Abstract
This study was undertaken to evaluate heart rate (HR) regulation during severe hemorrhage (HEM) at different rates of blood loss. Chronically instrumented male rats underwent HEM at one of three rates: slow (0.5 ml/min/kg; S-HEM), intermediate (1.0 ml/min/kg I-HEM), or 2.0 ml/min/kg (fast; F-HEM) until 30% of the estimated total blood volume (ETBV) was withdrawn. Heart rate variability analysis was performed and the absolute power within the low frequency (LF; 0.16-0.6 Hz) and high frequency (HF; 0.6 - 3 Hz) ranges were evaluated. During the first 15% of ETBV loss, arterial pressure (AP) was maintained while HR increased. The increase in HR was greatest in the S-HEM and I-HEM groups and was associated with a significant reduction in HF power in the S-HEM group only. As blood loss progressed AP and HR declined in all treatment groups. The decrease in HR was associated with a significant increase in HF power in the F-HEM and I-HEM groups only. Parasympathetic blockade with atropine methyl bromide eliminated all decreases in HR, independent of rate of hemorrhage. Blockade of parasympathetic activity also significantly increased the AP at ETBV losses ≥20% independent of the rate of hemorrhage. The effect of atropine on AP was most noticeable in the S-HEM and F-HEM groups. These results demonstrate that rate of blood loss has an important impact on autonomic regulation during severe HEM and support previous findings that neural strategies underlying autonomic control may vary depending on the rate of blood loss.
Keywords: autonomic regulation, compensation, sympatho-inhibition, heart rate variability
1. Introduction
Autonomic changes associated with mild to moderate blood loss, include an initial phase of reflex-induced sympathoexcitation which is critical for maintaining arterial pressure (AP). This initial compensatory response is baroreceptor-dependent and includes both an increase in vascular resistance and heart rate (HR) (10). However, if blood loss continues, and exceeds 15-20% of total blood volume, the body enters a second phase, characterized by sympatho-inhibition, bradycardia, and fall in AP (23, 51). The mechanism underlying the onset and maintenance of this second phase of severe hemorrhage (7, 21), or the hemorrhagic sympatho-inhibitory phase, is complex and remains to be clearly defined. There is, however, evidence that both central and peripheral factors contribute to hemorrhagic sympatho-inhibition, including a change in afferent activity from the heart, central activation of opioid-, γ-aminobutyric acid-(GABA), and/or serotonin-containing neurons in the brainstem (16, 17, 19-22, 46). Evidence from both our lab (1, 34) and that of Troy and colleagues has demonstrated (57) that the rate of blood loss also influences the characteristics of autonomic control during hemorrhage, including indicators of brain neuronal activation, the level of bradycardia observed at the time of peak blood withdrawal, and the HR sustained 40 min following the offset of blood loss, during the recovery phase. These observations suggest that the central neural circuits modulating sympathetic and parasympathetic recruitment activated during severe hemorrhage, may be different depending on the rate of blood loss.
Heart rate variability (HRV) analysis is a useful method for evaluating changes in autonomic regulation and has been used to study changes in autonomic balance during varying physiological states such as exercise, sleep, aging, and emotional disorders (6, 38, 42, 47). HRV analysis is performed by evaluating the frequency components of the interval between successive heart beats or R to R intervals (RRI) using a fast Fourier transform. Two main frequency components are recognized, including a low frequency (LF) and a high frequency (HF) component. The origin of these frequency components have been described by various autonomic blockade studies (2, 9, 12, 32, 40, 48, 53) and it is generally accepted that the HF component primarily represents parasympathetic influences (56, 62) whereas the LF component reflects a mixture of sympathetic and parasympathetic influences. The ratio of LF to HF power (LF/HF) is frequently used as an indicator of sympatho-vagal balance.
Based on the results of our previous study demonstrating that brain activation varied depending on the rate of blood loss between 0.5 ml/kg to 2.0 ml/kg in conscious rats, the present study was undertaken to evaluate how autonomic control varies during different rates of blood loss rate (1). We hypothesized that the change in both the HF and LF/HF ratio would increase as the rate of blood loss increased. Preliminary results from this study have been presented in abstract form (49).
2. Materials and Methods
2.1 Animal Preparation
All experimental procedures were approved by the Animal Care and Use Committee at the University of Florida. Male Sprague-Dawley rats (320-420 g, Harlan Industries, Minneapolis, IN) (n=33) were brought to the lab, weighed and then anesthetized with an intraperiotoneal (i.p.) injection of ketamine/xylazine/acepromazine (80-100/1-3/8-20 mg/kg, respectively; Phoenix Pharmaceutical, Inc., St. Joseph, MO). Once anesthetized to a surgical plane, small incisions were made in the hind limbs to expose the femoral arteries and veins. The femoral vasculature was separated from the surrounding connective tissue and nerves by blunt dissection. Following isolation, PE-10 catheters connected to PE-50 tubing (Braintree Scientific, Braintree, MA) were inserted into the left and right femoral arteries. In a subset of animals, a venous catheter was also inserted for subsequent fluid administration. Catheters were filled with heparinized saline (50-100 IU/ml), plugged with 23-gauge obturators and tunneled subcutaneously to exit through a small incision at the neck between the scapulae. Catheters were then secured with sutures to the surrounding skin and all incisions were closed. After surgery, incisions were covered with antibacterial ointment (Alpharma USPD Inc., Baltimore, MD) to prevent infection, and animals were allowed to recover on a heating pad. Rimadyl (Pfizer Animal Health, Exton, MD; 0.01 ml/Kg) and buprenorphine (Reckitt Benckiser Pharmaceuticals, Inc., Richmond, VA; 0.01 ml/kg) were administered subcutaneously prior to returning animals to their home cages. Rats were given food and water ad libitum and allowed 48 hours to recover prior to experimentation.
2.2 Experimental Protocol
The day following surgery, the animals returned to the lab, were weighed, gently handled, acclimated to the experimental container for 2-3 hours (9×9 inch bucket), and then returned to their home cages. On the day of the experiment, rats were weighed again and placed into the experimental container in a quiet room. The arterial catheters were connected to additional PE-50 tubing and passed through a swivel and tethering system (Instech Laboratories Inc.; Plymouth, MA) to allow free movement within the container. If a venous line was present it was also connected to additional PE tubing and attached to the outside of the swivel. One of the arterial lines was then flushed with heparinized saline (2 IU/ml) and connected to a pressure transducer (Stoelting Inc., Wood Dale, IL) in-series with a computer sampling system (Cambridge Electronics Design, Cambridge, England; CED). Pulsatile AP and mean arterial pressure (MAP) were recorded continuously at 100 Hz.
All rats were allowed a minimum of 60 min. of habituation prior to experimentation. Next, all animals underwent a fixed volume hemorrhage (30% of estimated total blood volume (ETBV)). ETBV was calculated using a previously reported equation for estimation of rat blood volume: (0.06 ml/g)*(body weight in g)+(0.77) (26, 37). The subset of animals instrumented with venous catheters also received an intravenous injection of atropine methyl bromide (0.6 mg in 200 μl) 7 min. prior to the onset of hemorrhage (30% ETBV). The animals were randomly assigned to one of six experimental groups: slow hemorrhage (S-HEM; 20 ml/kg/40 min.; n=6), atropine + S-HEM (n=5); intermediate hemorrhage (I-HEM; 20 ml/kg/20 min.; n=6), atropine + I-HEM (n=5); fast hemorrhage (F-HEM; 20 ml/kg/10 min.; n=6), and atropine + F-HEM (n=5). Blood volume was drawn from the second arterial catheter at the designated rate. Following the completion of treatment, data were continuously recorded for another 30-45 min. All rats were then euthanized with an injection of sodium pentobarbital (100-150 mg/kg, Ovation Pharmaceuticals, Inc., Deerfield, IL).
