Abstract
Introduction
Normal heart rhythms originate in the sinoatrial node. HCN-encoded funny current (If) and the Kir2-encoded inward rectifier (IK1) counteract each other by respectively oscillating and stabilizing the negative resting membrane potential, controlling action potential firing. Therefore, IK1 suppression and If overexpression have been independently exploited to convert cardiomyocytes (CMs) into AP-firing bioartificial pacemakers. Although the two strategies have been largely assumed synergistic, their complementarity has not been investigated.
Methods and Results
We explored the inter-relationships of automaticity, If and IK1 by transducing single left ventricular (LV) CMs isolated from guinea pig hearts with the recombinant adenoviruses Ad-CMV-GFP-IRES-HCN1-ΔΔΔ and/or Ad-CGI-Kir2.1 to mediate their current densities via whole-cell patch clamp technique at 37°C. Results showed that Ad-CGI-HCN1-ΔΔΔ- but not Ad-CGI-Kir2.1-transduction induced automaticity (181.1±13.1 bpm). Interestingly, Ad-CGI-HCN1-ΔΔΔ/Ad-CGI-Kir2.1 cotransduction significantly promoted the induced firing frequency (320.0±15.8 bpm; p<0.05). Correlation analysis revealed that the firing frequency, phase 4 slope and APD90 of AP-firing LV CMs were correlated to If (R2>0.7) only when -2>IK1>-4 pA/pF but not to IK1 over the entire If ranges examined (0.02<R2<0.4). Unlike If, IK1 displayed correlation to neither the phase 4 slope (R2=0.02) nor phase 4 length (R2=0.04) when -2>If>-4 pA/pF. As anticipated, however, APD90 was correlated to IK1 (R2=0.4).
Conclusion
We conclude that an optimal level of IK1 maintains a voltage range for If to operate most effectively during a dynamic cardiac cycle.
Keywords: If, IK1, bioartificial pacemaker, automaticity, synergism
Introduction
Normal heart rhythms originate in the sinoatrial node (SAN), a specialized cardiac tissue consisting of only a few thousand pacemaker cells. Funny current (If), encoded by the hyperpolarization-activated cyclic nucleotide-modulated (HCN) channel gene family plays a pivotal role in the generation of cardiac rhythms in nodal pacemaker cells. It serves as an intrinsic oscillator that drives diastolic depolarization to the action potential (AP) threshold after each excitation cycle.1-5 By contrast, the Kir2-encoded inwardly rectifying potassium current (IK1) has the dual function of stabilizing a negative resting membrane potential (RMP) and facilitating repolarization.6 Indeed, the robust expression of If and the lack of IK1 are a signature of rhythmically-firing nodal pacemaker cells.3,7 Conversely, IK1 but not If is robustly expressed in silent-yet-excitable adult atrial and ventricular cardiomyocytes (CMs).8,9 As such, the approaches of IK1 suppression10,11 and If over-expression,4,5,12-14 have been independently and, most commonly, exploited to convert normally quiescent CMs into spontaneously AP-firing cells as bioartificial pacemakers. Although the two strategies appear to be conceptually synergistic, their complementarity has not been experimentally investigated. Indeed, single-sided reproduction of either the If or IK1 profile of nodal cells in the atrial or ventricular CMs may not be optimal for inducing and modulating automaticity.15 Atrial and ventricular CMs have repertoires of ionic currents that are different from those of nodal pacemaker cells. Since If is activated by hyperpolarization and deactivated by depolarization, here we hypothesize that IK1, a presumptive opponent of If, synergistically enhances If by maintaining the voltage changes within a range where HCN channels can most effectively operate during a dynamic cardiac cycle.
Methods
Molecular biology
PCR-based mutagenesis of mouse HCN1 (generously provided by Dr. Steve Segalbaum, Columbia University) of the bicistronic adenovirus shuttle vector pAdCMV-GFP-IRES (or pAdCGI) was performed with overlapping oligos as described previously.16,17 The internal ribosomal entry site (IRES) allows the simultaneous translation of 2 transgenes, green fluorescence protein (GFP) and an engineered-HCN1 construct, with a single transcript. HCN1 was chosen since its structure-function properties have been extensively investigated in our previous studies.15-25 Adenoviruses were generated by Cre-lox recombination of purified ψ5 viral DNA and shuttle vector DNA using Cre4 cells.26 The recombinant products were plaque purified, amplified, and purified again by CsCl gradients, yielding concentrations on the order of 1010 plaque-forming units (PFU) ml-1.
