Abstract
The ability of positive and negative facial signals to influence attention orienting is crucial to social functioning. Given the dramatic developmental change in neural architecture supporting social function, positive and negative facial cues may influence attention orienting differently in relatively young or old individuals. However, virtually no research examines such age-related differences in the neural circuitry supporting attention orienting to emotional faces. We examined age-related correlations in attention-orienting biases to positive and negative face emotions in a healthy sample (N=37; 9-40 years old) using functional magnetic resonance imaging and a dot-probe task. The dot-probe task in an fMRI setting yields both behavioral and neural indices of attention biases towards or away from an emotional cue (happy or angry face). In the full sample, angry-face attention bias scores did not correlate with age, and age did not correlate with brain activation to angry faces. However, age did positively correlate with attention bias towards happy faces; age also negatively correlated with left cuneus and left caudate activation to a happy-bias fMRI contrast. Secondary analyses suggested age-related changes in attention bias to happy faces. The tendency in younger children to direct attention away from happy faces (relative to neutral faces) was diminished in the older age groups, in tandem with increasing neural deactivation. Implications for future work on developmental changes in attention-emotion processing are discussed.
1. Introduction
Attention biases noted in mood and anxiety disorders illustrate the complex manner in which emotion and attention interact. Research on attention biases indirectly suggests that such biases and their neural correlates change with development (Fox, Hane et al. 2007). However, minimal work has directly examined age-related differences in the neural correlates of emotion-attention interactions. Considerable evidence documents age-dependent changes in the neural circuitry supporting a range of social-emotional functions beyond attention-emotion interaction (Nelson, Leibenluft et al. 2005; Ernst and Fudge 2009). This evidence raises the possibility of developmental variation in how positive and negative emotional face cues influence attention. We address these possibilities with behavioral and functional magnetic resonance imaging (fMRI) data acquired using a classic attention-orienting paradigm, the dot-probe task (MacLeod, Mathews et al. 1986).
Most work on emotion-attention interactions examines links between attention and threat stimuli, emphasizing anxiety and associated patterns of perturbed amygdala function. However, attention is also shaped by rewards, reflecting their survival value (Holland and Gallagher 2004). Age-related changes in behavior, such as increased risk-taking and novelty-seeking in adolescence, may be accompanied by alterations in sensitivity to both rewards and threats (Nelson, Leibenluft et al. 2005; Ernst and Fudge 2009). Changes in threat sensitivity may reflect the maturation of neural structures implicated in processing threat during fear-provoking situations. This neural architecture is thought to encompass the amygdala and an associated network in the ventral prefrontal cortex and parietal lobes, a neural circuitry specifically implicated in threat-attention interactions (LeDoux 2000). Effects of reward on cognition, in contrast, are found in the striatum and mesolimbic dopamine pathway (Fudge, Breitbart et al. 2004; Fudge, Breitbart et al. 2005; Haber, Kim et al. 2006). Similar to the neuronal network associated with processing threat, these striatal regions are also thought to impact function in the ventral prefrontal cortex and parietal areas by modulating attention. Accordingly, age-related changes in this mesolimbic-striatal system may influence attention during reward processing.
Studies using the dot-probe task have the capacity to chart age-related changes in attention biases and associated neural circuitry function. This task assumes that reaction times to probes appearing in pre-cued locations are typically faster than to probes appearing in non-cued locations (Navon and Margalit 1983; Posner, Friedrich et al. 1983), thus providing an index of attention bias. In the version of the dot-probe task used here (Bradley, Mogg et al. 1998), two face-cues of the same actor, one either happy or angry and one neutral, appear simultaneously side-by-side. A probe appears in one location previously occupied by a face, and participants are asked to identify the probe's location via button press. This task generates “snap-shots” of attention deployment by measuring reaction times to probes replacing emotion cues indicating bias towards or away from that emotion. By including angry-neutral and happy-neutral pairs, this version of the task probes biases related to socially threatening and rewarding stimuli.
While psychopathology is not the focus of the current study, prior work comparing groups of anxious and non-anxious individuals, suggests developmental changes in attention bias for emotional information. Specifically, findings from studies of separate samples of non-anxious youths and adults suggest the presence of age-related changes. For example, Kindt et al. (1997; 2000) proposed that in general, young children have an attention bias towards threat information; and, during development, healthy (non-anxious) children learn to inhibit the threat bias. However, evidence is mixed with some studies showing no threat bias in either older or younger children (Morren, Kindt et al. 2003), and other studies suggesting a bias towards threat in healthy children and adolescents (Waters, Lipp et al. 2004; Monk, Nelson et al. 2006). Non-anxious adults more commonly either show no attention bias for threat cues, such as angry faces, or a tendency for a bias away from threat (Mogg and Bradley 1998; Mogg, Millar et al. 2000; Mogg and Bradley 2002; Monk, Nelson et al. 2004; Bar-Haim, Lamy et al. 2007; Frewen, Dozois et al. 2008). Thus, attention bias may differ between immature and mature humans (Pine, Cohen et al. 1998). However, a recent meta analysis suggested that non-anxious children and adults both lack a threat-related bias (Bar-Haim, Lamy et al. 2007), although it was also noted that there were insufficient child studies to clarify the developmental course of attention biases. Other data from comparisons of older and young adults, reviewed by Mather and Carstensen (2005), suggest that attention biases toward positive information increase with age, possibly due to improved ability to regulate emotion across the lifespan. However, the latter age-related differences manifested only for reward-related but not threat information, and there are little data directly comparing attention biases in adults and youths.
