Summary
The integrated signaling of insulin and gonadotropin-releasing hormone in the pituitary gonadotropes may have a profound bearing on reproductive function, although the cross-receptor signaling mechanisms are unclear. We demonstrate that the insulin receptor is constitutively localized to non-caveolar lipid raft microdomains in the pituitary gonadotrope cell line LβT2. The localization to rafts is consistent with similar localization of the GnRH receptor. Insulin receptor phosphorylation occurs in raft domains and activates the downstream signaling targets Insulin Receptor Substrate1 and Akt/Protein Kinase B. Although insulin alone does not strongly activate the extracellular signal-regulated kinase second messenger cascade, co-stimulation potentiates the phosphorylation of the extracellular signal-regulated kinase by gonadotropin-releasing hormone. The co-stimulatory effect of insulin and gonadotropin-releasing hormone is also evident in increased activation of cap-dependent translation. In contrast, co-stimulation attenuates Akt/Protein Kinase B activation. Our results show that both gonadotropin-releasing hormone and insulin are capable of mutually altering their respective regulatory signaling cascades. We suggest that this provides a mechanism to integrate neuropeptide and energy homeostatic signals to modulate reproductive function.
Keywords: insulin receptor, gonadotropin releasing hormone, translation, ERK
1. Introduction
The binding of the hypothalamic decapeptide gonadotropin releasing hormone (GnRH) to the pituitary GnRH Receptor (GnRHR) stimulates the synthesis and secretion of gonadotropin hormones, luteinizing hormone (LH) and follicle stimulating hormone (FSH) (Gharib et al., 1990, Clayton and Catt, 1981). In the absence of GnRH input to the pituitary gland, gonadotropin production, and consequently gonadal function in mammals, ceases (Mason et al., 1986a, Mason et al., 1986b). Upon ligand activation, the GnRHR couples mainly but not exclusively to Gαq/11, leading to stimulation of phospholipase C, elevation of intracellular free calcium and activation of one or more isoforms of protein kinase C (PKC) (Kaiser et al., 1997, Stanislaus et al., 1997, Liu et al., 2002b). These early events underlie GnRH activation of multiple mitogen activated protein kinase (MAPK) cascades, including extracellular signal regulated kinase (ERK) (Naor et al., 2000).
It has become clear that gonadotropin secretion can be modified by various inhibitory or stimulatory inputs including several metabolic factors (Schneider, 2004, Mahesh, 1985, Bilezikjian et al., 2004, Gregory and Kaiser, 2004). For example, clinical studies in humans and observations in knockout mouse models suggest a direct link between insulin action and the neuroendocrine control of reproduction (Kitamura et al., 2003, Withers, 2001). Emerging evidence suggests that insulin action may impact regulation of the reproductive axis at the level of the pituitary (Buggs et al., 2006). Past studies of insulin action in primary pituitary cultures in vitro show increased GnRH-induced release of LH after 4, 24, and 48 hours in the presence of insulin-like growth factor-1 (IGF-1), insulin, or both (Xia et al., 2001, Adashi et al., 1981, Soldani et al., 1994). Additionally, recent work has shown that insulin can augment GnRH-stimulated LHβ gene expression by Egr-1 in LβT2 cells under a specific treatment regime requiring GnRH pre-stimulation (Buggs et al., 2006). At this time, it appears that the integrated signaling by insulin and GnRH may have a profound bearing on the regulation of reproductive function, although the precise signaling mechanisms are unclear.
The insulin receptor (IR) is a member of the distinct subfamily of receptor tyrosine kinases (RTK) and is expressed ubiquitously throughout the body, including the pituitary (Buggs et al., 2006, Soldani et al., 1994, Unger and Lange, 1997). Upon insulin binding, the beta subunits of IR stimulate tyrosine auto-phosphorylation, leading to increased receptor tyrosine kinase activity. Following activation, the IR phosphorylates a variety of intracellular substrates including members of the IRS family, Shc, Gab1 and Gαq/11 (Bevan, 2001) which initiate several signaling cascades including phosphatidylinositol 3-kinase (PI3K) and ERK. IR signaling is thought to initiate in discrete caveolar containing membrane microdomains in various cell types including adipocytes and muscle (Ishikawa et al., 2005).
Regulation of translation by RTK, like insulin receptor, proceeds through the PI3K-Akt/PKB and/or ERK signaling pathways. Previous work from our lab has established that GnRH stimulation of LβT2 cells increases cap-dependent translation initiation through the ERK signaling cascade (Nguyen et al., 2004). Given the central role of insulin in translation, we have investigated the possibility that the initiation of insulin signaling in LβT2 cells may augment GnRH translational activation.
To elucidate potential mechanisms for IR crosstalk with the GnRHR in gonadotropes, we examined the localization of IR in gonadotrope plasma membranes. We report that, consistent with what has been found for the GnRH receptor, IR is localized to non-caveolar lipid rafts independent of hormone treatment in the gonadotrope derived LβT2 cell line. Additionally, IR is phosphorylated on tyrosine residues within these raft domains. We also examined the role of insulin in GnRH signaling. We first established that insulin signaling is functional in LβT2 cells. We show that insulin activates IRS-1, Akt/PKB, and is only a mild activator of the ERK signaling cascade. Interestingly, although insulin does not robustly activate ERK, we find that insulin enhances GnRH translational responses though the ERK map kinase pathway. Based on these findings, we suggest that translational regulation by combined GnRH and insulin treatment is an important component of the metabolic regulation of reproduction and is a target of convergent signaling of GnRH and insulin receptor activation.
2. Materials and Methods
2.1 Materials
The anti-Akt, anti-ERK1, anti-rabbit-HRP, and anti-mouse-HRP antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-p-Akt (473) antibody was purchased from Cell Signaling Technology (Danvers, MA). The anti-pan-caveolin antibody and anti-flotillin-1 were purchased from BD Transduction Laboratories (San Jose, CA). Insulin and GnRH were purchased from Sigma Aldrich (St. Louis, MO). The anti-GnRH receptor antibody was a generous gift from Dr. Donal Skinner (University of Wyoming). Phorbol 12-myristate 13-acetate (PMA) and PD98059 were purchased from Calbiochem (San Diego, CA)
2.2 Cell Culture
LβT2 cells were maintained in DMEM (Invitrogen, Carlsbad, CA) supplemented with 4.5 mg/ml glucose, 10% fetal bovine serum, 100 µg/ml penicillin, and 0.1 mg/ml streptomycin unless otherwise stated. All cells were grown in 5% CO2 at 37°C in a humidified environment. Cells were serum starved for 4–6 h before prior to the indicated treatments.
