MLP plays an important role in the regulation of the myocyte remodeling and hypertrophy and is required for adaptation to hypertrophic stimuli.
Keywords: hypertrophy, sarcomere remodeling, nucleocytoplasmic shuttling, mechanosensing, mechanotransduction
Introduction
Cells are constantly remodelling through a process of mRNA translation and protein turnover. This remodeling occurs in response to local changes and requires the activity of molecular sensors. There is growing evidence that a number of cytoplasmic, plasma membrane and cytoskeletal proteins shuttle constantly in and out of the nucleus in response to various stimuli [1]. These nuclear-cytoplasmic cycling proteins can act as messengers or transcription factors, allowing the nuclear machinery to respond rapidly to environmental changes.
In myocytes, the Z-disc within the sarcomere harbors a number of these shuttling proteins including CSRP3 or muscle LIM protein ( MLP). MLP is a member of a family of proteins containing one or more LIM domains, that mediate specific protein-protein interactions [2]. MLP interacts with multiple proteins within the Z-disc, including α-actinin and the titin-binding protein telethonin (T-cap) [3]. We have recently shown that MLP accumulates in the nucleus in response to human heart failure and in two animal models of cardiac disease [4]. In cultured neonatal myocytes we also showed that MLP translocated to the nucleolus in response to cyclic mechanical strain, thereby activating ribosomal protein synthesis.
MLP has at least two potential mechanisms of action in myocytes; through its structural role and interaction with other signaling molecules in the cytoplasm, and/or through its role as a transcription factor in the nucleus [5]. The protein is thought to shuttle to the nucleus via a putative 6 amino acid nuclear localization signal (NLS). Using synthetic cell permeable peptides containing this NLS it is possible to competitively inhibit the nuclear translocation of the endogenous protein. One such membrane permeable peptide is the fibroblast growth factor (FGF) membrane translocating peptide sequence (AAVALLPAVLLALLAP). This peptide has been used to import the NLS of the transcription factor NFκB, thereby blocking nuclear translocation and the subsequent activation of T-cells [6].
Various unsuccessful attempts have been made to determine the cellular function of MLP through mutagenesis and adenoviral transfection in cardiac myocytes [7][8]. We have previously shown that this is likely related to interference with MLP oligomerisation which is required for its function as a mechanosensor thus abrogating this approach for mechanistic studies [4]. Therefore, we use a new strategy by inhibiting MLP nuclear translocation, which takes advantage of the putative MLP-NLS sequence along with the cell permeable domain. We first show that treatment of cultured rat neonatal myocytes with this NLS-peptide prevents MLP-nuclear translocation confirming that this sequence is functionally effective. Inhibition of MLP nuclear translocation prevents the increased protein accumulation associated with phenylephrine treatment and cyclic strain in cardiac myocytes. When myocytes were cyclically strained in the absence of nuclear MLP, the sarcomeres were in disarray suggesting that cycling MLP or a factor associated with it is required for maintenance and remodelling of the myofilaments. These data suggest that MLP has a broad range of functions in cardiac myocytes and is required for adaptation to hypertrophic stimuli.
Material and methods
Cell culture
Myocytes were isolated from the cardiac ventricles of 1-2 day old Sprague Dawley rats by sequential collagenase digestion, as previously described [9]. Briefly, cells were pre-plated to reduce non-myocyte cell contamination and then plated (1 million cells/cm2) on fibronectin (25 μL/ml) coated silicone petri dishes in PC1 medium (BioWhittaker, Walkersville, MD) for 24 hours and transferred to a DMEM:M199 serum free medium. All drug treatments were done 24 hours after being placed in DMEM:M199 medium. To examine MLP expression in response to inhibition of transcription, cells were treated with 12.5mg/L actinomycin D for 10 hours, a concentration which inhibits both polymerase I and RNA polymerase II. These conditions were based on previous work [10]. A concentration of 1μM was used to inhibit RNA polymerase II for 24 hours. Some groups of cells were treated with 7.5 mM BDM, 10μM verapamil or phenylephrine for either 24 or 48 hours in DMEM:M199 medium. For inhibition of MLP nuclear translocation, synthetic peptides were used containing the fibroblast growth factor (FGF) membrane translocating peptide sequence (AAVALLPAVLLALLAP) and the NLS of MLP. The control peptide did not have the additional NLS. Cells were treated with these synthetic peptides for either 24 or 48 hours at a concentration ranging from 20 to 100μM depending on the experiment.
Cellular composition and subcellular fractionation
For subcellular fractionation of myocytes, the ProteoExtract Subcellular Proteome Kit from Calbiochem was used. This method uses a detergent-based protocol[11] and has been previously described [4]. Briefly, cellular proteins were sequentially extracted into four compartments: cytosolic, membrane/organelles, nuclear and cytoskeleton. Digitonin/ EDTA is used to remove the cytosol. Triton/EDTA is used to remove the membrane/organelle fraction. Tween/deoxycholate/benzonase removes the nuclei. Finally SDS is used to remove the cytoskeleton. Cells were briefly washed 3 times in PBS between each extraction fraction to prevent cross-contamination. After each fraction, cells were observed by microscopy to ensure that they were still attached to the dish. Cell integrity is maintained throughout the fractionation process. The fractions were then frozen at -80°C prior to analysis.
Western blotting for analysis of protein expression
Neonatal rat ventricular myocytes were rinsed with warm PBS and then scraped from the silicone membranes or dishes in lysis buffer containing 1% SDS and protease inhibitor cocktail (Sigma). The Bradford method was used to determine total protein using crystalline bovine serum albumin as standard. For whole heart protein analysis, tissue was ground in liquid nitrogen and added to lysis buffer containing 1% SDS, 50 mM NaF and protease inhibitor cocktail (Sigma). Samples were treated with β-mercaptoethanol and heated to 100°C for 5 minutes. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Hybond C, Amersham). Blots containing either whole cell lysates or fractionated cells were probed for anti-MLP (produced by Invitrogen using the last 14 amino acids from the carboxy terminus), phalloidin (Molecular Probes), fibrillarin (Abcam), histone 2B (Abcam), α-sarcomeric actinin (Abcam), α-sarcomeric actin (Abcam), desmin (BD Transduction Laboratories), Clock (Abcam) and myosin MF20 (Hybridoma Bank, Iowa). Horseradish peroxidase-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Research Diagnostics Inc) were used to visualize proteins by enhanced chemiluminescence (ECL, Amersham). The bands corresponding to the various proteins were quantified by laser densitometry. Protein bands were further standardized to total protein loading by using the amido black-stained nitrocellulose membrane as described previously [12].
