Abstract
In human kidneys, the mechanisms underlying angiotensinogen (AGT) augmentation by interleukin 6 (IL-6) are poorly understood and the only information available is in HK-2, immortalized human renal proximal tubular epithelial cells. Therefore, the present study was performed to elucidate the effects of IL-6 on AGT expression in primary cultured human renal proximal tubular epithelial cells (RPTEC) after characterization of HK-2 and RPTEC. RPTEC showed low basal AGT mRNA (11±1%) and protein (7.0±0.9%) expression, high IL-6 receptor (IL-6R) expression (282±17%), and low basal NF-κB (43±7%) and STAT3 (43±7%) activities compared to those in HK-2. In RPTEC, AGT mRNA and protein expressions were enhanced by IL-6 (172±31% and 378±39%, respectively). This AGT augmentation was attenuated by an IL-6R antibody. STAT3 phosphorylation (366±55% at 30 min) and translocation were enhanced by IL-6. The AGT augmentation was attenuated by a STAT3 inhibitor. These data indicate that IL-6 increases AGT expression via STAT3 pathway in RPTEC.
Keywords: IL-6, angiotensinogen, STAT3, renal proximal tubular epithelial cell
Introduction
Angiotensinogen (AGT) is regarded as an important factor for the systemic renin-angiotensin system (RAS) activity (Corvol and Jeunemaitre, 1997). Angiotensin II (Ang II) increases AGT expression via nuclear factor-κB (NF-κB) activation in hepatocytes (Brasier et al., 2000; Jamaluddin et al., 2000; Li and Brasier, 1996). Ang II induces secretion of cytokines including tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β)and interleukin 6 (IL-6) (Recinos et al., 2007). IL-6 augments AGT expression via signal transducer and activator of transcription 3 (STAT3) activation in hepatocytes (Jain et al., 2007; Ohtani et al., 1992; Ray et al., 2005). These data suggest that Ang II upregulates circulating AGT via IL-6 augmentation, which may worsen vascular inflammation (Brasier et al., 2002).
There is growing recognition that the intrarenal RAS is an independent mechanism of the systemic RAS and is involved in the regulation of individual organs in a tissue-specific manner (Dzau and Re, 1994; Paul et al., 2006; Re, 2004; Navar et al., 2002). An in vitro study showed that Ang II induces AGT expression in rat renal proximal tubular cells which is the main source of intrarenal AGT (Ingelfinger et al., 1999; Ingelfinger et al., 1990; Terada et al., 1993). Several in vivo studies also demonstrated that the expression of renal AGT mRNA and protein are enhanced in Ang II-infused rats and human renin/human AGT double transgenic mice (Schunkert et al., 1992; Kobori et al., 2001; Kobori et al., 2007b). These data provide a firm foundation for the hypothesis that the Ang II-induced AGT augmentation in renal proximal tubular cells contributes to further increases in intrarenal Ang II levels (Kobori et al., 2007a).
Intrarenal TNF-α and IL-6 levels are elevated in the kidneys of Ang II-infused hypertensive rats (Ruiz-Ortega et al., 2002). Moreover, Ang II stimulates IL-6 secretion from cultured mesangial cells (Moriyama et al., 1995). Enalapril, an angiotensin converting enzyme (ACE) inhibitor, abrogates enhanced expression of TNF-α, IL-1β and IL-6 in the renal cortex of diabetic rats (Navarro et al., 2006). In IL-6 knockout mice, the magnitudes of Ang II-induced hypertension and albumin excretion are attenuated (Lee et al., 2006). These findings suggest that the augmented intrarenal Ang II as well as circulating Ang II induces intrarenal cytokines which leads to the development of renal injury probably accompanied by the activation of Ang II-AGT augmentation mechanism. However, little is known about direct interaction between AGT expression and IL-6 in the kidney.
We recently reported that Ang II and IL-6 synergistically induce human AGT expression through the activation of NF-κB and STAT3 in HK-2 cells, immortalized human renal proximal tubular cells. In contrast, while augmentation of AGT by IL-6 alone (Jain et al., 2007; Ohtani et al., 1992; Ray et al., 2005) has been reported in hepatocytes, stimulation with IL-6 alone did not increase AGT expression in HK2 cells (Satou et al., 2008). However, HK-2 cells have high basal activity of NF-κB which may limit the ability of these cells to respond further to stimulatory agents (Satou et al., 2008; de Haij et al., 2003). In further studies, we observed that basal activities of NF-κB and STAT3 are much lower in primary cultured human renal proximal tubular epithelial cells (RPTEC). Thus, further studies were performed to compare the basal expression levels of AGT and IL-6 receptor (IL-6R) and basal activities of NF-κB and STAT3 in HK-2 cells and RPTEC. Then, we performed more detailed studies on action of IL-6 to augment AGT in RPTEC.
Methods
Cell culture
HK-2 cells were obtained from ATCC. The cells were cultured as previously described (Satou et al., 2008). RPTEC were obtained from Cambrex. The cells were grown in Renal Epithelial Cell Growth Medium (Cambrex) supplemented with 0.5% heat-inactivated fetal calf serum as recommended by the manufacturer. RPTEC were used within passage 7. Cells were plated at a density of 2×105 cells/well in 6-well plates. Prior to stimulation, the cells were exposed to serum-free medium for 24 hr in both cell lines. Thereafter, RPTEC were treated with 10 ng/ml human TNF-α (Pierce), 10 ng/ml human IL-1β (Peprotech), 0.625–20.0 ng/ml human IL-6 (Peprotech) and 10−7 M Ang II for up to 24 hr in a medium containing 0.05% serum. In addition, cells were treated with 10−7 M Ang II and 10 ng/ml for 24 hr to test synergistic effects of Ang II and IL-6 on AGT expression. To investigate the influence of STAT3 on human AGT expression, cells were treated with 0.2 μM JSI-124 (Calbiochem). The treatment with JSI-124 was started before 3 hr of stimulations with IL-6 because the inhibitory effect of JSI-124 is slower than STAT3 activation by IL-6 (van Kester et al., 2008). To determine the contribution of IL-6R to the change in AGT expression and STAT3 activation by IL-6, RPTEC was pretreated with 0.1–10 μg/ml anti-IL-6R antibody (R & D Systems) for 1 hr; thereafter, cells were treated with 10 ng/ml IL-6.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR (qRT-PCR) was performed to evaluate human AGT mRNA expression using the TaqMan PCR system. For total RNA isolation, treated cells were washed with 3 ml of PBS and harvested at each experimental time point. Total RNA was isolated from the cells using the BIO-ROBOT EZ 1 (Qiagen). Subsequently, qRT-PCR was performed as previously described (Kobori et al., 2005). In brief, 20 ng total RNA from each sample was applied to the Mx3000P System (Stratagene) equipped with the Brilliant Single-Step QRT-PCR Master Mix II Kit (Stratagene). All samples were analyzed in triplicate, and the data were normalized based on the expression level of the human GAPDH mRNA. The sequences are as follows: human AGT mRNA: forward primer, 5′-GAG AGA GCC CAC AGA GTC TA-3′; reverse primer, 5′-GCT TTG ATC ATA CAC AGC AA-3′; and probe, 5′/6-FAM/CCA ACA GCT TAA CAA GCC TGA GGT/BHQ1/3′ and human GAPDH mRNA; forward primer, 5′-ATC ATC CCT GCC TCT ACT GG-3′; reverse primer, 5′-CTG CTT CAC CAC CTT CTT GA-3′; and probe, 5′/HEX/ACC TGA CCT GCC GTC TAG AAA AAC CTG/BHQ2/3′.