2.3 RT-PCR and Primer Sequences
RNA was extracted with Trizol LS (Invitrogen, Carlsbad, CA) from LβT2 cells or adult male mouse liver or pituitary according to the manufacturer’s instructions. Tissues were harvested from nine-week old wild-type male mice (C57BL/6). Mice were sacrificed using CO2 asphyxiation followed by cervical dislocation, in accordance with UCSD Institutional Animal Care and Use Committee regulations. Murine IRS-1 was detected using the forward primer CCCTTCCTCATGCCAAACC and reverse primer CCTGCAAACCGTAGCAGACC detecting bases 3038–3837 of Genbank accession number NM010570.2. Murine IRS-2 was detected using the forward primer TACCCTCTACCCACAGAGC and reverse primer TACCTGATCCATGAGACTTAGC detecting sequences 927–1541 from Genbank accession number AF0907381.1. All primers were designed using OMIGA 2.0 software (Oxford Molecular Ltd., Cambridge) and reactions were performed using Omniscript RT-PCR reagents (Qiagen, Alhambra, CA).
2.4 Immunoprecipitations
For immunoprecipitation (IP), 800 µg of protein from cell extracts were diluted in IP lysis buffer (50 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 1% NP40, 0.5% sodium deoxycholate, Complete Mini protease inhibitor cocktail tablet (Roche Applied Science, IN), 0.7 µg/ml pepstatin, and incubated with protein specific antibody overnight at 4°C and immunoprecipitated using either a Protein A or G-agarose immunoprecipitation kit (Roche Applied Science, Indianapolis, IN) according to the manufacturers’ instructions. Samples were boiled for 5 min prior to separation by SDS-PAGE.
2.5 SELDI-TOF Mass Spectrometry
Approximately twenty million cells were plated in 55 cm2 dishes, cultured for 24 h, serum starved for 16 h, and treated with insulin at 10 nM for 5 minutes. Cells were washed with ice-cold PBS containing 1 mM Na3O4V, and 10 mM NaF, and harvested in 800 µl of lysis buffer. For immunoprecipitation, 800 µg of cell extract was incubated with 2 µg/ml IRS-1 antibody (sc-7200, Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C and immunoprecipitated as described above. Samples were digested with modified trypsin (Promega, Madison, WI) for 16 h at 37°C. A PS20 Protein Chip Array (Ciphergen, Fremont, CA) was spotted with 10 µl of 1 mg/ml of Protein A in PBS and incubated overnight at 4°C (Amersham Biosciences, Piscataway, NJ). After washing with PBS, phospho-tyrosine antibody (sc-508, Santa Cruz Biotechnology, Santa Cruz, CA) was added to the chip overnight at 4°C. Trypsin digested samples were added to the array and again incubated overnight at 4°C. For analysis, 10% alpha-cyano-4-hydroxy cinnamic acid (Ciphergen, Fremont, CA) was added to each spot of the array for crystallization and the array was analyzed using SELDI-TOF Mass Spectrometry (Ciphergen, Fremont, CA).
2.6 Isolation of Lipid Rafts
Approximately 20 million LβT2 cells were treated with insulin for the indicated times and then lysed in 750 µl of 500 mM sodium carbonate buffer (pH 11) and homogenized using a Wheaton loose fitting glass dounce homogenizer (10 strokes) followed by sonication (three 20 second bursts) on ice using a Branson 250 sonifier. 750 µl of 90% sucrose prepared in MES buffer (20% glycerol, 150 mM NaCl, 2mM EDTA, 25mM MES, pH 6.5) was added to the homogenized samples yielding a final concentration of 45% sucrose in a total volume of 1.5 ml. A discontinuous sucrose gradient was then layered on the surface of the 45% fraction (1.5 ml 35% sucrose, 1.5 ml 5% sucrose in MES containing 250 mM sodium carbonate). Isopycnic ultracentrifugation was then carried out at 46,000 rpm using a SW 55 rotor for 16–20 h at 4°C. Following ultracentrifugation, 250 µl samples were collected representing a total of 18 fractions. Proteins that migrated to the interface of the 5% and 35% gradients (approximately fractions 6 and 7) were considered to be raft-associated (Song et al., 1996).
2.7 Immunoprecipations Out of Lipid Raft Fractions
Aliquots (250 µl) of fractions containing suspensions of low-density or high density membranes were diluted in an equal volume of PBS. Anti- IRβ or nonimmune rabbit serum was added and the samples were rocked for 4 h at 4°C. Protein A/G agarose beads (30 µl) were added, and the samples were rocked an additional hour at 4°C. Beads were then washed three times in PBS with 0.01% Triton X-100, resuspended in SDS loading buffer, boiled, and separated by SDS-PAGE and probed with an anti-phosphotyrosine antibody (p-Tyr (PY20) sc-508, Santa Cruz Biotechnology, Santa Cruz, CA).
2.8 Western blots
Samples representing individual fractions from lipid raft fractions were subjected to SDS polyacrylamide gel electrophoresis (acrylamide:bis-acrylamide ratio of 29:1) and electro-blotted to PVDF (Bio-Rad Hercules, CA). Membranes were blocked in 2X casein solution (Vector Laboratories, Burlingame, CA) diluted in Tris buffered saline (TBS). Anti-IRβ, Anti-Flotillin, or Anti-transferrin antibodies were used at a 1:1000 dilution with an incubation time of 2 h. Anti-GnRHR was used at 1:1000 at 4°C overnight. Blots were washed with 0.2% Tween-TBS and then incubated with a 1:5000 dilution of the appropriate secondary antibody conjugated to HRP for 2 h at room temperature. All blots were washed for 60 min (6 × 10 min) with TBS after secondary antibody and then developed by enhanced chemiluminescence (Chemiglow, Alpha Innotech, San Leandro, CA). Membranes were visualized using the GeneSnap BioImaging System (Syngene, Frederick, MD).