Quantitative Real Time-PCR
Real-time quantitative polymerase chain reaction (RT-PCR) was performed using a LightCycler thermocycler (Roche Diagnostics) as previously described [13]. Briefly, total RNA was isolated from cell culture using TRIzol (Invitrogen) reagent. The RNA amplification kit with SYBR Green 1 (Roche Molecular Biochemical) used 100 ng in a one-step RT-PCR reaction. The reaction conditions for RT were 55°C for 15 min, which was followed by a four-step PCR amplification with signal acquisition at 80-89°C for 2 s (depending on the melting temperature for each PCR product, determined by the melting curve analysis) for 40 cycles. The RT-PCR reaction was quantified using in vitro transcribed mRNA standards, prepared for each gene, that were run alongside to develop a standard curve. The second derivative maximum (log linear phase) for each amplification curve was determined and plotted against the standard curve to calculate the amount of product [13]. Primers were: Brain natriuretic peptide (BNP): (F) 5’-GCTGGAGCTGATAAGAGAAA and (R) 5’-GGAATTTCGAAGTCTCTCCT and normalized against ribosomal protein L7: (F) 5’- GAAGCTCATCTATGAGAAGGC and (R) 5’-CAGACGGAGCAGCTGCAGCAC to ensure equal loading.
Immuno-cytochemistry and image analysis
After the various experimental protocols, cells for immuno-cytochemical staining were fixed in 4% paraformaldehyde for 3 minutes and then 100% methanol at -20°C for 1 minute. Fixed cells were immunostained with antibodies as described previously [14]. Rhodamine and Alexa Fluor-conjugated secondary antibodies (Molecular Probes) were used to visualize the specific proteins. Fluorescently-labeled cells were then viewed using a Zeiss Model LSM 510 laser scanning confocal microscope.
Biaxial mechanical strain of cardiac myocytes
Cyclic mechanical deformation of cultured neonatal rat ventricular myocytes was produced with a Flexcell Strain Unit (Model FX-4000, Flexcell International, McKeesport, PA). A sinusoidal cyclic strain at 10% maximum strain and 1 Hz for 48 hours was used without the posts for biaxial strain.
Fabrication of uniaxial scaffolds and cyclic strain
Neonatal myocytes were polarized on textured substrates and exposed to cyclic uniaxial strain (Flexcell) in either the transverse axis or in alignment with cells. Textured substrates for cell culture were fabricated as previously described [15]. Briefly, micron scaled architecture were sculpted in photosensitive resists exposed to patterns of high energy UV light. The resulting textured surface had grooves with a depth of 5 μm and widths of 10 μm of both the bottom grooves and top ridges. A parylene coat was then deposited on the surface to retain a master/negative mold of the original surface when peeled off. The parylene mold was then pressed against unpolymerized silicone (Dupont, Phoenix, AZ) over the strain region encircled by a 300μm thick polystyrene fence placed in the individual 35mm wells of the Uniflex™ strain device (Flexcell ® International, McKeesport, PA). The middle of the uniaxial dish had a strain region 15.25mm by 24.18mm for monolayer cultures with the strain orientation in the long axis. Once the silicone cured the parylene was peeled off and the fence removed. The resulting surface was ~300 μm thick substratum superficially imprinted with 5μm deep grooves. This textured silicone surface was treated with 11 mol/L hydrochloric acid (20 minutes), rinsed with distilled water, and dried at 37°C overnight. The following day substrates were sterilized with 70% ethanol and coated with 25mg/ml of fibronectin. The cells are oriented with the parallel grooves in the midsection of 15.25mm × 24.18mm membrane. The direction of strain application was either parallel or perpendicular yielding longitudinal or transverse strain, respectively.
Statistics
At least three separate primary cultures were averaged. Each culture used approximately 30 neonatal hearts. All values are means ± SEM. All values of significance were calculated using the appropriate comparisons: one way analysis of variance or the Students unpaired t-test. Differences among means were considered significant at p< 0.05. Data were analyzed using GraphPad and SigmaStat statistical software.
Results
MLP and myocyte contractility
Here, we have determined whether changes in myocyte contractility alter the subcellular distribution of MLP. Cells were treated for 24 hours with either the L-type calcium channel blocker verapamil at 10μM to inhibit contractility or the α-adrenergic agonist phenylephrine at 10μM to increase it. Cultured neonatal myocytes were immunostained for MLP and DAPI following drug treatment (Figure 1A-C). The contrast between MLP nuclear and cytoplasmic staining is reduced following verapamil treatment but is maintained following phenylephrine treatment. Some cell cultures were then fractionated and the nuclear fractions used to determine nuclear relative to total MLP protein levels by Western blotting. Nuclear MLP significantly increases following phenylephrine treatment but is reduced following verapamil treatment, (Figure 1D). Total MLP was measured from unfractionated myocytes and showed that although verapamil treated cells exhibited no change, phenylephrine treatment resulted in a 2 fold increase in total MLP, see Figure 1E. Treatment with either verapamil or phenylephrine alters both calcium cycling and myocyte contractility therefore we also treated cells with 7.5 mM BDM which inhibits myofilament crossbridge cycling but does not affect calcium transients [16]. With 24 hour BDM treatment there was a 40% reduction in nuclear MLP (controls 1.68±0.21 vs 0.95±0.21 n=3 cultures) suggesting that myocyte crossbridge cycling, not calcium regulates MLP nuclear shuttling.
Figure 1.

