Abstract
A central tenet of neuroscience is that the precise patterns of connectivity among neurons in a given brain area underlie its function. However, assigning any aspect of perception or behavior to the wiring of local circuits has been challenging. Here, we review recent work in sensory neocortex that demonstrates the power of identifying specific cell types when investigating the functional organization of brain circuits. These studies indicate that knowing the identity of both the pre- and postsynaptic cell type is key when analyzing neocortical circuits. Furthermore, identifying the circuit organization of particular cell types in the neocortex allows the recording and manipulation of each cell type’s activity and the direct testing of its functional role in perception and behavior.
The connectivity of local inhibitory neocortical circuits
Since the pioneering anatomical work of Ramon y Cajal, it has been clear that the neocortex contains a diverse population of neurons. These neurons can be divided into two broad categories, excitatory pyramidal neurons, representing the majority of neocortical neurons, and inhibitory interneurons, representing the remaining ~20%. Each category can be further subdivided into a number of different functional classes. Because the classification of inhibitory neurons is more advanced than that of pyramidal cells [1], the cell-type specific organization of inhibitory circuits within the neocortex has been most extensively studied. These studies show that both the patterns of connectivity of inhibitory neurons and the properties of their synaptic connections can depend on the cell type of the two synaptic partners.
Inhibitory neurons can be differentiated by a combination of anatomical, physiological and molecular criteria. For example, the most common inhibitory neurons in the neocortex, fast-spiking (FS) cells, are known to share a distinct overall morphology, target the somas and proximal dendrites of their synaptic partners, exhibit a signature electrophysiological profile, and express the protein parvalbumin [2]. Each class of inhibitory neuron shares a different complement of these characteristics including: 1) distinctive morphologies (e.g., the neurogliaform (NGF) cell), 2) defined target regions on their postsynaptic partners (e.g., the axonal initial segment of pyramidal neurons by chandelier cells or the apical dendritic tuft of pyramidal neurons by Martinotti cells), 3) different protein complements (e.g., cholecystokinin and cannabinoid receptor type 1).
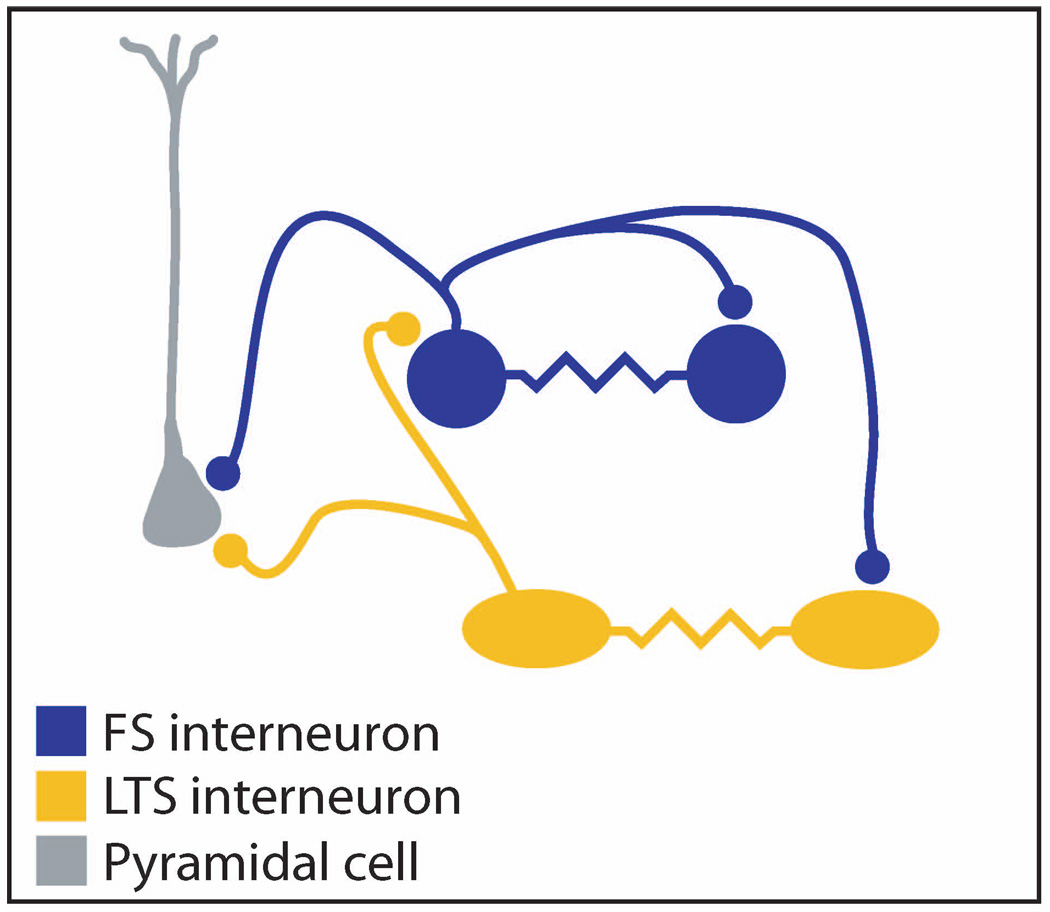
Using a combination of these properties, specific classes of inhibitory interneuron can be identified and targeted for physiologic recording. By recording simultaneously from two identified cells using whole-cell patch-clamp techniques, it was demonstrated that two classes of inhibitory neuron form cell-type specific connections with neighboring cells via both GABAergic and electrical synapses [3,4]. These experiments showed that neighboring FS cells are highly interconnected by GABAergic chemical synapses [3,4] while low-threshold spiking (LTS) cells are connected with pyramids and FS cells but rarely make GABAergic synapses onto other LTS cells [4]. Furthermore, these studies showed that neighboring FS cells connect via electrical synapses with very high probability (>60% of tested pairs) while avoiding neighboring non-FS cells [3,4]. Likewise, LTS cells are similarly interconnected via gap junctions while avoiding non-LTS cells [4] (see Fig. 1). Moreover, pairs of FS cells in adult animals are highly connected via electrical synapses [5], demonstrating that electrical coupling is not restricted to the immature neocortex.