Adenovirus-mediated gene transfer and isolation of LV CMs
Adult female guinea pigs (∼250-300g) were euthanized by intraperitoneal injection of pentobarbital (80mg/kg). The hearts were quickly excised, followed by perfusion with enzymatic solutions using a customized Langendorff apparatus. Left ventricular (LV) CMs were plated at 5×105 per laminin-coated glass coverslip in medium containing: 5 mM carnitine, 5 mM creatine, 5 mM taurine, 100 μg ml-1 penicillin-streptomycin and 10% fetal bovine serum in Medium 199 (Sigma-Aldrich Corp., St. Louis, MO, USA) in a 37°C incubator with 5% CO2 for 2 hrs. For transduction, LV CMs were incubated for 1 hour in serum-free medium containing adenoviral particles at a concentration of ∼2×109 PFU. A transduction efficiency of ∼70-80% could typically be achieved with this protocol. Such an in vitro system of adult guinea pig LV CMs has been previously used by both us 27 and others.28
Electrophysiology and data analysis
Electrical recordings were performed using the whole-cell patch-clamp technique at 37°C (HEKA Instruments Inc. Southboro, MA, USA). Successfully transduced cells were selected based on their epifluorescence at 488/530 nm (excitation/emission). Patch pipettes were prepared from 1.5 mm thin-walled borosilicate glass tubes using a Sutter micropipette puller P-97 and had typical resistances of 3-5 MΩ when filled with an internal solution containing (mM): 110 K+ aspartate, 20 KCl, 1 MgCl2, 0.1 Na-GTP, 5 Mg-ATP, 5 Na2-phosphocreatine, 1 EGTA, 10 HEPES, pH adjusted to 7.3 with KOH. The external Tyrode’s bath solution was composed of (mM): 140 NaCl, 5 KCl, 1 MgCl2, 1 CaCl2, 10 Glucose, 10 HEPES, pH adjusted to 7.4 with NaOH. Cell capacitance was measured by using the internal circuit for capacitance-current compensation, in which both cell capacitance and series resistance were compensated. Voltage-and current-clamp recordings were simultaneously performed on the same cells at 37°C within 24 to 36 hours after transduction. Absolutely no automaticity was detected from cultured control LV CMs recorded over the same period.
To elicit inward currents, cells were held at -30mV and pulsed from 0mV to -140mV with 10mV increments for 3 seconds. If was defined as 1mM Ba2+-insensitive, time-dependent currents. For recording action potentials, cells were held at 0pA without (for electrically-active cells) or with a stimulation of 0.1-1nA for 5ms to elicit a response. The steady-state current-voltage (I-V) relationship of If was determined by plotting the currents measured at the end of the 3-second test pulses of the above-mentioned protocol against the test potentials in the presence of 1mM BaCl2. Data were not corrected for the junction potential.
Statistical analysis
Correlation analysis was performed by linear regression. The larger the value of R2, the better correlation of the two parameters in comparison. . All data reported were means ± S.E.M. with P<0.05 indicating statistical significance as determined using an unpaired Student’s t test.
Results
Adenovirus-mediated overexpression of If and/or IK1 in adult LV CMs
For If and/or IK1 overexpression, we generated the recombinant adenoviruses Ad-CGI-HCN1-ΔΔΔ and Ad-CGI-Kir2.1 that mediate ectopic expression of HCN1-ΔΔΔ and Kir2.1 channels, respectively. The engineered HCN1 construct, HCN1-ΔΔΔ whose S3-S4 linker has been shortened by deleting residues 235-237 to favor channel opening,16,17,23 was chosen to best mimic native heteromultimeric If in adult LV CMs without having to consider poorly defined factors such as accessory subunits and cellular context as we previously described.4,5 Figure 1A shows that all control LV CMs were electrically quiescent but capable of generating a single AP upon stimulation by a depolarizing current pulse (800 pA for 2 to 5 ms; n=17). Consistent with our previous results,4,5 robust IK1 (-32.1±1.2 pA/pF at -140mV) but no detectable If (-2.5±1.0 pA/pF at -140mV) could be recorded from the same control LV CMs.
Figure 1.
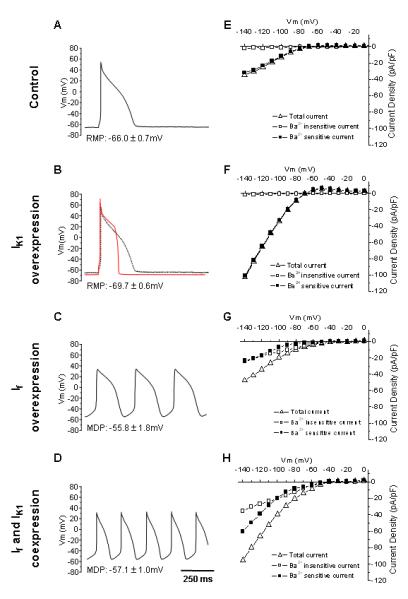
Representative action potential waveforms and averaged steady-state I-V relationships of control (A, E), Ad-CGI-Kir2.1- (B, F), Ad-CGI-HCN1ΔΔΔ- (C, G), as well as Ad-CGI-HCN1ΔΔΔ/Ad-CGI-Kir2.1 (D, H)-tranduced LV CMs.