2.9 Akt and ERK Activation Assays
A monolayer of LβT2 cells (2 × 106) in 6-well tissue culture plates were used in the ERK and Akt activation assays. LβT2 cells were serum starved for 4 h and administered either 0 or 10 nM GnRH for a 15 min incubation. Cells were washed in ice cold PBS and lysed in RIPA buffer containing 20mM Tris (pH 8.0), 137 mM NaCl, 10% glycerol, 1% NP-40, 0.1% SDS, 0.5% deoxycholate and 0.2 mM PMSF. 6X sample buffer (300 mM Tris-HCl, pH 6.8, 60% glycerol, 30 mM DTT, 6% SDS) was added to yield a final concentration of 1X. Aliquots (30 µl) of each lysate were heated to 95°C for 5 min and subjected to SDS-PAGE and Western analysis. PVDF membranes were incubated for 2 h with either a phospho-Akt (Millipore/Upstate, Billerica, MA 1:1,000 dilution) or a phospho-ERK antibody (Santa Cruz biotechnology, Santa Cruz, CA) followed by a 2 h incubation with a 1:5,000 dilution of an HRP conjugated secondary antibody. Phospho-Akt or phosho-ERK blots were then stripped at room temperature with 100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl (pH 6.7) heated to 50°C for 30 min. After stripping, membranes were washed twice for 15 min with TBS and blocked with 2x casein for 1 h. Blots were then re-probed with a 1:1,000 dilution of either an anti-Akt or anti ERK1 antibody that recognizes the specified proteins independent of phosphorylation state. Following washing in TBS, blots were incubated with a 1:5,000 dilution of anti-rabbit HRP secondary antibody and then developed by enhanced chemiluminescence (Chemiglow, Alpha Innotech, San Leandro, CA). Membranes were visualized using the GeneSnap BioImaging System and Gene Tools software (Syngene, Frederick, MD). Statistical analysis of at least 4 independent experiments was performed as described below in the statistical analysis section of the materials and methods.
2.10 Plasmids and Transfections
The bicistronic reporter plasmid (pGL3-CMV-Luc-EMCV-Gal) was previously described (Nguyen et al., 2004, Sosnowski et al., 2000). The plasmid directs the synthesis of a single transcript encoding the luciferase reporter followed by the encephalomyocarditis virus 5’ untranslated region containing the internal ribosome entry site and the β-galactosidase coding sequence. Each reading frame is translated independently by either cap-dependent (luciferase) or cap-independent (β-galactosidase) translation initiation mechanisms (Pelletier and Sonenberg, 1988).
Cells (2 ×105) were plated in 24 well dishes and incubated 24 h prior to transfection. Cells were then transfected with the bicistronic reporter using Fugene 6 (Roche Applied Science, Indianapolis, IN) according to the manufacturers’ instructions and incubated 16–18 h prior to treatment. Cells were serum starved for 4 h. Cells that received PD98059 (50 uM) were pretreated for 30 minutes prior to a 6 h incubation of the indicated combination of treatments (GnRH 10 nM), insulin (10 nM) or 1nM PMA. Cells were then lysed and assayed directly for luciferase (Luciferase Assay System, Promega, Madison, WI) and β-galactosidase (Galacto-Light Plus, Tropix, Bedford, MA) activity according to manufacturer’s instructions in a 96-well plate using a veritas luminometer (Turner Biosystems, Sunnyvale, CA).
2.11-Statistical Analysis
All statistical analysis was performed using JMP 7 (SAS Institute, Cary, NC). Data are expressed as means ± SEM of at least 3 independent experiments. Results were analyzed for significance by ANOVA. Post-hoc group comparisons were made using Student’s T-test, Dunnett’s comparison to control test, or Tukey’s Honestly Significant Difference (HSD) as appropriate using the critical value p=0.05 for declaring significance. Analysis was conducted using untransformed data or data optimally transformed by the method of Box and Cox (Box and Cox, 1964) as implemented in JMP.
3. Results
3.1 Insulin receptor activation occurs in non-caveolar cholesterol-enriched raft membrane microdomains in LβT2 cells
The insulin receptor is commonly found in caveolin-associated raft subdomains in 3T3-L1 adipocytes as well in non-caveolar domains in some hepatocytes (Muller and Frick, 1999, Ishikawa et al., 2005). However, in some systems, insulin receptor is thought to migrate to these domains after ligand activation (Remacle-Bonnet et al., 2005). Previous work has established the presence of insulin receptors (IR) in LβT2 gonadotrope cells (Buggs et al., 2006), although the precise biochemical organization of the IR within the plasma membrane of these cells has not been established. To assess the membrane sub-localization of the insulin receptor, LβT2 cells were administered either vehicle or 10 nM insulin (Ins) for 5 min, lysed and fractionated in a discontinuous sucrose gradient. Fractions were isolated and subjected to SDS-PAGE followed by western blotting using an anti-insulin receptor β, anti-transferrin receptor, anti-GnRHR, or an anti-flotillin antibody (Fig. 1). Using this protocol, lanes 6 and 7 represent the interface of the 5% and 35% sucrose fractions where raft-associated proteins would migrate. Flotillin is a marker protein for low-density membrane microdomains, and appropriately localized to low-density fractions within the sucrose gradient (Morrow et al., 2002). Transferrin receptor, not considered a raft-associated membrane protein did not partition into membrane rafts in these conditions (Batista et al., 2004). Immunodetectable IR localized to low-density fractions within the gradient independent of insulin treatment. IR localization to lipid rafts is similar to what has already been established for the GnRHR in a number of cell types (Navratil et al., 2003). To confirm IR localization to lipid rafts, an independent approach based on resistance to detergent solubilization (Triton-X 100) was utilized to prepare samples prior to isopycnic ultracentrifugation. This technique takes advantage of the relative insolubility of lipid rafts in non-ionic detergents at 4°C (London and Brown, 2000). Similar to the detergent-free raft preparations, IR localized to the low-density fractions, consistent with its presence in lipid rafts.