Figure 1.
Inhibitory interneurons of the neocortex show cell-type specific patterns of GABAergic and electrical connections. Low threshold spiking (LTS; orange) cells form GABAergic connections with neighboring fast spiking (FS; blue) and pyramidal neurons (gray), but only rarely synapse onto other LTS cells. FS cells, in contrast, form GABAergic connections with neighboring FS cells as well as with LTS cells and pyramids. LTS are highly interconnected via electrical connections as are FS (blue) cells, but each cell type rarely forms gap junctions with other classes of inhibitory neuron.
These specific patterns of connectivity among different classes of inhibitory neuron demonstrate the importance of identifying cell types when trying to understand the organization of local neocortical circuits. Electrical coupling among FS cells and LTS cells is very common. However, because electrical coupling is mainly found among cells of the same type, and each population represents only a small fraction of all neocortical cells, recordings from randomly selected pairs of neocortical cells would only rarely reveal electrical connections, and the principles governing the pattern of these connections would remain obscure. Only by targeting these specific cell types for recording was their pattern of connectivity revealed.
Genetic labeling of specific cell types
Initially, FS and LTS cells were identified based on the appearance of the cell body and proximal dendrites in living neocortical slices, the cells’ electrophysiological responses, and their post-hoc morphological and immunohistochemical characterization [3,4]. The advent of several transgenic lines of mice in which fluorescent markers label specific classes of inhibitory neurons greatly accelerated the study of inhibitory circuits in the neocortex [6–11]. These studies revealed a variety of patterns of GABAergic chemical connections among inhibitory neurons. For example, although FS cells have a high probability of forming GABAergic connections with neighboring FS cells, they avoid neighboring multipolar bursting (MB) cells that express both parvalbumin and calbindin [8]. Neighboring FS cells also have a three-fold higher probability of connection than neighboring cannabinoid receptor-expressing, irregular spiking (CB1-IS) interneurons [11]. Like FS and CB1-IS cells, two types of calretinin-containing inhibitory neurons also exhibit cell-type specific patterns of GABAergic connections [7].
Studies of eight distinct types of GABAergic neuron revealed that all form electrical synapses with neighboring GABAergic neurons [3,4,6–8,11–13]. The majority of these inhibitory cell types form electrical synapses exclusively with cells of the same type, although there are some exceptions. Neurogliaform (NGF) cells in layer 2/3 (L2/3) are extensively electrically coupled to other NGF cells. However, they also form electrical synapses with neighboring FS cells, although with lower probability [13]. In a similar vein, the multipolar calretinin-expressing (MCR) GABAergic cells are electrically coupled to MB cells rather than to neighboring MCR cells [7].
