Abstract
Maternal cocaine administration during gestation caused a down-regulation of PKCε expression in the heart of adult offspring resulting in an increased sensitivity to ischemia and reperfusion injury. The present study investigated the direct effect of cocaine in epigenetic modification of PKCε gene repression in the fetal heart. Hearts were isolated from gestational day 17 fetal rats and treated with cocaine in an ex vivo organ culture system. Cocaine treatment for 48 h resulted in significant decreases in PKCε protein and mRNA abundance and increases in CpG methylation at two SP1 binding sites in the PKCε promoter region (−346 and −268). Electrophoretic mobility shift assays demonstrated that CpG methylation of both SP1 sites inhibited SP1 binding. Consistently, chromatin immunoprecipitation assays showed that cocaine treatment significantly decreased binding of SP1 to the SP1 sites in the intact fetal heart. Reporter gene assays revealed that site-directed mutations of CpG methylation at both SP1 sites significantly reduced the PKCε promoter activity while methylation of a single site at either −346 or −268 did not have a significant effect. The causal effect of increased methylation in the cocaine-induced down-regulation of PKCε was demonstrated with the use of DNA methylation inhibitors. The presence of either 5-aza-2’-deoxycytodine or procainamide blocked the cocaine-induced increase in SP1 sites methylation and decrease in PKCε mRNA. The results demonstrate a direct effect of cocaine in epigenetic modification of DNA methylation and programming of cardiac PKCε gene repression linking prenatal cocaine exposure and pathophysiological consequences in the heart of adult offspring.
Keywords: SP1, fetal programming, epigenetic, DNA methylation, gene regulation
Introduction
Acute ischemic injury and myocardial infarction resulting from coronary artery disease is a major cause of death among people in the western world. In addition to traditional cardiovascular risk factors, an adverse intrauterine environment can predispose a person to adult cardiovascular disease [1, 2]. While this effect has been mainly studied in malnutrition and intrauterine growth restriction models, recent studies have demonstrated that other adverse factors during fetal development can increase the risk of ischemic heart disease in the adulthood [3–6]. Fetal cocaine exposure has detrimental effects on the developing heart at both the structural and molecular levels [7]. Children born to mothers with a history of cocaine abuse show a high incidence of congenital cardiovascular malformations, including abnormalities of ventricular structure and function, arrhythmias, and intracardiac conduction abnormalities, which persist beyond the period of exposure to cocaine [8–15].
Recent animal studies have demonstrated that maternal cocaine administration causes myocardiocyte apoptosis in neonate rats [16] and increases the myocardial sensitivity to ischemic and reperfusion injury in adult males [4]. Additionally, cocaine administration during pregnancy abolished ischemic preconditioning-induced cardioprotection due to down-regulation of protein kinase C ε (PKCε) in adult offspring [17]. It has been well demonstrated that PKCε plays a pivotal role of cardioprotection during cardiac ischemia and reperfusion injury [18–21]. Recently, we have found that maternal cocaine administration causes a decrease in PKCε expression in the heart of offspring, which appears to be mediated by increased methylation of the PKCε promoter [22]. This finding suggests an in utero epigenetic modification and programming of PKCε gene repression in the heart. However, it remains unclear if these outcomes are a result of cocaine acting directly on the fetal heart or are the result of secondary effects induced by maternal cocaine treatment. While maternal cocaine administration may cause fetal hypoxia and other forms of fetal stress, cocaine can cross the placenta from the maternal circulation to rapidly enter the fetal circulation and has been found to accumulate in the organs at a concentration several times higher than that in the blood [15, 23–26]. Herein we present evidence that cocaine acts directly on the fetal heart to increase DNA methylation in the PKCε promoter resulting in programming of PKCε gene repression in the heart.
Materials and Methods
Animals
Time-dated pregnant Sprague–Dawley rats were purchased from Charles River Laboratories (Portage, MI). Isolated fetal hearts were studied. All procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee of Loma Linda University, and followed the guidelines by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cardiomyocyte culture
Myocardial cells were isolated from gestational day 20 fetal rat hearts, as previously described [27]. Cells were plated at a density of 25,000 cells/ml in 6-well tissue culture plate in DMEM supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1% antibiotics (10,000 U/ml penicillin and 10,000 µg/ml streptomycin), and were cultured at 37 °C in 95% air/5% CO2. BrdU (0.1 mM) was added in the medium to prevent fibroblast proliferation. Cells were observed to spontaneously contract 24 h after collection. As reported previously, >95% of the cells manifested spontaneous contractions and were α-cardiac sarcomeric actin positive. Studies were conducted in ~80% confluent cells. Additionally, an embryonic rat heart cell line H9c2 [28] obtained from ATCC (Rockville, MD) were studied in the 4th to 6th passage.
Intact heart culture
Hearts were isolated from gestational day 17 fetal rats and were cultured in M199 media (Hyclone) supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in 95% air/5% CO2. As reported previously, the intact fetal rat hearts can live and beat in M199 media for at least 6 days [29]. Hearts were given 24 hours to recover before the treatment and observed to spontaneously beat 24 hours after collection.
Drug treatment
Cocaine hydrochloride (Sigma, St. Louis, MO) was dissolved in H2O to create a concentrated stock solution (1 mM) which was added to culture media to the desired concentration. Unless otherwise noted all experiments used a cocaine concentration of 10 µM for 48 h. DNA methylation inhibitors procainamide and 5-aza-2’-deoxycytodine (Sigma) were added to the media at a concentration of 300 µM and 1 µM, respectively. Media was changed at 24 h intervals during the treatment.
Western blotting
Cells or hearts were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris.HCl, 10 mM EDTA, 0.1% Tween-20, 0.1% β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 5 µg/ml leupeptin, and 5 µg/ml aprotinin, pH 7.4. Homogenates were centrifuged at 4 °C for 10 min at 10,000g, and supernatants collected. Protein concentration was measured in the supernatant using a protein assay kit (Bio-Rad, Hercules, CA). Samples with equal amounts of protein were loaded onto 10% polyacrylamide gel with 0.1% sodium dodecyl sulfate and separated by electrophoresis at 100 V for 2 h. Proteins were then be transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked by 1 h incubation at 4 °C in a Tris-buffered saline solution containing 5% dry-milk. The membranes were incubated with primary antibodies against PKCε (Santa Cruze Biotechnology; Santa Cruz, CA). α-Sacromeric actin antibody (Sigma) was used to normalize the loading. After washing, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. The results were quantified with the Kodak electrophoresis documentation and analysis system and Kodak ID image analysis software.
Real-time RT-PCR
RNA was isolated using TRIzol reagent following the manufacturer’s instructions (Invitrogen, Carlsbad, USA). PKCε mRNA abundance was determined using real-time RT-PCR in the Icycler Thermal cycler (Bio-Rad, Hercules, CA), as previously described [17]. PKCε primers sequence was 5’-gcgaagcccctaagacaat-3’ (forward) and 5’-caccccagatgaaatccctac-3’ (reverse). Real-time RT-PCR was performed in a final volume of 25 µl. Each PCR reaction mixture consisted of 600 nM of primers, 33 units of M-MLV reverse transcriptase (Bio-Rad, Hercules, CA), and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) containing 0.625 unit Taq polymerase, 400 µM each of dATP, dCTP, dGTP, and dTTP, 100 mM KCl, 16.6 mM ammonium sulfate, 40 mM Tris-HCl, 6 mM MgSO4, SYBR Green I, 20 nM fluoresein and stabilizers. Our RT-PCR protocol is: 42 °C for 30 min, 95 °C for 5 min, followed by 40 cycles of 95 °C for 15 s, 52 °C for 30 s. GAPDH was used as an internal reference. Serial dilutions of the positive control were done on each plate to create a standard curve. PCR was done in triplicate and threshold cycle numbers were averaged.