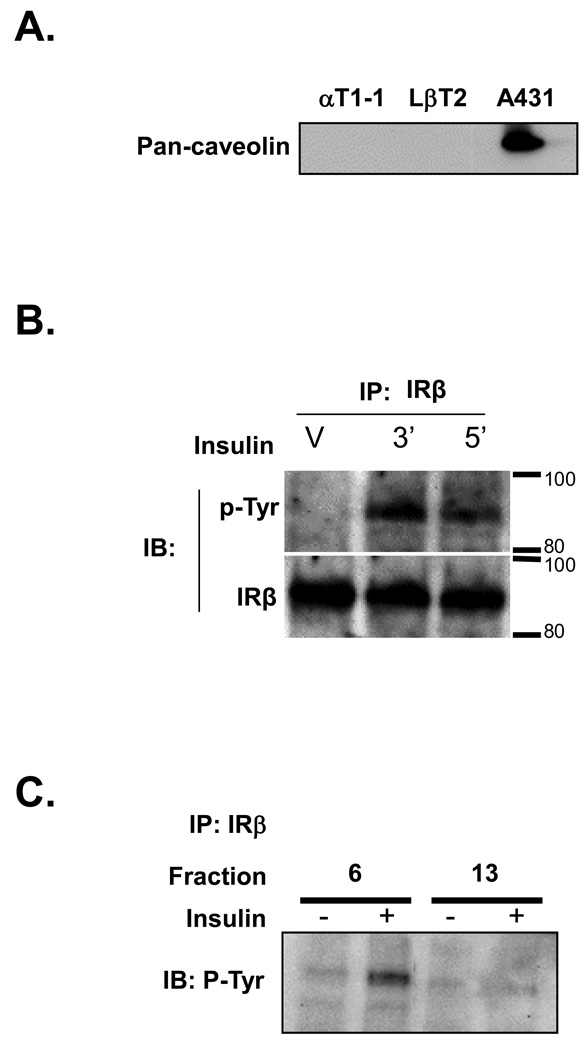
Figure 1. Insulin receptor is localized to lipid rafts in LβT2 cells.
(A.) LβT2 cells were incubated in the presence or absence of 10 nM Insulin for 5 min at 37°C. Raft samples were then prepared using a detergent free sodium carbonate buffer and separated by isopycnic ultracentrifugation through a non-linear sucrose gradient (45%, 35%, 5%). Fractions were collected from the top of the gradient and separated by SDS-PAGE. Western blots were conducted using antibodies directed against the IRβ subunit, transferrin receptor (Tfn R), GnRH receptor (GnRH R) or flotillin-1 (Flotillin). (B.) LβT2 cells were prepared using a 0.1% TX-100 based raft preparation and separated by isopycnic ultracentrifugation through a non-linear sucrose gradient (45%, 35%, 5%). Fractions were collected and electrophoresis and immunoblotting were performed as above. M. molecular weight marker.
It has been found that the gonadotrope derived αT3-1 cell line does not contain caveolin associated raft subdomains (Navratil et al., 2003). Although the αT3-1 cells, a line representing a committed, but immature gonadotrope cell approximating day e13 of mouse development, does not express caveolin, we were interested in testing whether caveolin was differentially expressed at various developmental stages within the gonadotrope. To test the possibility of differential caveolin expression, we examined whole cell lysates from the αT1-1 cell line, representing a less differentiated cell of the thyrotrope-gonadotrope lineage approximating embryonic day 11 of mouse development, or the LβT2 cell line, which approximates a fully differentiated gonadotrope cell representing at least embryonic day 17. Western blot analysis was carried out with a pan-caveolin antibody that detects caveolin isoforms 1, 2, and 3. Similar to the αT3-1 cell line, neither the αT1-1 nor the LβT2 cell lines express these isoforms of caveolin (Fig. 2A). The human epithelial carcinoma cell line A431 was used as a caveolin-expressing positive control. Thus, it appears that caveolin is not differentially expressed at various developmental ages and the IR, like the GnRHR, is localized to a non caveolar-containing raft domain in the LβT2 cell line.
Figure 2. Insulin receptor is phosphorylated in a non-caveolar lipid raft compartment.
(A.) Whole cell RIPA lysates were prepared from either αT1-1, LβT2 or A431 cells. Western blots were conducted using a pan-caveolin antibody that detects caveolin-1, caveolin-2 and caveolin-3. (B.) Western Blot analysis of IRβ immunoprecipitated from LβT2 cell protein extracts. Cells were treated with vehicle (V) for 5 min or insulin for 3 or 5 min and then harvested. Extracts were immunoprecipitated with anti-IRβ and subjected to SDS-PAGE and Western Blotting with anti phospho-tyrosine (α-p-Tyr) antibody. The presence of IRβ was confirmed by stripping the membrane and re-blotting with anti-IRβ (IRβ). (C.) Western blot analysis of IRβ immunoprecipitated from LβT2 lipid raft fractions treated for 5 min with either vehicle or 10 nM insulin. Fraction 6 represents low-density (raft associated) proteins and fraction 13 represents high-density (non-raft associated) proteins.
Next, we assessed the functionality of the insulin receptor by testing the ability of insulin to induce phosphorylation of the IRβ-subunit. LβT2 cellular lysates were immunoprecipitated using an anti-IRβ-subunit antibody. Following SDS-PAGE, membranes were immunoblotted with an anti-phosphotyrosine antibody. The blots identified a protein of approximately 90 kDa, consistent with phosphorylation of the insulin receptor (Fig. 2B). To determine whether IR was phosphorylated in lipid rafts, both raft (fraction 6) and non-raft (fraction 13) associated fractions were isolated and subjected to immunoprecipitation using an anti-IRβ-subunit antibody. Immunoblotting using an anti-phosphotyrosine antibody revealed a protein of approximately 90 kDa, consistent with our previous results (Fig. 2C). Thus, it appears that the functional IR is phosphorylated in a non-caveolar raft domain in LβT2 cells.