Human AGT ELISA
To quantify human AGT protein in the cell lysate and the culture medium, human AGT ELISA was performed. HK-2 and RPTEC was cultured in 6-well plates with 1.5 ml of medium/well. The media were collected into tubes, and the supernatants were collected after centrifugation of the media for 5 min at 5,000 rpm. Cell lysates of RPTEC were prepared as previously described (Satou et al., 2008). The ELISA for AGT was performed as previously described (Katsurada et al., 2007). Total protein concentrations in the media were also quantified using Micro BCA Protein Assay Kit (Pierce). For comparison of basal AGT expressions between HK-2 cells and RPTEC, data from human AGT ELISA were normalized based on total volume of medium. To examine the effect of IL-6 on AGT expression in RPTEC, data were normalized based on total protein concentration of the media.
Western blot analysis
To detect the protein expression levels of IL-6R, western blots were performed as previously described (Satou et al., 2008). After treatment with serum-free medium for 24 hr, HK-2 cells and RPTEC were washed 3 times with 3 ml PBS. The cells were harvested with 80 μL lysis buffer containing 1% Triton X-100, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Nonidet P-40, 1 mmol/L Na3VO4, and 0.25% Protease Inhibitor Cocktail (Sigma). The lysates were sonicated 3 times for 10 sec each and centrifuged at 13,000 rpm at 4°C for 30 min. Total protein concentration of the supernatant was quantified using Micro BCA Protein Assay Kit (Pierce). Then, 20 μg of total protein was applied to a pre-cast NuPAGE 4–12% gel (Invitrogen). The separated proteins were transferred to a nitrocellulose membrane (Bio-Rad). A goat anti-IL-6R antibody (1:150, R & D Systems) and an IRDye labeled anti-goat IgG antibody (1:15000, Li-Cor) were used for the detection of human IL-6R. Detection was performed using the Odyssey System (Li-Cor). Data were normalized based on human β-actin protein expression levels. To determine NF-κB activity, a mouse anti-phospho-p65 (Ser 536) antibody (1:1000, Cell Signaling) and an IRDye labeled anti-mouse IgG antibody (1:15000, Li-Cor) were used for the detection of phosphorylated p65. Expression level of total p65 protein was evaluated using a rabbit anti-p65 antibody (1:1000, Cell Signaling) and an IRDye labeled anti-rabbit IgG antibody (Li-Cor). The level of phosphorylated p65 was normalized based on total p65.
Phosphorylation of STAT3 was detected using western blot analysis in order to elucidate participations of the transcriptional factor in the effects mediated by IL-6 on AGT expression in RPTEC. Cells were treated with 10 ng/ml IL-6 for up to 60 min and for 24 hr. A mouse anti-phospho-STAT3 (Tyr 705) antibody (1:250, BD Transduction Laboratories) and an IRDye labeled anti-mouse IgG antibody (1:15000, Li-Cor) were used for the detection of phosphorylated STAT3. A rabbit anti-STAT3 antibody (1:250, Thermo) and an IRDye labeled anti-rabbit IgG antibody (Li-Cor) were used to detect total STAT3. The level of phosphorylated STAT3 was normalized based on total STAT3.
Immunofluorescence staining
RPTEC were cultured in 4-well chamber (Lab-Tek). After treatment with 10 ng/ml IL-6 for 30 min, cells were rinsed with PBS, then, fixed for 20 min by 4% formaldehyde. After incubated with 0.2% Triton X-100 for 4 min, the chambers were blocked by Image-iT FX signal enhancer (Invitrogen). The cells were incubated with a rabbit anti-STAT3 antibody (1:100, Thermo) for 30 min. After washing with PBS, the cells were incubated with an Alexa Fluor 488 goat anti-rabbit antibody (Invitrogen). ProLong Gold anti-fade reagent with DAPI (Invitrogen) was used to stain nuclei and as a mount reagent. Localization of STAT3 in RPTEC was observed and photographed under a fluorescence microscope (Olympus BX51, Olympus Optical Co. Ltd.).
Statistical analysis
Data are expressed as mean ± SD. The data were analyzed using student t test or one-way ANOVA followed by Bonferroni/Dunn multiple comparison post hoc test. P<0.05 was considered statistically significant.