Quantitative methylation-specific PCR
DNA was isolated from hearts using a GenElute Mammalian Genomic DNA Mini-Prep kit (Sigma), denatured with 2 N NaOH at 42 °C for 15 min, and treated with sodium bisulfite at 55 °C for 16 h, as previously described [22]. DNA was purified with a Wizard DNA clean up system (Promega) and resuspended in 120 µl of H2O. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR (MSP) using primers created to amplify promoter binding sites containing possible methylation sites based on our previous sequencing of rat PKCε promoter [22]. GAPDH was used as an internal reference gene. Real-time MSP was performed using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad).
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared from hearts using NXTRACT CelLytic NuCLEAR Extraction Kit (Sigma) following the manufacturer’s directions. The oligonucleotide probes with CpG and mCpG at the two SP1 binding sites (−346 and −268) in rat PKCε promoter region were labeled and EMSA was performed by Biotin 3’ end labeling kit and LightShift Chemiluminescent EMSA Kit (Pierce Biotechnology, Rockford, IL), as previously described [22]. Dot blot and series of controls were performed to ensure sufficient labeling and successful shift. Binding reactions were performed in 20 µl containing 50 fmol oligo probes, 1× binding buffer, 1 µg of poly(dI-dC), and 3 µg of nuclear extracts. Cold competition was added to samples using increasing concentrations of unlabeled oligos. For super-shift assays, 2 µg of SP1 antibody (Active Motif, Carlsbad CA) were added and further incubated for 10 min at 25 °C. Protein-DNA complexes were resolved with 5% non-denaturing polyacrylamide mini-gels (29:1 cross-linking ratio). Nylon membranes were used for transferring DNA-protein complex followed by UV crosslinking to the membrane. After applying chemiluminescent substrate, the membranes were exposed to Kodak X-Ray film for autoradiography.
Chromatin Immunopercipitation (ChIP)
ChIP was performed using the ChIP-IT kit (Active Motif), as previously described [17]. Briefly, hearts were minced and fixed with 1% formaldehyde. Cross-linking was stopped by the addition of glycine to a final concentration of 125 mM. Chromatin extracts were prepared and sonicated to produce DNA fragments between 100 and 500 bp in length. Antibody-pulled chromatin extracts were used as templates for PCR and DNA from an aliquot of nonprecipitated lysates was used as template for total input. Two sets of primers flanking the two SP1 binding sites at −346 and −268 were used: 5’-accatttcctctcgacatgc-3’ (forward) and 5’-agatttcaacccggatcctc-3’ (reverse); 5’-agaggatccgggttgaaatc-3’ (forward) and 5’-ctcacctacctttccgaaaca-3’ (reverse), which yielded products of 117 bp and 116 bp in length, respectively. PCR amplification products were visualized on 1% agarose gel stained with ethidium bromide. To quantify PCR amplification, 45 cycles of real-time PCR were carried out with 3 min initial denaturation followed by 95 °C for 30 s, 54 °C for 30 s, and 72 °C for 30 s, using the iQ SYBR Green Supermix with iCycler real-time PCR system (Bio-Rad).
Site-directed mutagenesis and reporter gene assay
A 1941-bp fragment of rat PKC promoter region spanning −1941 to −1 bp relative to the transcriptional starting site of the PKCε was amplified by PCR and inserted into the pDrive Cloning Vector, as previously described [22]. The KpnI/HindIII fragment flanking the PKCε promoter region was then inserted into the luciferase reporter gene plasmid, pGL3 (Promega) to yield the full-length promoter-reporter plasmid denoted as pPKCε1941. To generate a SacI cutting site at the 3’ downstream of the SP1 at −268, a single nucleotide cytosine was introduced to the sequence GAGTC becoming GAGCTC using QuikChange II Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). In order to perform site-directed mutation of cytosine methylation at CpG dinucleotides in core regions of the two SP1 binding sequences at −346 and −268, three customized 152-bp NsiI/SacI oligonucleotide fragments with methylation at each sites and both sites, respectively, were synthesized, and were then ligated back to the pPKCε1941 plasmid. Amount of ligation product was determined by real-time PCR analysis and equal amount of plasmid was used in a transfection assay. Cell transfection was performed using H9c2, as described previously [22]. Cells were seeded in six-well plates (2 × 106 cells/plate) and transiently co-transfected with 1 µg of promoter/reporter vector along with 0.05 µg of internal control pRL-SV40 vector using Tfx-20 transfection reagents for eukaryotic cells (Promega) following manufacturer’s instructions. After 48 h, firefly and Renilla reniformis luciferase activities in cell extracts were measured in a luminometer using a dual-luciferase reporter assay system (Promega). The truncated promoter activities were then calculated by normalizing the firefly luciferase activities to R. reniformis luciferase activity.
Statistical analysis
Data are shown as mean ± SEM. Statistical significance was taken to be p < 0.05. A Student t-test was used when comparing two groups. When comparing more than two groups at a time, data were analyzed by ANOVA, followed by Neuman-Keuls post hoc testing.
Results
Direct cocaine exposure decreases myocardial PKCε protein and mRNA expression
Direct cocaine (10 µM) exposure produced a time-dependent decrease in PKCε protein abundance in H9c2 cells with the effect seen at 48 h and 72 h treatments (Fig. 1A). The decreased protein abundance was accompanied by a significant decrease in PKCε mRNA levels at the 48 h treatment. Consistent with the finding in H9c2 cells, cocaine treatment for 48 h significantly decreased PKCε protein abundance in primary fetal rat myocardiocytes (Fig. 1A). The same findings were obtained in the intact fetal rat hearts as shown in Fig. 1B, in which cocaine treatment for 48 h produced a concentration-dependent decrease in PKCε protein and mRNA abundance with a greater effect at 10 µM as compared with 3 µM. Subsequent studies were performed with 10 µM cocaine treatment for 48 h in the fetal heart.
Figure 1. Effect of cocaine on PKCε protein and mRNA.
PKCε protein and mRNA abundance was determined by Western blot and real time RT-PCR, respectively. A) PKCε protein and mRNA were measured in H9c2 cells and freshly isolated rat myocardiocytes (FRMC) after treatments with 10 µM of cocaine for 24–72 h. B) PKCε protein and mRNA were measured in intact fetal hearts treated with 3 or 10 µM of cocaine for 48 h. Data are means ± SEM. * P < 0.05 vs. control, n = 5.