2.3 Data Analysis
HR was determined offline by detection of the interval between systolic peak pulses in the AP signal (Spike 2, CED). For HRV analysis, four time periods (4.5-5 minute duration each) were chosen for analysis (see Fig. 1): baseline (within 10 min. prior to the onset of hemorrhage), peak (the 4.5-5 min. interval just preceding the drop in AP and HR associated with hemorrhagic hypotension), nadir (last 5 min. just preceding the offset of hemorrhage), and recovery (30 min. after the offset of hemorrhage). Within each chosen segment, HR was then converted into a tachogram, a record of time between heart beats or RRI. The filtered tachogram was then analyzed in the frequency domain using HRV software (Biosignal Analysis Group; University of Kuopio, Finland (45)). In the software used, the tachogram was interpolated at 10 Hz and detrended via the smoothness priors formulation (alpha=1000; (45, 55)). The autoregressive model was set to the 40th order. The Welch's Periodogram window width was designated to 512 points with an overlap of 256 points in the Hanning window. In the rat, the frequency components of HRV are designated by the following frequency ranges: 0.16-0.6 Hz (LF), and 0.6-3.0 Hz (HF) (32). Frequency domain characteristics analyzed included the power or area under the curve for the LF and HF components and the ratio of LF/HF power.
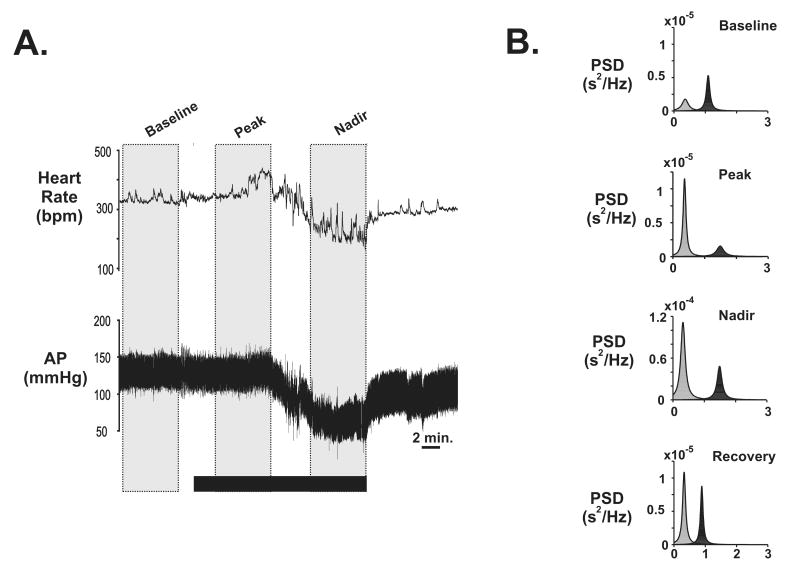
Fig. 1. Example of arterial pressure (AP), heart rate (HR) and power spectral analysis of R-R intervals before, during and after a moderate rate of hemorrhage (1 ml/kg/min) for an individual animal.
A. Black horizontal bar indicates the duration of hemorrhage (30% loss of estimated total blood volume). The vertical gray boxes indicate three time points used for heart rate variability analysis (baseline, transition, and nadir). B. Power spectral density (PSD) levels measured in the low frequency (LF; lightly shades peaks) and high frequency (HF; darkly shaded peaks) ranges from the different time points before and during hemorrhage for the animal illustrated in A. Recovery time point reflects data from 30 min. following the offset of hemorrhage.
For evaluation of the effect of atropine, 1 min. averages of MAP and HR were calculated at 2 min. prior to the onset of hemorrhage (baseline) and every 5% increment of ETBV loss during hemorrhage. The absolute change from the initial baseline was then determined. Additionally, HRV analysis was performed on 5 min. HR averages taken 10 min. prior to atropine administration and 2 min. following atropine injection (prior to any blood loss), and the 5 min. just prior to the offset of blood withdrawal.
2.4 Statistical Analysis
Within treatment groups, data were averaged and reported as the mean ± SEM. To evaluate the effect of blood loss rate on HRV, data were analyzed by a two-way analysis of variance (ANOVA) with repeated measures comparing treatment groups against the designated time points or percentage of blood withdrawal. Atropine data was analyzed using either a three-way ANOVA with repeated measures (%ETBV withdrawal data) or a two-way analysis of variance (atropine vs. no-atropine HRV data). When indicated, the test was followed by either a one-way ANOVA with a Scheffe Post Hoc. Significance determined as p<0.05.
3. Results
3.1 Cardiovascular responses to different rates of hemorrhage
Figure 1A illustrates the typical cardiovascular response to an intermediate rate (I-HEM) of hemorrhage. After the onset of hemorrhage, AP remained steady and was paralleled by an increase in HR. The rise in HR peaked approximately half way through the hemorrhage protocol or at 15% ETBV loss. As blood loss continued beyond 15% ETBV, there was a sudden decrease in AP and HR, which reached a plateau or nadir just prior to the offset of hemorrhage. Following the termination of hemorrhage, both AP and HR slowly returned to pre-hemorrhage levels.
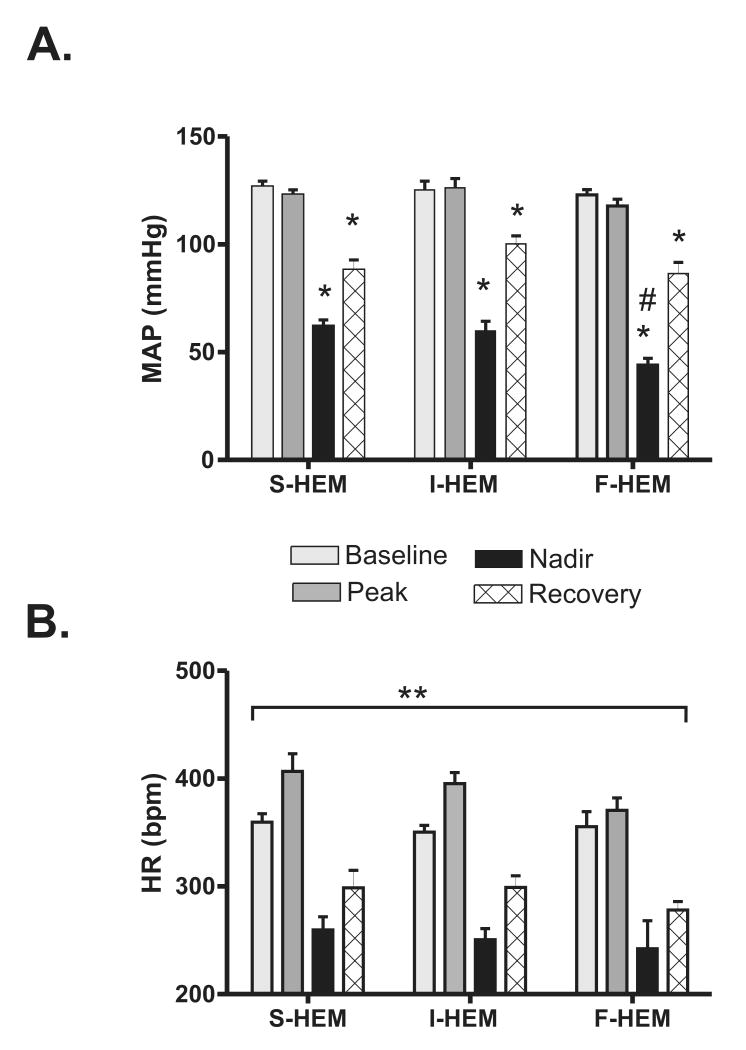
The averaged cardiovascular response from each of the four measurement periods selected for HRV analysis is shown in Figure 2. Prior to the onset of hemorrhage there was no significant difference in baseline MAP or HR between groups (p=0.81). Repeated measures analysis of variance (Fig. 2A) identified a significant interaction between the effect of rate of blood loss and time on MAP (p<0.03). Further analysis within groups identified that MAP at both the nadir time point and during the recovery period was significantly different from the baseline MAP (p<0.001) in all groups. Additionally, analysis between groups identified that the MAP of the F-HEM group at the nadir time point was significantly different from the other two groups at the same time point. Although the drop in MAP was greater in the F-HEM group, the transition point from compensation to hemorrhagic sympatho-inhibition occurred around 15% ETBV loss (Table 1) for all rates of blood loss. Analysis of HR as a function of blood loss rate and time (Fig. 2B) did not identify any significant effect of rate (p=0.26). There was, however, a significant effect of time (p<0.0001). With all groups combined, the average HR at the peak, nadir, and recovery time points were all significantly different from baseline.