With regard to neuroimaging, five studies have used face-based dot-probe tasks to examine neural correlates of emotion-attention interactions; none have examined age-related variation. Two of these studies examined non-anxious adults (Monk, Nelson et al. 2004; Pourtois, Schwartz et al. 2006); three others examined youths (Monk, Nelson et al. 2006; Monk, Telzer et al. 2008; Telzer, Mogg et al. 2008). The first study in adults did not find evidence of an attention bias in research participants, but it did reveal greater temporal-parietal and occipito-parietal cortex engagement in contrasts involving fearful relative to neutral faces (Pourtois, Schwartz et al. 2006). Data from the second adult study suggested that healthy subjects implicitly learn to avoid threats without changing activation in fear-related circuitry (Monk, Nelson et al. 2004). Two dot-probe studies examined adolescents, one using an unmasked face presentation (Monk, Nelson et al. 2006) and the other using a masked face presentation (Monk, Telzer et al. 2008). The latter two studies did not directly compare various age groups, given the focus in these studies on individual differences in frontal-amygdalar function and anxiety. A third study on adolescents using the dot-probe task found a positive association between state anxiety scores and both behavioral bias toward angry faces and associated neural activation in right dorsolateral prefrontal cortex (Telzer, Mogg et al. 2008). Taken together, the results from these five imaging studies, much like the above-noted behavioral studies, raise the possibility that age moderates the neural correlates of attention-emotion interactions (Fox, Hane et al. 2007; Pine 2007). This possibility raises many related, specific questions on the precise manner in which age influences attention (e.g., by increasing or decreasing bias to threats or rewards in particular age groups and/or by influencing different brain regions). These questions remain premature, however, since none of the five brain-imaging studies directly examined age-related variations in task performance or neural activation patterns.
The present study examines the correlation between age and attention biases both to positive and negative affective stimuli in an fMRI experiment. The study uses a version of the dot-probe task frequently employed in both behavioral and imaging studies (Bradley, Mogg et al. 1999). Prior work shows this particular task to be sensitive to hypothesized associations between individual differences in mood or anxiety symptoms and individual differences in attention bias. Indeed, past findings using this precise task indicate different patterns of attention bias for threat and reward-related information; for example, increased bias for threat relates most consistently to anxiety, whereas reduced bias for rewards relates most consistently to more general measures of negative affect (Frewen, Dozois et al. 2008). As a result, the current study maps age-related associations with attention biases to both threats, represented by angry faces, and rewards, represented by happy faces. Finally, because our study is the first to assess developmental trends in neuro-functional patterns during performance on the dot-probe task, we apply whole brain analyses to begin to describe age-related correlations with neural response across the entire brain.
2. Results
Whole Sample Behavioral Data
Table 1 presents mean reaction times (RTs), attentional bias scores, and error rates for each condition. A one sample t-test indicated that overall, independent of age, participants displayed a small (9 ms) but significant attention bias toward threat, t(36) = 3.09, p = 0.05. No such bias was detected for the happy faces, t(36) < 1, p = 0.8.
Table 1.
Mean reaction time and accuracy rates for angry and happy trials on the dot-probe task across all participants (N=37).
| Mean RT | SD | % error | SD | |
|---|---|---|---|---|
| Angry Congruent | 504.81 | 87.16 | 1.7 | 0.06 |
| Angry Incongruent | 513.98 | 87.59 | 5.1 | 0.10 |
| Happy Congruent | 509.07 | 92.40 | 3.9 | 0.09 |
| Happy Incongruent | 508.18 | 87.34 | 4.9 | 0.99 |
| Across All Trials | 508.00 | 87.19 | 3.9 | 0.05 |
| Angry Bias | 9.17 | 18.06 | -- | -- |
| Happy Bias | -0.89 | 19.05 | -- | -- |
Note: Angry Bias calculated by subtracting response to Angry Congruent trials from response to Angry Incongruent trials. Happy Bias calculated by subtracting response to Happy Congruent trials from response to Happy Incongruent trials.
The main behavioral analyses examined correlations between age and each bias score. Angry face bias scores did not correlate with age, r(37) = 0.17, p = 0.94. Happy face bias scores correlated positively with age, r(37) = 0.32, p = 0.05, such that older participants showed greater bias towards happy, relative to neutral, faces.
Whole Sample fMRI Data
To explore associations between age and brain activity during performance on the dot-probe task, we first used age as a predictor of activation, across the brain, in two contrasts: angry incongruent vs. angry congruent and happy incongruent vs. happy congruent (i.e., the same events used to calculate angry bias and happy bias). Following a whole-brain Monte Carlo procedure correcting for multiple comparisons at the p < 0.05 level, no associations were found between brain activation and age for the angry-bias contrast. However, for the happy-bias contrast, activation within the left caudate and left cuneus correlated negatively with age (Figure 1).
Figure 1.