Direct cocaine exposure increases methylation of SP1 binding sites in the PKCε promoter
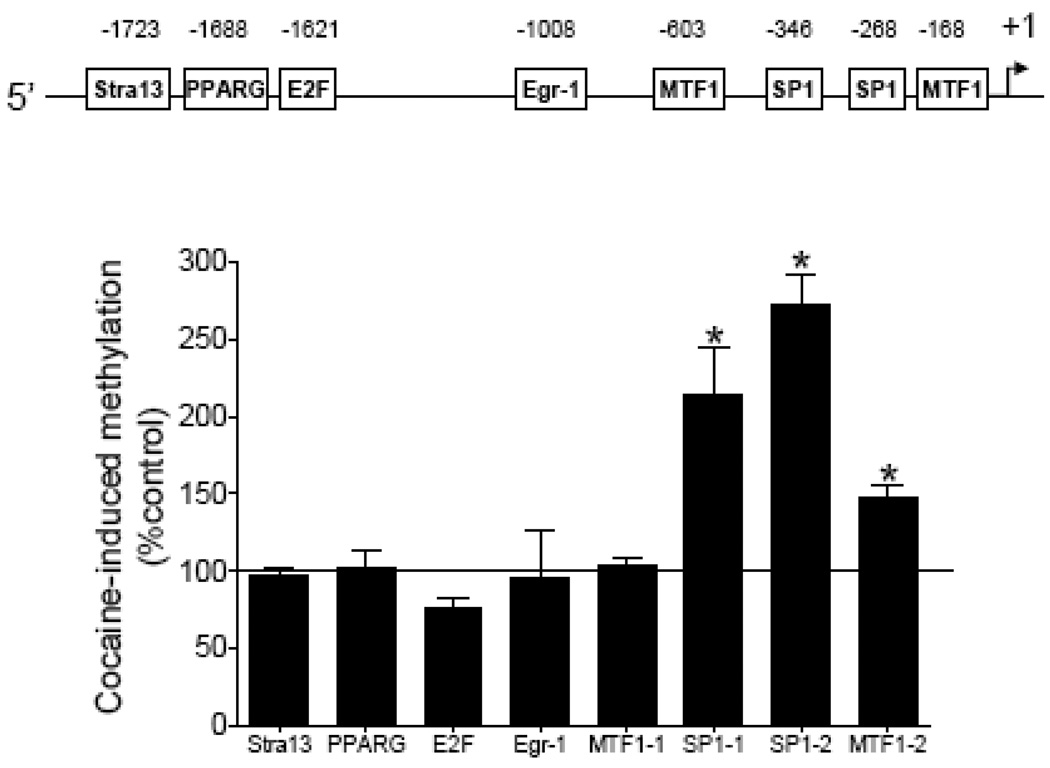
Our previous study identified eight putative transcription factor binding sites that contain CpG dinucleotides in their core binding sequences in rat PKCε promoter: Stra13 at −1723, PPARG at −1688, E2F at −1621, Egr1 at −1008, MTF1 at −603, SP1 at −346, SP1 at −268, and MTF1 at −168 [22]. Direct cocaine exposure had no significant effects on CpG dinucleotide methylation at binding sequences of Stra13, PPARG, E2F, Egr1, and MTF1 at −603 in fetal hearts, but significantly increased CpG methylation at the two putative SP1 binding sites and the MTF1 site at −168 (Fig. 2).
Figure 2. Effect of cocaine on DNA methylation of the PKCε promoter.
Intact fetal hearts were treated with 10 µM cocaine for 48 h, after which DNA was isolated and methylation levels determined by methylation specific real time PCR. Data are means ± SEM. * P < 0.05 vs. control, n = 5.
Methylation of the SP1 binding sites inhibits SP1 binding
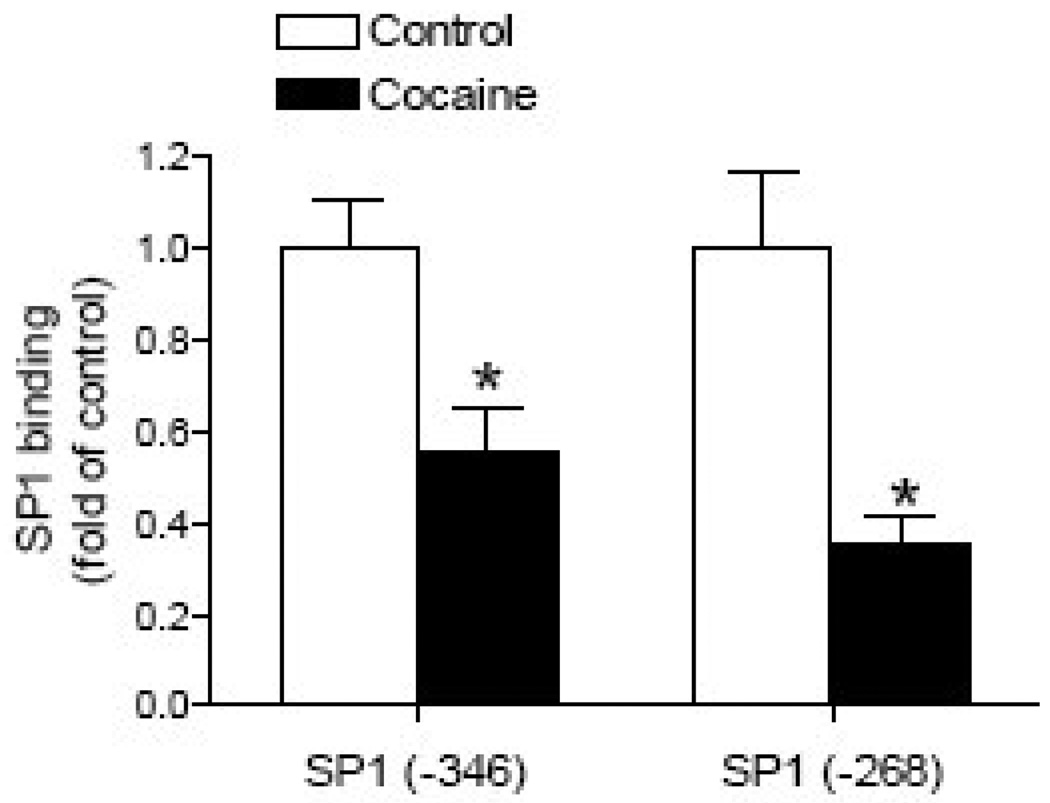
Given the finding that a deletion of the MTF1 binding site at −168 had no significant effect on the PKCε promoter activity [22], our further investigation focused on the two SP1 binding sites. To evaluate the binding of fetal heart nuclear proteins to the two putative SP1 elements at −346 and −268, EMSA was performed. Incubation of nuclear extracts from fetal rat hearts with double-stranded oligonucleotide probes encompassing the putative SP1 binding elements at −346 and −268, respectively, resulted in the appearance of one major DNA-protein complex, which was supershifted by a SP1 antibody (Fig. 3A). To determine if methylation of the SP1 binding sites inhibits SP1 binding from fetal heart nuclear extracts, EMSA was performed with methylated and unmethylated oligonucleotide probes containing the SP1 sites at −346 and −268. As shown in Fig. 3B, nuclear extracts from fetal rat hearts bound and shifted the double-stranded unmethylated SP1 oligonucleotides at both sites, but failed to cause a shift of the methylated SP1 oligonucleotides. Additionally, ChIP assays were performed to determine whether cocaine-mediated increases in methylation of the SP1 binding sites inhibits SP1 binding to the PKCε promoter in vivo in the context of intact chromatin. Fig. 4 shows that direct cocaine exposure caused significant decreases in the SP1 binding to both SP1 binding sites at −346 and −268, respectively, in the fetal hearts.