Taken together, these data demonstrate that several networks of inhibitory neurons are formed within the neocortex by selective chemical and electrical synapses. The patterns of activity generated in the cortex resulting from the selective connections made by different types of inhibitory neuron are not understood. It has been suggested, for example, that neighboring FS cells form networks via both electrical and GABAergic synapses and facilitate γ-band oscillations (30–70 Hz) while neighboring LTS cells are interconnected via gap junctions and can generate lower frequency oscillations (3–10 Hz) [10,14,15]. However, it is important to note that these networks do not operate as isolated units but rather interact with pyramidal cells and other types of interneurons. Determining their role in shaping neocortical activity remains an active area of research.
The local connectivity of different classes of pyramidal neuron
Pyramidal neurons are the principal neurons of the neocortex, and their excitatory synaptic connections represent the main type of synaptic connection in the neocortex. The overall average connection probability among pyramidal cells is relatively low [16], and several hypotheses have been put forth on how interactions among pyramids are mediated. The connectivity among pyramidal neurons and certain types of inhibitory neurons, including FS cells, is high, suggesting that GABAergic neurons could mediate interactions among pyramids [17]. Recent work has shown that high-frequency trains of action potentials initiated in a single pyramidal cell can generate IPSPs in neighboring pyramids by recruiting local inhibitory Martinotti neurons [18,19]. These Martinotti cells modulate the sensory responses of the apical dendrites of layer 5 pyramids in vivo [20]. Furthermore, several lines of evidence suggest that single action potentials in a pyramidal neuron can also elicit network activity in the neocortex mediated by neighboring chandelier cells which paradoxically produce spikes in pyramids via depolarizing GABAergic synapses on the initial segment [21].
Another hypothesis is that, although the average connectivity among pyramids is quite low, the connection probability among selected classes of pyramidal neurons may actually be high. Several lines of evidence suggest that connections among pyramidal cells form subnetworks within the neocortex. It was shown that action potentials in layer 5 (L5) corticotectal pyramids elicited spikes in a limited repertoire of postsynaptic neurons [22]. Simultaneous whole-cell recordings from up to four neurons showed that once a synaptic connection has been identified in a small group of L5 pyramids, the likelihood of finding additional connections within this group is greater than expected from the average rate of connectivity [23,24]. Moreover, the probability of connection between a L2/3 pyramid and a pair of L5 pyramids is higher when the L5 cells are synaptically connected and when the L5 cells share similar firing patterns [25,26]. Similarly, pairs of connected L2/3 pyramidal cells are more likely to share excitatory input from L2/3 and L4 as compared with unconnected cells [27]. Furthermore, recent work has shown that radial clones of pyramidal neurons that are developmentally descended from the same mother cell are preferentially connected as compared to randomly selected neighboring pyramids [28].
The local connection patterns of pyramids correlate with their long-range targets
Unlike interneurons, the axons of pyramidal cells project both to local neighbors and to numerous distant brain areas with distinct functional roles. A morphological analysis of local intracortical axons showed that the local axonal trajectories of pyramidal neurons are less tortuous than those of neocortical interneurons suggesting that local axons of pyramids may be less selective than axons of interneurons [29]. However, the long-range projections of pyramids are thought to be quite selective and divide pyramids into different functional classes. Anatomical studies have demonstrated that neighboring pyramids intermingled within a single cortical layer can have distinct long-range projections. Do the patterns of local connections among these neighbors correlate with their patterns of long-range projections?
The projection patterns of the long-range axons of these different pyramidal cell classes can be used to label the different functional classes by injecting a retrograde neuronal tracer into the cells’ axonal targets. These retrogradely labeled cells can then be targeted for electrophysiological recording in cortical slices. Using this approach, several authors have shown that one class of pyramidal neuron can have a different probability of interconnecting relative to another class [30,31]. However, these findings do not necessarily imply that excitatory connections are selective; they are also consistent with the hypothesis that the probability of connection is a global property specific to each pyramidal cell type.