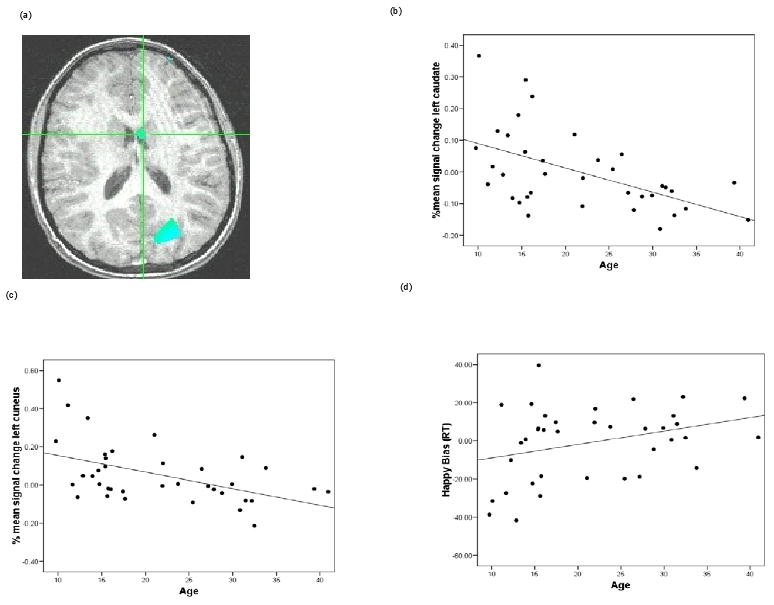
(a) Activation map depicting a negative correlation between age and activation in the left cuneus and left caudate for the contrast that yields happy bias (happy incongruent vs. happy congruent trials). Cross-hairs are centered on the left caudate (x = -1.8, y = 7.7, z = 16.9); also shown is the left cuneus (x = -24.4, y = -74.1, z = 19.4). Percent signal change values were extracted from each region of interest and graphed for illustrative purposes. (b) Correlation between age and percent signal change in the caudate for the happy bias contrast across all participants, Pearson r (37) = -0.54 (c) Correlation between age and percent signal change in the cuneus for the happy bias contrast across all participants, Pearson r (37) = -0.49 (d) Scatter plot of age and happy bias scores derived from RT data (happy incongruent – happy congruent).
Secondary analyses clarified factors contributing to age-related neural patterns associated with happy bias. Analyses for mean percent signal change within the two largest clusters (see all clusters in Table 2), the caudate and the cuneus, indicated that as age increased, the activation decreased in these regions (Pearson rs(37) = -0.54 and -0.49, for the left caudate and left cuneus, respectively). It is important to note that extracted estimates from the ROIs are taken from non-independent voxels, which can lead to biases in additional statistical analyses. Thus, the extracted data are used solely for illustrative purposes; associated r-values are used to depict the direction of any significant correlation effect, as detected in the primary analysis. Follow-up analyses were conducted to decompose the nature of this effect by examining the correlation between age and BOLD signal in the happy congruent condition (i.e., trials in which the target probe replaced the happy face of a happy-neutral face pair) and the happy incongruent condition (i.e., trials in which the target probe replaced the neutral face of a happy-neutral face pair) separately. For both areas, although non-significant, the correlation with the happy congruent trials was negative and with the happy incongruent trials was positive (all p > 0.1). Finally, we used these extracted BOLD values to examine correlations between RT measures of bias and brain activation(Poldrack and Mumford 2009). There was no significant correlation between the happy-face attention bias (derived from the RT data) and the corresponding contrast of BOLD values in the left cuneus and left caudate (Pearson rs(37) = -0.16 and -0.08, ps = 0.35 and 0.63, for the left caudate and left cuneus, respectively).
Table 2.
List of active clusters over 100 voxels in regions showing an age correlation with activation to happy bias contrast (happy incongruent vs. happy congruent). Asterisk (*) indicates regions with significant activation using whole-brain corrected threshold p<0.05.
| Brain region | BA | Voxels | Side | Talairach coordinates | T-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Cuneus* | 18 | 3169 | Left | 21 | 80 | 21 | -3.09 |
| Caudate body* | 940 | Center | 1 | -4 | 16 | -3.76 | |
| Posterior cingulate | 30 | 374 | Left | 8 | 40 | 7 | -3.06 |
| Inferior temporal gyrus | 37 | 313 | Left | 42 | 67 | 2 | -2.96 |
| Superior frontal gyrus | 6 | 285 | Right | -3 | -30 | 53 | -3.08 |
| Uncus | 28 | 225 | Left | 18 | 6 | -24 | 3.82 |
| Medial frontal gyrus | 9 | 147 | Right | -4 | -57 | 42 | -3.00 |
| Middle frontal gyrus | 6 | 140 | Right | -41 | -2 | 55 | -2.99 |
| Parahippocampal gyrus | 35 | 132 | Right | -25 | 26 | -21 | 3.02 |
Behavioral and fMRI Data in Narrowly-Defined Age Groups
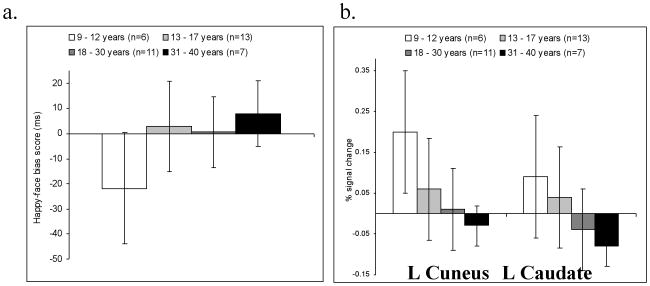
Results from initial analyses revealed age-related associations with reaction time and with neural response to reward cues in the cuneus and caudate. These associations manifest across the entire sample, considered as a whole. A final set of analyses used one-sample t-tests in smaller sub-samples. These analyses examined the degree to which one or another behavioral or fMRI pattern manifested in more specific, narrowly-constructed, age-delimited samples. These final analyses divided the subjects into four unique age subgroups. Results from these analyses appear in Figure 2. For behavioral data, the youngest subjects (9 -12 years old) showed a trend (t(5) = -2.3; p = 0.06; d = 1.87, r = 0.68) for an attention bias away from happy faces (as indicated by RT data), but bias did not differ significantly from zero in any other subgroup. For fMRI data, the oldest subjects (31-40 years old) showed a statistically significant caudate deactivation and the happy bias (i.e., lower signal in incongruent relative to congruent happy-face trials) (t(6) = -4.6; p = 0.004; d = -3.75, r = 0.88). No other subgroup showed significant activations in response to attention bias contrasts in fMRI data.