Figure 3. Effect of methylation on SP1 binding.
A) Nuclear extracts from fetal rat hearts were incubated with double-stranded oligonucleotide probes containing the PKCε gene consensus SP1 binding motif at −346 and −268, in the absence (Lane 3) or presence (Lane 1) of a SP1 antibody. Lane 2 is oligo alone without nuclear extracts. B) The nuclear extracts were incubated with double-stranded oligonucleotide probes containing either unmethylated (UM) or methylated (M) CpG dinucleotides at the consensus SP1 binding motif at −346 and −268.
Figure 4. Effect of cocaine on SP1 binding in the intact fetal heart.
Intact fetal hearts were treated with 10 µM cocaine for 48 h, and SP1 binding to the PKCε promoter at −346 and −268 was determined by ChIP assays. Data are means ± SEM. * P < 0.05 vs. control, n = 5.
Effect of cocaine on SP1 abundance and activity
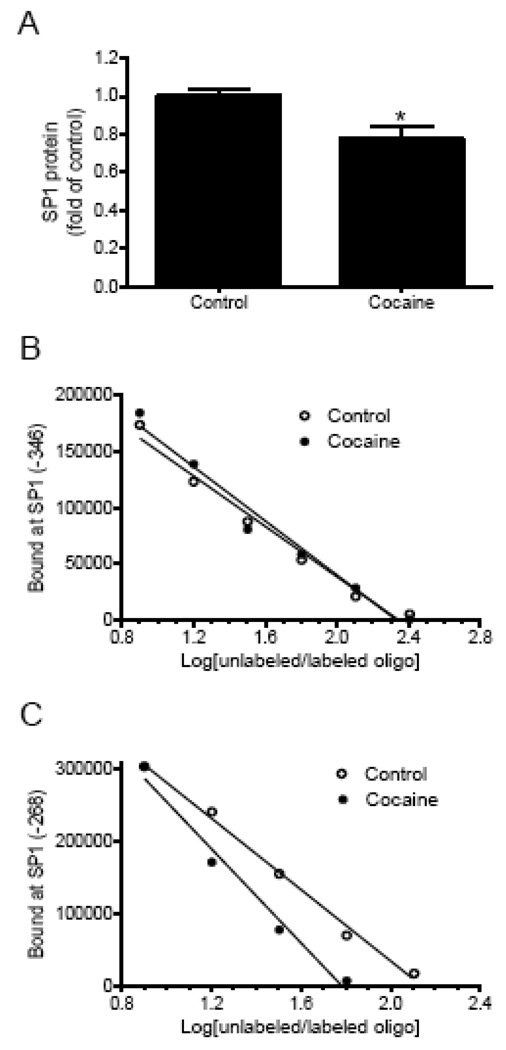
Western blot analyses showed a small but significant decrease in SP1 protein abundance in cocaine-treated fetal hearts (Fig. 5A). The binding affinity of SP1 to the unmethylated SP1 binding sites was determined in competition studies performed in pooled nuclear extracts from fetal hearts with increasing ratios of unlabeled/labeled oligonucleotides encompassing the SP1 sites at −346 and −268, respectively. As shown in Fig. 5B and 5C, cocaine treatment had no significant effect on the binding of nuclear extracts to the site at −346, but decreased their binding affinity at the site −268.
Figure 5. Effect of cocaine on SP1 abundance and activity.
Intact fetal hearts were treated with 10 µM cocaine for 48 h. A) SP1 protein abundance in nuclear extracts prepared from the fetal hearts was determined by Western blot using a SP1 antibody. Data are mean ± SEM. * P < 0.05 vs. control, n = 5. B, C) Pooled nuclear extracts from the fetal hearts were incubated with labeled double-stranded oligonucleotide probes containing the PKCε promoter consensus SP1 binding motif at −346 and −268, respectively, in the presence of 1, 2, 4, 8, and 16 folds of the unlabeled oligonucleotides.
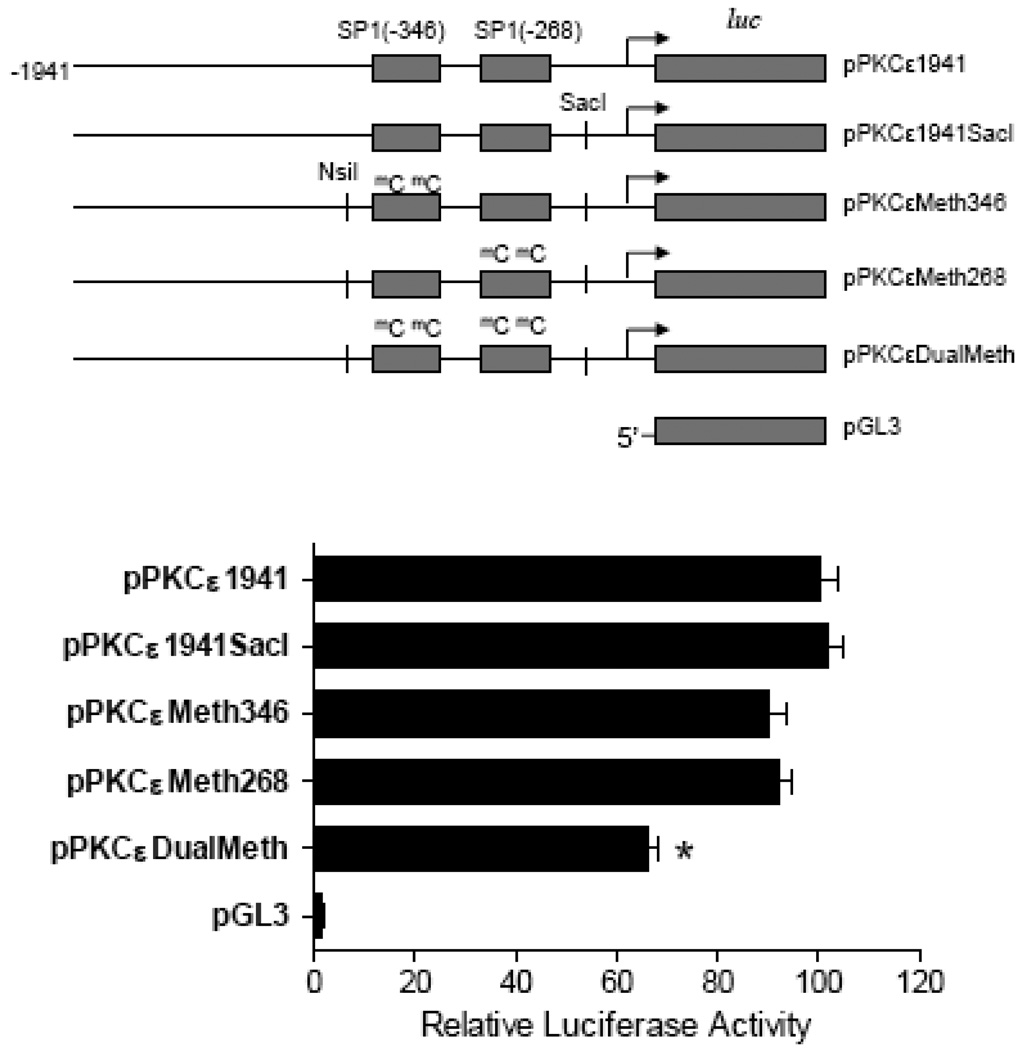
Methylation of SP1 sites reduces the PKCε promoter activity
Given that cocaine increased CpG methylation of SP1 binding sites resulting in an inhibition of SP1 binding, the involvement of SP1 site methylation in the regulation of PKCε promoter activity was determined. To create the site-directed mutations of CmG at the two SP1 binding sites in the PKCε promoter, a single nucleotide was inserted at the 3’ downstream of the SP1 site −268 to generate a SacI cutting site (pPKCε1941SacI). As shown in Fig. 6, the insertion of a single nucleotide (pPKCε1941SacI) had no significant effect on the activity, as compared with the control full-length promoter activity (pPKCε1941), which was over 50-fold greater than that in vector alone in the high glucose medium. The mutation of CmG at either SP1 site −268 (pPKCεMeth268) or −346 (pPKCεMeth346) alone had no significant effect on the promoter activity. However, the mutation of CmG at the both SP1 binding sites (pPKCεDualMeth) significantly reduced the promoter activity.