Results
Comparison of basal AGT and IL-6R expressions between HK-2 cells and RPTEC
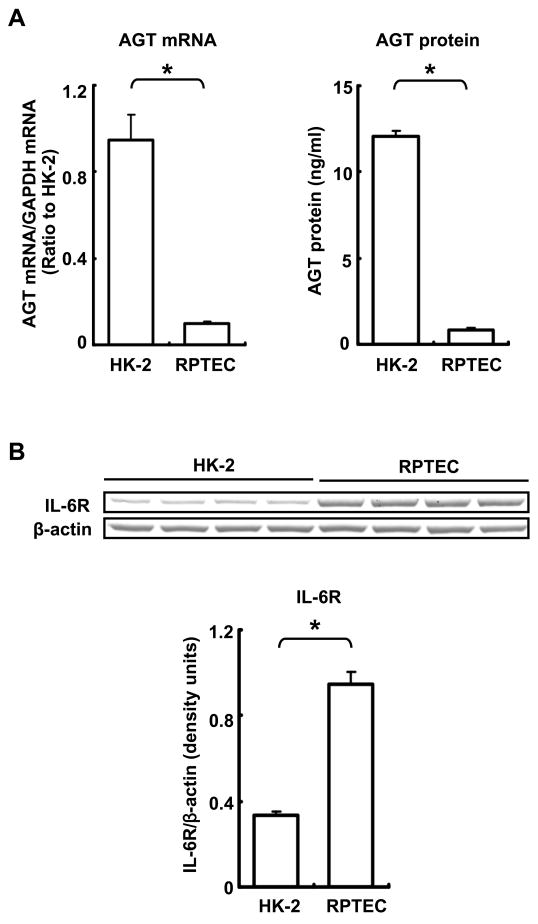
Basal AGT mRNA expression in RPTEC was markedly lower than in HK-2 cells (0.11 ± 0.01, ratio to HK-2, Fig. 1A). This lower expression level of AGT in RPTEC than in HK-2 cells was also observed at the protein level (HK-2: 12.04 ± 0.35 ng/ml, RPTEC: 0.84 ± 0.11 ng/ml, Fig. 1A). IL-6R protein expression in RPTEC was higher than in HK-2 cells (282 ± 17%, compared with HK-2, Fig. 1B).
Figure 1.
Comparison of basal expression levels of AGT and IL-6R between untreated HK-2 cells and RPTEC. Basal AGT mRNA levels were compared between HK-2 cells and RPTEC by real time RT-PCR (A, left panel, n=4). Expression levels of AGT mRNA were normalized based on GAPDH. Basal AGT protein was compared between two cell lines by human AGT ELISA (A, right panel, n=4). Data from human AGT ELISA were normalized based on total volume of media, respectively. Basal expression level of IL-6R was compared between two cell lines by western blot analysis (B, n=4). Data were normalized based on the expression level of each β-actin protein in the two cell lines. Data are expressed as mean ± SD. Asterisk indicates significant difference between the two cell lines (P < 0.05).
Comparison of basal activities of NF-κB and STAT3 between HK-2 cells and RPTEC
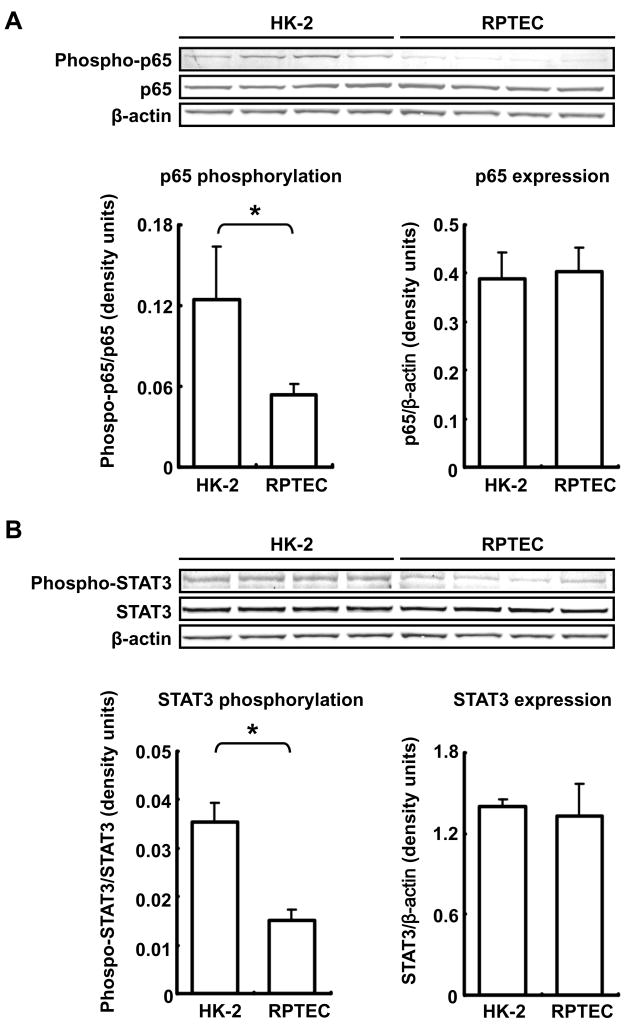
NF-κB activation involves phosphorylation of p65, a subunit of NF-κB (Sakurai et al., 1999) Basal phosphorylation level of p65 in RPTEC was lower than in HK-2 cells (43 ±7%, compared with HK-2) although total p65 expression levels were similar in both cell lines (Fig. 2A). Moreover, basal phosphorylation level of STAT3 in RPTEC was lower than in HK-2 cells (43 ± 7, compared with HK-2) although total STAT3 expression levels were similar in both cell lines (Fig. 2B).
Figure 2.
Comparison of basal activities of NF-κB and STAT3 between untreated HK-2 cells and RPTEC. Basal NF-κB activity and expression level were compared between HK-2 cells and RPTEC by western blot analysis (A, n=4). Phosphorylation level of NF-κB (p65) was normalized based on expression level of each total p65 in the two cell lines. Expression level of total p65 was normalized based on the expression levels of each β-actin protein in the two cell lines. Basal STAT3 activity and expression level were compared between the two cell lines by western blot analysis (B, n=4). Phosphorylation level of STAT3 was normalized based on expression level of each total STAT3 in the two cell lines. Expression level of total STAT3 was normalized based on the expression level of each β-actin protein in the two cell lines. Data are expressed as mean ± SD. Asterisk indicates significant difference between two cell lines (P < 0.05).