Figure 2.
(a) Happy face attention bias score derived from RT data and (b) % signal change in the caudate and cuneus for the happy bias contrast in the age defined groups.
3. Discussion
Our study used the dot-probe task to examine associations between age and either reward-related or threat-related attention bias. Two main sets of findings emerged. First, reward-related, happy-face bias showed a positive correlation with age, a finding consistent with previous literature (Carstensen, Pasupathi et al. 2000; Mather and Carstensen 2005). Age also showed a negative correlation with neural activation for happy-face/reward bias, specifically in the striatum and cuneus, two brain regions previously implicated in attention control (Pessoa, McKenna et al. 2002). Second, while a threat-related attention bias emerged behaviorally across the entire sample, there was no evidence of an association between threat bias and age. Moreover, age showed no association with neural activation on angry incongruent versus angry congruent events, the events from which the measure of threat bias emerges. In summary, age related to both behavioral and neural indicators of biased attention allocation to rewarding, happy faces, but not to attention allocation to threatening, angry faces.
Analyses of both behavioral and fMRI data for angry faces were consistent in that neither revealed an association with age. Interpretation of null results is qualified by many constraints, particularly small sample sizes. Nevertheless, the null finding cannot be completely discounted, given that the failure to observe associations with age emerged in the context of a significant overall threat bias in the sample as a whole. This finding supports suggestions that attention to threat is a core function that facilitates survival and adaptive social behavior (Damasio 1999; Ohman 2002). Although the present finding of an overall threat bias is consistent with this view, it contrasts results from a meta-analysis of face-based dot-probe tasks, indicating no bias for angry faces in non-anxious participants (Bar-Haim, Lamy et al. 2007). Yet, previous work also indicates that state-related factors (e.g., situational stressors) can increase threat bias (Bar-Haim, Lamy et al. 2007). In addition, Helfinstein et al. (2008) found that even mildly aversive social threat primes can induce a bias towards threat in healthy non-anxious subjects. Similar effects may occur in the context of fMRI research. Specifically, past work, conducted among healthy adolescents, suggests that increases in state anxiety associated with the experience of being in an MRI scanner might induce a bias towards angry faces in non-anxious participants (Monk, Nelson et al. 2006; Pine 2007; Monk, Telzer et al. 2008; Telzer, Mogg et al. 2008). Thus, a behavioral attention bias is not typically detected in the laboratory, when factors may make the environment a low-threat context. However, increases in state anxiety associated with MRI scanning might induce an attention bias towards threat in healthy subjects. Finally, in the current study, repeated measures of state anxiety were not made. As a result, future work examining contextual influences on attention bias is needed. Such work might specifically examine associations between changes in state anxiety and changes in threat bias when the same individuals are studied outside and inside the MRI scanner.
Unlike for angry faces, age did correlate with behavioral and neural responses to happy face-bias. Thus, associations emerged with age, positively with the behavioral measure of attention bias to happy faces and negatively with brain regions engaged by the happy-incongruent, relative to the happy-congruent event types. A recent quantitative review of dot-probe performance data documented a bias towards happy faces in behavioral studies of healthy adults, based on data from face-based dot-probe paradigms (Frewen, Dozois et al. 2008); however, child samples were not included in this meta-analysis, so developmental questions were not considered in detail in this review. Other work finds evidence of increasing positive mood and attention bias to positive information with age, in older adults relative to younger adults, possibly reflecting associations with cognitive and neural maturity (Mather and Carstensen 2005).
The current findings, demonstrating age-related differences in caudate engagement to happy-face dot-probe events, fit within a broader context of studies examining brain systems engaged by evocative stimuli. Considerable neuroimaging research demonstrates the capacity of diverse approach-related cues to engage the caudate and other structures within the striatum. Similarly, prior studies find age-related differences in engagement of the caudate and other striatal regions to reward cues. However, the direction of these associations varies across studies and across experimental tasks. Some studies find increased striatal reactivity, as well as increased reactivity in prefrontal structures within the brain's reward circuit, to pleasant stimuli in youth relative to adults (Hare, Tottenham et al. 2005; Ernst, Pine et al. 2006; Eshel, Nelson et al. 2007). Other studies find the opposite (Bjork, Knutson et al. 2004). The current study found less caudate reactivity in response to the happy bias contrast (i.e., happy-incongruent versus happy-congruent trials) with increasing age.