3.2 Expression and activation of IRS isoforms in LβT2 cells
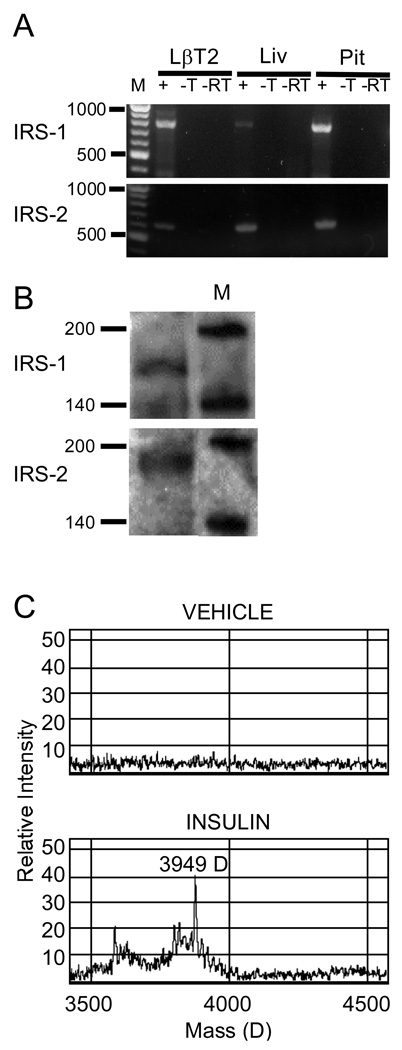
IRS family members play a significant role in insulin signaling and have been implicated as a point of insulin insensitivity through feedback regulation by intracellular kinases and steroids (Morelli et al., 2003, Richards et al., 1996). Given their central role in insulin action, we sought to confirm their presence in the LβT2 cell line. Analysis of total RNA by RT-PCR and western blot revealed that IRS-1 and IRS-2 mRNAs are present in LβT2 cells (Fig. 3A, 3B). When insulin binds to its receptor it causes receptor-mediated phosphorylation of IRS-1 at residue Y608, activating downstream signaling intermediates. Analysis of tyrosyl phosphorylated tryptic peptides by surface-enhanced laser desorption/ionization time-of-flight (SELDI-TOF) mass spectrometry of immunoprecipitated IRS-1 from insulin-treated LβT2 cells revealed the presence of the 3949 Da peptide predicted by trypsin cleavage of IRS-1 phosphorylated at Y608 (Fig. 3C). We conclude that LβT2 cells express IRS proteins and that insulin treatment results in activation of IRS-1 by tyrosine phosphorylation at an appropriate regulatory site. Thus, it appears that LβT2 cells appropriately respond to insulin treatment by activation of known downstream signaling targets such as IRS proteins.
Figure 3. Expression of IRS family members and activation by insulin in LβT2 cells.
(A.) Both IRS-1 and IRS-2 mRNA were confirmed by RT-PCR using primers described in Materials and Methods. M, 100 bp Ladder, +, RT-PCR with template, -T, without template, -RT, without reverse transcriptase. Liv, mRNA template isolated from mouse liver, Pit, template isolated from mouse pituitary. (B.) Extracts of LβT2 cells were immunoprecipitated with anti–IRS-1 or anti-IRS-2 antibody, resolved by SDS-PAGE, and blotted onto PVDF membrane. Blots were probed with anti-IRS-1 or anti-IRS-2 antibody to confirm the presence of protein. M, protein ladder. (C.) Extracts of LβT2 cells treated with vehicle or 10 nM insulin for 5 min were immunoprecipitated with anti-IRS-1 antibody and subjected to trypsin digestion to yield defined peptides. Tryptic peptides were incubated with anti-phosphotyrosine antibody bound to Protein A-linked chips as described in Materials and Methods. Bound peptides were subjected to SELDI-TOF Mass Spectrometry. Insulin treatment of LβT2 cells resulted in the appearance of the 3949 D tyrosyl phosphorylated tryptic peptide predicted by digest of Y608-phosphorylated IRS-1.
3.3 Insulin and GnRH differentially activate AKT and ERK
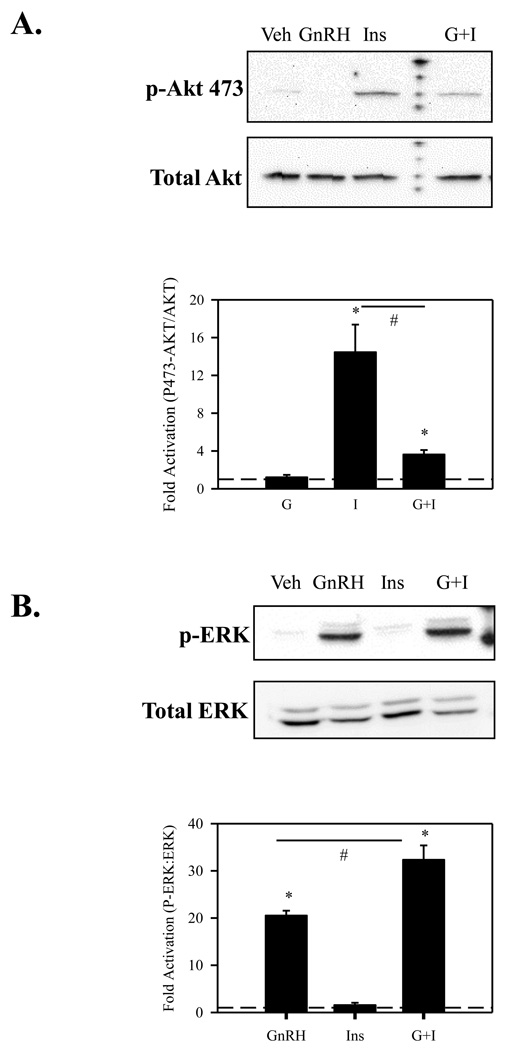
We next focused our attention on two main signaling intermediates utilized by IR, activation of Akt/PKB and phosphorylation of ERK (Bevan, 2001). Akt/PKB is robustly activated in response to insulin and plays a critical role in cell survival and proliferation. To directly test if Akt is activated by insulin in LβT2 cells, we preformed both time and dose response studies. We find that LβT2 cells phosphorylate Akt/PKB in both a time and dose dependent manner in response to insulin. In contrast, 10nM GnRH did not activate Akt in either a time or dose dependent fashion (data not shown). Because both IR and GnRHR occupy non-caveolin raft domains in LβT2 cells, we assessed whether there exists any signaling interaction between these receptors. To this end, we co-stimulated LβT2 cells with 10 nM GnRH and 10 nM insulin. There is a severe attenuation of Akt phosphorylation with GnRH and insulin co-stimulation in comparison to insulin stimulation alone (Fig. 4A). This finding is consistent with what has previously been seen with IGF and GnRH co-treatment in αT3-1 cells (Rose et al., 2004).
Figure 4. GnRH attenuates insulin induced Akt activation while insulin accentuates GnRH induced ERK activation.