We next studied the effect of transducing LV CMs with Ad-CGI-Kir2.1 (Figure 1B), Ad-CGI-HCN1-ΔΔΔ (Figure 1C) and both (Figure 1D). For Ad-CGI-Kir2.1-transduced LV CMs, there was a significant increase in IK1 relative to control (-101.2±3.2 pA/pF at -140 mV; p<0.05), but If was still absent (-1.8±1.1 pA/pF at -140 mV; p<0.05). By contrast, IK1 in Ad-CGI-HCN1-ΔΔΔ-trasnduced LV CMs (-34.8±5.5 pA/pF at -140 mV; n=5) was not different from control (p>0.05), but hyperpolarization-activated time-dependent If could be readily recorded (-28.5±3.9 pA/pF at -140 mV; p<0.05). For LV CMs simultaneously cotransduced by Ad-CGI-HCN1-ΔΔΔ and Ad-CGI-Kir2.1-WT, both IK1 (-46.6±5.5 pA/pF at -140 mV; n=9) and If (-40.7±6.4 pA/pF at -140 mV; n=9) were substantially expressed. The corresponding current-voltage relationships are given (Figure 1E-H).
Coexpression of If and IK1 promoted induced automaticity
To directly probe the functional consequences of overexpressing If or IK1 alone or both in the AP waveform, we subjected the same cells presented above to current-clamp recordings. While Ad-CGI-Kir2.1-transduction significantly hyperpolarized the RMP (-69.7±0.7 mV vs. -66.0±0.7 mV of WT; p<0.05) (Figure 2A) and shortened the duration of AP elicited upon a depolarizing current stimulus (by ∼65%, n=7; p<0.05) (Figure 2B and Table 1), Ad-CGI-HCN1-ΔΔΔ uniquely induced ventricular automaticity (n=10), even without stimulation, with a firing rate of 181.1±13.1 bpm, a relatively depolarized maximum diastolic potential (MDP = -55.8±1.8 mV) and phase-4 depolarization (180±20 mV/s), consistent with what we recently reported.4,5 Spontaneous APs were never observed in either control or Ad-CGI-Kir2.1-transduced LV CMs. Interestingly, simultaneous overexpression of If and IK1 significantly promoted the induced automaticity by increasing the AP-firing frequency by ∼1.8-fold to 320.0±15.8 bpm (n=9; p<0.05) (Figure 2C). Relative to single Ad-CGI-HCN1-ΔΔΔ transduction, cotransduced LV CMs had significantly (p<0.05) hyperpolarized MDP (-57.1±0.9 mV, Figure 2A), hastened phase 4 depolarization (280±16 mV/s, Figure 2D) and shortened APD90 (by 56% to 97.3±7.9 ms from 199.5±8.7 ms). All these AP parameters as well as cycle length and APD50 of control and transduced LV CMs are summarized in Table 1.
Figure 2.
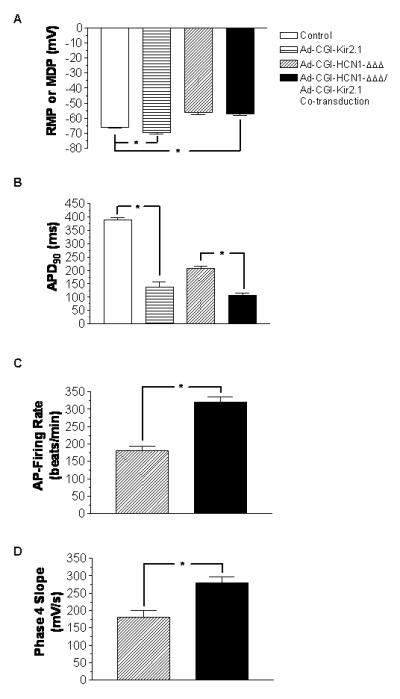
Bar charts showing (A) RMP or MDP; (B) APD90; (C) beating rate; and (D) phase 4 slope of control, Ad-CGI-Kir2.1-transduced, Ad-CGI-HCN1ΔΔΔ-transduced, as well as Ad-CGI-HCN1ΔΔΔ/Ad-CGI-Kir2.1-cotranduced LV CMs. *, P<0.05.
Table 1.