Effects of TNF-α, IL-1β, IL-6 and Ang II on AGT mRNA expression in RPTEC
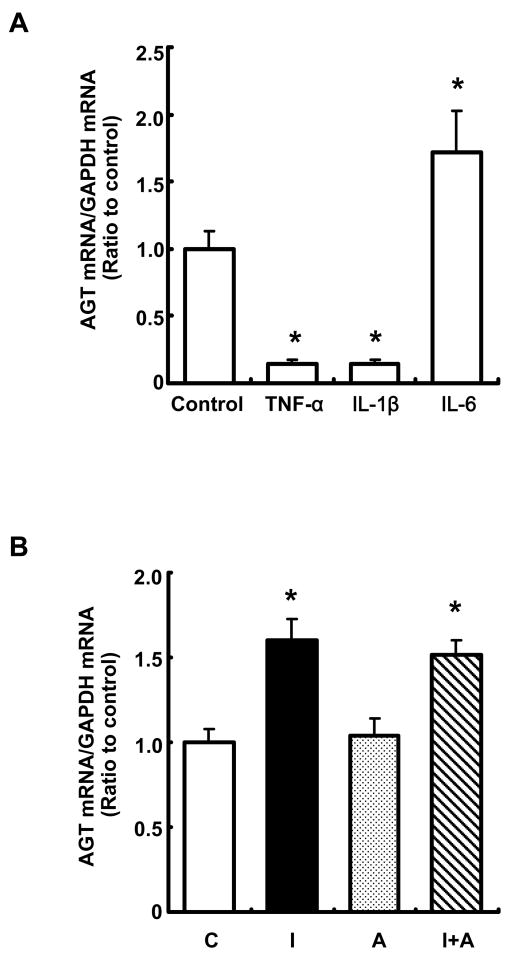
Treatment with 10 ng/ml TNF-α suppressed AGT mRNA expression at 24 hr (Fig. 3A, 0.14 ± 0.03, ratio to control). Furthermore, the same concentration of IL-1β decreased AGT mRNA expression (Fig. 3A, 0.14 ± 0.03, ratio to control). Only IL-6 (10 ng/ml) augmented AGT mRNA expression (1.72 ± 0.31, ratio to control) in cytokines tested in this study (Fig. 3A). Treatment with 10−7 M Ang II did not change AGT mRNA expression. Additionally, Ang II did not further augment IL-6-increased AGT expression (Fig. 3B).
Figure 3.
Effects of TNF-α, IL-1β, IL-6 and Ang II on AGT mRNA expression in RPTEC. After treatment with TNF-α, IL-1β and IL-6 (10 ng/ml each cytokine) for 24 hr (A, n=4). To test the effect of Ang II on AGT expression, cells were treated with 10 ng/ml IL-6 and/or 10−7 M Ang II for 24 hr (B, n=4). AGT mRNA was measured by real time RT-PCR. Expression levels of AGT mRNA were normalized based on GAPDH. C: control, I: IL-6, A: Ang II, I+A: IL-6+Ang II. Data are expressed as relative values compared with the control and represent the mean ± SD. Asterisk indicates significant difference compared to the control (P < 0.05).
Time- and dose-dependencies of IL-6 effects on AGT mRNA expression in RPTEC
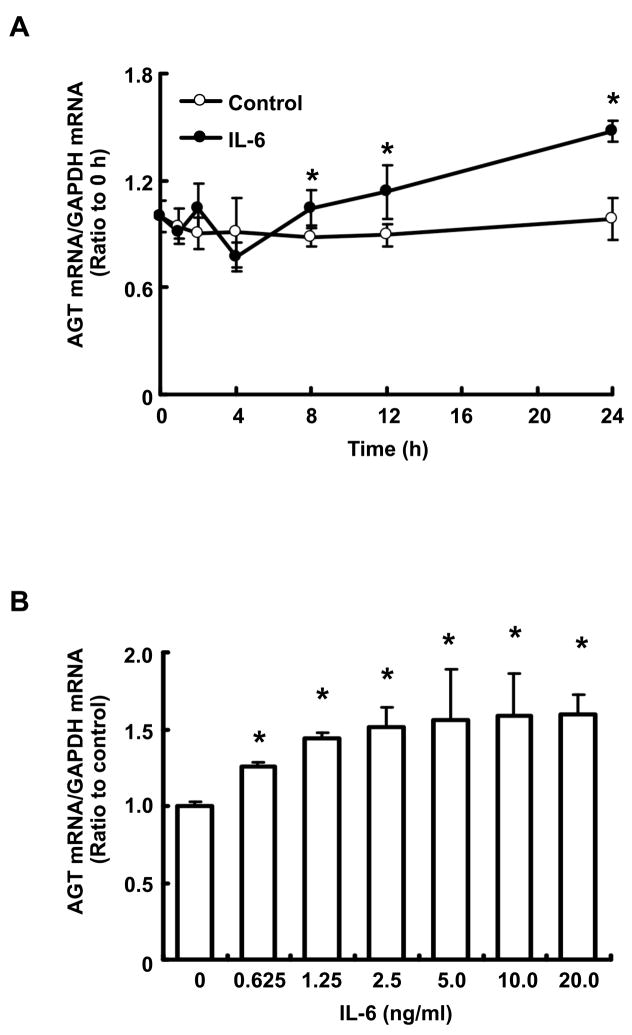
Treatment with 10 ng/ml IL-6 increased AGT mRNA expression in a time-dependent manner (Fig. 4A). A significant increase compared to the control was observed after 8 hr. This increment in AGT mRNA expression induced by IL-6 was dose-dependent at 24 hr (Fig. 4B).
Figure 4.
Time- and dose-dependencies of effect of IL-6 on AGT mRNA expression in RPTEC. RPTEC were treated with 0–20.0 ng/ml IL-6 (B, n=4) for up to 24 hr (A, n=4). After the treatments, AGT mRNA was measured by real-time RT-PCR. Expression levels of AGT mRNA were normalized based on GAPDH. Data are expressed as relative values compared with 0 hr or the control and represent the mean ± SD. Open circle indicates result in control group and closed circle indicates result in IL-6 treatment group. Asterisk indicates significant difference compared to the control (P < 0.05).