Beyond the caudate, the cuneus emerged in the current study as the other brain region showing a strong negative relationship between the happy attention bias contrast and age. As with the striatum, some evidence implicates medial parietal cortex more generally, and the cuneus specifically, in attention-orienting behavior (Corbetta and Shulman 2002). Although we are aware of no prior study that implicates this region in reward-related cuing specifically, some work implicates the parietal cortex in reward-attention interactions as well as in broader facets of attention and its modulation by emotion (Platt and Glimcher 1997; Bendiksby and Platt 2006).
Attention bias scores for happy faces were positively associated with age, as there was a tendency to direct attention away from happy faces (relative to neutral faces) in the younger subjects, which diminished across the age groups, with no significant bias in the older subjects. In contrast, corresponding neural response to happy faces showed a shift from activation to deactivation as age increased. Such an opposing pattern for behavioral and neural data, in terms of associations with age, might reflect diverse underlying factors. For example, the shift in attention bias towards happy faces occurring in tandem with a shift in neural engagement in the opposite direction might reflect age-related differences in efficiency of activation-behavior associations. From this perspective, lower fMRI activation with increasing age, in the context of similar or even opposite-appearing behavioral response patterns, may reflect the requirement of greater brain activation to support one or another behavioral repertoire in the younger individuals, whose engagement of brain regions may be “less efficient” (Bunge, Dudukovic et al. 2002). This pattern might suggest that rewards have the capacity to engage the brain differentially at particular points in development. This idea is consistent with prior work underscoring the importance of developmental change in the salience of rewarding social stimuli (Nelson, Leibenluft et al. 2005).
Alternatively, features of our paradigm complicate efforts to understand the precise factors that account for the observed associations with age. Our design relied on event-related parameters, which allowed us to contrast brain regions engaged by specific event-types, such as correctly performed trials including congruent or incongruent emotional events. We chose to use a rapid, event-related design to reduce the length of our task so that we could study young individuals while also acquiring sufficient data on event type, controlling for specific trial parameters and potential performance confounds. With this design, engagement of neural regions is modeled based on all features contained within each stimulus-event class; as such, we cannot dissociate the hemodynamic response to cues and dot-probes appearing within each event. Alternative designs, using considerably longer tasks, or alternative neuroimaging techniques with better temporal resolution, such as magnetoencephalography (MEG) or event-related potentials (ERP) (Pourtois, Grandjean et al. 2004; Bar-Haim, Lamy et al. 2005; Eldar and Bar-Haim in press), could disambiguate the response to cues and probes. Further work using such designs and tools, perhaps with a restricted range of event-types to minimize the overall study length, might clarify factors contributing to age-related variation that emerged in the current study. Of note, such questions are understandable given the paucity of research in this area; the current report is the first imaging study to document age-related variation in neural responses to happy faces in the context of an attention-emotion interaction experiment.
The observation of contrasting patterns for behavioral and brain imaging data also raises broader questions about the sensitivity of behavior and fMRI to age-related differences. Some groups have argued that brain imaging data, relative to behavioral measures, generate more sensitive indices of underlying between-group differences (Meyer-Lindenberg and Weinberger 2006). Prior fMRI studies using the dot-probe task provide some support for this position (Monk, Telzer et al. 2008). While the current findings document age-related variations with behavior and brain activation, differential sensitivity in brain-based and behavioral measures could account for some other aspects of our findings. For example, in the secondary, age-delimited group analysis (Figure 2) we separated subjects into four age-defined groups (9-12, 13-17, 18-30 and 31-40 years old). In this analysis, we observed a statistically significant attention-modulation effect on neural activation in happy-face trials (i.e., contrast of happy-incongruent and happy-congruent trials), based on measures of caudate deactivation in older but not younger age groups. For behavioral data, however, the youngest group showed a near significant trend towards attention bias away from happy faces, with an overall trend for bias scores to shift with increasing age from negative to positive across all age groups. This failure to observe significant bias in behavioral data in the secondary analysis could result from many factors, most prominently low statistical power, given our small sample sizes and the small magnitude of bias typically generated by the dot-probe task (Bar-Haim, Lamy et al. 2007). Regardless, the findings do echo different conclusions in studies of between-group differences that frequently emerge based on behavioral as opposed to imaging data. Moreover, the significant relationship between age and various indices of reward processing (i.e. both behavioral and neural indices) might also encourage future work of larger samples within narrow age groups, which might detect unique associations between reward bias and associated brain activation profiles.
The current study should be considered in light of some limitations. One significant limitation, as noted above, relates to the somewhat brief nature of our task; we did not use a slow event-related design or a longer task, presenting isolated trials with only cues or only dot-probes. As a result, it remains unclear how age-related variation affects the responses to specific event features (e.g., face-pair cues, dot-probe cues). Second, while our sample is relatively large for a developmental fMRI study (N=37), the overall sample size remains small compared to other research on development. As a result, negative results (i.e., the absence of age-related changes in angry bias) should be interpreted with caution. The limited sample size also clearly affects the interpretation of the results from the secondary, age-delimited analysis. Third, we examined associations between age and performance on the dot-probe task in dimensional analyses that treated age continuously to maximize statistical power. Thus, studies using other designs and larger groups of individuals in relatively narrow age-bands are needed. Beyond being more sensitive to bias in individual groups, such studies also might search for nonlinearity in the age-by-attention-orienting association. Finally, prior work on neural underpinnings of social development emphasizes the importance of puberty (Nelson, Leibenluft et al. 2005), but the current study did not consider the relationship between puberty and attention bias or activation. Future studies should attempt to dissociate the effects of age and puberty, though such studies will require very large sample sizes.