Using fluorescent beads to retrogradely label pyramids whose long-range axons targeted different brain regions, we recently compared the probability of connection among different classes of pyramidal neuron and demonstrated that the connectivity among neighboring pyramids reflects the identity of both the presynaptic and postsynaptic cell type [32]. The probability of connection of a L5 corticocortical (CC) pyramid with a neighboring L5 corticotectal (CT) pyramid was almost four-fold higher than its probability of connection with another L5 CC pyramid (see Fig. 2). These results suggest that the local connections among pyramids correlate with their long-range connections. Furthermore, given that each cortical region contains many cell types with distinct long-range projections, these results suggest that a number of different pyramidal cell networks exist within the cortex.
Figure 2.
The patterns of local connections among layer 5 pyramidal neurons reflect their long-range axonal targets. The probability of identifying a synaptic connection from pyramids projecting to the contralateral cortex (red) onto pyramids projecting to the ipsilateral superior colliculus (green) is almost fourfold the probability of identifying a connection between neighboring corticocortical pyramids.
Cell-type specificity and Peters’ rule
One model of the organization of pyramidal cell circuits is often described as a generalization of Peters’ rule [33,34]. Peters first suggested that geniculocortical axons synapse onto neocortical neurons in proportion to the availability of all of the neuronal elements in the thalamorecipient area of the neocortex [35]. Under this scheme, synaptic connections among pyramids reflect the geometric overlap between the presynaptic axon and the postsynaptic dendrite, or the axodendritic overlap. Recent laser scanning photostimulation of pyramids in different layers of barrel cortex showed that, although the strength of interactions among layers of barrel cortex correlated with the axodendritic overlap of pyramidal cells in those layers, some notable exceptions were also identified [36]. In addition, a recent paper showed that the dendritic structure of the postsynaptic neurons could not account for the relative strength of different input pathways to the neocortex [37]. Using quadruple recordings among heterogeneous populations of identified pyramids, we showed that the probability of connection among different classes of pyramidal neuron does not solely reflect the axodendritic overlap between pyramidal cell types [32]. Taken together, these results suggest that axodendritic overlap alone is insufficient to describe both the strength and the patterns of synaptic connection in the neocortex.
The synaptic properties of local neocortical circuits
Just as the connection patterns among neocortical neurons can reflect the identity of the presynaptic and postsynaptic cell type, so can the strength and dynamics of neocortical synaptic connections. The properties of GABAergic synapses among different types of inhibitory neuron can vary greatly. For example, FS cell interconnections are very reliable whereas synapses among interneurons expressing cannabinoid receptors (CB1-IS cells) are highly unreliable [11]. Moreover, postsynaptic GABAergic responses can be mediated by GABAA or GABAB receptors. For example, while action potentials in FS cells generate fast GABAA responses in their postsynaptic partners, action potentials in neurogliaform (NGF) cells produce a mixed GABAA-mediated and GABAB-mediated response in neighboring pyramids and other inhibitory cells [38].
The properties of pyramidal cell synapses may also show cell-type specificity. For example, the strength and dynamic properties of excitatory synaptic connections can depend on the type of postsynaptic cell [39–41]. Furthermore, a recent paper showed that synaptic properties correlate with the dendritic morphology of pyramidal neurons in prefrontal cortex (PFC) [42]. The main type of pyramidal cell in the PFC is the ‘complex’ pyramid. These cells have a much higher rate of reciprocal connections than ‘simple’ pyramids in the same region, and these connections are strongly facilitating rather than undergoing the more typical depression described for pyramid-pyramid synapses [42]. Taken together, these findings indicate that both the patterns of connectivity and the synaptic dynamics of neocortical connections show cell-type specificity.
Future directions
Cell identification
Central to the success of the work we describe here is the concept of cell identity. Focusing on particular cell types creates a common language among different laboratories who study neocortical circuits. It also allows for the comparison of data across individual animals. For example, the precise connections of an individual neuron within the brain of a single subject could be idiosyncratic, but the patterns of connectivity among different cell types are likely to recur across both individuals and across species. Importantly, cell type identification allows data from in vivo and in vitro experiments to be pooled thus taking advantage of the complementary information provided by these approaches.