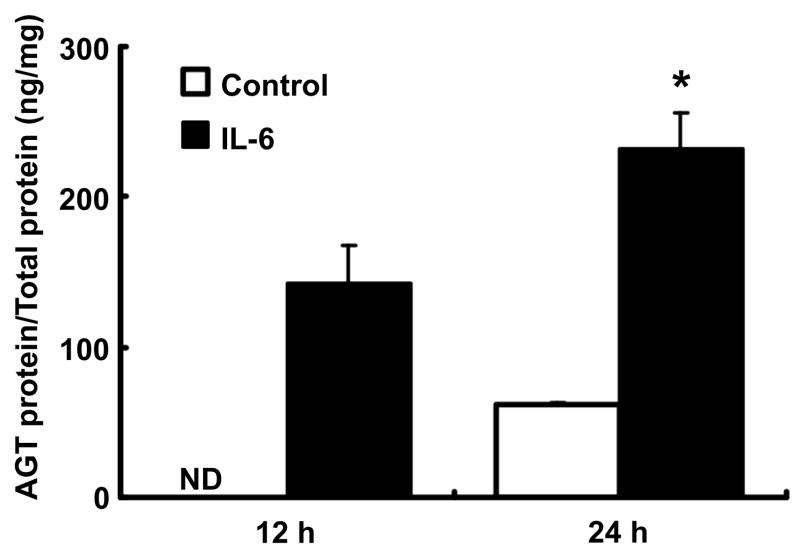
Effects of IL-6 on AGT protein expression in RPTEC
In control cells, accumulated AGT protein in the medium was not detectable at 12 hr. In contrast, AGT protein level following treatment with 10 ng/ml IL-6 was detectable at the same time point (Fig. 5, 141.9 ± 25.6 ng AGT/mg total protein). AGT protein levels were detectable in 24 hr accumulation in both groups. AGT protein levels were significantly increased by treatment with IL-6 in medium at the time (Fig. 5, 61.4 ± 1.0 and 232.4 ± 23.9 ng AGT/mg total protein, control vs. IL-6 treatment). However, intracellular AGT protein was below the detection level both in control and IL-6 treatment groups at any time point tested.
Figure 5.
Effects of IL-6 on AGT protein expression in RPTEC. RPTEC were treated with 10 ng/ml IL-6 for 12 and 24 hr (n=4). After treatment, AGT protein levels in medium were measured by human AGT ELISA. Expression levels of AGT protein were normalized based on total volume of the medium. Data are expressed as mean ± SD. Open column indicates result in control group and black column indicates result in IL-6 treatment group. Asterisk indicates significant difference compared to the control (P < 0.05).
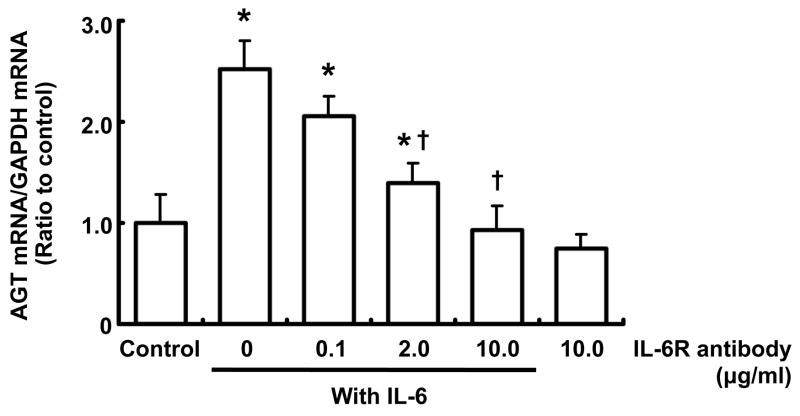
Effects of IL-6R antibody on AGT mRNA augmentation by IL-6 in RPTEC
Treatment with 10 ng/ml IL-6 alone increased AGT mRNA expression. This augmentation of AGT was attenuated by 2 and 10 μg/ml IL-6R antibody at 24 hr (Fig. 6). Treatment with 10 μg/ml IL-6R antibody alone did not have any effect on AGT mRNA.
Figure 6.
Effects of IL-6R antibody on AGT mRNA augmentation by IL-6 in RPTEC. RPTEC were treated with anti-IL-6R antibody and/or 10 ng/ml IL-6 for 24 hr (n=4). After the treatments, AGT mRNA was measured by real-time RT-PCR. Expression levels of AGT mRNA were normalized based on GAPDH. Data are expressed as relative values compared to the control and expressed as mean ± SD. Asterisk indicates significant difference compared to the control (P < 0.05). Dagger indicates significant difference compared to treatment with IL-6 alone (P < 0.05).
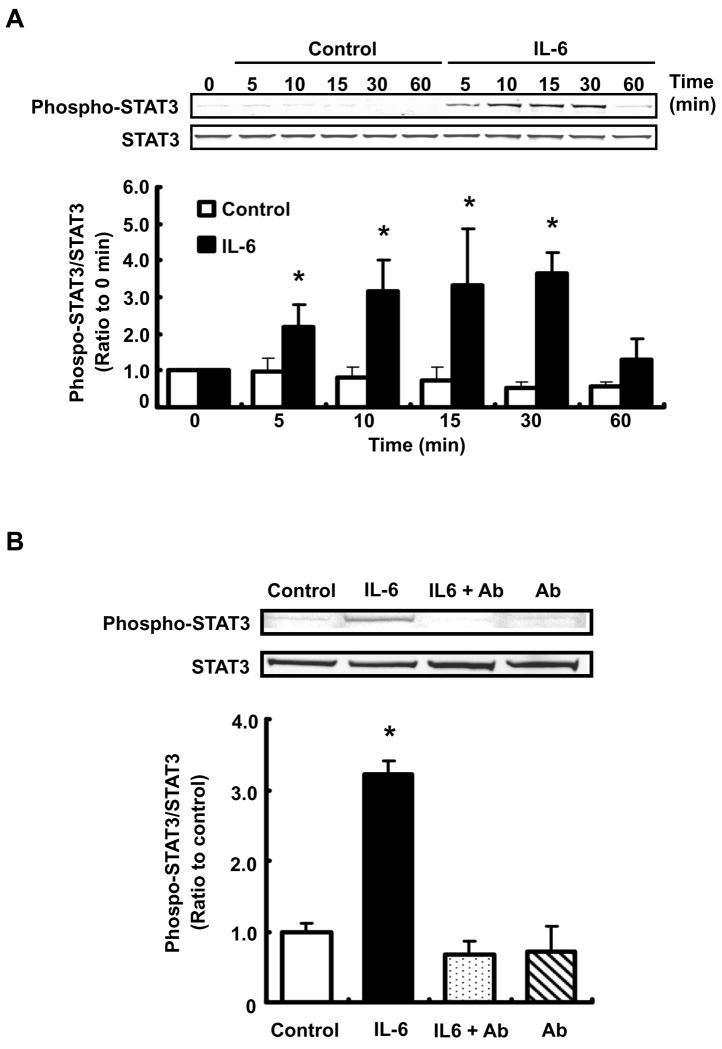
Effect of IL-6 on STAT3 activity in RPTEC
STAT3 phosphorylation (Tyr705) by 10 ng/ml IL-6 was increased compared to control at 5, 10, 15 and 30 min (Fig 7A, 2.21 ± 0.61, 3.15 ± 0.87, 3.34 ± 1.54 and 3.36 ± 0.55, respectively, ratio to 0 min). The difference of phosphorylation level between control and IL-6 treatment was not observed at 24 hr (data not shown).