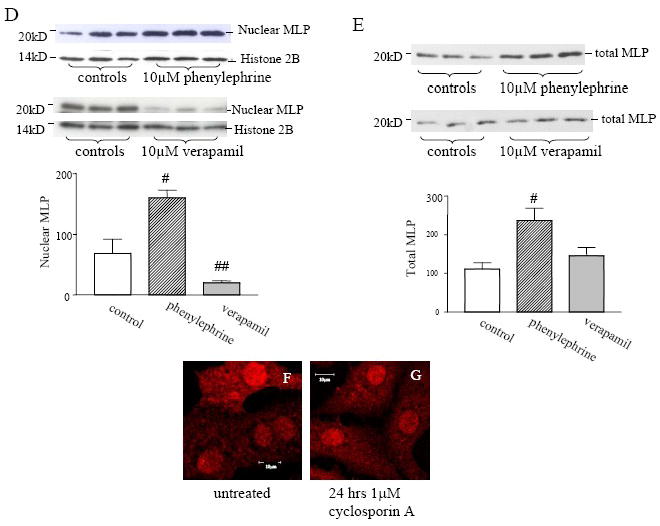
Regulation of MLP subcellular localization in cultured neonatal rat myocytes. (A, B and C) Neonatal rat ventricular myocytes MLP (red) and (A’, B’ and C’) nuclei with DAPI (blue). Myocytes are (A) unstrained, or treated for 24 hours with (B) 10μM verapamil or (C) with 10μM phenylephrine. (D) Western blot of nuclear fractions myocytes compared to untreated control for MLP and normalized to the nuclear protein histone 2B. 24h phenylephrine treatment increased nuclear MLP (p<0.05 n=3) but verapamil decreased it (p<0.01 n=3). (E) Western blot of total MLP from myocytes treated with either verapamil (not significant) or phenylephrine (p<0.05, n=3). Panels F and G show cultured myocytes with and without 1μM treatment of the calcineurin inhibitor cyclosporin A for 24 hours. There is strong nuclear staining for MLP in both control and treated cells.
The calcium binding protein calcineurin interacts with MLP at the Z-disk [17] and inhibition of calcineurin with either cyclosporin A or FK506 prevents cellular hypertrophy [18]. To determine whether calcineurin played a part in the nuclear localization of MLP, cultured myocytes were treated with 1μM cyclosporin A for 24 hours, then fixed and stained for MLP, Figure 1F and 1G. There is strong nuclear staining in both untreated control cells and cyclosporin A treated cells. This suggests that MLP translocation to the nucleus is not dependent upon the activity of calcineurin alone.
Inhibition of MLP nuclear translocation with synthetic peptides
In order to prevent MLP nuclear translocation, various blocking peptides were synthesized, (Figure 2A). One peptide consisted of the putative NLS of MLP and the membrane translocating motif of fibroblast growth factor, enabling the whole peptide to cross the myocyte plasma membrane. Once inside the cell, the synthetic NLS competes with the endogenous NLS in MLP thereby competitively inhibiting nuclear entry of the native protein as shown in the diagram (Figure 2B). Cultured neonatal myocytes were treated with either 50 or 100μM of MLP-NLS containing peptide, or a control peptide containing only the plasma membrane translocating motif. Some peptides were synthesized conjugated to FITC to determine their subcellular localization with 50-100μM to visualize the peptide in cultured myocytes. Myocytes take up both the control and NLS containing peptides and are visible within 24 hours of treatment (Figure 3A and 3D). In the control group the distribution of the peptide is mainly cytoplasmic. However, the NLS containing peptide localizes to the nucleus. Cells treated with both peptides were also immunostained with MLP and DAPI. MLP strongly localizes to the nucleus but is significantly reduced in cells treated with the MLP-NLS containing peptide (Figure 3C and 3F). This shows that the methodology successfully prevents MLP nuclear translocation and that the MLP-NLS is functional.
Figure 2.
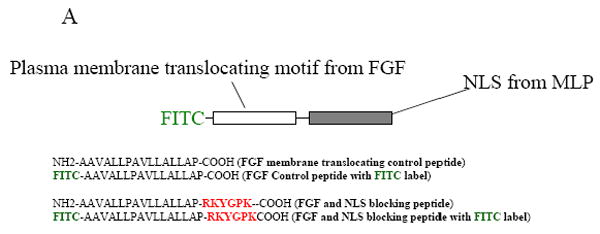
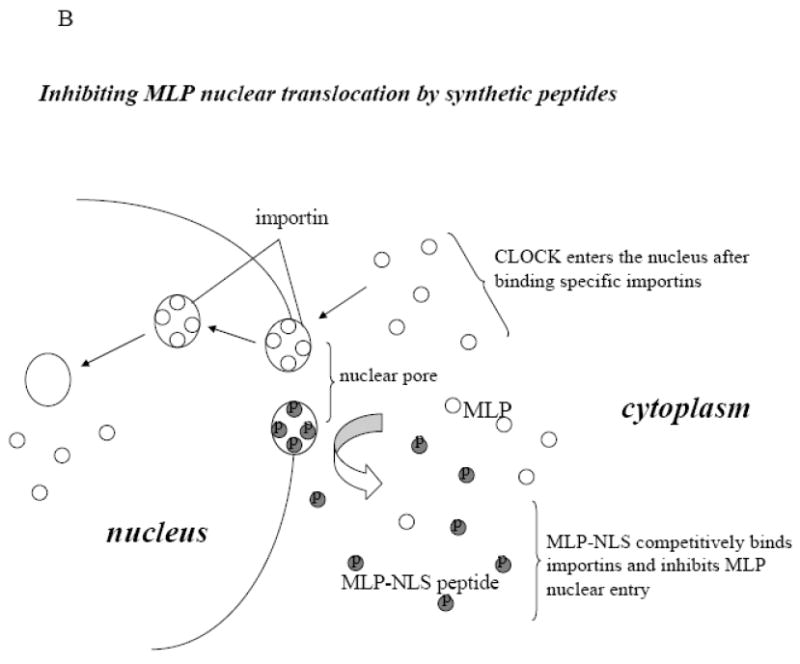
Inhibition of MLP nuclear translocation by synthetic peptides. (A) Diagram of sequences for the membrane translocating motif from FGF (16 amino acids) with or without the FITC label (green) and attached the putative NLS sequence of MLP (6 amino acid) (red). (B) Diagram showing how cell permeable peptides can competitively inhibit the nuclear translocation of endogenous MLP. FITC-labeled MLP-NLS containing synthetic peptide (dark circles) and endogenous MLP (open circles).
Figure 3.