Despite these limitations, the current report also contains some notable strengths. First, the present study includes a reasonably large sample of rigorously ascertained subjects studied with fMRI methods. Performance was monitored closely in the scanner, using a task with strong theoretical importance based on prior behavioral data. Second, despite the inconsistent direction of age-related findings for behavior and brain engagement data, the current study provides some evidence of age-related associations in two data streams, one for behavioral measures of attention and another for brain engagement. While relatively few previous fMRI studies provide such evidence, it is important to continue efforts to capture simultaneously age-related variations in behavior and brain function. Finally, while a series of reports focuses on brain regions engaged in threat-based dot-probe tasks (Monk, Nelson et al. 2006; Pourtois, Schwartz et al. 2006; Monk, Telzer et al. 2008), the current study is the first to report regions correlated with the response to the happy faces of the dot-probe task. The findings generated here fit within a broader context demonstrating age-related modulation of reward responses, as reflected in both behavior and striatal engagement.
4. Experimental Procedures
Participants
Participants were 37 healthy paid volunteers (18 males, mean age: 21.51 years old, range: 9-40 years old, SD= 8.69). These volunteers were recruited from a larger sample of 49 volunteers who had participated. A total of 12 subjects were excluded from the study either due to movement of 2.5 mm in any direction, failure to perform under a 25% error rate on the dot-probe task or technical complications in the acquisition of data.
All participants were recruited via advertisements. The National Institute of Mental Health (NIMH) Institutional Review Board approved the study and all participants/parents provided written informed consent/assent. All participants were free of any medical illness, based on history and physical exam, and free of any psychiatric illness. Psychiatric history was assessed using a structured psychiatric interview, the Kiddie-Schizophrenia-and-Affective-Disorders-Schedule (K-SADS) (Kaufman, Birmaher et al. 1997) in youth and the Structured Clinical Interview for DSM-IV-TR (SCID) (Spitzer, Williams et al. 1992) in adults. Experienced clinicians trained to achieve high reliability for all diagnoses (kappa>0.75) administered these interviews to each participant, as well as to one parent of each participating youth. Each participant had an IQ > 70 based on the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler 1999), mean IQ = 115, range = 87-142. Other exclusion criteria were any lifetime use of a psychoactive substance or contraindications to fMRI, such as braces.
Stimuli
The same procedures and stimuli were used as described previously (Mogg and Bradley 1999). Briefly summarized, stimuli were achromatic face pairs of the same actor appearing side-by-side. Thus, one photograph of the same actor appeared in each of two hemi-fields. In each face-pair, one face displayed an emotion (i.e., anger, happy), while the other face was non-emotional (i.e., neutral). The location of the emotional face was counterbalanced across trials. There were a total of 80 face-pairs, comprising 32 happy-neutral pairs, 32 angry-neutral pairs, and 16 neutral-neutral pairs. An equal number of male and female were presented in all face-pair combinations.
The Dot-Probe Task
Each trial began with a centrally-located fixation cross displayed for 500 ms, followed by a pair of faces that appeared side by side on the screen for 500 ms. Faces were replaced by an asterisk-probe in either the right or left visual field location just vacated by one of the faces. Participants were instructed to press one of two buttons as quickly and as accurately as possible to indicate the location of the probe (left or right) using a response button system developed by MRI Devices (Waukesha, WI). The probe was displayed for 1100 ms and the inter-trial interval was 2100ms (Figure 3).
Figure 3.
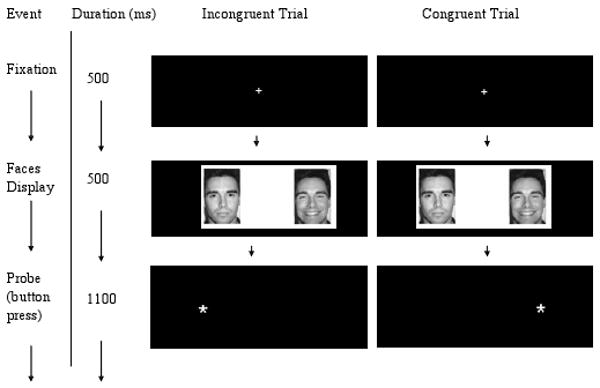
Participants viewed a central fixation cross for 500ms. Afterwards, a set of faces of the same identity were presented side by side. One face displayed a neutral expression and the other an emotional expression (happy or angry). Following the face presentation, an asterisk probe was presented on the right or left side of the screen and participants indicated the location by a button press. Congruent trials occurred if the probe was located on the same side as the emotional face and incongruent trials occurred if the probe was located on the opposite side as the emotional face.
In total, 200 trials were presented. To measure attention bias, both congruent and incongruent trials were administered. In the 64 congruent trials, the probe replaced the happy (or angry) face in the face pair. In the 64 incongruent trials, the probe replaced the neutral face in the happy-neutral or angry-neutral face pair. For the neutral-neutral face pairs (32 trials), the target appeared on the left or right sides of the display with equal probability. The location of the probe was counterbalanced across the experiment. Trial presentation order was fully randomized for each participant.