Fig. 2. Effect of rate of hemorrhage on cardiovascular response to 30% estimated total blood volume.
(A) Mean arterial pressure (MAP) and B) heart rate (HR) at different time points during slow (S-HEM; 0.5 ml/kg/min; n=6), intermediate (I-HEM, 1 ml/kg/min; n=6), and fast (F-HEM, 2 ml/kg/min, n=6) hemorrhage (30% estimated total blood volume loss (ETBV)). *P<0.05 indicates a significant change from baseline within treatment group. #P<0.05 indicates a significant difference from F-HEM at the nadir time point between groups. **P<0.05 indicates a significant effect of time (all rates of HEM were combined).
Table 1. Effect of rate of hemorrhage on HSI onset parameters.
| Parameter | S-HEM (n=6) |
I-HEM (n=6) |
F-HEM (n=6) |
|---|---|---|---|
| Time to HSI onset (min) | 19.3±1.5 * | 11.5±1.1 * | 4.7±0.5 |
| Volume loss at HSI onset (percent ETBV) | 14.5±1.1 % | 17.2±1.2 % | 14.1±1.6 % |
indicates significantly different from F-HEM value.
3.2 HRV analysis of hemorrhage response
The typical changes in the LF and HF components observed following frequency analysis at each time point during I-HEM are shown in Figure 1B. Prior to hemorrhage onset (baseline), the highest peak was observed in the HF range, however the peak power shifted to the LF range during the compensatory phase (peak time point). At the nadir time point there was a large increase (∼10 fold) in peak amplitude of both the LF and HF components, but the LF peak remained above the HF peak. Finally, the peak amplitudes of both the HF and LF components were reduced during the recovery time point relative to the nadir of hemorrhage, but still remained above baseline 30 min. following the offset of hemorrhage.
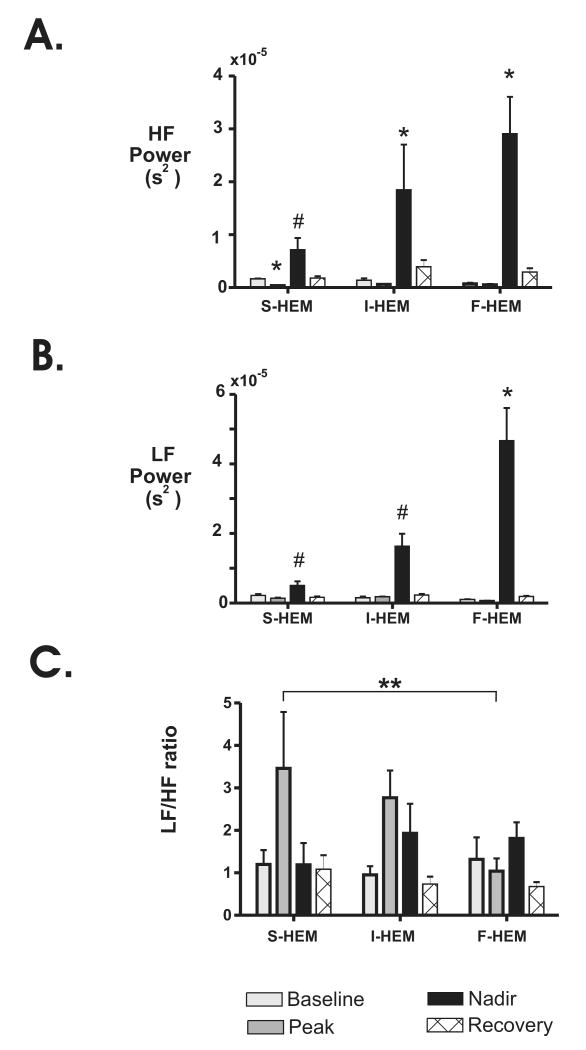
For comparison between groups, the average power of the LF and HF frequency components are plotted in Fig. 3. For all three rates of HEM, the increase in power for both the HF and LF components was greatest during the nadir time point relative to baseline. Analysis of the HF component identified a significant interaction between time point and rate of blood loss (p<0.0001; Fig. 3A). Comparisons within groups identified that the HF power at the nadir time point was significantly different from baseline for both I-HEM and F-HEM (p<0.004). In contrast, the HF power was only significantly different from baseline at the peak time point for the S-HEM group (p<0.003). Comparisons between groups further identified that the increase HF power observed in the F-HEM group at the nadir time point was significantly greater (p<0.02) than that of the nadir for the S-HEM group.
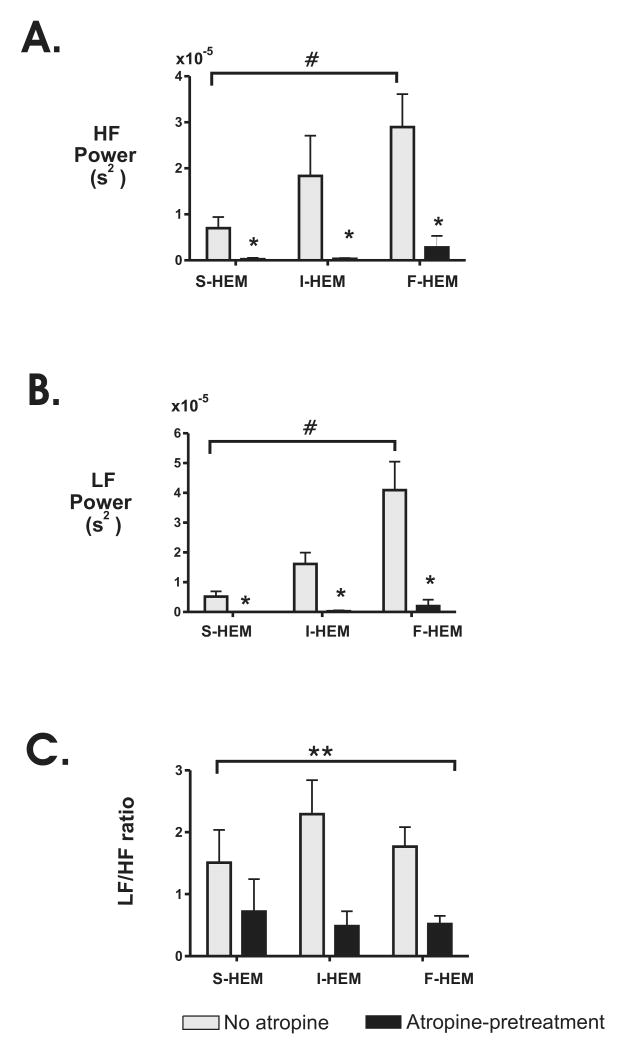
Fig. 3. Effect of rate of blood loss on heart rate variability parameters at different time points during severe hemorrhage.