Figure 6. Effect of site-directed mutation of CmG at SP1 sites on the PKCε promoter activity.
Full-length wild type (pPKCε1941), a single nucleotide insertion (pPKCε1941SacI), methyaltion at SP1 −346 (pPKCεMeth346), methylation at SP1 −268 (pPKCεMeth268), and dual methylation at −346 and −268 (pPKCεDualMeth), PKCε promoter-reporter gene constructs were transiently co-transfected with pRL-SV40 driven R. reniformis luciferase in a rat embryonic heart-derived myogenic cell line H9c2. After 48 h, firefly and R. reniformis luciferase activities in cell extracts were measured using a dual-luciferase reporter assay system. The truncated promoter activities were then calculated by normalizing the firefly luciferase activities to R. reniformis luciferase activity. Data are mean ± SEM. * P < 0.05 vs. pPKCε1941, n = 6.
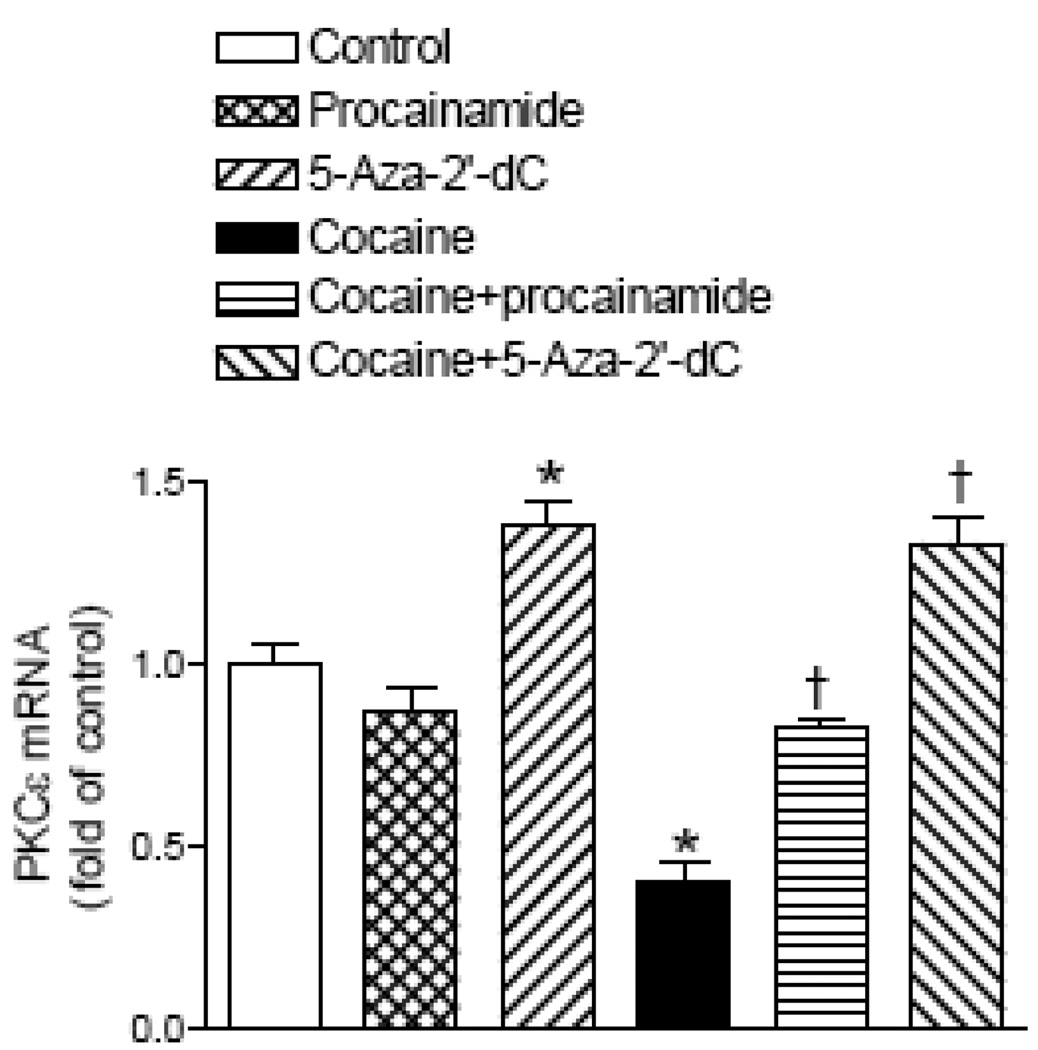
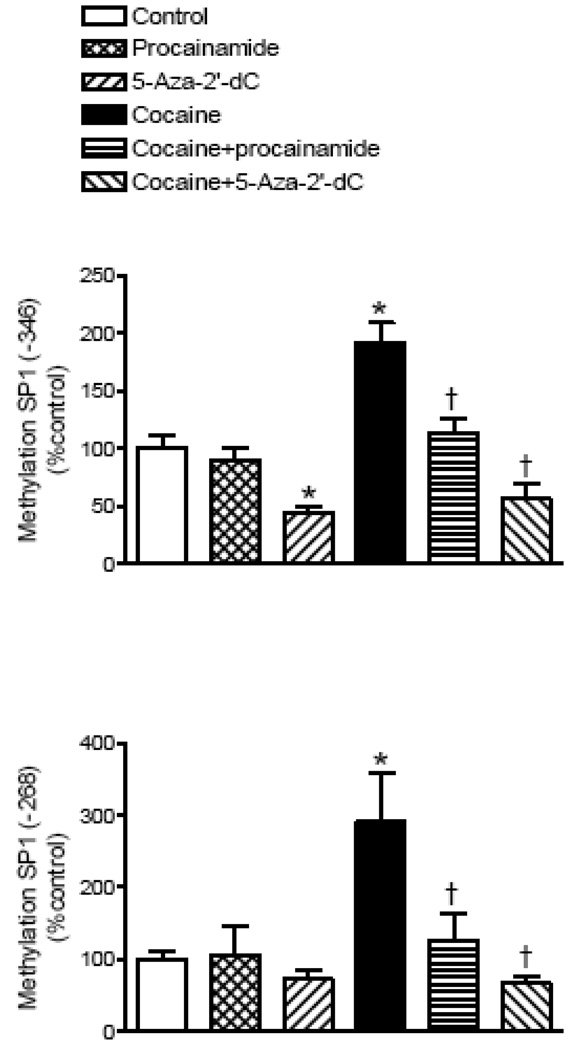
Methylation inhibitors reverse the cocaine’s effect
We further determined the cause-and-effect relation of cocaine-mediated increases in methylation of the SP1 elements and PKCε gene repression in the fetal hearts. As shown in Fig. 7 and Fig. 8, the treatment of intact fetal rat hearts with the DNA methylation inhibitor procainamide had no significant effect on PKCε mRNA levels or the methylation of the two SP1 binding sites in the PKCε promoter. Unlike procainamide, the DNA methylation inhibitor 5-aza-2’-deoxycytodine decreased the methylation of the SP1 binding site at −346 and increased PKCε mRNA levels. In the presence of either methylation inhibitor, cocaine had no significant effects on CpG methylation of the SP1 binding elements or the expression of PKCε mRNA levels when compared to the inhibitor alone (Fig. 7 and Fig. 8).