Prior to scanning, all participants received practice trials on the dot-probe task using a different set of faces than the stimulus set used in the experiment. Practice continued until participants were comfortable performing the task properly (typically 10-30 practice trials).
As in a prior fMRI study of development (Guyer, Monk et al. 2008), we relied on a rapid-event-related design, as opposed to a slow-event-related design. In this rapid design, jitter in the timing of fMRI acquisition is accomplished by the inclusion of randomly-appearing null events or so-called “blank trials”. We included 40 such blank trials, randomly interspersed among the face trials. Beyond ensuring jittering of timing, these trials also provide a comparison condition with minimal stimulation to serve as the implicit baseline for analyses. In these blank trials, a fixation cross was presented for 500 ms followed by a blank screen for 1600 ms. Thus, with this design, the fMRI activation during each trial type can be randomly sampled, so that unbiased comparisons can be made of brain regions engaged across specific trial types. The task was programmed using E-prime version 1.0 (Psychological Software Tools, Pittsburgh, PA) and displayed on the Avotec Silent Vision Glasses (Stuart, FL).
Behavioral Data Analysis
Participants were required to perform correctly on at least 75% of task trials; subjects unable to meet this standard were excluded. Individual task trials were excluded from analyses if participants responded in less than 200 ms or more than 800 ms after probe presentation (Monk, Nelson et al. 2006; Monk, Telzer et al. 2008). As in prior studies, attention bias for emotionally-evocative faces was calculated by subtracting the mean reaction time (RT) of trials in which the target probe appeared at the location of the emotional face (i.e., congruent trials) from the mean RT of trials in which the target appeared at the location of the neutral face (i.e., incongruent trials). Positive values indicate bias towards emotion, whereas negative values indicate bias away from emotion. Separate bias scores were calculated for the angry and happy face-event types.
For the primary analysis, associations between attention bias and age were tested using Pearson correlations, with a p < 0.05 threshold. On an exploratory basis, additional secondary analyses were conducted. One secondary analysis generated correlations between age and performance for behavioral parameters: overall mean RT and percent error. This analysis was considered secondary since most prior research on behavioral indices obtained from dot probe tasks examines bias scores. Another secondary analysis used one-sample t-tests on happy bias scores in smaller sub-samples formed by grouping subjects into relatively narrow age ranges (age ranges: 9-12, 13-17, 18-30, 31-40). These analyses were considered secondary in light of low statistical power, given the small sample sizes and expectation of small point estimates for bias scores, based on prior work with the dot-probe task.
fMRI Data Acquisition
Images were collected on a General Electric 3 Tesla scanner (Waukesha, WI). During the scanning session, both anatomical and functional images were obtained. Parameters for T2 weighted functional echo planar image (EPI) data collection were: 240mm field of view (FOV), matrix size of 64 × 64, 29 continuous slices 3.3mm thick, repetition time (TR) of 2300ms, echo time (TE) of 23ms, and a 90 degree flip angle. The parameters for the anatomical images were: 256mm field of view (FOV), 256 × 256 matrix size, 180 continuous slices, 1.0 mm slice thickness, TR: 11.4 ms, TE: 4.4 ms, time to return to equilibrium (TI) 300ms. Imaging data were processed and analyzed using AFNI software version 2, 2006_06_30_1332 (Cox 1996). Movement was monitored for each participant and data from those participants who moved more than 2.5mm in any plane were excluded from the analysis.
FMRI Data Processing and Analysis
Each participant's EPI data were motion corrected, registered to the anatomical image, and concatenated across runs. Functional data were smoothed with a 6 mm full width at half maximum isotropic Gaussian filter. After preprocessing, individual-level data were analyzed using multiple regression. Five conditions were specified as regressors: angry-congruent, angry-incongruent, happy-congruent, happy-incongruent, and neutral. Trials with errors were included as events of no interest. Contrast values were created based on the five conditions. Given the prior literature on attention bias, our main interest was on contrasts of incongruent relative to congruent conditions for both angry and happy emotions. These contrasts focused on the happy and angry attention biases. After individual models were created, participant's anatomical and functional data were converted into Talairach space (Talairach and Tournoux 1988).