Changes in (A) high frequency (HF) and (B) low frequency (LF) power spectrum density (PSD) before, during and following S-HEM (n=6), I-HEM (n=6) and F-HEM (n=6) are shown. C. Changes in the LF/HF ratio within individual time points. *P<0.05 indicates a significant change from baseline within treatment group. #P<0.05 indicates a significant difference from F-HEM at the nadir time point between treatment groups. **P<0.05 indicates a significant effect of time (all rates of HEM were combined).
Analysis of the LF component (Fig. 3B) also identified a significant interaction between time and rate of hemorrhage (p<0.0001). Comparisons within groups demonstrated that the LF power at the nadir time point was only significantly different from baseline in the F-HEM group (p<0.009). Furthermore, the increase in LF power observed at the nadir time point in the F-HEM group was also significantly different from the LF power in both the I-HEM and S-HEM groups at the nadir time point (p<0.009). No other significant differences were identified.
Finally, analysis of the LF/HF ratio (Fig. 3C) only identified a significant effect of time (p<0.003). When the LF/HF ratio of all groups were combined (S-HEM, I-HEM, F-HEM), the mean LF/HF ratio at the peak time point (2.4± 0.53 au) was significantly different from both baseline (1.1±0.2 au; p<0.03) and recovery time points (0.86± 0.12; p<0.01).
3.3 Effect of parasympathetic blockade on MAP and HR response to HEM
Because the greatest effect of hemorrhage was associated with the HF component, presumably reflecting changes in parasympathetic drive, the response to severe HEM was evaluated following peripheral administration of atropine methyl bromide in a separate group of animals (n=15). Prior to the onset of hemorrhage, parasympathetic blockade induced a significant increase in baseline HR (365±5 pre-atropine vs 411±5 bpm post-atropine; p<0.001) but had no effect on MAP (126±3 pre-atropine vs. 121±7 mmHg post-atropine).
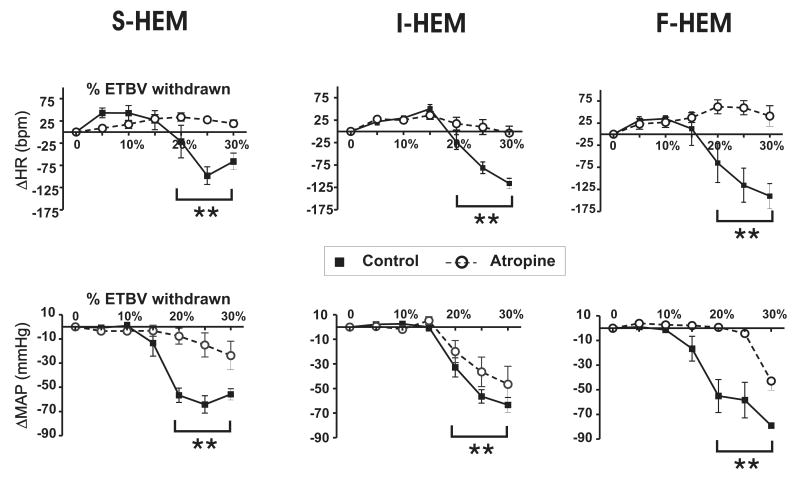
Figure 4 illustrates the effect of atropine administration on the cardiovascular response to severe hemorrhage induced at different rates. Atropine administration eliminated all decreases in HR in response to severe hemorrhage independent of rate of hemorrhage. Using a three way analysis of variance with repeated measures, the effect of atropine treatment, the rate of hemorrhage and %ETBV withdrawn was evaluated. For both MAP and HR there was no significant interaction between all three factors. Independent of the rate of hemorrhage, however, there was a significant interaction between atropine treatment and %ETBV withdrawn (p<0.001) on HR. Further analysis with all rates of blood loss combined identified the drop in HR following parasympathetic blockade was significantly different from HR in the absence of parasympathetic blockade (p<0.001) at blood loss volumes ≥20%. Analysis of MAP also identified a significant interaction between atropine treatment and %ETBV withdrawn (p<0.001) when all rates of blood loss combined. Following parasympathetic blockade the drop in MAP at blood loss volumes ≥20% were significantly from animals without parasympathetic blockade (p<0.001).
Fig. 4. Effect of parasympathetic blockade on the cardiovascular response to different rates of hemorrhage.
Average change from pre-hemorrhage baseline for HR (top panels) and mean arterial pressure (MAP; bottom panels) during severe hemorrhage in untreated (n=6 per group) versus atropine-treated animals (n=5). Data represent one min. averages taken during increments of 5% of estimated total blood volume (ETBV) loss within treatment groups (S-HEM, I-HEM, and F-HEM) until at total of 30% ETBV loss. ** P<0.05 indicates significant difference between atropine and no-atropine animals at %ETBV loss points greater than or equal to 20% independent of rate of HEM groups (p<0.05).
To further evaluate the effect of atropine administration on the hemorrhage response, HRV parameters were compared both before and after atropine administration. As shown by Table 2, atropine significantly reduced LF and HF power and the LF/HF ratio. Using a two-way analysis of variance (rate of hemorrhage × atropine administration) the effect of atropine treatment on HRV variables at the nadir time point was also evaluated. As shown in Figure 5, atropine administration significantly reduced both LF and HF power and LF/HF ratio in all three treatment groups at the nadir time point compared to non-atropine treated animals. For both HF and LF power there was a significant interaction between rate of hemorrhage and atropine administration identified (p<0.0003). Further analysis identified a significant effect of atropine treatment for each rate of hemorrhage. Within the atropine treated animals, however, there was no significant effect of rate of hemorrhage on either LF or HF power (P>0.18). Analysis of the effect of rate of HEM and atropine administration on the LF/HF ratio did not identify a significant interaction. There was, however, a significant effect of atropine administration when all HEM groups were combined (P<0.002).
Table 2. Effect of atropine on baseline HRV parameters.
| Pre-atropine (n=15) |
Post-Atropine (n=15) |
|
|---|---|---|
| LF power (s2 × 10-7) | 7.6±1.4 | 0.76±.15* |
| HF power (s2 × 10-7) | 9.9±2.2 | 2.8±0.8* |
| LF/HF ratio | 1.08±.2 | 0.4±.0.08* |
indicates significantly different (p<0.01) from no atropine group for same rate of hemorrhage.
Fig. 5. Average effect of atropine pre-treatment on HRV parameters at the nadir time point of hemorrhage.
Changes in (A) high frequency (HF) and (B) low frequency (LF) power spectrum density (PSD) in untreated S-HEM (n=6), I-HEM (n=6) and F-HEM (n=6) animals (lightly shaded bars) versus atropine treated animals (n=5/group) following 30% ETBV withdrawal. C. Comparison of the LF/HF ratio between atropine-treated and untreated individuals. *P<0.05 indicates a significant difference from no-atropine value within treatment group. ** P<0.05 indicates a significant effect of atropine treatment with all rates of HEM combined. # P<0.05 indicates a significant effect of rate of HEM on HF or LF power in non-atropine treated animals.