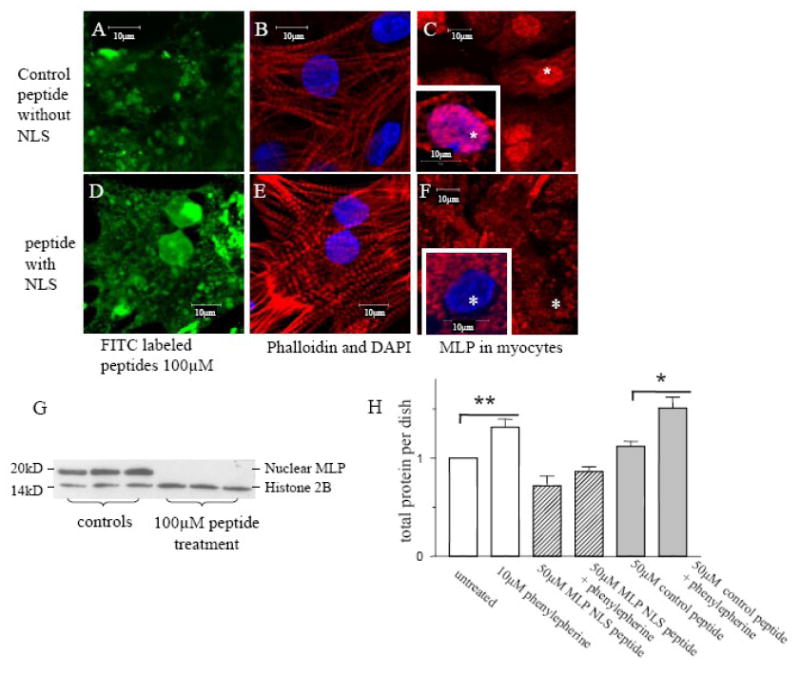
Inhibition of MLP nuclear translocation by synthetic peptides. Myocytes treated for 24 h with 100μM FITC labeled membrane translocating peptide (green) (A) without MLP-NLS (control) or (D) with the MLP-NLS. (B, E) Myocytes stained for actin phalloidin (red). (C) Myocytes without and (F) with MLP-NLS treatment stained for MLP (red). (E, F) Nuclei stained by DAPI (blue). (G) Western blot of nuclear extracts from myocytes treated with the control or MLP NLS peptides and probed for MLP and the nuclear protein histone 2B. (H) Total protein from myocyte cultures with and without 10μM phenylephrine treatment and 50μM peptide. Phenylephrine significantly increases total protein in control myocytes (**p<0.01 n=6 cultures) and in the presence of the control peptide (*p<0.05 n=6 cultures).
Some of these treated cells were fractionated and the nuclear samples used to measure MLP protein. MLP was undetectable from nuclear samples taken from myocytes treated with the MLP-NLS peptide compared with the control peptide (Figure 3G). However, in the same samples, the DNA associated protein histone 2B was unchanged. These results correlate well with the immunocytochemical results in Figure 3, panels 3C and 3F.
Specificity of the MLP-NLS
In myocytes, the peptide localized to the nucleus and nucleolus indicating that the peptide sequence RKYGPK in MLP is important for their localization there and may be myocyte specific (Figure 4A-D). The previous data shows that MLP nuclear entry can be successfully blocked by the NLS containing peptide. However, to determine whether other nuclear shuttling proteins were unaffected by this treatment we examined the localization of Clock protein in these cells. Myocytes were immunostained for the circadian protein Clock, a histone acetyl transferase that shuttles to the nucleus in response to phenylephrine treatment [19]. Nuclear shuttling of Clock protein continues in the presence of 50μM MLP-NLS suggesting nuclear entry may not be inhibited for other proteins too (Figures 4E-H).
Figure 4.
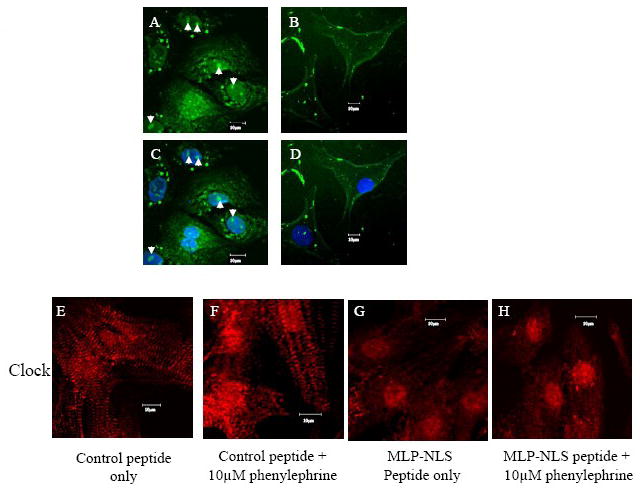
Specificity of the MLP-NLS peptide. (A) Myocytes treated for 24 h with 100μM of MLP-NLS-FITC peptide (green) seen in the cytoplasm, nucleus and nucleolus (arrows). (B) Fibroblasts treated for 48 h with 100μM of the MLP-NLS-FITC peptide (green) only present in the cytoplasm. (C, D) DAPI staining for nuclei (blue). Effect of MLP-NLS on the circadian protein Clock (immunostained red), (E) control peptide only or (F) with 10μm phenylephrine treatment; (G) MLP-NLS peptide only or (H) 10μm phenylephrine treatment. Clock protein translocates to the nucleus with phenylephrine treatment in both absence and presence of MLP blocking peptide.
Transcriptional activity, MLP-NLS peptide and nuclear translocation
In order to determine whether MLP nuclear translocation was dependent upon nuclear transcriptional activity, cells were treated either with α-amanitin or actinomycin D. α-amanitin is a specific inhibitor of RNA polymerase II, whilst actinomycin D can inhibit RNA polymerase I and also polymerase II at concentrations above 5mg/L, (Figure 5). Following 24-hour drug treatment of either 1μM α-amanitin or 12.5mg/L actinomycin D, neonatal rat cardiac myocytes were stained for MLP and fibrillarin. The presence of fibrillarin within the nucleolus depicts active ribosomal RNA transcription [10]. Myocytes treated with the RNA polymerase II blocker α-amanitin have some nuclear MLP staining indicating that the translocation of the protein to the nucleus is not totally dependent on general transcriptional activity alone, Figure 5B. Fibrillarin also remains in the nucleolus showing that nucleolar transcription is unaffected by this treatment.
Figure 5.