Improved methods for identifying specific classes of neuron promise to greatly enhance our ability to dissect brain circuits. To date, the few studies of pyramidal cell types have largely relied on anatomically based methods to identify the cells. However, this approach is limited by the large number of pyramids for which the projection patterns remain unknown [43]. Increasing our knowledge of the morphology and projection patterns of pyramidal neurons will facilitate the analysis of the network organization of the neocortex.
This knowledge can be harnessed by viral and other approaches to express proteins in particular subsets of neurons to probe the functional organization of the neocortex. For example, Petreanu and colleagues recently used a variety of techniques to selectively express the light-gated cation channel, channelrhodopsin-2 (ChR2), throughout the axonal arbor of different cortical afferents [37]. Light was used to selectively stimulate these afferents to determine the strength of different inputs onto L3, L5A and L5B pyramidal neurons. Rabies and herpes virus-based vectors can be stereotaxically injected in the efferent long-range targets of pyramids to retrogradely transport fluorescent and light-gated proteins to the desired cell bodies [44]. This approach will allow particular subsets of pyramids to be targeted genetically (like the corticotectal pyramid). Further refinements to these approaches, including using specific promoters or combining these approaches with systems such as the Cre/loxP system, will allow additional specificity of expression.
Mapping neuroanatomical descriptions of cell types onto molecular definitions and developing appropriate transgenic mouse lines that express selected proteins (e.g. a fluorescent protein or Cre recombinase) in subsets of pyramidal neurons will further accelerate progress in this area, although much work will need to be done to determine or confirm the class or classes of pyramidal neuron identified in these lines [45]. These lines can then be shared among laboratories, forming a common platform upon which to study neocortical circuits, much like the studies of inhibitory networks using transgenic lines of mice that express fluorescent proteins in known subsets of inhibitory neurons.
Functional organization of the brain
A number of laboratories are working to provide a synapse-level wiring diagram of whole brains termed the connectome [46]. To take advantage of connectome-derived information, it will be necessary to identify the cell types whose connections are determined anatomically. Cell type identification of a subset of interneurons may be determined based on their local morphology (e.g., chandelier and Martinotti cells). However, the determination of pyramidal cell types will require, in addition, reconstruction of their long-range connections. By identifying the cell types, recurring patterns of connections can be compared across individual reconstructed brains. Tools for cell-type specific labeling will then allow targeted recording from these cells to determine the physiological properties of their synaptic interactions.
Making the leap from circuits to function
Ultimately, the effort to fully describe brain circuitry will need to be mapped to the extensive body of work on the response properties of neurons recorded in vivo. Currently, a large gap exists between the circuit level description derived from anatomical and in vitro experiments and the functional description developed from in vivo experiments. For example, how the response properties of cortical sensory neurons map onto the morphological classes is still an open question. Uncovering the circuit organization of particular cell types, as described here, will help to provide the groundwork for detecting and manipulating each cell type’s activity and testing the circuit’s operation in the intact brain. A number of approaches have recently been used to identify and manipulate specific cell types in in vivo experiments, including two-photon imaging of fluorescently tagged cells in transgenic animals [47,48], juxtacellular recording of individual neurons followed by morphological and histological characterization [49,50], and cell-type specific optogenetic manipulations of neural activity [14,15].
Taken together, this recent work in sensory neocortex underscores how productive marrying anatomical and physiological approaches to brain circuitry can be. By studying the functional connectivity of specific cell types within the neocortex, these studies have revealed recurring patterns of connection among both inhibitory interneurons and pyramids. These studies are laying the groundwork for manipulating each cell type’s activity and testing the circuit’s effect on perception and behavior. Recent efforts to develop synapse-level large-scale anatomical approaches for defining brain circuitry, as well as parallel efforts to develop similarly scaled functional approaches, will allow us to fully exploit the potential of this approach. Combined with a number of new genetic techniques expressing selected proteins in defined cell types, these developments promise to accelerate our understanding of the functional organization of the brain.