4.1 Discussion
To our knowledge, the present study is the first to evaluate the impact of different rates of constant volume blood withdrawal (30% ETBV) on autonomic control of HR using HRV analysis in the conscious animal. There were two main findings in this study. First, during the initial compensatory phase, HRV analysis indicated that only the slowest rate of hemorrhage (0.5 ml/kg/min) elicited a significant reduction in parasympathetic drive as indicated by changes in HF power. The second major finding of this study was that as blood loss approached 30% of the ETBV, the sympatho-inhibitory phase of hemorrhage was characterized by indicators of elevated parasympathetic drive to the heart, but the magnitude of change in autonomic balance varied depending on the rate of hemorrhage. Together these observations support previous results from both our lab and Troy and colleagues (1, 57) demonstrating that brain mechanisms underlying autonomic control during severe hemorrhage are not constant, but appear to vary depending upon the rate of blood loss.
4.2 Methodological Considerations
The first two rates of hemorrhage chosen for evaluation in the present study (0.5 and 1.0 ml/kg/min) were based on rates commonly used by other investigators studying the neural origins of hemorrhagic sympatho-inhibition and those rates used in recent studies by both Troy and colleagues and ourselves (1, 8, 16, 57). Additionally, we chose to evaluate a faster rate of blood withdrawal (2 ml/kg/min) since many hemorrhage studies begin with a higher rate of blood loss (>1.5 ml/kg/min) and then progress to a slower rate of hemorrhage, to sustain a fixed pressure or to meet a fixed volume of withdrawal (5, 52). Associated with this faster rate of hemorrhage in the present study is the acknowledgement that 4.5-5 min. time windows utilized for HRV analysis represented different percentages of the total time of hemorrhage. For example, analysis of the compensatory phase of hemorrhage for F-HEM group occurred during the first 5 min. of hemorrhage and encompassed a time period during which 15% ETBV was lost. Alternatively, for the I-HEM and S-HEM groups, the period chosen for analysis during the compensatory phase occurred in the second and fourth 5 min. period of the hemorrhage protocol, respectively, and represented time periods when 7.5 to 15% or 11.25 to 15% of ETBV was lost, correspondingly. Thus, for the F-HEM group, the periods chosen for analysis of the two phases of hemorrhage represented a time of great autonomic fluctuation which may not have been optimal for comparison of HRV parameters. Nonetheless, autonomic blockade appeared to confirm the HRV findings, suggesting our comparisons were appropriate.
Another consideration is the fact that the present study was done in conscious animals following a two-day recovery period from surgery. In our study, the sympatho-inhibitory phase of hemorrhage was characterized by a marked bradycardia and hypotension. This characteristic slowing of heart rate following 15% ETBV withdrawal has been reported by other investigators in conscious rats and rabbits, but is markedly different from the response reported by Troy and colleagues (8, 41, 51, 57). In their study, HR continued to increase, while MAP declined following >15% ETBV withdrawal. Their observation of a sustained tachycardia during hemorrhagic hypotension is likely to reflect the lingering impact of halothane (which was withdrawn only ∼ 70 min. prior to the onset of the experiment) on vagal drive (29). Nonetheless, the results from their study demonstrated that the heart rate response to a faster rate of hemorrhage was more dependent upon the recruitment of pre-collicular brain regions compared to a slower rate of hemorrhage. This compliments our results which suggest that the pattern of autonomic drive recruited also depends upon the rate of hemorrhage.
Finally, the results of the present study need to be interpreted with the consideration that in addition to changes in parasympathetic drive, changes in respiration can have a profound impact on HRV parameters. In fact, HF power has been shown to be significantly influenced by the mechanical effects of breathing (15), with power in the HF range increasing as tidal volume increases, independent of changes in parasympathetic drive (28). Thus, it is possible that the differences in HF power we observed during the nadir time point of hemorrhage reflected in part changes in respiratory control induced by the different rates of hemorrhage rather than just rate-dependent increases in parasympathetic drive. Unfortunately, in the present study we did not monitor respiration and to our knowledge the impact of severe hemorrhage on respiratory control in the conscious rat has not been previously evaluated. In the conscious male rabbit, however, there is evidence that hemorrhagic hypotension is coupled to an increase in respiratory frequency and not tidal volume changes (54). But whether this pattern of respiratory control is the same in rodents or during different rates of hemorrhage is unknown. Thus, future studies are needed to evaluate the role of respiration in HRV changes associated with severe hemorrhage in the rodent. Moreover, to truly isolate the contribution of parasympathetic drive to changes in HRV, future studies should be also be evaluated in the presence of a beta blocker to eliminate sympathetic contributions (33, 36, 43).
4.3 Effect of rate of hemorrhage on the compensatory phase
The first main finding of this study was that the pattern of autonomic balance elicited during the compensatory phase was markedly different in the S-HEM group compared to I-HEM and F-HEM. During the peak time point, S-HEM was characterized by an increase in HR and a significant decrease in HF power. Parasympathetic blockade suggested that the rise in HR during the compensatory phase in the S-HEM group may have been mediated in part by parasympathetic withdrawal since there was no noticeable change in HR until 10% ETBV loss in the presence of atropine compared to a marked rise in HR in the unblocked animal. This pattern of vagal withdrawal during the compensatory phase of S-HEM may indicate that baroreflex modulation of HR in response to hypovolemia was the primary mechanism underlying the response to S-HEM. Conversely, neither I-HEM nor F-HEM induced a significant change in the HF power during the compensatory phase. Furthermore, the rise in HR following atropine administration was similar to that induced in the absence of atropine for both rates of hemorrhage. This suggests the compensatory response in response to I-HEM and F-HEM may have been primarily mediated by sympathetic recruitment. This putative change in sympathetic drive, however, may not have been sufficient to be detectable via HRV analysis (ie. changes in LF/HF ratio). Alternatively, several autonomic blockade studies have questioned whether the use of the LF component is a valid measure of sympathetic drive since this component is so significantly reduced by parasympathetic blockade (18, 33, 36, 43). Thus, to truly elucidate how changes in HRV parameters during the compensatory phase of hemorrhage reflect changes in sympathetic/parasympathetic balance, additional studies in the presence of beta-adrenergic blockers are needed (24, 33, 36).
4.4 Effect of rate of hemorrhage on the sympatho-inhibitory phase
The second major finding of the present study was that the rate of hemorrhage influenced autonomic balance during the sympatho-inhibitory phase of hemorrhage. First, the decline in MAP measured during the nadir time point was greatest in the F-HEM group. This was coupled with a significant increase in both LF and HF power in the HR signal. Atropine administration corroborated that parasympathetic drive to the heart was significantly elevated during the sympathoinhibitory phase above baseline in all groups when blood loss was greater than or equal to 20% ETBV. Furthermore, although no significant effect of rate of hemorrhage was identified, the elevation in HR following atropine treatment was greatest in the F-HEM group, suggesting that this rate of hemorrhage may have been associated with some level of simultaneous beta-adrenergic stimulation. This observation is in agreement with the results of a study by Gonzalez-Gonzalez et al., which demonstrated that beta-blockade during a rapid rate of severe hemorrhage (∼2.2 ml/kg/min) induced a greater drop in HR compared to saline treated animals at the time of 30% ETBV withdrawal (25). Unfortunately, in that study, additional rates of hemorrhage were not examined, thus it remains to be determined if indeed there is a significant effect of rate of hemorrhage on cardiac sympatho-excitation during blood loss of volumes >20% ETBV. There is some evidence that increases in sympathetic drive to the heart during severe hemorrhage may be mediated by increases in circulating catecholamines (60). Thus, the results of the present study suggest that reflex regulation of adrenal activation during hemorrhage may be dependent upon the rate of blood loss. However, to further elucidate the role of sympathetic stimulation in mediating changes in HRV during the decompensatory phase of hemorrhage and to clearly isolate the effects of parasympathetic drive on the HF component, additional studies are need to be performed in the presence of a beta-adrenergic receptor blocker (33, 43).