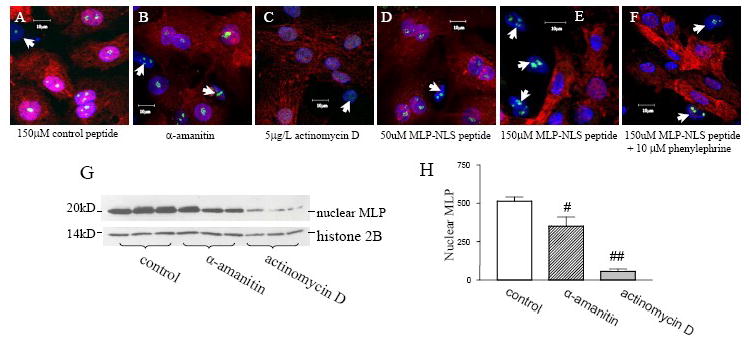
Transcriptional activity and MLP nuclear translocation. (A-F) Cardiac myocytes and fibroblasts treated with synthetic peptides and inhibitors of RNA polymerase I or both RNA polymerase I and polymerase II dependent transcription: (A) 150μM control peptide for 48h, (B) 1μM of the RNA polymerase II inhibitor α-amanitin for 24 h, (C) 12.5mg/L actinomycin D for 10 hours, a concentration which inhibits both polymerase I in addition to RNA polymerase II, (D) 50μM MLP-NLS peptide for 48h, (E) 150μM of MLP-NLS peptide for 48 h and (F) 150μM of MLP-NLS peptide and 10μM phenylephrine for 48 h. Cells were stained for MLP (red) and nuclei with DAPI (blue). Active nucleolar transcription is detected by presence of fibrillarin (green) and found only in fibroblasts (white arrows) that do not express MLP. (G) Western blot of nuclear MLP from myocytes treated with either α-amanitin or actinomycin D with histone 2B as a nuclear loading control. (H) Quantification of MLP from nuclear fractions after treatment with α-amanitin (# vs. control p<0.05 n=3) and actinomycin D (## vs. control p<0.01 n=3).
Myocytes were also treated with 12.5mg/L actinomycin D which inhibits both nuclear and nucleolar RNA transcription. Under these conditions, MLP was significantly reduced in the nucleus and fibrillarin no longer locates to the nucleus or nucleolus alone but is distributed throughout the myocytes. This demonstrates that nucleolar transcription has now ceased [10][20] Nuclear extracts from the various treatment groups were used to quantify nuclear MLP by Western blotting (Figure 5G and 5H). Both drugs significantly reduced nuclear MLP but the greatest reduction occurred in response to inhibition of both nuclear and nucleolar transcription. These effects demonstrate that the presence of MLP in the nucleus is dependent upon the activity of RNA polymerase I and II.
When cultured cardiac cells are treated with 50μM MLP-NLS peptide (a concentration that blocks MLP nuclear transport), nucleolar transcription is unaffected (as determined by the presence of fibrillarin in the nucleolus). However, if the concentration is increased to 150μM, fibrillarin is lost from the nucleolus of myocytes (as it is with actinomycin D) but not in the adjacent fibroblasts (Figure 5E). This inhibition is maintained in the presence of phenylephrine treatment (Figure 5F). These data suggest that the effects of the MLP-NLS peptide are likely to be myocyte specific.
Directional strain and MLP nuclear translocation
We have previously shown that MLP translocates to the nucleus and nucleolus in response to cyclic biaxial stretch [4]. However, it is unknown whether MLP is sensitive to directional strain. Using microtopography, we have aligned and strained cardiac myocytes either transversely or longitudinally to determine whether nuclear translocation is differentiated under these conditions. The data show that the protein does not translocate in response to uniaxial but only to biaxial strain (Figure 6). With uniaxial strain nuclear MLP appears to be reduced compared with control. However, immunostaining also suggests that the cytoplasmic distribution and alignment of the protein changes with directional strain. In untreated cells MLP has a punctate appearance but with transverse strain the protein aligns longitudinally with the fiber axis, i.e. perpendicular to the axis of the strain with lower nuclear staining (Figure 6F). Following longitudinal strain the protein becomes more striated (Figure 6G).
Figure 6.
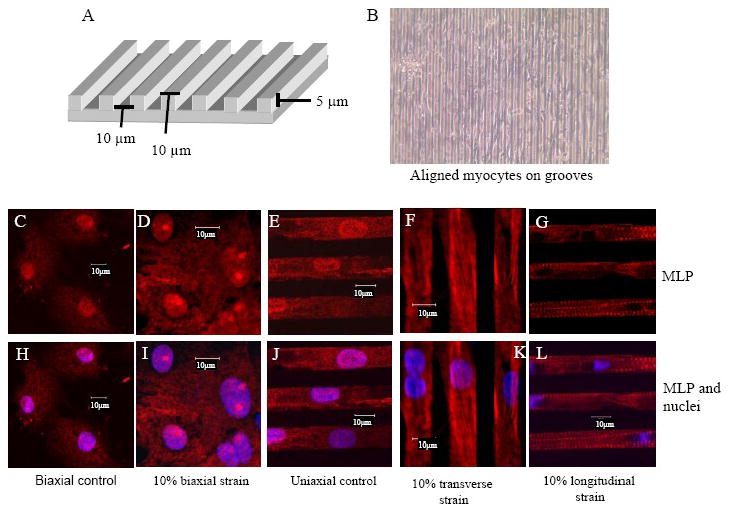
MLP nuclear translocation and directional strain. (A) Diagram of the microtopography silicone surface with groove dimensions of 5μm height,10 μm width in a parallel array pattern with 10 μm spacing between them. (B) Phase image of aligned myocytes cultured on the grooved silicon membranes. (C) Unstrained myocytes cultured on a flat silicone membrane with nuclear MLP (red). (D) Myocyte with 10% cyclic biaxial strain, 1Hz, 48h have punctate nuclear MLP. (E) Unstrained myocytes on grooved surface with nuclear MLP. (F) Myocytes after 10% cyclic transverse stretch, 1Hz for 48h have decreased nuclear MLP but have longitudinal myofibrillar streaks. (G) Myocytes after 10% cyclic longitudinal strain, 1Hz for 48h decreased nuclear MLP but with striations of sarcomeres. (C-G) MLP (red), (H-L) MLP and DAPI (blue).