Acknowledgements
This work was supported by NIH grants EY009120 and EY012114 (S.H.) and by NIH grant NS049706 and the Stanford Neuro-Innovate Fund (S.P.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uematsu M, Hirai Y, Karube F, Ebihara S, Kato M, Abe K, Obata K, Yoshida S, Hirabayashi M, Yanagawa Y, et al. Quantitative chemical composition of cortical GABAergic neurons revealed in transgenic venus-expressing rats. Cereb Cortex. 2008;18:315–330. doi: 10.1093/cercor/bhm056. [DOI] [PubMed] [Google Scholar]
- 3.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 4.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 5.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer AH, Katona I, Blatow M, Rozov A, Monyer H. In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J Neurosci. 2002;22:7055–7064. doi: 10.1523/JNEUROSCI.22-16-07055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caputi A, Rozov A, Blatow M, Monyer H. Two calretinin-positive GABAergic cell types in layer 2/3 of the mouse neocortex provide different forms of inhibition. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn175. [DOI] [PubMed] [Google Scholar]
- 8.Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron. 2003;38:805–817. doi: 10.1016/s0896-6273(03)00300-3. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galarreta M, Erdelyi F, Szabo G, Hestrin S. Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex. 2008;18:2296–2305. doi: 10.1093/cercor/bhm253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu Z, Galarreta M, Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci. 2003;23:96–102. doi: 10.1523/JNEUROSCI.23-01-00096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon A, Olah S, Molnar G, Szabadics J, Tamas G. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25:6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009 doi: 10.1038/nature07991. The authors show that efficient and specific Cre-recombinase-dependent expression of light-gated ion channels in fast spiking (FS) cells and pyramids, respectively, can be achieved using an adenovirus-associated virus expression system and a double-floxed inverse open reading frame (DIO-AAV). Using this approach, they investigate the role of FS cells in the generation of γ-band oscillations in the neocortex. Their results include a demonstration that inhibiting FS cells can reduce the power of γ-band oscillations in vivo.
- 15. Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009 doi: 10.1038/nature08002. By selectively expressing a light-gated cation channel in either fast-spiking (FS) interneurons or a subset of pyramidal neurons in vivo, these authors showed that stimulating FS cells preferentially enhanced γ-band oscillations relative to pyramidal cell stimulation. Furthermore, they showed that the sensory response to whisker stimulation could be modulated by the temporal relationship between these oscillations and the sensory stimulus.
- 16.Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci. 2007;1:19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. These authors report that trains of high-frequency action potentials in individual L5 pyramidal neurons can reliably elicit action potentials in postsynaptic Martinotti cells which in turn produce IPSPs in neighboring pyramidal cells. Morphological reconstruction of pyramidal cells and Martinotti cells connected via this disynaptic pathway showed that the inhibitory synapses are often located on the distal apical dendrites of the postsynaptic pyramidal cells.
- 19. Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. These authors show that a train of action potentials in a single layer 2/3 pyramidal cell in the somatosensory cortex can generate disynaptic inhibition in neighboring pyramidal cells. This disynaptic inhibition increased disproportionately with the number of active pyramidal neurons. This supralinear increase of inhibition resulted from the incremental recruitment of somatostatin-expressing inhibitory interneurons located in layers 2/3 and 5, likely including Martinotti cells.
- 20.Murayama M, Perez-Garci E, Nevian T, Bock T, Senn W, Larkum ME. Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature. 2009;457:1137–1141. doi: 10.1038/nature07663. [DOI] [PubMed] [Google Scholar]
- 21.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 22.Kozloski J, Hamzei-Sichani F, Yuste R. Stereotyped position of local synaptic targets in neocortex. Science. 2001;293:868–872. doi: 10.1126/science.293.5531.868. [DOI] [PubMed] [Google Scholar]
- 23.Markram H. A network of tufted layer 5 pyramidal neurons. Cereb Cortex. 1997;7:523–533. doi: 10.1093/cercor/7.6.523. [DOI] [PubMed] [Google Scholar]
- 24.Song S, Sjostrom PJ, Reigl M, Nelson S, Chklovskii DB. Highly nonrandom features of synaptic connectivity in local cortical circuits. PLoS Biol. 2005;3:e68. doi: 10.1371/journal.pbio.0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kampa BM, Letzkus JJ, Stuart GJ. Cortical feed-forward networks for binding different streams of sensory information. Nat Neurosci. 2006;9:1472–1473. doi: 10.1038/nn1798. [DOI] [PubMed] [Google Scholar]
- 26.Otsuka T, Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J Neurosci. 2008;28:11186–11195. doi: 10.1523/JNEUROSCI.1921-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- 28. Yu YC, Bultje RS, Wang X, Shi SH. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458:501–504. doi: 10.1038/nature07722. These authors showed that pyramidal neurons that are developmentally descended from the same mother cell are preferentially connected by excitatory synapses as compared to randomly selected pyramids separated by similar distances.