Independent of the rate of blood pressure withdrawal, atropine administration also attenuated the drop in MAP associated with %ETBV losses greater than or equal to 20%. Two previous studies have evaluated the effect of atropine administration on MAP in the conscious rat. Interestingly, the study that utilized hemorrhage rates closer to 1 mg/kg/min reported no effect of atropine administration on MAP (4). Alternatively the other study utilizing a faster rate of blood loss (∼2.2.ml/kg/min) demonstrated a significant elevation of MAP following parasympathetic blockade and severe hemorrhage (25). A comparison of our results from the I-HEM and F-HEM groups support these observations; the average drop in MAP following atropine administration in the I-HEM group was not remarkably different from that observed in the absence of atropine versus during F-HEM the drop in MAP was markedly attenuated by atropine administration. Previous results from our laboratory and others suggest that different rates of hemorrhage may differentially activate select regions of the brain, which in turn, may impact the pattern of autonomic recruitment (11) or respiratory pattern (54). A respiratory pattern of elevated tidal volume versus one of elevated respiratory rate might be expected to have profoundly different effects on the role of HR in the maintenance of MAP (15). Future studies with a larger sample size are needed to be to further elucidate whether statistical differences across different rates of hemorrhage can identify the impact of parasympathetic drive on MAP during the later stages of hemorrhage. Moreover, as mentioned above, simultaneous evaluation of changes in respiratory pattern is necessary for the interpretation of mechanical versus autonomic contributions to HRV.
Only a few other groups have utilized spectral analysis to evaluate changes in autonomic control during hemorrhage (3, 25, 39, 41). In one study, spectral analysis of MAP and renal sympathetic nerve activity (RSNA) was performed over consecutive 5-min. segments during a 20-min. hemorrhage (rate 1.35 ml/kg/min) in conscious rabbits. Similar to our results, a decline in MAP and HR was noted following the withdrawal of approximately 15% of ETBV. During the nadir (15-20 min) time point, when MAP was lowest, spectral analysis of both MAP and RSNA reported a significant elevation of all frequency components (LF and HF) relative to control. This agrees with our observation that both LF and HF components of HRV were elevated during the nadir time point in both the I-HEM and F-HEM groups. Conversely, in a recent study by Batchinsky and colleagues, it was noted that 40% ETBV loss in isoflurane anesthetized sheep induced a significant decrease in HF power and sustained increase in LF/HF ratio (3). These changes in HRV parameters were observed in parallel to sudden withdrawal of renal sympathetic nerve activity and it was concluded that changes in sympathetic drive do not correlate well with changes in HRV during hemorrhage. It should be noted that their study was done under anesthesia and the presence of even small amounts of anesthesia can strongly attenuate parasympathetic outflow (58). The exact origin of the effect of anesthesia on parasympathetic integration has not been defined, but it is likely that a component of this change takes place in the brainstem, possibly at the level of the vagal preganglionic neurons (61). The results of our study however suggest that in the absence of anesthesia, the magnitude of the power in the HF component may be predictive of the rate or severity of blood loss.
Critical to the induction of the sympatho-inhibitory phase of hemorrhage is activation of ventrolateral periaqueductal gray (VLPAG) neurons in the midbrain (8, 57). Previously we identified that the number of c-Fos positive neurons in the VLPAG increases as the rate of hemorrhage rises (1). In the context of the present study, this raises the possibility that the level of VLPAG activation directly determines the magnitude of hemorrhagic hypotension and sympatho-vagal drive to the heart. In a recent study by Vagg and colleagues, the output from the VLPAG to the medulla was carefully characterized and was shown to involve a projection to the caudal midline medullary nuclei, a region known to induce sympatho-inhibition (27, 59). However, since the bradycardic response to severe hemorrhage can be blocked by vasopressin antagonists with only modest effects on the hypotensive response to hemorrhage (30, 31), convergent input from other regions, such as the area postrema, the nucleus of the solitary tract and/or the parabrachial nucleus, must also play an important role in cardiac sympatho-vagal balance changes during severe hemorrhage. It is well documented that the parabrachial nucleus plays an important role in autonomic control of cardio-neurogenic responses and when activated by certain inputs can selectively increase parasympathetic drive (44, 50). In a previous study, we identified that several rostral brainstem regions, including the locus coeruleus and the rostral parabrachial nucleus showed selective increases in c-Fos levels during both F-HEM and I-HEM, but not S-HEM compared to control conditions (1). The results of the present study suggest that these regions may play an important role in recruiting autonomic circuits needed to modulate sympathetic and parasympathetic drive outside of normal baroreflex circuitry, as the rate of blood loss rises above 0.5 ml/kg/min. Interestingly, the VLPAG has been identified to send a dense descending projection to the parabrachial nucleus (35), but the function of this projection has not been defined.
Summary
The results of the present study evaluated the impact of three rates of blood loss on autonomic control of HR during severe hemorrhage. Our findings demonstrated that, even though the sympatho-inhibitory phase of hemorrhage occurred consistently following the loss of ∼15% ETBV, the rate of blood loss markedly affected the pattern of autonomic activation, both before and after this point, as indicated by changes in HRV. The F-HEM group showed the greatest change HF power during hemorrhage. The S-HEM group demonstrated similar changes in overall AP and HR, yet HRV analysis demonstrated that the level of autonomic activation, as indicated by HF power, was significantly less at the nadir compared to faster rates of blood loss. Although some of the differences in HRV could be explained by changes in respiratory pattern (increases in tidal volume vs. increases in respiratory frequency) which was not monitored in the present study, these observations support recent results suggesting that the central pathways activated in response to hemorrhage are dependent upon the rate of blood loss (1, 57). Additionally, our results suggest that comparisons of forebrain regions activated by F-HEM vs. S-HEM may be useful in identifying regions critical in initiating the sympatho-inhibitory phase of hemorrhage and the onset of elevated parasympathetic drive. Our results also provide new information to a growing body of literature evaluating changes in HRV following trauma as a potential predictive index of survival (13, 14). In that regard, the results of the present study suggest that excessive elevations in HF power, in the absence of any significant change in LF/HF ratio, may be indicative of a fast rate of blood loss. With further study, HRV may be a useful guide in determining an appropriate therapeutic method or survival potential for trauma patients.