Mechanical strain and nuclear MLP
To determine whether MLP mediates gene expression and cellular hypertrophy in response to strain, cells were cyclically strained in the presence of 50μM MLP-NLS- containing peptide. Following this period, total protein, S6 ribosomal protein, α-actinin protein and the mRNA expression of brain natriuretic peptide (BNP) were measured. Strain stimulates an increase in cellular protein in untreated and control peptide treated cells, (Figure 7A). However, in the absence nuclear MLP, this increase in protein synthesis is completely blunted indicating that the protein is necessary for the increased myocyte protein accumulation following mechanical strain. Next, BNP mRNA was measured following cyclic strain in the absence of nuclear MLP. In untreated cells there was a significant increase in the expression of this hypertrophic marker, see Figure 7B. However, in the absence of nuclear MLP shuttling, even the basal levels of BNP were significantly reduced and did not increase following cyclic strain. As a measure of ribosomal protein synthesis, ribosomal S6 protein was measured by Western blotting. Ribosomal protein synthesis increases following mechanical strain in all groups, including those that had been treated with the MLP-NLS peptide. This demonstrates that the concentration of peptide that prevents MLP nuclear translocation does not inhibit ribosomal protein synthesis in response to stretch (see Figure 7C). Finally we measured the expression of α-actinin protein in the same groups. Following cyclic strain there was no a significant change in protein levels although these trended upwards (see Figure 7D). However, in the presence of the MLP NLS peptide, α-actinin levels were significantly diminished and did not increase following cyclic strain. These results from the total protein, BNP mRNA and α-actinin protein data would strongly suggest that MLP is needed for the myocyte hypertrophic response and sarcomeric organization following mechanical stimulation.
Figure 7.
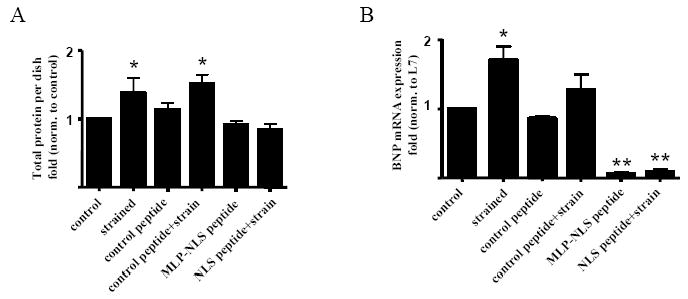

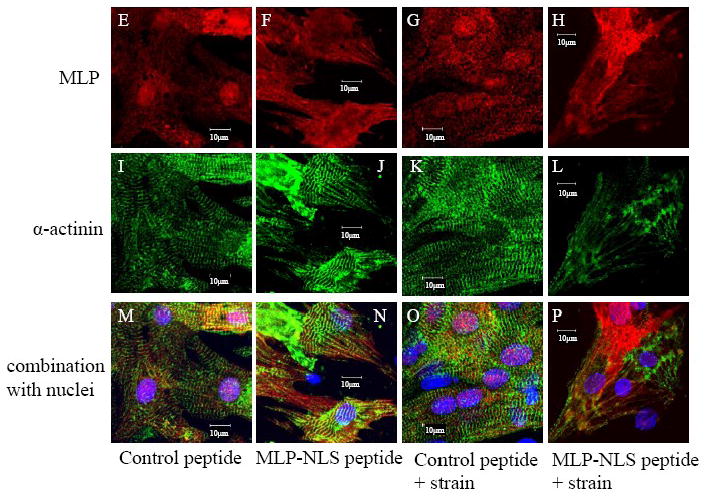
Cyclic strain and nuclear MLP in cultured neonatal myocytes. (A) Total protein from cyclically strained myocytes increased significantly in untreated cells and control peptide treated cells. *Control vs. strained, p<0.05. *Control peptide vs. NLS peptide, p<0.01. *Control peptide+strain vs. NLS peptide + strain, p<0.01, n=5 separate cultures. There was no change following cyclic strain in the presence of MLP nuclear blockade. (B) BNP mRNA increased significantly in control myocytes but not in the MLP nuclear blocked group. *Control vs. strained, p<0.05. *Control peptide vs. control peptide+ strain p<0.05, n=5 separate cultures. Similar results were obtained when the expression of BNP was standardized to either GAPDH (data not shown). (C) Western blots quantified show S6 ribosomal protein expression increased significantly following strain in all groups. *Control vs. strained, p<0.05. *Control peptide vs. control peptide+ strain p<0.05, *NLS peptide vs. NLS peptide+ strain p<0.05, n=5 separate cultures. (D) Western blots quantified show α-actinin protein expression in the same treatment groups. Following treatment with the MLP NLS peptide α-actinin decreased significantly. These levels did not increase following cyclic strain. *Control vs. NLS peptide, p<0.05 and *Control vs. NLS peptide+ strain p<0.05, n=4 separate cultures. Images of myocytes treated for 48 hours with (E,I,M) 50μM control peptide, (F,J, N) 50μM MLP-NLS peptide, (G,K, O) control peptide and cyclically strained at 10% maximum strain, 1 Hz for 48 hours, and (H,L,P) 50μM MLP-NLS peptide and cyclically strained at 10% maximum strain, 1 Hz. (E-P) Cells immunostained for MLP (red), α-actinin (green) and nuclei with DAPI (blue).
Cultures were also fixed and examined with immunocytochemistry for cell morphology following cyclic strain. In the presence of control peptide, myocytes responded to cyclic strain by producing more prominent striations when probed for α-actinin (Figure 7E-P). However, with blockade of MLP nuclear cycling, most myocyte striations were lost in response to cyclic strain. This suggests that nuclear MLP is important for strain dependent remodeling of the sarcomeric architecture.
Discussion
For the first time, we have isolated the nuclear function of MLP by inhibiting nuclear translocation of the endogenous protein. Our novel findings suggest that nucleocytoplasmic shuttling of MLP plays an important role in the regulation of myocyte remodelling and adaptation to hypertrophic stimuli. We have previously shown that MLP translocates to the nucleus and nucleolus in response to cyclic strain [4]. Its presence there stimulates ribosomal protein synthesis, an important step in the hypertrophic response in myocytes. It has been previously shown that adult myocytes isolated from MLP knockout hearts have functional abnormalities [21]. This provides important new information distinct from the signaling roles in the cytoplasm [17]. In contrast to a previous study where MLP was suggested to be mostly cytosolic [22], we show here that the protein plays an important functional role in the nuclei of cardiac myocytes.
MLP translocates to the nucleus in response to phenylephrine treatment but the opposite is true with verapamil and BDM treatment which blocked the nuclear translocation of MLP, showing that myocyte contractility may influence MLP activity. This is consistent with the previous finding that the distribution and amounts of mitochondria around the myofilaments were altered in the MLP knockout mice, indicating that the myocytes had impaired energy sensing possibly linked to aberrant sensing of cross-bridge activity [23]. The exact mechanism by which MLP responds to contractility and mechano-stimulation remains to be elucidated. It has been shown that MLP interacts with the calcium dependent phosphatase calcineurin in the Z-disk [17]. Blocking the nuclear translocation of calcineurin has been shown to prevent myocyte hypertrophy in response to angiotensin II [24]. However, we found that inhibition of calcineurin with cyclosporine A did not prevent MLP nuclear translocation suggesting that the process is independent of calcineurin activity.