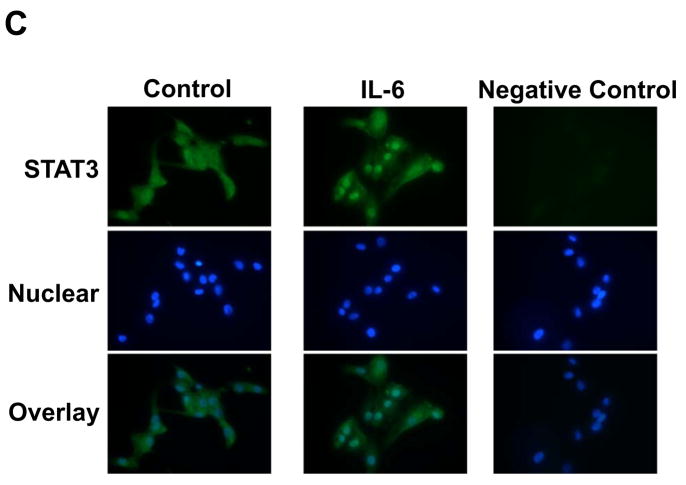
Figure 7.
Effect of IL-6 on STAT3 activity in RPTEC. RPTEC were treated with 10 ng/ml IL-6 for 0–60 min (A, n=4). Additionally, cells were pretreated with 10 μg/ml anti-IL-6R antibody for 1 hr; thereafter, cells were treated with 10 ng/ml IL-6 for 30 min (B, n=4). Ab: anti-IL-6R antibody. After these treatments, STAT3 phosphorylation level was evaluated by western blot analysis. Phosphorylation level of STAT3 was normalized based on expression level of total STAT3. Data are expressed as relative values compared with 0 hr in panel A, and expressed as mean ± SD. Asterisk indicates significant difference compared to the control (P < 0.05). Translocation of STAT3 by IL-6 was examined by immunofluorescence staining (C). After the treatment with 10 ng/ml IL-6 for 30 min, localization of STAT3 in RPTEC was observed and photographed under a fluorescence microscope. Left pictures indicate STAT3 staining and nuclear staining in the control. Center pictures indicate STAT3 staining and nuclear staining in IL-6-treated cells. Right pictures indicate STAT3 staining without primary antibody and nuclear staining as a negative control. Lower panels indicate merged images.
To determine the contribution of IL-6R to the IL-6-induced STAT3 phosphorylation, cells were pretreated with anti-IL-6R antibody for 1hr before treatment with IL-6 for 30 min. The STAT3 phosphorylation by IL-6 was attenuated by pretreatment with 10 μg/ml IL-6 antibody (Fig. 7B).
Using immunofluorescence staining, we observed translocation of STAT3 by IL-6. In control condition, STAT3 was localized primarily in cytoplasm (Fig. 7C left panels). Translocation of STAT3 into nuclei occurred with IL-6 treatment (10 ng/ml) was confirmed by merged images (Fig. 7C, center and lower panels). In the absence of primary antibody against STAT3, there was no fluorescence except for nuclei by 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining (Fig. 7C, right panels).
Effect of STAT3 inhibitor on AGT augmentation by IL-6 in RPTEC
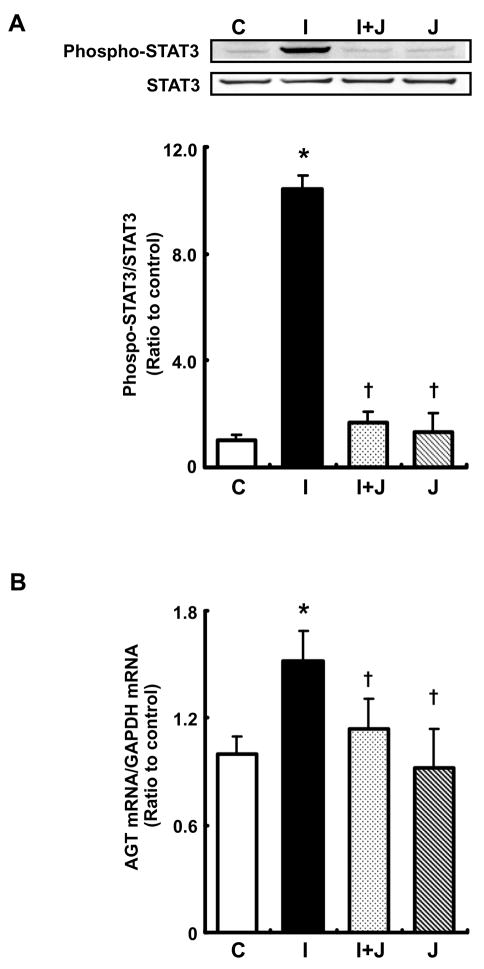
JSI-124, an inhibitor of STAT3 activation (Blaskovich et al., 2003), inhibited STAT3 phosphorylation by treatment with 10 ng/ml IL-6 for 30 min (Fig. 8A). Treatment with JSI-124 alone did not affect STAT3 phosphorylation compared with control. The inhibitor attenuated increment in AGT mRNA expression by treatment with 10 ng/ml IL-6 (Fig. 8B). Treatment with JSI-124 alone did not show effect on basal AGT expression.
Figure 8.
Effect of STAT3 inhibitor on AGT augmentation by IL-6 in RPTEC. Cells were treated with 10 ng/ml IL-6 and/or 0.2 μM JSI-124 for 30 min and 24 hr. STAT3 phosphorylation level was evaluated by western blot analysis (A, n=3). Phosphorylation level of STAT3 was normalized based on expression level of total STAT3. AGT mRNA was measured by real-time RT-PCR (B, n=4). Expression levels of AGT mRNA were normalized based on GAPDH. C: control, I: IL-6, I+J: IL-6+JSI-124, J: JSI-124. Data are expressed as relative values compared with the control and represent the mean ± SD. Asterisk indicates significant difference compared to the control (P < 0.05). Dagger indicates significant difference compared to treatment with IL-6 alone (P < 0.05).