Summary of AP parameters of control, singly- and co-transduced LV CMs. All values are means ± S.E.
| RMP (mV) |
MDP (mV) |
APD (ms) |
APD50 (ms) |
APD90 (ms) |
Phase 4 Length (ms) |
Phase 4 Slope (mV/s) |
Cycle Length (ms) |
Beating Rate (Beats/ min) |
|
|---|---|---|---|---|---|---|---|---|---|
|
Control
(17) |
-66.0 ± 0.7 |
-- | 387.2 ± 10.4 |
299.5 ± 12.0 |
357.3 ± 10.0 |
-- | -- | -- | -- |
|
Kir 2.1
(7) |
-69.7 ± 0.6 |
-- | 138.6 ± 17.6 |
116.1 ± 17.2 |
127.8 ± 17.8 |
-- | -- | -- | -- |
|
HCN1-ΔΔΔ
(10) |
-- | -55.8 ± 1.8 |
207.4 ± 9.4 |
166.9 ± 6.9 |
199.5 ± 8.7 |
88.3 ± 9.6 |
180.0 ± 20.0 |
343.7 ± 24.9 |
181.1 ± 13.1 |
|
HCN1-ΔΔΔ +
Kir2.1-WT (9) |
-- | -57.1 ± 1.0 |
107.37 ± 7.8 |
72.7 ± 7.8 |
97.3 ± 7.9 |
55.0 ± 4.5 |
280.0 ± 16.0 |
182.3 ± 6.8 |
320.0 ± 15.8 |
n numbers are given in bracket.
As IK1 plays important roles in setting the resting membrane potential and in influencing the final phase of repolarization, the accelerated firing rate in cotransduced LV CMs may be a consequence of a greater magnitude of If due to the more negative membrane potential, or of a shorter AP duration due to a more rapid repolarization, or both. In order to dissect these IK1-dependent effects contributing to the accelerated firing rate, we measured firing rate, action potential duration, phase-4 slope and phase-4 duration of automatic cotransduced LV CMs by pharmacological blockade of IK1 with external Ba2+ at concentration of 0.1 mM and 0.3 mM. At baseline, the cycle length was 257.8±6.0 ms, i.e., 233.9±5.4 bpm with APD90 of 94.8±1.0 ms and phase-4 length of 68.7±2.7 ms. As expected, external application of Ba2+(0.1 mM) shifted the maximal diastolic potential from -45.9±0.1 mV to -29.1±0.3 mV, which was associated with a reduction of firing rate for 26.8%. Although the APD90 was lengthened by 58 ms, i.e., 61.2% increment, the phase-4 duration were prolonged by67.4 ms (98.2%) due to the flattening of the phase-4 slope by 51.2%. However, further increase in external Ba2+ concentration to 0.3 mM resulted in a complete abortion of automaticity. These data suggests that IK1 accelerates the firing rate of If induced automaticity by enhancing the phase-4 slope as well as shortening AP duration.
Inter-relationships between IK1, If and automaticity
For shedding mechanistic insights, we next investigated the intricate inter-relationships among IK1, If and induced automaticity. To obtain a more global and accurate view, we plotted the various biophysical parameters recorded from all of control, singly- and doubly-transduced LV CMs to generate broader ranges for correlation analyses (i.e., the different experimental groups were included on the same plots in different symbols, but the entire data sets were fitted to liner regression). In spontaneously AP-firing cells, the frequency did not depend on the amplitude of If at -60 mV (If, -60mV) when IK1 at -60 mV (IK1, -60mV) was ≥-2 pA/pF (R2=0.03; Figure 4A, solid squares). However, the induced firing rate displayed a strong positive correlation to If, -60mV when -2>IK1, -60mV >-4 pA/pF (R2=0.8; Figure 4A, open circles). Such a positive correlation diminished (to R2=0.3) as IK1, -60mV increased to ≤ -4 pA/pF (Figure 4A, solid triangle). In stark contrast to If, the induced automaticity did not depend on IK1, -60mV when If, -60mV was similarly fixed (i.e. If, -60mV ≤ 2pA/pF, -2 < If, -60mV ≤ 4pA/pF and If, -60mV >4pA/pF; Figure 4B).
Figure 4.
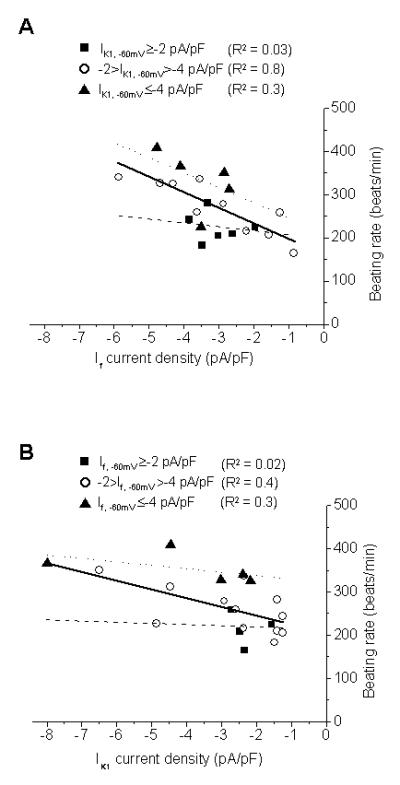
(A) Correlations between If and the frequency of spontaneously AP-firing cells when IK1, -60mV ≥-2 pA/pF (■), -2>IK1, -60mV >-4 pA/pF (○) and IK1, -60mV ≤-4 pA/pF (▲). (B) Correlations between IK1 and the firing frequency rate when If, -60mV ≥-2 pA/pF (■),-2>If, -60mV >-4 pA/pF (○) and If, -60mV ≤-4 pA/pF (▲).