Our study has demonstrated for the first time that the putative NLS sequence RKYGPK is likely to be the sequence by which the protein enters the nucleus since the sequence attached to FITC localizes to the nucleus. Treatment of cardiac fibroblasts with this peptide sequence did not result in nuclear localization suggesting that the sequence is not recognized by general non-myocytic nuclear importins. We also found that the histone acetlyase Clock was still able to enter and HDAC5 could exit the nucleus (data not shown) in the presence of the MLP-NLS peptide suggesting that nucleus transport of other proteins was functional. Nuclear localization sequences can be highly specific to a cell type and even to the stage of development or differentiation of the same cell type [25, 26]. Surprisingly, MLP NLS was found to strongly localize to the nucleolus, raising the possibility that the sequence is also important for nucleolar localization. A small number of these sequences have been found in other proteins [27-29]. Whether this is the means by which MLP is able to target the nucleolus following strain also remains to be elucidated. Interestingly, inhibition of MLP nuclear transport did not block the increase in ribosomal protein associated with cyclic strain. This may suggest that although MLP can activate ribosomal protein synthesis in response to strain, there are MLP independent pathways to achieve this. The import of MLP into the nucleus is also regulated by transcriptional activity as inhibition of RNA polymerase II or both RNA polymerase I and II reduced amounts there. For many nuclear cytoplasmic cycling proteins their presence in the nucleus is dependent upon active nuclear transcription [30, 31].
Inhibition of MLP nuclear translocation in combination with cyclic strain leads to significant loss of myocyte sarcomeric structure along with decreased α-actinin expression. These findings, combined with the inhibition of strain and phenylephrine-induced myocyte hypertrophy suggest that nuclear MLP is important for myocyte remodelling in response to hypertrophic stimuli. In humans with the K69R MLP mutation, arginine is replaced with a lysine in the NLS region resulting in a cardiomyopathy as a result of aberrant MLP localization [32]. Those findings confirm that nuclear MLP has an important function in the human heart. In MLP knockout mice, cyclically strained cardiac myocytes did not induce BNP transcription suggesting that the protein is an important component of the mechanosensing mechanism in myocytes [33].
In conclusion, in cultured neonatal myocytes, inhibition of MLP nuclear translocation prevents myocyte hypertrophy and alters sarcomeric remodeling in response to cyclic strain and phenylephrine treatment. The presence of MLP in the nucleus also appears to be dependent upon active transcription. These data suggest that both the functions and regulation of MLP are complex and play an important role in myocyte remodeling in response to hypertrophic stimuli.
Acknowledgments
This research is supported by the National Institutes of Health HL-62426 and HL 77995 and AHA-0630307N. The authors thank Dr Wilma Hoffman for helpful discussions about cellular nuclear shuttling proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benmerah A, Scott M, Poupon V, Marullo S. Nuclear functions for plasma membrane-associated proteins? Traffic. 2003;4(8):503–11. doi: 10.1034/j.1600-0854.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 2.Wixler V, Geerts D, Laplantine E, Westhoff D, Smyth N, Aumailley M, et al. The LIM-only protein DRAL/FHL2 binds to the cytoplasmic domain of several alpha and beta integrin chains and is recruited to adhesion complexes. Journal of Biological Chemistry. 2000;275(43):33669–78. doi: 10.1074/jbc.M002519200. [DOI] [PubMed] [Google Scholar]
- 3.Hoshijima M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am J Physiol Heart Circ Physiol. 2006;290(4):H1313–25. doi: 10.1152/ajpheart.00816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boateng SY, Belin RJ, Geenen DL, Margulies KB, Martin JL, Hoshijima M, et al. Cardiac dysfunction and heart failure are associated with abnormalities in the subcellular distribution and amounts of oligomeric muscle LIM protein. American Journal of Physiology - Heart & Circulatory Physiology. 2007;292(1):H259–69. doi: 10.1152/ajpheart.00766.2006. [DOI] [PubMed] [Google Scholar]
- 5.Gehmlich K, Geier C, Milting H, Furst D, Ehler E. Back to square one: what do we know about the functions of Muscle LIM Protein in the heart? J Muscle Res Cell Motil. 2008 Dec 30; doi: 10.1007/s10974-008-9159-4. [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Liu XY, Robinson D, Burnett C, Jackson C, Seele L, et al. Suppression of Staphylococcal Enterotoxin B-induced Toxicity by a Nuclear Import Inhibitor. Journal of Biological Chemistry. 2004;279(18):19239–46. doi: 10.1074/jbc.M313442200. [DOI] [PubMed] [Google Scholar]
- 7.Henderson JR, Pomies P, Auffray C, Beckerle MC. ALP and MLP distribution during myofibrillogenesis in cultured cardiomyocytes. Cell Motil Cytoskeleton. 2003;54(3):254–65. doi: 10.1002/cm.10102. [DOI] [PubMed] [Google Scholar]
- 8.Arber S, Halder G, Caroni P. Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell. 1994;79(2):221–31. doi: 10.1016/0092-8674(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 9.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. American Journal of Physiology - Cell Physiology. 2003;285(1):C171–82. doi: 10.1152/ajpcell.00013.2003. [DOI] [PubMed] [Google Scholar]
- 10.Chen M, Jiang P. Altered subcellular distribution of nucleolar protein fibrillarin by actinomycin D in HEp-2 cells. Acta Pharmacol Sin. 2004;25(7):902–6. [PubMed] [Google Scholar]
- 11.Ramsby ML, Makowski GS, Khairallah EA. Differential detergent fractionation of isolated hepatocytes: biochemical, immunochemical and two-dimensional gel electrophoresis characterization of cytoskeletal and noncytoskeletal compartments. Electrophoresis. 1994;15(2):265–77. doi: 10.1002/elps.1150150146. [DOI] [PubMed] [Google Scholar]