A random effects model was used to analyze fMRI data at the group level using a general linear model with a two-tailed, whole-brain corrected p < .05. The AFNI 3dRegAna program was used to regress whole brain contrast values with age for the selected contrasts. These analyses examined two contrasts: i) happy incongruent vs. happy congruent (i.e., happy bias) and ii) angry incongruent vs. angry congruent (i.e., angry bias). Analyses relied on an initial whole-brain at p < 0.005 for each contrast of interest, examining specifically the regressions between age and activation. Using this initial p < 0.005 threshold and 1000 Monte Carlo simulations, the whole-brain-corrected significant p < 0.05 threshold was determined to require a cluster size of 809 voxels. Thus, the final t-maps are corrected at the whole-brain level. After defining whole-brain significant clusters, mean percent signal change values were extracted from the caudate and cuneus clusters for both contrasts corresponding to happy and threat bias and correlated with age for illustrative purposes and to exclude the possibility of an outlier-driven effect. Secondary analyses of fMRI data were designed to parallel secondary analyses of behavioral data in smaller sub-samples, grouping subjects into relatively narrow age ranges (age ranges: 9-12, 13-17, 18-30, 31-40 years old). As with the associated behavioral analyses, these analyses are considered secondary due to low statistical power.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bar-Haim Y, Lamy D, et al. Attentional bias in anxiety: A behavioral and ERP study. Brain and Cognition. 2005;59(1):11–22. doi: 10.1016/j.bandc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, et al. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bendiksby MS, Platt ML. Neural correlates of reward and attention in macaque area LIP. Neuropsychologia. 2006;44(12):2411–20. doi: 10.1016/j.neuropsychologia.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, et al. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci. 2004;24(8):1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, et al. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition & Emotion. 1998;12(6):737–753. [Google Scholar]
- Bradley BP, Mogg K, et al. Attentional bias for emotional faces in generalized anxiety disorder. Br J Clin Psychol. 1999;38(Pt 3):267–78. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, et al. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, et al. Emotional experience in everyday life across the adult life span. J Pers Soc Psychol. 2000;79(4):644–55. [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens. New York: Harcourt Brace; 1999. [Google Scholar]
- Eldar S, Bar-Haim Y. Neural plasticity in response to attention training in anxiety. Psychological Medicine. doi: 10.1017/S0033291709990766. in press. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci Biobehav Rev. 2009;33(3):367–82. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, et al. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med. 2006;36(3):299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, et al. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45(6):1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Hane AA, et al. Plasticity for affective neurocircuitry - How the environment affects gene expression. Current Directions in Psychological Science. 2007;16(1):1–5. [Google Scholar]
- Frewen PA, Dozois DJ, et al. Selective attention to threat versus reward: meta-analysis and neural-network modeling of the dot-probe task. Clin Psychol Rev. 2008;28(2):307–37. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, et al. Selective attention to threat versus reward: Meta-analysis and neural-network modeling of the dot-probe task. Clinical Psychology Review. 2008;28(2):307–337. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, et al. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490(2):101–18. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, et al. Amygdaloid inputs define a caudal component of the ventral striatum in primates. J Comp Neurol. 2004;476(4):330–47. doi: 10.1002/cne.20228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, et al. A Developmental Examination of Amygdala Response to Facial Expressions. J Cogn Neurosci. 2008;20:1565–82. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, et al. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci. 2006;26(32):8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, et al. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57(6):624–32. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, White LK, et al. Affective primes suppress attention bias to threat in socially anxious individuals. Behaviour Research and Therapy. 2008;46(7):799–810. doi: 10.1016/j.brat.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14(2):148–55. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kindt M, Bierman D, et al. Cognitive bias in spider fear and control children: assessment of emotional interference by a card format and a single-trial format of the stroop task. J Exp Child Psychol. 1997;66(2):163–79. doi: 10.1006/jecp.1997.2376. [DOI] [PubMed] [Google Scholar]
- Kindt M, Van Den Hout M, et al. Cognitive bias for pictorial and linguistic threat cues in children. Journal of Psychopathology and Behavioral Assessment. 2000;22(2):201–219. [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, et al. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends Cogn Sci. 2005;9(10):496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36(9):809–48. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: a replication study using a modified version of the probe detection task. Behav Res Ther. 1999;37(6):595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40(12):1403–14. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, et al. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J Abnorm Psychol. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163(6):1091–7. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, et al. Experience-dependent plasticity for attention to threat: Behavioral and neurophysiological evidence in humans. Biol Psychiatry. 2004;56(8):607–10. doi: 10.1016/j.biopsych.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morren M, Kindt M, et al. Anxiety and the processing of threat in children: Further examination of the cognitive inhibition hypothesis. Behaviour Change. 2003;20(3):131–142. [Google Scholar]
- Navon D, Margalit B. Allocation of Attention According to Informativeness in Visual Recognition. Quarterly Journal of Experimental Psychology Section a-Human Experimental Psychology. 1983;35(Aug):497–512. doi: 10.1080/14640748308402484. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, et al. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Ohman A. Automaticity and the amygdala: Nonconscious responses to emotional faces. Current Directions in Psychological Science. 2002;11(2):62–66. [Google Scholar]
- Pessoa L, McKenna M, et al. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99(17):11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine Research Review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007 doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55(1):56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Platt ML, Glimcher PW. Responses of intraparietal neurons to saccadic targets and visual distractors. J Neurophysiol. 1997;78(3):1574–89. doi: 10.1152/jn.1997.78.3.1574. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience. 2009;4(2):208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Friedrich FJ, et al. Neural Control of Covert Visual Orienting. Bulletin of the Psychonomic Society. 1983;21(5):349–349. [Google Scholar]
- Pourtois G, Grandjean D, et al. Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cerebral Cortex. 2004;14(6):619–633. doi: 10.1093/cercor/bhh023. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Schwartz S, et al. Neural systems for orienting attention to the location of threat signals: An event-related fMRI study. Neuroimage. 2006;31(2):920–933. doi: 10.1016/j.neuroimage.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, et al. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49(8):624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Altas of the Human Brain. Stuttgart: Georg Thieme Verlag; 1988. [Google Scholar]
- Telzer EH, Mogg K, et al. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79(2):216–22. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Lipp OV, et al. Attentional bias toward fear-related stimuli: An investigation with nonselected children and adults and children with anxiety disorders. Journal of Experimental Child Psychology. 2004;89(4):320–337. doi: 10.1016/j.jecp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment, Inc; 1999. [Google Scholar]