To further dissect the inter-relationship, we similarly plotted the Phase 4 slope, Phase 4 length, and APD90 against If, -60mV with fixed ranges of IK1, -60mV. Similar to the firing frequency, all these AP parameters were also correlated to If, -60mV only when -2>IK1>-4 pA/pF. Figure 5A shows that steeper Phase 4 slopes could be attained with higher If densities (R2=0.7). Both the Phase 4 length (Figure 5C; R2=0.6) and APD90 (Figure 5E; R2=0.7) were negatively correlated to If, -60mV. Unlike If, -60mV, IK1, -60mV displayed correlation to neither the Phase 4 slope (to R2=0.02) nor Phase 4 length (to R2=0.04) when -2> If, -60mV >-4 pA/pF (Figures 5B & D). As anticipated, however, APD90 was correlated to IK1, -60mV (to R2=0.4; Figure 5F).
Figure 5.
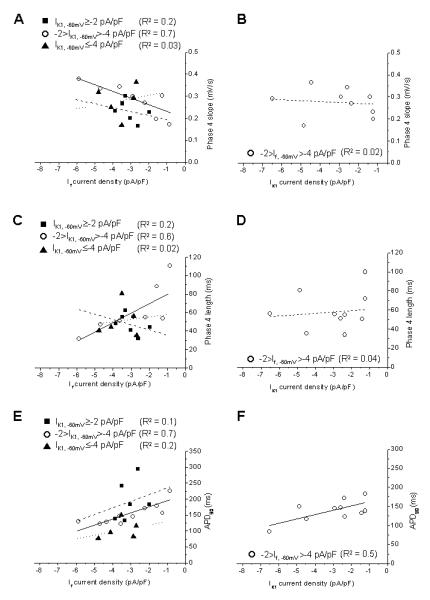
(A) Correlations between If and the phase 4 slope of spontaneously AP-firing cells when IK1, -60mV ≥-2 pA/pF (■), -2>IK1, -60mV >-4 pA/pF (○) and IK1, -60mV ≤-4 pA/pF (▲). (B) Correlations between IK1 and the phase 4 slope when If, -60mV ≥-2 pA/pF (■), -2>If, -60mV >-4 pA/pF (○) and If, -60mV ≤-4 pA/pF (▲). (C) Correlations between If and the phase 4 length of spontaneously AP-firing cells when IK1, -60mV ≥-2 pA/pF (■), -2>IK1, -60mV >-4 pA/pF (○) and IK1, -60mV ≤-4 pA/pF (▲). (D) Correlations between IK1 and the phase 4 length when If, -60mV ≥-2 pA/pF (■), -2>If, -60mV >-4 pA/pF (○) and If, -60mV ≤-4 pA/pF (▲). (E) Correlations between If and the APD90 of spontaneously AP-firing cells when IK1, -60mV ≥-2 pA/pF (■), -2>IK1, -60mV >-4 pA/pF (○) and IK1, -60mV ≤-4 pA/pF (▲). (F) Correlations between IK1 and the APD90 when If, -60mV ≥-2 pA/pF (■), -2>If, -60mV >-4 pA/pF (○) and If, -60mV ≤-4 pA/pF (▲).
Isoproterenol accelerates firing rate of Ad-CGI-HCN1-ΔΔΔ transduced LV CMs
To test the functionality of automatic Ad-CGI-HCN1-ΔΔΔ-transduced LV CMs as a pacemaker, we investigated their response to adrenergic stimulation by superfusion of 1 μM isoproterenol for 5 minutes. The cycle length reduced from 377±8.7 to 318±10.1 ms (p=0.001; n=3) representing 18% increase in the firing rate (Figure 6). This is largely due to the increase of phase-4 slope (166±9.5 mV/s to 265±11.9 mV/s, p=0.003), while the APD90, maximal diastolic potential and take-off potential were largely unchanged after isoproterenol treatment.
Figure 6.