- 12.Boateng SY, Seymour AM, Bhutta NS, Dunn MJ, Yacoub MH, Boheler KR. Sub-antihypertensive doses of ramipril normalize sarcoplasmic reticulum calcium ATPase expression and function following cardiac hypertrophy in rats. J Mol Cell Cardiol. 1998;30(12):2683–94. doi: 10.1006/jmcc.1998.0830. [DOI] [PubMed] [Google Scholar]
- 13.Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL, et al. Protein kinase Cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res. 2004 Aug 20;95(4):424–32. doi: 10.1161/01.RES.0000138299.85648.92. [DOI] [PubMed] [Google Scholar]
- 14.Boateng SY, Lateef SS, Mosley W, Hartman TJ, Hanley L, Russell B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am J Physiol Cell Physiol. 2005;288(1):C30–8. doi: 10.1152/ajpcell.00199.2004. [DOI] [PubMed] [Google Scholar]
- 15.Deutsch J, Motlagh D, Russell B, Desai TA. Fabrication of microtextured membranes for cardiac myocyte attachment and orientation. J Biomed Mater Res. 2000;53(3):267–75. doi: 10.1002/(sici)1097-4636(2000)53:3<267::aid-jbm12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Motlagh D, Alden KJ, Russell B, Garcia J. Sodium current modulation by a tubulin/GTP coupled process in rat neonatal cardiac myocytes. J Physiol. 2002 Apr 1;540(Pt 1):93–103. doi: 10.1113/jphysiol.2001.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heineke J, Ruetten H, Willenbockel C, Gross SC, Naguib M, Schaefer A, et al. Attenuation of cardiac remodeling after myocardial infarction by muscle LIM protein-calcineurin signaling at the sarcomeric Z-disc. Proc Natl Acad Sci U S A. 2005;102(5):1655–60. doi: 10.1073/pnas.0405488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281(5383):1690–3. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 19.Qi L, Boateng SY. The circadian protein Clock localizes to the sarcomeric Z-disk and is a sensor of myofilament cross-bridge activity in cardiac myocytes. Biochemical & Biophysical Research Communications. 2006;351(4):1054–9. doi: 10.1016/j.bbrc.2006.10.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, von Mikecz A. Specific inhibition of rRNA transcription and dynamic relocation of fibrillarin induced by mercury. Exp Cell Res. 2000 Aug 25;259(1):225–38. doi: 10.1006/excr.2000.4923. [DOI] [PubMed] [Google Scholar]
- 21.Su Z, Yao A, Zubair I, Sugishita K, Ritter M, Li F, et al. Effects of deletion of muscle LIM protein on myocyte function. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280(6):H2665–73. doi: 10.1152/ajpheart.2001.280.6.H2665. [DOI] [PubMed] [Google Scholar]
- 22.Geier C, Gehmlich K, Ehler E, Hassfeld S, Perrot A, Hayess K, et al. Beyond the sarcomere: CSRP3 mutations cause hypertrophic cardiomyopathy. Hum Mol Genet. 2008 Sep 15;17(18):2753–65. doi: 10.1093/hmg/ddn160. [DOI] [PubMed] [Google Scholar]
- 23.van den Bosch BJ, van den Burg CM, Schoonderwoerd K, Lindsey PJ, Scholte HR, de Coo RF, et al. Regional absence of mitochondria causing energy depletion in the myocardium of muscle LIM protein knockout mice. Cardiovascular Research. 2005;65(2):411–8. doi: 10.1016/j.cardiores.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 24.Hallhuber M, Burkard N, Wu R, Buch MH, Engelhardt S, Hein L, et al. Inhibition of nuclear import of calcineurin prevents myocardial hypertrophy. Circulation Research. 2006;99(6):626–35. doi: 10.1161/01.RES.0000243208.59795.d8. [DOI] [PubMed] [Google Scholar]
- 25.Heilman DW, Teodoro JG, Green MR. Apoptin nucleocytoplasmic shuttling is required for cell type-specific localization, apoptosis, and recruitment of the anaphase-promoting complex/cyclosome to PML bodies. Journal of Virology. 2006;80(15):7535–45. doi: 10.1128/JVI.02741-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hozumi Y, Ito T, Nakano T, Nakagawa T, Aoyagi M, Kondo H, et al. Nuclear localization of diacylglycerol kinase zeta in neurons. European Journal of Neuroscience. 2003;18(6):1448–57. doi: 10.1046/j.1460-9568.2003.02871.x. [DOI] [PubMed] [Google Scholar]
- 27.Martel C, Macchi P, Furic L, Kiebler MA, Desgroseillers L. Staufen1 is imported into the nucleolus via a bipartite nuclear localization signal and several modulatory determinants. Biochemical Journal. 2006;393(Pt 1):245–54. doi: 10.1042/BJ20050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohrum MA, Ashcroft M, Kubbutat MH, Vousden KH. Identification of a cryptic nucleolar-localization signal in MDM2. Nat Cell Biol. 2000;2(3):179–81. doi: 10.1038/35004057. [DOI] [PubMed] [Google Scholar]
- 29.Kakuk A, Friedlander E, Vereb G, Jr, Lisboa D, Bagossi P, Toth G, et al. Nuclear and nucleolar localization signals and their targeting function in phosphatidylinositol 4-kinase PI4K230. Exp Cell Res. 2008 Aug 1;314(13):2376–88. doi: 10.1016/j.yexcr.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Neumann M, Stearman R, Stauber R, Pause A, Pavlakis GN, et al. Transcription-dependent nuclear-cytoplasmic trafficking is required for the function of the von Hippel-Lindau tumor suppressor protein. Molecular & Cellular Biology. 1999;19(2):1486–97. doi: 10.1128/mcb.19.2.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valgardsdottir R, Prydz H. Transport signals and transcription-dependent nuclear localization of the putative DEAD-box helicase MDDX28. Journal of Biological Chemistry. 2003;278(23):21146–54. doi: 10.1074/jbc.M300888200. [DOI] [PubMed] [Google Scholar]
- 32.Mohapatra B, Jimenez S, Sanchez X, Faulkner G, Perles Z, Sinagra G, et al. Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis. Journal of the American College of Cardiology. 2003;42(11):2014–27. doi: 10.1016/j.jacc.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Knoll R, Hoshijima M, Hoffman HM, Person V, Lorenzen-Schmidt I, Bang ML, et al. The cardiac mechanical stretch sensor machinery involves a Z disc complex that is defective in a subset of human dilated cardiomyopathy. Cell. 2002;111(7):943–55. doi: 10.1016/s0092-8674(02)01226-6. [DOI] [PubMed] [Google Scholar]