(A.) LβT2 cells were serum starved for 4 h followed by treatment with either vehicle, 10nM GnRH (GnRH), 10 nM insulin (Ins), or both (G+I) for 15 min. Cells were then harvested in RIPA buffer and subjected to SDS-PAGE and western blotted using an anti-phosho-Akt (473) antibody. After probing with anti-p-Akt, blots were stripped and re-probed with an antibody that detects Akt independent of phosphorylation. (B.) LβT2 cells were serum starved for 4 h followed by treatment with either vehicle, 10nM GnRH (GnRH), 10nM insulin (Ins), or both (G+I) for 15 min. Cells were then harvested in RIPA buffer and subjected to SDS-PAGE and western blotted using an anti-phospho-ERK1/2 antibody. After probing with anti-p-ERK, blots were stripped and re-probed with an antibody that detects ERK 1/2 independent of phosphorylation.
Recently it has been shown that GnRH stimulation in the presence of insulin leads to heightened activation of LH promoter activity in LβT2 cells (Buggs et al., 2006). LH promoter activity is attributed largely to activation of MAPK cascades, particularly through the activation of ERK (Liu et al., 2002a). We therefore assessed the activation of ERK in response to insulin alone and to insulin and GnRH co-stimulation. We found that that the combination of GnRH and insulin significantly augments ERK activation (Fig 4B). Interestingly, insulin alone does not robustly activate ERK within the 15 minute time-frame examined, though some slight activation does occur.
3.4 Insulin enhances GnRH-induced cap-dependent translation
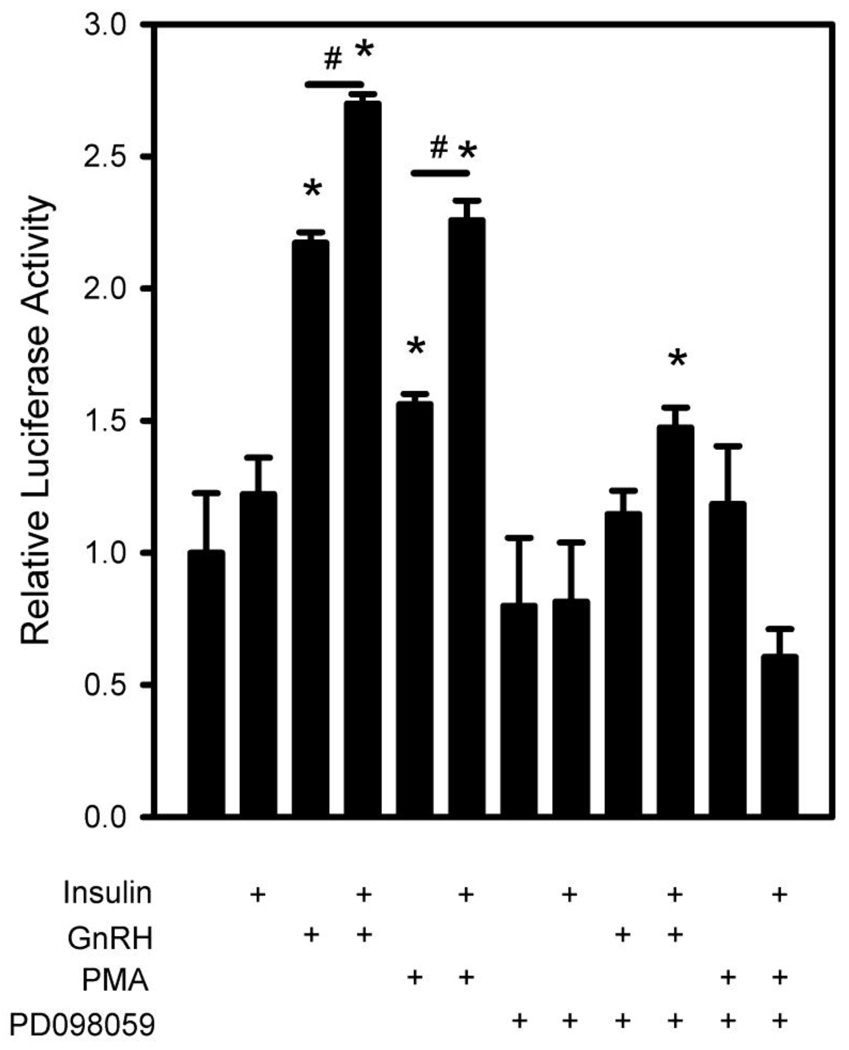
Synthesis of LH protein is increased in LβT2 cells by acute GnRH treatments (Liu et al., 2002a). Recent work from our lab has shown that the acute increase in LH synthesis is due to activation of cap-dependent translation by GnRH, in addition to an acute increase in gonadotropin mRNA synthesis (Nguyen et al., 2004). These translational events are dependent on the activation of ERK. Given the role of insulin in potentiating the protein synthesis capacity of cells and the increased activation of ERK in Fig. 4, we investigated the possibility that insulin may accentuate GnRH induced cap-dependent translation. To directly test this, we examined the effect of co-treatment with insulin and GnRH on the activation of a bicistronic reporter gene described previously (Sosnowski et al., 2000). This reporter directs synthesis of a single transcript encoding two independently translated reading frames and can detect increases in cap-dependent translational activity that are not evident in measurements of overall protein synthesis. The first reading frame is translated by the cap-dependent initiation mechanism and directs synthesis of luciferase. The second reading frame is translated independently from the first via an internal ribosomal entry site derived from the murine cardiovirus, encephalomyocarditis virus and directs synthesis of E. coli β-galactosidase. Measurement of increased cap-dependent translation activity can be made by comparison of the ratios of luciferase to β-galactosidase gene expression. Consistent with our previous report, we find that GnRH leads to an approximately 2.25 fold induction of reporter activity. Insulin stimulation of cap-dependent translation appears to be around 1.5 fold. Interestingly, when GnRH and insulin are combined, there is an additive effect on cap-dependent translation (approx 2.75 fold) (Fig. 5). Thus it appears that GnRH and insulin can work cooperatively to stimulate cap-dependent translation in LβT2 cells.
Figure 5. Blockade of ERK attenuates the additive insulin effect on GnRH induced translation.