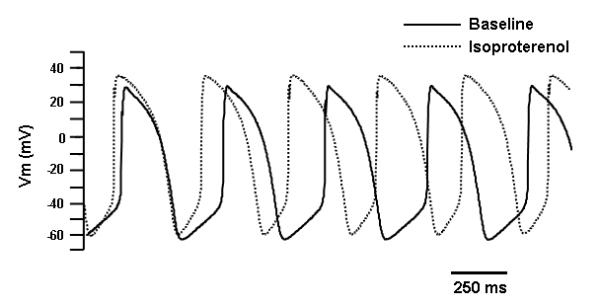
Effects of adrenergic stimulation by superfusion of 1 μM isoproterenol for 5 minutes (3 cells from a single animal) on HCN1-ΔΔΔ-transduced LV CMs.
Discussion
In recent years, efforts to generate bioartificial pacemakers as potential alternatives or supplements to electronic devices for treating cardiac rhythm disorders have been pursued. Experimentally, the one-sided approaches of maximizing If 4,12 or minimizing its “antagonizing” IK1 10,29 to convert nonpacing CMs to spontaneously firing pacemaker cells have been investigated. Our previous in silico and in vivo HCN-gene-transfer experiments of LV CMs demonstrate that both If and IK1 play critical roles in the induction of automaticity 4, 5, 21. While the two strategies appear to be conceptually synergistic, their complementarity has not been investigated. Indeed, although the primary functions of IK1 and If have been largely presumed to antagonize each other, our present study provides novel experimental supports that they do not necessarily counter-balance each other. Firstly, adenovirus-mediated expression of If (or IK1) in LV CMs did not alter IK1 (or If). These observations suggest that the two “opposing” currents do not undergo compensatory electrophysiological changes against each other when their relative expression is altered. As such, the resultant AP phenotypes can be principally attributed to the changes of either If or IK1 alone. Secondly, simultaneous overexpression of If and IK1 in LV CMs generates the highest oscillation frequency of automaticity. Correlation analysis to dissect the intricate mechanistic relationships among various If, IK1 and AP biophysical parameters indicates that, mechanistically, the frequency of induced AP-firing cells was dependent upon If when IK1, -60mV was between -2 and -4 pA/pF. However, the induced automaticity did not rely on IK1 when If, -60mV was similarly fixed. Thus, the data were in accordance with the notion that IK1 functions only as a binary switch for automaticity without providing a direct means for frequency modulation4,5, and further indicate that the strategies of IK1 suppression and If overexpression are not necessarily synergistic in conferring automaticity on quiescent cardiac muscles.
While the level of induced automaticity by simultaneous If and IK1 overpression is tachycardic and, as such, not desirable for bioartificial pacing, the findings have significant implications. Clinically, the implantation site for bioartificial pacemaker needs to be carefully considered because the different extents of innervations and the differential responses of IK1 and If to endogenous sympathetic and parasympathetic inputs might significantly alter the If/IK1 ratio from the original level, thereby leading to unanticipated tachyarrhythmias. Mechanistically, it is clear that the If/IK1 ratio is a more effective parameter to engineer for automaticity induction to generate bioartificial pacemakers than manipulating either If or IK1 per se. As a complex and heterogeneous tissue, the SAN displays gradual changes in phenotypic properties such as ionic current densities, potential profile and gap junction expression from the center (dominant pacemaker range) to the periphery (subsidiary pacemaker range) of the nodal cells.30 While the maximum diastolic potential is more negative, the AP is shorter with faster spontaneous rate in the center than in the periphery, AP is first initiated in the center and then transmitted to the periphery of the SAN.31 Such gradients ensure normal AP propagation in appropriate directions. Our present findings therefore raise the possibility that bioengineered cells that mimic the central and peripheral SA nodal cells showing native AP gradient can be best engineered by targeted firing rate engineering via virus cotransduction to improve the functional efficacy.
Of note, the current study has certain limitations. The choice of a gate-engineered version of HCN1 instead of wild-type HCN4 (the predominant HCN in genuine biological pacemakers) appears nonphysiological. However, this is because overexpression of wild-type HCN4 alone does not suffice to cause automaticity in adult LV CMs, probably due to the vast differences in ion channel panoplies as well as certain contentious β-subunits between sinoatrial pacemaker cells and normally quiescent adult LV CMs.13 Consequently, the use of this gate-engineered version of HCN1 may compensate for any context-dependent gating effects such as other modifying subunits and factors that may be present in SA nodal cells but not CMs. Second, the expression of If and IK1 in the present work were sporadic in nature due to the available transduced method, which may render a large variation in certain physiological measurement such as APD.
Conclusion
Based on the present results, we conclude that the induction of automaticity in LV CMs requires a fine balance of IK1 and If. Indeed, IK1 synergistically interacts with If by maintaining the voltage changes within a range where HCN channels can most effectively operate during a dynamic cardiac cycle.
Figure 3.