Figure 7. Effect of DNA methylation inhibitors on PKCε mRNA.
Intact fetal hearts were treated with 10 µM cocaine for 48 h in the absence or presence of either 300 µM procainamide or 1 µM 5-aza-2’-deoxycytodine (5-Aza-2’-dC). PKCε mRNA levels were measured with real time RT-PCR. Data are mean ± SEM. * P < 0.05 vs. control; † P < 0.05 vs. cocaine alone; n = 5.
Figure 8. Effect of DNA methylation inhibitors on methylation of SP1 binding sites in the PKCε promoter.
Intact fetal hearts were treated with 10 µM cocaine for 48 h in the absence or presence of either 300 µM procainamide or 1 µM 5-aza-2’-deoxycytodine (5-Aza-2’-dC). CpG methylation of SP1 binding motifs at −346 and −268 was determined by methylation specific real time PCR. Data are mean ± SEM. * P < 0.05 vs. control; † P < 0.05 vs. cocaine alone; n = 5.
Discussion
The findings of the present study show that cocaine directly impacts the fetal heart to increase DNA methylation at specific transcription factor binding sites in the PKCε promoter region thereby causing a decrease in the expression of PKCε protein and mRNA. We also demonstrate that these effects are reversed by the addition of methylation inhibitors concurrently with cocaine.
The present study builds on our previous work that has shown an epigenetic down-regulation of PKCε in the fetus and adult male in response to maternal cocaine treatment [22] by investigating the direct effects of cocaine on the fetal heart. The concentration of cocaine (10 µM) used in the present study is consistent with those often used in H9c2 studies [30–32] and is within the range of plasma and fetal tissue levels among human cocaine abusers [15, 23–26]. The findings of the decreased PKCε protein and mRNA expression in response to cocaine exposure in the intact fetal heart are consistent with the impact of maternal cocaine administration on the heart in the fetus in vivo [22], demonstrating that the effect of cocaine on PKCε gene repression in the fetus is primarily due to a direct action of cocaine on the heart. This finding is both a first step to understanding the mechanism of action of maternal cocaine exposure on the fetal heart and gives us a model to further investigate both the effect of cocaine on the heart and the mechanism responsible for these effects.
The majority of the data in the present study was generated using an intact fetal heart model. This model was used in conjunction with data gathered from an embryonic myocyte cell line and isolated primary fetal myocytes. The cell line we used, H9c2, is an embryonic rat myocyte cell [28] that has been widely used in many myocardiocyte studies. However, the use of a cell line has some clear drawbacks. The H9c2 cells do not spontaneously contract as do normal myocardiocytes and have been modified to divide indefinitely. Furthermore, the response of an isolated cell type to a stimulus such as cocaine can be different than the response of the intact organ. Previous work has found that the intact heart can survive and beat for 6 days in M199 media and up to three weeks with in ideal conditions [29]. These hearts in organ culture did not suffer from a loss of contractile function or any dedifferentiation [29]. In the present study, the intact fetal hearts maintained spontaneous contraction and beating throughout the study period. Using these models, we have demonstrated that cocaine produces comparable responses in down-regulation of PKCε in H9c2 cells, freshly isolated fetal myocardiocytes, and intact fetal hearts, suggesting that the primary action of cocaine is on the myocardiocytes.
The decrease in PKCε protein was accompanied by a similar decrease in PKCε mRNA suggesting that the decrease is due primarily to transcriptional repression. The finding of increased CpG methylation at both SP1 binding sites in the PKCε promoter in the fetal heart was consistent with the previous finding in the heart of adult offspring after prenatal cocaine exposure [22]. This provides clear evidence that cocaine has a direct effect in epigenetic modification of SP1 binding sites resulting in fetal programming of PKCε gene repression in the heart. While methylation is generally associated with decreased gene expression, current literature is conflicted regarding the impact of methylation of SP1 sites on SP1 binding with some studies suggesting the methylation can decrease binding [33, 34] while others suggest that methylation does not affect SP1 binding [35]. Consistent with the previous study in the adult heart [22], the present study demonstrated that the SP1 probes with methylated-CpG dinucleotides at the core of the consensus SP1 elements in the PKCε promoter abolished the binding of SP1 in the fetal heart, indicating that CpG methylation in non-CpG islands and sequence-specific SP1 binding sites can directly inhibit the DNA binding of SP1 and result in down-regulation of PKCε gene expression in the heart. This is further demonstrated in vivo in the context of intact chromatin by ChIP assays showing that cocaine-mediated increase in methylation of SP1 sites inhibits the recruitment of SP1 to the PKCε promoter in both sites at −346 and −268 in the fetal heart.
In the previous study, we have shown that in male offspring maternal cocaine administration causes an increased methylation and decreased SP1 binding to the PKCε promoter at both binding sites of −346 and −268, which is associated with the decreased PKCε mRNA and protein abundance in the left ventricle [22]. In contrast, in females, the increased methylation and decreased SP1 binding at the site of −268 alone were not associated with significant decreases in PKCε mRNA and protein levels in the left ventricle of cocaine-treated animals [22]. This finding raises a question that changes in CpG methylation and SP1 binding at the SP1 binding site at −268 may not play a key role in epigenetic modification of PKCε expression in the heart. Alternatively, an increase in CpG methylation and decrease in SP1 binding of SP1 binding sites at either −268 or −346 alone may not be sufficient for PKCε gene repression in the heart, and methylation of the both sites may be required. To address these questions, we have developed site-directed methylation of PKCε promoter-luciferase constructs selectively at SP1 binding sites at −268 and −346, and have shown indeed that the mutation of CmG at either SP1 site −268 or −346 alone has no significant effect on the promoter activity, but the mutation of CmG at the both SP1 binding sites significantly reduces the promoter activity.