Acknowledgments
Grants: This work was supported by AHA-Florida Puerto Rico (0555226B), NIH (HL-76518), and AHA-Florida Puerto Rico (0715455B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahlgren J, Porter K, Hayward LF. Hemodynamic responses and c-Fos changes associated with hypotensive hemorrhage: standardizing a protocol for severe hemorrhage in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1862–1871. doi: 10.1152/ajpregu.00325.2006. [DOI] [PubMed] [Google Scholar]
- 2.Akselrod S, Gordon D, Madwed JB, Snidman NC, Shannon DC, Cohen RJ. Hemodynamic regulation: investigation by spectral analysis. Am J Physiol. 1985;249:H867–875. doi: 10.1152/ajpheart.1985.249.4.H867. [DOI] [PubMed] [Google Scholar]
- 3.Batchinsky AI, Cooke WH, Kuusela TA, Jordan BS, Wang JJ, Cancio LC. Sympathetic nerve activity and heart rate variability during severe hemorrhagic shock in sheep. Auton Neurosci. 2007;136:43–51. doi: 10.1016/j.autneu.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Blair ML, Mickelsen D. Activation of lateral parabrachial nucleus neurons restores blood pressure and sympathetic vasomotor drive after hypotensive hemorrhage. Am J Physiol Regul Integr Comp Physiol. 2006;291:R742–750. doi: 10.1152/ajpregu.00049.2006. [DOI] [PubMed] [Google Scholar]
- 5.Blair ML, Want A, Olschowka JA, Piekut D. Role of paraventricular nucleus parvicellular neurons in the compensatory responses to graded hemorrhage. Am J Physiol. 1998;275:R278–285. doi: 10.1152/ajpregu.1998.275.1.R278. [DOI] [PubMed] [Google Scholar]
- 6.Burgess HJ, Penev PD, Schneider R, Van Cauter E. Estimating cardiac autonomic activity during sleep: impedance cardiography, spectral analysis, and Poincare plots. Clin Neurophysiol. 2004;115:19–28. doi: 10.1016/s1388-2457(03)00312-2. [DOI] [PubMed] [Google Scholar]
- 7.Burke S, Dorward PK. Influence of endogenous opioids and cardiac afferents on renal nerve activity during hemorrhage in conscious rabbits. J Physiol. 1988;402:9–27. doi: 10.1113/jphysiol.1988.sp017191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavun S, Millington WR. Evidence that hemorrhagic hypotension is mediated by the ventrolateral periaqueductal gray region. Am J Physiol Regul Integr Comp Physiol. 2001;281:R747–752. doi: 10.1152/ajpregu.2001.281.3.R747. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti C, Gustin MP, Paultre CZ, Lo M, Julien C, Vincent M, Sassard J. Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am J Physiol. 1991;261:H1292–1299. doi: 10.1152/ajpheart.1991.261.4.H1292. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers JP, Korner PI, White SW. The effects of haemorrhage in the unanaesthetized rabbit. J Physiol. 1967;189:367–391. doi: 10.1113/jphysiol.1967.sp008174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann N Y Acad Sci. 2001;940:1–19. doi: 10.1111/j.1749-6632.2001.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiu HW, Wang TH, Huang LC, Tso HW, Kao T. The influence of mean heart rate on measures of heart rate variability as markers of autonomic function: a model study. Med Eng Phys. 2003;25:475–481. doi: 10.1016/s1350-4533(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 13.Cooke WH, Salinas J, Convertino VA, Ludwig DA, Hinds D, Duke JH, Moore FA, Holcomb JB. Heart rate variability and its association with mortality in prehospital trauma patients. J Trauma. 2006;60:363–370. doi: 10.1097/01.ta.0000196623.48952.0e. discussion 370. [DOI] [PubMed] [Google Scholar]
- 14.Cooke WH, Salinas J, McManus JG, Ryan KL, Rickards CA, Holcomb JB, Convertino VA. Heart period variability in trauma patients may predict mortality and allow remote triage. Aviat Space Environ Med. 2006;77:1107–1112. [PubMed] [Google Scholar]
- 15.Cottin F, Medigue C, Lepretre PM, Papelier Y, Koralsztein JP, Billat V. Heart rate variability during exercise performed below and above ventilatory threshold. Med Sci Sports Exerc. 2004;36:594–600. doi: 10.1249/01.mss.0000121982.14718.2a. [DOI] [PubMed] [Google Scholar]
- 16.Dean C. Hemorrhagic sympathoinhibition mediated through the periaqueductal gray in the rat. Neurosci Lett. 2004;354:79–83. doi: 10.1016/j.neulet.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 17.Dean C, Bago M. Renal sympathoinhibition mediated by 5-HT(1A) receptors in the RVLM during severe hemorrhage in rats. Am J Physiol Regul Integr Comp Physiol. 2002;282:R122–130. doi: 10.1152/ajpregu.2002.282.1.R122. [DOI] [PubMed] [Google Scholar]
- 18.Elghozi JL, Julien C. Sympathetic control of short-term heart rate variability and its pharmacological modulation. Fundam Clin Pharmacol. 2007;21:337–347. doi: 10.1111/j.1472-8206.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- 19.Evans RG, Haynes JM, Ludbrook J. Effects of 5-HT-receptor and alpha 2-adrenoceptor ligands on the haemodynamic response to acute central hypovolaemia in conscious rabbits. Br J Pharmacol. 1993;109:37–47. doi: 10.1111/j.1476-5381.1993.tb13528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans RG, Ludbrook J. Effects of mu-opioid receptor agonists on circulatory responses to simulated haemorrhage in conscious rabbits. Br J Pharmacol. 1990;100:421–426. doi: 10.1111/j.1476-5381.1990.tb15822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans RG, Ludbrook J, Ventura S. Role of vagal afferents in the haemodynamic response to acute central hypovolaemia in unanaesthetized rabbits. J Auton Nerv Syst. 1994;46:251–260. doi: 10.1016/0165-1838(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 22.Evans RG, Ludbrook J, Woods RL, Casley D. Influence of higher brain centres and vasopressin on the haemodynamic response to acute central hypovolaemia in rabbits. J Auton Nerv Syst. 1991;35:1–14. doi: 10.1016/0165-1838(91)90033-y. [DOI] [PubMed] [Google Scholar]
- 23.Evans RG, Ventura S, Dampney RA, Ludbrook J. Neural mechanisms in the cardiovascular responses to acute central hypovolaemia. Clin Exp Pharmacol Physiol. 2001;28:479–487. doi: 10.1046/j.1440-1681.2001.03473.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldberger JJ, Challapalli S, Tung R, Parker MA, Kadish AH. Relationship of heart rate variability to parasympathetic effect. Circulation. 2001;103:1977–1983. doi: 10.1161/01.cir.103.15.1977. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez Gonzalez J, Cordero Valeriano JJ, Feria Rodriguez M. Autonomic mediation of short-term cardiovascular oscillations after acute hemorrhage in conscious rats. J Auton Nerv Syst. 1995;55:123–130. doi: 10.1016/0165-1838(95)00038-y. [DOI] [PubMed] [Google Scholar]
- 26.Hauptman J, DeJong GK, Blasko KA, Chaudry IH. Measurement of hepatocellular function, cardiac output, effective blood volume, and oxygen saturation in rats. Am J Physiol. 1989;257:R439–R444. doi: 10.1152/ajpregu.1989.257.2.R439. [DOI] [PubMed] [Google Scholar]
- 27.Heslop DJ, Keay KA, Bandler R. Haemorrhage-evoked compensation and decompensation are mediated by distinct caudal midline medullary regions in the urethane-anaesthetised rat. Neuroscience. 2002;113:555–567. doi: 10.1016/s0306-4522(02)00161-6. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch JA, Bishop B. Volume, flow, and timing of each breath during negative airway pressure in humans. J Appl Physiol. 1981;50:552–560. doi: 10.1152/jappl.1981.50.3.552. [DOI] [PubMed] [Google Scholar]