LβT2 cells were transfected with a bicistronic reporter gene encoding firefly luciferase and β-galactosidase translated by cap-dependent and -independent mechanisms, respectively for 24 h. Cells were then serum starved for 4 h and pretreated with either vehicle or 50 µM PD098059 (PD) to block MAPK activation for 30 min. Cells were further treated for 6 h with vehicle, 10 nM GnRH (GnRH), 10 nM insulin (Ins), GnRH + Insulin, 1n M PMA, or 1nM PMA + insulin. Cells were then harvested and assayed for reporter gene activity. The histogram shows the ratio of luciferase to β-galactosidase activity. (#) indicates significant differences (p<0.05) between pairs connected by solid lines. (*) indicates significant increase above the control vehicle treated group as determined by ANOVA and post-hoc evaluation using either Students T test or Dunnett’s comparison to control test as appropriate.
Our results above show that insulin co-treatment of LβT2 gonadotropes can augment GnRH-induced ERK activation and cap-dependent translational activity. Blockade of ERK activation would cause a loss of cap-dependent translational stimulation by either GnRH or insulin, as well as a loss of additive stimulation. To test this, we examined the effect of PD098059 blockade of ERK activation on cap-dependent translational stimulation. After transfection with the bicistronic reporter, LβT2 cells were treated with vehicle or 50 µM PD098059 for 30 min prior to stimulation with GnRH, insulin, or both. Interestingly, cells pre-treated with PD098059 did not show increased stimulation compared to control (GnRH and insulin co-stimulation alone) (Fig. 5).
To further confirm these results, we also used phorbol ester (PMA) to pharmacologically activate the MEK-ERK cascade independent of GnRH. We find that PMA alone mimics the effect of GnRH. additionally; PMA in combination with insulin also leads to an additive effect on cap-dependent translation similar to that seen with GnRH treatment. Both the GnRH and PMA effects are blocked with PD098059.
We conclude from these multiple observations that ERK is a common target of GnRH and insulin signaling, and this is reflected in the regulation of translational control targeted by ERK activation and ultimately in the degree of cap-dependent translational activation.
4. Discussion
Based on numerous studies, it is clear that the integrated signaling by insulin and GnRH can impact the regulation of reproductive function, although at his time, the precise signaling mechanisms and downstream consequences at the cellular level are still unclear (Adashi et al., 1981, Soldani et al., 1994, Xia et al., 2001). To address this issue, we first attempted to identify the sub-membrane localization of the insulin receptor in gonadotrope cells. Intracellular signaling for cell-surface receptors is often achieved through compartmentalization and scaffolding of proteins into discrete subcellular domains in the plasma membrane termed lipid rafts. Many raft-associated proteins are cell-surface receptors and components of intracellular signaling cascades. Thus, lipid rafts appear to serve as both spatial and temporal signaling platforms in the plasma membrane (Brown and London, 1998). Herein, we report that the insulin receptor is constitutively localized to non-caveolar low-density raft membrane microdomains within the plasma membrane of LβT2 cells. Typically, insulin receptor signaling is initiated in discrete caveolar containing membrane microdomains in a number of cell types (Inokuchi, 2006, Saltiel and Pessin, 2003). Caveolae are a subset of membrane microdomains particularly abundant in adipocytes and muscle. It has also been found that the β subunit of IR contains a binding motif specific for caveolin (Nystrom et al., 1999) and mutation of this motif results in inhibition of insulin signaling (Inokuchi, 2006). Interestingly, pituitary gonadotropes do not express the caveolin proteins (Fig. 2). Thus, IR localization, phosphorylation, and signaling in the pituitary gonadotrope occurs in discrete membrane microdomains independent of caveolin association.
Previous reports suggest that the GnRHR resides constitutively within low-density membrane domains in a cholesterol-dependent manner (Navratil et al., 2003). In addition to the GnRHR, a number of its downstream signaling intermediates, such as Gαq/11, ERK, and c-raf are also present in lipid rafts. The organization of the GnRHR in lipid rafts appears to be critical for the ability of GnRH to propagate an intracellular signal to the level of MAP kinase (Navratil et al., 2003, Bliss et al., 2007). Given the central role of lipid rafts in GnRH and insulin signaling, it is tempting to speculate that one of the common foci for signaling overlap between insulin receptor and GnRH receptor within lipid raft domains.
Following IR phosphorylation it appears that the receptor is capable of initiating the activation of known downstream signaling intermediates, IRS and Akt/PKB (Nystrom et al., 1999). Our results suggest that when GnRH and insulin are co-treated, there is a dramatic attenuation in insulin induced Akt activation in the LβT2 cell line. This is not without precedent. Previous work has shown that when IGF-1 and GnRH are co-treated in the gonadotrope derived αT3-1 cell line there is a significant reduction in Akt/PKB phosphorylation (Rose et al., 2004). It has long been known that Akt/PKB plays a pivotal role in cell survival and proliferation and that the unregulated activation of the PI3K/Akt pathway is a prominent feature of many human cancers (Bjornsti and Houghton, 2004). What is particularly interesting in our studies is that co-treatment of GnRH and insulin causes an increase in cap-dependent translational activity even with significantly attenuated Akt. This suggests that an alternative pathway than the well-characterized Akt signaling cascade pathway, which targets translational activation through the cap-dependent translational control factors mTOR and eIF 4E binding protein is utilized in the corporative activation of translation.
Insulin is only a very modest activator of MAPK signaling cascades in LβT2 cells. Unlike the Akt/PKB data, when GnRH and insulin are added in combination, there is a significant additive effect on ERK activation. This is consistent with other work that has shown a similar effect in αT3-1 cells with IGF-1 and GnRH (Rose et al., 2004). At this time the exact mechanism by which insulin can accentuate GnRH activation of ERK 1/2 is unclear. One possibility is that insulin may sufficiently block negative feedback to ERK 1/2 activation, thereby allowing further activation through other signaling cascades. Alternatively, insulin may recruit other signaling intermediates to participate in GnRH receptor signaling events. The GnRH receptor activates ERK 1/2 via G-protein mediated induction of phospholipase activity and subsequent activation of protein kinase C, Ras, and Raf (Liu et al., 2003, Liu et al., 2002c, Liu et al., 2002a), and possibly via activation of FAK and PYK family members of the large protein tyrosine kinases (Naor et al., 2000, Maudsley et al., 2007) which are also known to signal to MAPK cascades through Grb (Blaukat et al., 1999, Litvak et al., 2000). Recruitment of Grb, Ras and Raf to insulin signaling complexes may allow the subsequent shared utilization by GnRH receptor signaling, thus enhancing overall signaling by the GnRH receptor. This scenario would require proximal activation of both receptors in the same raft structures. Further investigation of the mechanisms of insulin on GnRH signaling is required to determine the impact of signal intermediate recruitment by insulin.