The cause and effect relation in cocaine-mediated increase in methylation of SP1 binding sites and PKCε gene repression in the fetal heart was further demonstrated with two widely used DNA methylation inhibitors, procainamide and 5-aza-2’-deoxycytodine in the present study. 5-Aza-2’-deoxycytodine binds nonreversibly to DNA methyltransferase 1 causing its depletion and preventing DNA methylation and can cause DNA hypomethylation [36] while procainamide is a class IA antiarrythmic drug that inhibits DNA methylation through an unknown mechanism [37, 38]. The doses of both inhibitors used in the present study are consistent with previous studies using procainamide or 5-aza-2’-deoxycytodine to inhibit DNA methylation [39–42]. Both inhibitors blocked cocaine-induced increases in CpG methylation of SP1 binding sites at −346 and −268, and reversed the effect of cocaine in down-regulation of PKCε mRNA, indicating that increased DNA methylation at the SP1 binding sites is required for the effect of cocaine on PKCε repression in the fetal heart. While procainamide treatment alone did not significantly affect methylation of the SP1 binding sites or the level of PKCε mRNA, 5-aza-2’-deoxycytodine treatment decreased methylation of SP1 and increased PKCε mRNA levels. The finding that 5-aza-2’-deoxycytodine, but not procainamide, decreased basal methylation is consistent with previous studies showing that procainamide is a less effective DNA methylation inhibitor [34, 38]. Additionally, in some cases procainamide cannot reactivate genes that have been silenced by methylation while 5-aza-2’-deoxycytocine can [39].
We previously cloned the PKCε promoter and found that it has many areas rich in CpG suggesting that DNA methylation is an important regulatory mechanism of the gene’s expression [22]. The present finding that 5-aza-2’-deoxycytodine increases expression of PKCε mRNA while decreasing methylation of the −346 SP1 binding site supports this hypothesis. In the fetal heart, the basal level of methylation of the −346 SP1 binding site is approximately 15% and that of the −268 SP1 site is approximately 5%. 5-aza-2’-deoxycytodine treatment reduced the methylation of the −346 site by approximately 50% while the reduction in methylation of the −268 site did not reach statistical significance. While cocaine treatment only increased methylation of the SP1 binding sites in the fetal heart, other transcription factor binding sites in the PKCε promoter contain CpGs and some of these sites are heavily methylated up to 75%. Thus, 5-aza-2’-deoxycytodine may also reduce methylation at other binding sites besides the −346 SP1 site, which contribute to its effect on basal PKCε expression. The correlation between the decreased methylation and increased the level of PKCε mRNA when 5-aza-2’-deoxycytodine is added suggests that methylation of the promoter region is at least one of the mechanisms that controls basal PKCε expression in the fetal heart.
In addition to the increased methylation of SP1 binding sites, we have observed a small but significant decrease in SP1 protein abundance in the cocaine-treated fetal hearts. Although the decreased SP1 protein may contribute to the cocaine-mediated decrease in PKCε expression in the fetal heart, this effect is not sustained in the offspring that showed no significant difference in SP1 protein abundance in the heart between control and cocaine-treated animals [22]. Unlike the changes in protein abundance, the cocaine-mediated decrease in SP1 binding affinity to the SP1 site at −268 in the fetal heart persists into the heart of adult offspring, although its significance in PKCε gene repression is not clear given the finding that the deceased SP1 binding at −268 was not associated with a decrease in PKCε expression in the heart [22].
The present study provides insight into the direct effect of cocaine in an epigenetic modification of gene expression pattern in the fetal heart. Our collective observations indicate that an increase in CpG methylation at the core of the consensus SP1 elements in the PKCε promoter caused by cocaine is necessary and sufficient for a decrease in SP1 binding to the PKCε promoter and repression of PKCε gene expression in the fetal heart. This demonstrates that cocaine acts as a potent programming agent and is capable of inducing epigenetic changes in the rat heart. While this study focused exclusively on PKCε regulation in response to fetal cocaine exposure, further study is needed to determine if cocaine causes epigenetic modifications in other parts of the genome that affect the expression of additional genes that play a role in the risk for cardiovascular disease. As cocaine has a direct effect on cardiac myocytes causing an increase in oxidative stress [31, 32] and oxidative stress modulates epigenetic regulation of gene expression through changes in DNA methylation [43], further study is needed in the role of oxidative stress in the cocaine-mediated DNA methylation in the fetal heart
Acknowledgements
This work was supported in part by NIH grants HL82779 (LZ) and HL83966 (LZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker D, Gluckman P, Godfrey K, Harding J, Owens J, Robinson J. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 2.Barker D. The fetal origins of coronary heart disease. Eur Heart J. 1997;18:883–884. doi: 10.1093/oxfordjournals.eurheartj.a015368. [DOI] [PubMed] [Google Scholar]
- 3.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10(5):265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 4.Bae S, Gilbert RD, Ducsay CA, Zhang L. Prenatal cocaine exposure increases heart susceptibility to ischaemia–reperfusion injury in adult male but not female rats. J Physiol. 2005;565(1):149–158. doi: 10.1113/jphysiol.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodic M, Samuel C, Moritz K, Wintour EM, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89:623–629. doi: 10.1161/hh1901.097086. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence J, Xiao D, Xue Q, Rejali M, Yang S, Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J Pharmacol Exp Ther. 2008;324:331–341. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer KD, Zhang L. Short- and long-term adverse effects of cocaine abuse during pregnancy on the heart development. Ther Adv Cardiovasc Dis. 2009;3(1):7–16. doi: 10.1177/1753944708099877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frassica JJ, Orav EJ, Walsh EP, Lipshultz SE. Arrhythmias in children prenatally exposed to cocaine. Arch Pediatr Adolesc Med. 1994;148(11):1163–1169. doi: 10.1001/archpedi.1994.02170110049008. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Frassica JJ, Orav EJ. Cardiovascular abnormalities in infants prenatally exposed to cocaine. J Pediatr. 1991;118:44–51. doi: 10.1016/s0022-3476(05)81842-6. [DOI] [PubMed] [Google Scholar]
- 10.Mehta SK, Finkelhor RS, Anderson RL, Harcar-Sevcik RA, Wasser TE, Bahler RC. Transient myocardial ischemia in infants prenatally exposed to cocaine. J Pediatr. 1993;122(6):945–949. doi: 10.1016/s0022-3476(09)90025-7. [DOI] [PubMed] [Google Scholar]