- 29.Holobotovskyy VV, Arnolda LF, McKitrick DJ. Effect of anaesthetic and rat strain on heart rate responses to simulated haemorrhage. Acta Physiol Scand. 2004;180:29–38. doi: 10.1046/j.0001-6772.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- 30.Imai Y, Kim CY, Hashimoto J, Minami N, Munakata M, Abe K. Role of vasopressin in neurocardiogenic responses to hemorrhage in conscious rats. Hypertension. 1996;27:136–143. doi: 10.1161/01.hyp.27.1.136. [DOI] [PubMed] [Google Scholar]
- 31.Japundzic-Zigon N. Effects of nonpeptide V1a and V2 antagonists on blood pressure fast oscillations in conscious rats. Clin Exp Hypertens. 2001;23:277–292. doi: 10.1081/ceh-100102667. [DOI] [PubMed] [Google Scholar]
- 32.Japundzic N, Grichois ML, Zitoun P, Laude D, Elghozi JL. Spectral analysis of blood pressure and heart rate in conscious rats: effects of autonomic blockers. J Auton Nerv Syst. 1990;30:91–100. doi: 10.1016/0165-1838(90)90132-3. [DOI] [PubMed] [Google Scholar]
- 33.Jokkel G, Bonyhay I, Kollai M. Heart rate variability after complete autonomic blockade in man. J Auton Nerv Syst. 1995;51:85–89. doi: 10.1016/0165-1838(95)80010-8. [DOI] [PubMed] [Google Scholar]
- 34.Kapusta DR, Knardahl S, Koepke JP, Johnson AK, DiBona GF. Selective central alpha-2 adrenoceptor control of regional haemodynamic responses to air jet stress in conscious spontaneously hypertensive rats. J Hypertens. 1989;7:189–194. [PubMed] [Google Scholar]
- 35.Krout KE, Jansen AS, Loewy AD. Periaqueductal gray matter projection to the parabrachial nucleus in rat. J Comp Neurol. 1998;401:437–454. [PubMed] [Google Scholar]
- 36.Kuwahara M, Yayou K, Ishii K, Hashimoto S, Tsubone H, Sugano S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J Electrocardiol. 1994;27:333–337. doi: 10.1016/s0022-0736(05)80272-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26:72–76. [PubMed] [Google Scholar]
- 38.Legramante JM, Galante A, Massaro M, Attanasio A, Raimondi G, Pigozzi F, Iellamo F. Hemodynamic and autonomic correlates of postexercise hypotension in patients with mild hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1037–1043. doi: 10.1152/ajpregu.00603.2001. [DOI] [PubMed] [Google Scholar]
- 39.Malpas S, Evans R, Head G, Lukoshkova E. Contribution of renal nerves to renal blood flow variability during hemorrhage. 1988:R1283–R1294. doi: 10.1152/ajpregu.1998.274.5.R1283. [DOI] [PubMed] [Google Scholar]
- 40.Malpas SC. Neural influences on cardiovascular variability: possibilities and pitfalls. Am J Physiol Heart Circ Physiol. 2002;282:H6–20. doi: 10.1152/ajpheart.2002.282.1.H6. [DOI] [PubMed] [Google Scholar]
- 41.Malpas SC, Burgess DE. Renal SNA as the primary mediator of slow oscillations in blood pressure during hemorrhage. Am J Physiol Heart Circ Physiol. 2000;279:H1299–1306. doi: 10.1152/ajpheart.2000.279.3.H1299. [DOI] [PubMed] [Google Scholar]
- 42.McCraty R, Atkinson M, Tomasino D, Stuppy WP. Analysis of twenty-four hour heart rate variability in patients with panic disorder. Biol Psychol. 2001;56:131–150. doi: 10.1016/s0301-0511(01)00074-6. [DOI] [PubMed] [Google Scholar]
- 43.Medigue C, Girard A, Laude D, Monti A, Wargon M, Elghozi JL. Relationship between pulse interval and respiratory sinus arrhythmia: a time- and frequency-domain analysis of the effects of atropine. Pflugers Arch. 2001;441:650–655. doi: 10.1007/s004240000486. [DOI] [PubMed] [Google Scholar]
- 44.Mraovitch S, Kumada M, Reis DJ. Role of the nucleus parabrachialis in cardiovascular regulation in cat. Brain Res. 1982;232:57–75. doi: 10.1016/0006-8993(82)90610-2. [DOI] [PubMed] [Google Scholar]
- 45.Niskanen J, Taravainen M, Ranta-aho P, Karjalainen P. Software for advanced HRV analysis. Computer Methods and Programs in Biomedicine. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Pelaez NM, Schreihofer AM, Guyenet PG. Decompensated hemorrhage activates serotonergic neurons in the subependymal parapyramidal region of the rat medulla. Am J Physiol Regul Integr Comp Physiol. 2002;283:R688–697. doi: 10.1152/ajpregu.00154.2002. [DOI] [PubMed] [Google Scholar]
- 47.Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol. 2003;90:317–325. doi: 10.1007/s00421-003-0953-9. [DOI] [PubMed] [Google Scholar]
- 48.Perlini S, Giangregorio F, Coco M, Radaelli A, Solda PL, Bernardi L, Ferrari AU. Autonomic and ventilatory components of heart rate and blood pressure variability in freely behaving rats. Am J Physiol. 1995;269:H1729–1734. doi: 10.1152/ajpheart.1995.269.5.H1729. [DOI] [PubMed] [Google Scholar]
- 49.Porter K, Ahlgren J, Castellanos M, Hayward L. Heart rate variability and the rate of hemorrhage: a partnership in characterizing autonomic regulation. Society of Neuroscience Conference. 2005 [Google Scholar]
- 50.Saleh TM, Connell BJ. Estrogen-induced autonomic effects are mediated by NMDA and GABAA receptors in the parabrachial nucleus. Brain Res. 2003;973:161–170. doi: 10.1016/s0006-8993(03)02432-6. [DOI] [PubMed] [Google Scholar]
- 51.Schadt JC, Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Am J Physiol. 1991;260:H305–318. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 52.Scrogin KE, Veelken R, Johnson AK. Central methysergide prevents renal sympathoinhibition and bradycardia during hypotensive hemorrhage. Am J Physiol. 1998;274:H43–51. doi: 10.1152/ajpheart.1998.274.1.h43. [DOI] [PubMed] [Google Scholar]
- 53.Stauss HM. Heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2003;285:R927–931. doi: 10.1152/ajpregu.00452.2003. [DOI] [PubMed] [Google Scholar]
- 54.Strittmatter RR, Schadt JC. Sex differences in the respiratory response to hemorrhage in the conscious, New Zealand white rabbit. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1963–1969. doi: 10.1152/ajpregu.00494.2006. [DOI] [PubMed] [Google Scholar]
- 55.Tarvainen MP, Ranta-Aho PO, Karjalainen PA. An advanced detrending method with application to HRV analysis. IEEE Trans Biomed Eng. 2002;49:172–175. doi: 10.1109/10.979357. [DOI] [PubMed] [Google Scholar]
- 56.Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. Am J Physiol Heart Circ Physiol. 2001;280:H2804–2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- 57.Troy BP, Heslop DJ, Bandler R, Keay KA. Haemodynamic response to haemorrhage: distinct contributions of midbrain and forebrain structures. Auton Neurosci. 2003;108:1–11. doi: 10.1016/S1566-0702(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 58.Tzeng YC, Galletly DC, Larsen PD. Paradoxical respiratory sinus arrhythmia in the anesthetized rat. Auton Neurosci. 2005;118:25–31. doi: 10.1016/j.autneu.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 59.Vagg DJ, Bandler R, Keay KA. Hypovolemic shock: critical involvement of a projection from the ventrolateral periaqueductal gray to the caudal midline medulla. Neuroscience. 2008;152:1099–1109. doi: 10.1016/j.neuroscience.2007.10.070. [DOI] [PubMed] [Google Scholar]
- 60.Victor RG, Thoren P, Morgan DA, Mark AL. Differential control of adrenal and renal sympathetic nerve activity during hemorrhagic hypotension in rats. Circ Res. 1989;64:686–694. doi: 10.1161/01.res.64.4.686. [DOI] [PubMed] [Google Scholar]
- 61.Wang X, Huang ZG, Dergacheva O, Bouairi E, Gorini C, Stephens C, Andresen MC, Mendelowitz D. Ketamine inhibits inspiratory-evoked gamma-aminobutyric acid and glycine neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Anesthesiology. 2005;103:353–359. doi: 10.1097/00000542-200508000-00019. [DOI] [PubMed] [Google Scholar]
- 62.Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125:683–690. doi: 10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]