- 29.Stepanyants A, Tamas G, Chklovskii DB. Class-specific features of neuronal wiring. Neuron. 2004;43:251–259. doi: 10.1016/j.neuron.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Le Be JV, Silberberg G, Wang Y, Markram H. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex. 2007;17:2204–2213. doi: 10.1093/cercor/bhl127. [DOI] [PubMed] [Google Scholar]
- 31.Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457:1133–1136. doi: 10.1038/nature07658. By simultaneously recording from different classes of retrogradely labeled pyramidal neurons, these authors showed that the patterns of local intracortical connectivity among pyramids can reflect their long-range axonal targets. They also showed that the probability of connection cannot solely be explained by the axodendritic overlap among pyramids.
- 33.Braitenberg V, Schuz A. Cortex: Statistics and Geometry of Neuronal Connectivity. edn Second. Heidelberg: Springer-Verlag; 1998. [Google Scholar]
- 34.Binzegger T, Douglas RJ, Martin KA. A quantitative map of the circuit of cat primary visual cortex. J Neurosci. 2004;24:8441–8453. doi: 10.1523/JNEUROSCI.1400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters A. Thalamic input to the cerebral cortex. Trends in Neurosciences. 1979;2:183–185. [Google Scholar]
- 36.Shepherd GM, Stepanyants A, Bureau I, Chklovskii D, Svoboda K. Geometric and functional organization of cortical circuits. Nat Neurosci. 2005;8:782–790. doi: 10.1038/nn1447. [DOI] [PubMed] [Google Scholar]
- 37. Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. These authors developed a novel method for mapping synaptic inputs onto the somatodendritic membrane of postsynaptic cells. This method termed sCRACM (subcellular channelrhodopsin2-assisted circuit mapping) is based on action potential-independent local transmitter release by light-induced depolarization of small axonal regions expressing channelrhodopsin2 in the presence of the sodium channel blocker, TTX. By selectively expressing a light-gated cation channel in several different afferent pathways into the barrel cortex, these authors demonstrated that each afferent pathway targets different locations along the postsynaptic pyramidal cells’ dendrites, and that the dendritic structure of the postsynaptic neurons could not account for the relative strength of the input pathways.
- 38.Tamas G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- 39.Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nat Neurosci. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- 40.Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- 41.Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Markram H, Goodman PH, Berger TK, Ma J, Goldman-Rakic PS. Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat Neurosci. 2006;9:534–542. doi: 10.1038/nn1670. [DOI] [PubMed] [Google Scholar]
- 43.Bohland JW, Wu C, Barbas H, Bokil H, Bota M, Breiter HC, Cline HT, Doyle JC, Freed PJ, Greenspan RJ, et al. A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLoS Comput Biol. 2009;5:e1000334. doi: 10.1371/journal.pcbi.1000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hattox AM, Nelson SB. Layer V neurons in mouse cortex projecting to different targets have distinct physiological properties. J Neurophysiol. 2007;98:3330–3340. doi: 10.1152/jn.00397.2007. [DOI] [PubMed] [Google Scholar]
- 46.Lichtman JW, Sanes JR. Ome sweet ome: what can the genome tell us about the connectome? Curr Opin Neurobiol. 2008;18:346–353. doi: 10.1016/j.conb.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi SP, Yanagawa Y, Stryker MP. Delayed plasticity of inhibitory neurons in developing visual cortex. Proc Natl Acad Sci U S A. 2008;105:16797–16802. doi: 10.1073/pnas.0806159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J Neurosci. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puig MV, Ushimaru M, Kawaguchi Y. Two distinct activity patterns of fast-spiking interneurons during neocortical UP states. Proc Natl Acad Sci U S A. 2008;105:8428–8433. doi: 10.1073/pnas.0712219105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Kock CP, Bruno RM, Spors H, Sakmann B. Layer- and cell-type-specific suprathreshold stimulus representation in rat primary somatosensory cortex. J Physiol. 2007;581:139–154. doi: 10.1113/jphysiol.2006.124321. [DOI] [PMC free article] [PubMed] [Google Scholar]