- 11.Mone S, Gillman M, Miller T, Herman E, Lipshultz S. Effects of environmental exposures on the cardiovascular system: prenatal period through adolescence. Pediatrics. 2004:1058–1069. [PubMed] [Google Scholar]
- 12.Norris M, Hill C. Assessing congenital heart defects in the cocaine-exposed neonate. Dimens Crit Care Nurs. 1992;11:6–12. doi: 10.1097/00003465-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Shaw G, Malcoe L, Lammer E, Swan S. Maternal use of cocaine during pregnancy and congenital cardiac anomalies. J Pediatr. 1991;118:167–168. doi: 10.1016/s0022-3476(05)81888-8. [DOI] [PubMed] [Google Scholar]
- 14.van de Bor M, Walther FJ, Ebrahimi M. Decreased cardiac output in infants of mothers who abused cocaine. Pediatrics. 1990;85:30–32. [PubMed] [Google Scholar]
- 15.Wiggins R. Pharmacokinetics of cocaine in pregnancy and effects on fetal maturation. Clin Pharmacokinet. 1992;22(85–93) doi: 10.2165/00003088-199222020-00001. [DOI] [PubMed] [Google Scholar]
- 16.Bae S, Zhang L. Prenatal cocaine exposure increases apoptosis of neonatal rat heart and heart susceptibility to ischemia-reperfusion injury in 1-month-old rat. Br J Pharmacol. 2005;144:900–907. doi: 10.1038/sj.bjp.0706129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer KD, Zhang H, Zhang L. Prenatal Cocaine Exposure Abolished Ischemic Preconditioning-Induced Protection in Adult Male Rat Hearts: Role of PKCε. Am J Physiol Circ Physiol. 2009;296:H1566–H1576. doi: 10.1152/ajpheart.00898.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ping P, Zhang J, Pierce W, Bolli R. Functional proteomic analysis of protein kinase C ε complexes in normal heart and during cardioprotection. Circ Res. 2001;88:59–62. doi: 10.1161/01.res.88.1.59. [DOI] [PubMed] [Google Scholar]
- 19.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilonPKC in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420(2):246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Hahn H, Wu G, Chen C, Liron T, Schechtman D, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cross HR, Murphy E, Bolli R, Ping P, Steenberge C. Expression of Activated PKC Epsilon (PKC ε) Protects the Ischemic Heart, without Attenuating Ischemic H+ Production. J Mol Cell Cardiol. 2002;34:361–367. doi: 10.1006/jmcc.2001.1518. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Meyer K, Zhang L. Fetal Exposure to Cocaine Causes Programming of Prkce Gene Repression in the Left Ventricle of Adult Rat Offspring. Biol Reprod. 2009;80(3):440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein J, Greenwald M, Becker L, Koren G. Fetal distribution of cocaine: case analysis. Pediatr Pathol. 1992;12:462–468. doi: 10.3109/15513819209023326. [DOI] [PubMed] [Google Scholar]
- 24.DeVane C, Simpkins J, Miller R, Braun S. Tissue distribution of cocaine in the pregnant rat. Life Sci. 1989;45:1271–1276. doi: 10.1016/0024-3205(89)90129-x. [DOI] [PubMed] [Google Scholar]
- 25.Poklis A, Mackell M, Graham M. Disposition of cocaine in fetal poisoning in man. J Anal Toxicol. 1985;9:227–229. doi: 10.1093/jat/9.5.227. [DOI] [PubMed] [Google Scholar]
- 26.Schenker S, Yang Y, Johnson R, Downing J. The transfer of cocaine and its metabolites across the term human placenta. Clin Pharmacol Ther. 1993;53:329–339. doi: 10.1038/clpt.1993.29. [DOI] [PubMed] [Google Scholar]
- 27.Li G, Xiao Y, Zhang L. Cocaine induces apoptosis in fetal rat myocardial cells through the p38 MAPK and mitochondrial/cytochrome c pathways. J Pharmacol Exp Ther. 2005;312:112–119. doi: 10.1124/jpet.104.073494. [DOI] [PubMed] [Google Scholar]
- 28.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological biochemical and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 29.Wildenthal K. Long-term maintenance of spontaneously beating mouse hearts in organ culture. J Appl Physiol. 1970;30(1):153–157. doi: 10.1152/jappl.1971.30.1.153. [DOI] [PubMed] [Google Scholar]
- 30.Wu S, Chang D, Sung R. Cocaine-induced inhibition of ATP-sensitive K+ channels in rat ventricular myocytes and in heart-derived H9c2 cells. Basic Clin Pharmacol Toxicol. 2006;98:510–517. doi: 10.1111/j.1742-7843.2006.pto_354.x. [DOI] [PubMed] [Google Scholar]
- 31.Fan L, Sawbridge D, George V, Lei T, Bailey A, Kitchen I, et al. Chronic cocaine-induced cardiac oxidative stress and MAPK activation: The role of Nox2 oxidase. J Pharmacol Exp Ther. 2008 doi: 10.1124/jpet.108.145201. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lattanzio FJ, Tiangco D, Osgood C, Beebe S, Kerry J, Hargrave B. Cocaine increases intracellular calcium and reactive oxygen species, depolarizes mitochondria, and activates genes associated with heart failure and remodeling. Cardiovasc Toxicol. 2005;5(4):377–390. doi: 10.1385/ct:5:4:377. [DOI] [PubMed] [Google Scholar]
- 33.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJL. Epigenetic Modification of the Renin-Angiotensin System in the Fetal Programming of Hypertension. Circ Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alikhani-Koopaei R, Fouladkou F, Frey F, Frey B. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114:1146–1157. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tate P, Bird A. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr Opin Genet Dev. 1993;3:226–231. doi: 10.1016/0959-437x(93)90027-m. [DOI] [PubMed] [Google Scholar]
- 36.Compere SJ, Palmiter RD. DNA methylation controls the inducibility of the mouse metallothionein-I gene in lymphoid cells. Cancer Cell. 1980;25:233–240. doi: 10.1016/0092-8674(81)90248-8. [DOI] [PubMed] [Google Scholar]
- 37.Cornacchia E, Golbus J, Maybaum J, Strahler J, Hanash S, Richardson B. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol. 1988;140:2197–2200. [PubMed] [Google Scholar]
- 38.Lin X, Asgari K, Putzi M, Gage W, Yu X, Cornblatt B, et al. Reversal of GSTP1 CpG island hypermethylation and reactivation of pi-class glutathione S-transferase (GSTP1) expression in human prostate cancer cells by treatment with procainamide. Cancer Res. 2001;61:8611–8616. [PubMed] [Google Scholar]
- 39.Chuang JC, Yoo CB, Kwan JM, Li TWH, Liang G, Yang AS, et al. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2'-deoxycytidine. Mol Cancer Ther. 2005;4:1515–1520. doi: 10.1158/1535-7163.MCT-05-0172. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen CT, Weisenberger DJ, Velicescu M, Gonzales FA, Lin JCY, Liang G, et al. Histone H3-Lysine 9 Methylation Is Associated with Aberrant Gene Silencing in Cancer Cells and Is Rapidly Reversed by 5-Aza-2'-deoxycytidine. Cancer Res. 2002;62:6456–6461. [PubMed] [Google Scholar]
- 41.Bender CM, Pao MM, Jones PA. Inhibition of DNA Methylation by 5-Aza-2'-deoxycytidine Suppresses the Growth of Human Tumor Cell Lines. Cancer Res. 1998;58:95–101. [PubMed] [Google Scholar]
- 42.Lee BH, Yegnasubramanian S, Lin X, Nelson WG. Procainamide Is a Specific Inhibitor of DNA Methyltransferase 1. J Biol Chem. 2005;280(49):40479–40756. doi: 10.1074/jbc.M505593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco R, Schoneveld O, Georgakilas A, Panayiotidis M. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]