Discussion
The present data demonstrates the effects of IL-6 on human AGT expression in primary cultured human renal proximal tubular epithelial cells. Several in vivo studies in rodents have demonstrated Ang II-induced augmentation of renal AGT expression (Schunkert et al., 1992; Kobori et al., 2001; Kobori et al., 2007b). Moreover, Ang II increases renal IL-6 levels (Ruiz-Ortega et al., 2002). It has also been demonstrated that NF-κB and STAT3 activation contribute to the AGT augmentation by Ang II in hepatocytes (Brasier et al., 2000; Jamaluddin et al., 2000; Li and Brasier, 1996). In the present study, we compared basal expression levels of AGT and IL-6R, and basal activities of NF-κB and STAT3 between HK-2 cells and RPTEC to characterize these cell lines. AGT mRNA and protein expression levels in HK-2 cells were higher than those in RPTEC (Fig. 1A). Furthermore, basal NF-κB and STAT3 activities in HK-2 cells were higher than those in RPTEC (Fig. 2A and B). The high basal NF-κB activity in HK-2 cells has been shown previously (Navarro et al., 2006; Satou et al., 2008). These results indicate that high basal NF-κB and STAT3 activities contribute to high basal AGT expression in HK-2 cells. The basal NF-κB and STAT3 activities in HK-2 cells might be caused by immortalization or culture conditions of this cell line.
Basal IL-6R expression in RPTEC was higher than in HK-2 cells (Fig. 1B); in contrast, basal STAT3 activity in RPTEC was lower than in HK-2 cells (Fig. 2B). Based on these characteristics of RPTEC, we examined the contribution of IL-6 to AGT regulation and its mechanism in this cell line. In comparing the effects of cytokines on AGT expression in RPTEC, we observed TNF-α suppressed AGT mRNA expression (Fig. 3). Cardiac restricted overexpression of TNF-α reduced cardiac AGT expression (Flesch et al., 2003). In human adipocytes, TNF-α suppressed AGT expression (Wang et al., 2005). The present results are consistent with these reports. In contrast, it has been reported that TNF-α contributes to an augmentation of AGT in several tissues (Nyui et al., 1997). Therefore, the effects of TNF-α on AGT expression may be cell- and tissue-specific. Endogenous AGT expression was not affected by IL-1β in primary cultured rat hepatocytes (Ohtani et al., 1992). Recently, it was reported that IL-1β abrogated Ang II-induced connective tissue growth factor production in mesangial cells (Sanchez-Lopez et al., 2008). In contrast to the effects of IL-6, we observed that IL-1β decreased AGT mRNA expression (Fig. 3). These data suggest a possible antagonistic activity of IL-1β on RAS activity.
Of the cytokines examined in this study, only IL-6 increased AGT mRNA expression (Fig. 3). The effects of IL-6 on AGT expression were in time- and dose-dependent (Fig. 4). Moreover, AGT protein expression and accumulation in medium was elevated by IL-6 (Fig. 5). These results suggest that intrarenal IL-6 increases AGT expression in human renal proximal tubular cells. Because renal proximal tubular cells are the main source of AGT in the kidney (Ingelfinger et al., 1990; Terada et al., 1993), IL-6-AGT axis in the cells may be able to play an important role in intrarenal Ang II generation. Increases in blood pressure and albumin excretion elicited by Ang II infusions in IL-6 knockout mice were weaker than those in wild type mice although it was shown that the increase in blood pressure was not due to renal injury caused by Ang II infusion (Lee et al., 2006). Therefore, the intrarenal IL-6-AGT axis suggested by the present results may help explain the development of hypertension and/or renal injury.
Our previous study showed that co-stimulation with Ang II and IL-6 increased AGT expression (Satou et al., 2008). Moreover, stimulation with IL-6 alone did not have any effect on AGT expression in HK-2 (Satou et al., 2008). The present study demonstrated that Ang II did not cause further augmentation of IL-6-induced AGT upregulation. Although more detailed experiments will be required to elucidate effect of Ang II on IL-6-induced AGT upregulation, the different effects of Ang II and IL-6 on AGT expression in these two human renal proximal tubular epithelial cell lines, HK-2 and RPTEC, may be due to differences in basal NF-κB and STAT3 activities and in basal AGT expression level. In particular, the difference in basal IL-6R expression is strongly associated with the differences in response to IL-6 because anti-IL-6R antibody completely neutralized the AGT augmentation by IL-6 in RPTEC (Fig. 6). On the other hand, expression levels of β-actin, NF-κB and STAT3 were the same in both cell lines indicating that basal characteristics of the two cell lines are similar as human renal proximal tubular cells. These findings suggest that the mechanism underlying the contribution of IL-6 to the augmentation may be dependent on the activities of signal transducers and the receptor expression levels in renal proximal tubular cells. However, further study will be necessary to elucidate mechanisms underlying AGT augmentation by IL-6 in human kidneys under different clinical status.
After binding of IL-6 to membrane IL-6R or soluble IL-6R, the complex and GP130 protein synergistically stimulate Janus kinase 2. Thereafter, the activated kinase activates STAT3 (Hodge et al., 2005). In human hepatocytes, IL-6 augments AGT expression via STAT3 activation (Jain et al., 2007; Ohtani et al., 1992; Ray et al., 2005). In the present study, STAT3 was phosphorylated by IL-6 in RPTEC (Fig. 7A). The anti-IL-6R antibody attenuated the STAT3 phosphorylation by IL-6 (Fig. 7B) indicating that IL-6R participates in the STAT3 activation by IL-6 in RPTEC. Furthermore, STAT3 was translocated into nuclei by the treatment (Fig. 7C). An inhibitor of STAT3 activation attenuated STAT3 phosphorylation (Fig. 8A) and increment in AGT mRNA expression (Fig. 8B) by treatment with 10 ng/ml IL-6. These data indicate that the AGT augmentation by IL-6 is mediated by STAT3 activation in RPTEC as well as in hepatocytes.
In summary, we have demonstrated that IL-6 stimulates human AGT expression through STAT3 activation in human renal proximal tubular epithelial cells, suggesting that IL-6 plays an important role in augmentation of AGT by Ang II in human renal proximal tubular epithelial cells which may contribute to the development of hypertension and renal injury. Therefore, elucidation of the mechanisms underlying this augmentation may lead to new strategies to treat hypertension, and cardiovascular and renal diseases.
Acknowledgments
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408), National Center for Research Resources (P20RR017659), and National Heart, Lung, and Blood Institute (R01HL026371). The authors acknowledge the valuable comments and excellent technical assistance from Toshie Saito, MD and Akemi Katsurada, MS (Tulane University).