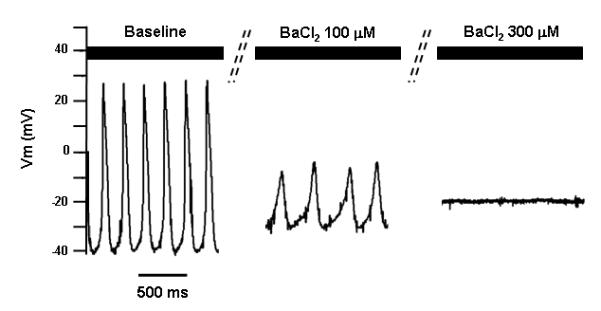
The effect of external application of Ba2+ on the automaticity in Ad-CGI-HCN1ΔΔΔ/Ad-CGI-Kir2.1-cotranduced LV CMs. Representative action potential waveforms at baseline, with 0.1 mM BaCl2, and 0.3 mM BaCl2 are shown, respectively.
Acknowledgments
This work was supported by grants from the NIH (R01 HL72857 to R.A.Li), the Stem Cell Program of the University of California (to R.A.Li), and the Hong Kong Research Grant Council (HKU 7459/04M to C.P.Lau, H.F.Tse, and R.A.Li). C.W.Siu was supported by a postdoctoral fellowship award from the Croucher Foundation.
References
- 1.DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–72. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- 2.Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- 3.Siu CW, Lieu DK, Li RA. HCN-encoded pacemaker channels: from physiology and biophysics to bioengineering. J Membr Biol. 2006;214(3):115–22. doi: 10.1007/s00232-006-0881-9. [DOI] [PubMed] [Google Scholar]
- 4.Tse HF, Xue T, Lau CP, Siu CW, Wang K, Zhang QY, Tomaselli GF, Akar FG, Li RA. Bioartificial sinus node constructed via in vivo gene transfer of an engineered pacemaker HCN Channel reduces the dependence on electronic pacemaker in a sick-sinus syndrome model. Circulation. 2006 Sep 5;114(10):1000–11. doi: 10.1161/CIRCULATIONAHA.106.615385. [DOI] [PubMed] [Google Scholar]
- 5.Xue T, Siu CW, Lieu DK, Lau CP, Tse HF, Li RA. Mechanistic role of I(f) revealed by induction of ventricular automaticity by somatic gene transfer of gating-engineered pacemaker (HCN) channels. Circulation. 2007 Apr 10;115(14):1839–50. doi: 10.1161/CIRCULATIONAHA.106.659391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhamoon AS, Jalife J. The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm. 2005 Mar;2(3):316–24. doi: 10.1016/j.hrthm.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007 Apr 10;115(14):1921–32. doi: 10.1161/CIRCULATIONAHA.106.616011. [DOI] [PubMed] [Google Scholar]
- 8.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002 May 17;90(9):939–50. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 9.Yu H, Chang F, Cohen IS. Pacemaker current exists in ventricular myocytes. Circ Res. 1993 Jan;72(1):232–6. doi: 10.1161/01.res.72.1.232. [DOI] [PubMed] [Google Scholar]
- 10.Miake J, Marban E, Nuss HB. Functional role of inward rectifier current in heart probed by Kir2.1 overexpression and dominant-negative suppression. J Clin Invest. 2003 May;111(10):1529–36. doi: 10.1172/JCI17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milanesi R, Baruscotti M, Gnecchi-Ruscone T, DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006 Jan 12;354(2):151–7. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- 12.Bucchi A, Plotnikov AN, Shlapakova I, Danilo P, Jr., Kryukova Y, Qu J, Lu Z, Liu H, Pan Z, Potapova I, KenKnight B, Girouard S, Cohen IS, Brink PR, Robinson RB, Rosen MR. Wild-type and mutant HCN channels in a tandem biological-electronic cardiac pacemaker. Circulation. 2006 Sep 5;114(10):992–9. doi: 10.1161/CIRCULATIONAHA.106.617613. [DOI] [PubMed] [Google Scholar]
- 13.Qu J, Barbuti A, Protas L, Santoro B, Cohen IS, Robinson RB. HCN2 overexpression in newborn and adult ventricular myocytes: distinct effects on gating and excitability. Circ Res. 2001 Jul 6;89(1):E8–14. doi: 10.1161/hh1301.094395. [DOI] [PubMed] [Google Scholar]
- 14.Qu J, Plotnikov AN, Danilo P, Jr., Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circulation. 2003 Mar 4;107(8):1106–9. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 15.Lieu DK, Chan YC, Lau CP, Tse HF, Siu CW, Li RA. Overexpression of HCN-encoded pacemaker current silences bioartificial pacemakers. Heart Rhythm. 2008 Sep;5(9):1310–7. doi: 10.1016/j.hrthm.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Lesso H, Li RA. Helical secondary structure of the external S3-S4 linker of pacemaker (HCN) channels revealed by site-dependent perturbations of activation phenotype. J Biol Chem. 2003 Jun 20;278(25):22290–7. doi: 10.1074/jbc.M302466200. [DOI] [PubMed] [Google Scholar]