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- Brasier AR, Jamaluddin M, Han Y, Patterson C, Runge MS. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol Cell Biochem. 2000;212:155–69. [PubMed] [Google Scholar]
- Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–66. doi: 10.1161/01.atv.0000021412.56621.a2. [DOI] [PubMed] [Google Scholar]
- Corvol P, Jeunemaitre X. Molecular genetics of human hypertension: role of angiotensinogen. Endocr Rev. 1997;18:662–77. doi: 10.1210/edrv.18.5.0312. [DOI] [PubMed] [Google Scholar]
- de Haij S, Adcock IM, Bakker AC, Gobin SJ, Daha MR, van Kooten C. Steroid responsiveness of renal epithelial cells. Dissociation of transrepression and transactivation. J Biol Chem. 2003;278:5091–8. doi: 10.1074/jbc.M209836200. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–8. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- Flesch M, Hoper A, Dell’Italia L, Evans K, Bond R, Peshock R, Diwan A, Brinsa TA, Wei CC, Sivasubramanian N, Spinale FG, Mann DL. Activation and functional significance of the renin-angiotensin system in mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2003;108:598–604. doi: 10.1161/01.CIR.0000081768.13378.BF. [DOI] [PubMed] [Google Scholar]
- Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Ingelfinger JR, Jung F, Diamant D, Haveran L, Lee E, Brem A, Tang SS. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: autocrine ANG II feedback. Am J Physiol. 1999;276:F218–27. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–23. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Li Y, Patil S, Kumar A. HNF-1alpha plays an important role in IL-6-induced expression of the human angiotensinogen gene. Am J Physiol Cell Physiol. 2007;293:C401–10. doi: 10.1152/ajpcell.00433.2006. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M, Meng T, Sun J, Boldogh I, Han Y, Brasier AR. Angiotensin II induces nuclear factor (NF)-kappaB1 isoforms to bind the angiotensinogen gene acute-phase response element: a stimulus-specific pathway for NF-kappaB activation. Mol Endocrinol. 2000;14:99–113. doi: 10.1210/mend.14.1.0400. [DOI] [PubMed] [Google Scholar]
- Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–60. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007a;59:251–87. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- Kobori H, Ozawa Y, Satou R, Katsurada A, Miyata K, Ohashi N, Hase N, Suzaki Y, Sigmund CD, Navar LG. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007b;293:F938–45. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–80. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H935–40. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- Li J, Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the Rel A (nuclear factor-kappaB p65) transcription factor: one mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol Endocrinol. 1996;10:252–64. doi: 10.1210/mend.10.3.8833654. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Fujibayashi M, Fujiwara Y, Kaneko T, Xia C, Imai E, Kamada T, Ando A, Ueda N. Angiotensin II stimulates interleukin-6 release from cultured mouse mesangial cells. J Am Soc Nephrol. 1995;6:95–101. doi: 10.1681/ASN.V6195. [DOI] [PubMed] [Google Scholar]
- Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro JF, Milena FJ, Mora C, Leon C, Garcia J. Renal pro-inflammatory cytokine gene expression in diabetic nephropathy: effect of angiotensin-converting enzyme inhibition and pentoxifylline administration. Am J Nephrol. 2006;26:562–70. doi: 10.1159/000098004. [DOI] [PubMed] [Google Scholar]
- Nyui N, Tamura K, Yamaguchi S, Nakamaru M, Ishigami T, Yabana M, Kihara M, Ochiai H, Miyazaki N, Umemura S, Ishii M. Tissue angiotensinogen gene expression induced by lipopolysaccharide in hypertensive rats. Hypertension. 1997;30:859–67. doi: 10.1161/01.hyp.30.4.859. [DOI] [PubMed] [Google Scholar]
- Ohtani R, Yayama K, Takano M, Itoh N, Okamoto H. Stimulation of angiotensinogen production in primary cultures of rat hepatocytes by glucocorticoid, cyclic adenosine 3′, 5′-monophosphate, and interleukin-6. Endocrinology. 1992;130:1331–8. doi: 10.1210/endo.130.3.1311238. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Ray S, Boldogh I, Brasier AR. STAT3 NH2-terminal acetylation is activated by the hepatic acute-phase response and required for IL-6 induction of angiotensinogen. Gastroenterology. 2005;129:1616–32. doi: 10.1053/j.gastro.2005.07.055. [DOI] [PubMed] [Google Scholar]
- Re RN. Tissue renin angiotensin systems. Med Clin North Am. 2004;88:19–38. doi: 10.1016/s0025-7125(03)00124-x. [DOI] [PubMed] [Google Scholar]
- Recinos A, 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR. Angiotensin II induces IL-6 expression and the Jak-STAT3 pathway in aortic adventitia of LDL receptor-deficient mice. Atherosclerosis. 2007;194:125–33. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002:12–22. doi: 10.1046/j.1523-1755.62.s82.4.x. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–6. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez E, Rodriguez-Vita J, Cartier C, Ruperez M, Esteban V, Carvajal G, Rodrigues-Diez R, Plaza JJ, Egido J, Ruiz-Ortega M. Inhibitory effect of interleukin-1beta on angiotensin II-induced connective tissue growth factor and type IV collagen production in cultured mesangial cells. Am J Physiol Renal Physiol. 2008;294:F149–60. doi: 10.1152/ajprenal.00129.2007. [DOI] [PubMed] [Google Scholar]
- Satou R, Gonzalez-Villalobos RA, Miyata K, Ohashi N, Katsurada A, Navar LG, Kobori H. Costimulation with angiotensin II and interleukin 6 augments angiotensinogen expression in cultured human renal proximal tubular cells. Am J Physiol Renal Physiol. 2008;295:F283–9. doi: 10.1152/ajprenal.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263:E863–9. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–9. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- van Kester MS, Out-Luiting JJ, von dem Borne PA, Willemze R, Tensen CP, Vermeer MH. Cucurbitacin I inhibits STAT3 and induces apoptosis in Sezary cells. J Invest Dermatol. 2008;128:1691–5. doi: 10.1038/sj.jid.5701246. [DOI] [PubMed] [Google Scholar]
- Wang B, Jenkins JR, Trayhurn P. Expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture: integrated response to TNF-alpha. Am J Physiol Endocrinol Metab. 2005;288:E731–40. doi: 10.1152/ajpendo.00475.2004. [DOI] [PubMed] [Google Scholar]