- 17.Tsang SY, Lesso H, Li RA. Critical intra-linker interactions of HCN1-encoded pacemaker channels revealed by interchange of S3-S4 determinants. Biochem Biophys Res Commun. 2004 Sep 17;322(2):652–8. doi: 10.1016/j.bbrc.2004.07.167. [DOI] [PubMed] [Google Scholar]
- 18.Au KW, Siu CW, Lau CP, Tse HF, Li RA. Structural and functional determinants in the S5-P region of HCN-encoded pacemaker channels revealed by cysteine-scanning substitutions. Am J Physiol Cell Physiol. 2008 Jan;294(1):C136–44. doi: 10.1152/ajpcell.00340.2007. [DOI] [PubMed] [Google Scholar]
- 19.Azene EM, Xue T, Li RA. Molecular basis of the effect of potassium on heterologously expressed pacemaker (HCN) channels. J Physiol. 2003 Mar 1;547(Pt 2):349–56. doi: 10.1113/jphysiol.2003.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azene EM, Sang D, Tsang SY, Li RA. Pore-to-gate coupling of HCN channels revealed by a pore variant that contributes to gating but not permeation. Biochem Biophys Res Commun. 2005 Feb 25;327(4):1131–42. doi: 10.1016/j.bbrc.2004.12.127. [DOI] [PubMed] [Google Scholar]
- 21.Azene EM, Xue T, Marban E, Tomaselli GF, Li RA. Non-equilibrium behavior of HCN channels: insights into the role of HCN channels in native and engineered pacemakers. Cardiovasc Res. 2005 Aug 1;67(2):263–73. doi: 10.1016/j.cardiores.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Henrikson CA, Xue T, Dong P, Sang D, Marban E, Li RA. Identification of a surface charged residue in the S3-S4 linker of the pacemaker (HCN) channel that influences activation gating. J Biol Chem. 2003 Apr 18;278(16):13647–54. doi: 10.1074/jbc.M211025200. [DOI] [PubMed] [Google Scholar]
- 23.Tsang SY, Lesso H, Li RA. Dissecting the structural and functional roles of the S3-S4 linker of pacemaker (hyperpolarization-activated cyclic nucleotide-modulated) channels by systematic length alterations. J Biol Chem. 2004 Oct 15;279(42):43752–9. doi: 10.1074/jbc.M408747200. [DOI] [PubMed] [Google Scholar]
- 24.Xue T, Marban E, Li RA. Dominant-negative suppression of HCN1- and HCN2-encoded pacemaker currents by an engineered HCN1 construct: insights into structure-function relationships and multimerization. Circ Res. 2002 Jun 28;90(12):1267–73. doi: 10.1161/01.res.0000024390.97889.c6. [DOI] [PubMed] [Google Scholar]
- 25.Xue T, Li RA. An external determinant in the S5-P linker of the pacemaker (HCN) channel identified by sulfhydryl modification. J Biol Chem. 2002 Nov 29;277(48):46233–42. doi: 10.1074/jbc.M204915200. [DOI] [PubMed] [Google Scholar]
- 26.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps ML. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997 Mar;71(3):1842–9. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ennis IL, Li RA, Murphy AM, Marban E, Nuss HB. Dual gene therapy with SERCA1 and Kir2.1 abbreviates excitation without suppressing contractility. J Clin Invest. 2002 Feb;109(3):393–400. doi: 10.1172/JCI13359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li RA, Ennis IL, Tomaselli GF, Marban E. Structural basis of differences in isoform-specific gating and lidocaine block between cardiac and skeletal muscle sodium channels. Mol Pharmacol. 2002 Jan;61(1):136–41. doi: 10.1124/mol.61.1.136. [DOI] [PubMed] [Google Scholar]
- 29.Miake J, Marban E, Nuss HB. Biological pacemaker created by gene transfer. Nature. 2002 Sep 12;419(6903):132–3. doi: 10.1038/419132b. [DOI] [PubMed] [Google Scholar]
- 30.Dobrzynski H, Li J, Tellez J, Greener ID, Nikolski VP, Wright SE, Parson SH, Jones SA, Lancaster MK, Yamamoto M, Honjo H, Takagishi Y, Kodama I, Efimov IR, Billeter R, Boyett MR. Computer three-dimensional reconstruction of the sinoatrial node. Circulation. 2005 Feb 22;111(7):846–54. doi: 10.1161/01.CIR.0000152100.04087.DB. [DOI] [PubMed] [Google Scholar]
- 31.Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000 Sep;47(4):658–87. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]