It has been known for some time that activation of Egr-1, a factor essential for long term induction of LHβ subunit transcription, is subject to control through ERK 1/2 by GnRH (Liu et al., 2002a, Maudsley et al., 2007). Others have shown that insulin can augment GnRH-stimulated Egr-1 expression and LHβ gene transcription on LβT2 cells (Buggs et al., 2006). These data, coupled with our findings showing accentuated ERK activation, are entirely consistent with enhanced GnRH signaling by insulin. We suggest that the additive effect on ERK by GnRH and insulin is a likely candidate for the additive downstream effects on Egr-1 and LHβ.
The consequence of insulin modulation of GnRH signaling in gonadotrope cells is at present not clear. Studies in animal model systems (Todd et al., 2003, Valdes et al., 1991) and more recently in humans (Lawson et al., 2008) have suggested that excessive insulin may suppress reproductive function or the pituitary response to GnRH. However, experiments performed in vitro using primary pituitary cells or gonadotrope cell lines have suggested that insulin promotes GnRH responses (Adashi et al., 1981, Buggs et al., 2006, Hashizume et al., 2002, Rose et al., 2004, Soldani et al., 1994, Xia et al., 2001). This discrepancy may be due to the significant differences in the study of cultured cell model systems versus in vivo studies, the different levels of insulin used, or in the manner of insulin treatment. Nevertheless, the primary observation of both in vivo and in vitro studies is that insulin and GnRH interact at the level of the pituitary, and gonadotrope responses are modified by this interaction. A clear understanding of the interaction between metabolic and reproductive signals will be important to further our understanding of the normal and pathophysiological consequences of insulin action on reproduction. The evidence presented here suggests that there are specific, mechanistic interactions of GnRH and insulin signaling in the pituitary gonadotrope, and these molecular interactions may have a broad impact on reproductive function.
In summary, our results show that the IR is constitutively localized to non-caveolar microdomains in gonadotrope cells. Additionally, following IR phosphorylation in rafts, IR is capable of initiating the activation of downstream signaling targets, such as IRS proteins and Akt/PKB. Although insulin does not strongly activate ERK signaling alone, it potentiates the phosphorylation of ERK by GnRH. Our results suggest that this increase in ERK from insulin and GnRH co-treatment leads to direct increase in the cap-dependent translation response in LβT2 cells. Our results agree with previous reports showing that insulin can directly modulate gonadotrope responses to GnRH stimulation. We further show that there is a regulatory effect of insulin on GnRH activation of MAP kinase and translation, providing a mechanism for the gonadotrope to integrate neuropeptide and energy homeostatic signals to regulate reproductive function.
Acknowledgements
This work was also supported by the National Institute of Child Health and Human Development and National Institute of Diabetes, Digestive, and Kidney Disease/National Institutes of Health (NIH) through NIH Grants R01 HD 043758 and R21 DK 068167 (to M.A.L.) A. M. N. and B.D.C. were supported in part by NIH Grant T32 HD 007203 and J.B.H. was supported by NIH Grant R25 GM 064107. M.T.D. and J.M.L. were supported in part by T32 GM 008666. This work was also supported by National Institute of Child Health through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction Research (to M.A.L.). We thank Dr. Emily Rickert for critical reading of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adashi EY, Hsueh AJ, Yen SS. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology. 1981;108:1441–1449. doi: 10.1210/endo-108-4-1441. [DOI] [PubMed] [Google Scholar]
- Batista A, Millan J, Mittelbrunn M, Sanchez-Madrid F, Alonso MA. Recruitment of transferrin receptor to immunological synapse in response to TCR engagement. J Immunol. 2004;172:6709–6714. doi: 10.4049/jimmunol.172.11.6709. [DOI] [PubMed] [Google Scholar]
- Bevan P. Insulin signalling. J. Cell Sci. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- Bilezikjian LM, Blount AL, Leal AM, Donaldson CJ, Fischer WH, Vale WW. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol. Cell. Endocrinol. 2004;225:29–36. doi: 10.1016/j.mce.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. Lost in translation: dysregulation of cap-dependent translation and cancer. Cancer Cell. 2004;5:519–523. doi: 10.1016/j.ccr.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- Bliss SP, Navratil AM, Breed M, Skinner DC, Clay CM, Roberson MS. Signaling complexes associated with the type I gonadotropin-releasing hormone (GnRH) receptor: colocalization of extracellularly regulated kinase 2 and GnRH receptor within membrane rafts. Mol Endocrinol. 2007;21:538–549. doi: 10.1210/me.2006-0289. [DOI] [PubMed] [Google Scholar]
- Box GEP, Cox DR. An analysis of transformations. Journal of the Royal Statistical Society Series B. 1964;26:211–252. [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Buggs C, Weinberg F, Kim E, Wolfe A, Radovick S, Wondisford F. Insulin augments GnRH-stimulated LHbeta gene expression by Egr-1. Mol Cell Endocrinol. 2006;249:99–106. doi: 10.1016/j.mce.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton RN, Catt KJ. Gonadotropin-releasing hormone receptors:characterization, physiological regulation, and relationship to reproductive function. Endocr Rev. 1981;2:186–209. doi: 10.1210/edrv-2-2-186. [DOI] [PubMed] [Google Scholar]
- Gharib SD, Wierman ME, Shupnik MA, Chin WW. Molecular biology of the pituitary gonadotropins. Endocrine Rev. 1990;11:177–199. doi: 10.1210/edrv-11-1-177. [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Kaiser UB. Regulation of gonadotropins by inhibin and activin. Semin. Reprod. Med. 2004;22:253–267. doi: 10.1055/s-2004-831901. [DOI] [PubMed] [Google Scholar]
- Hashizume T, Kumahara A, Fujino M, Okada K. Insulin-like growth factor I enhances gonadotropin-releasing hormone- stimulated luteinizing hormone release from bovine anterior pituitary cells. Anim. Reprod. Sci. 2002;70:13–21. doi: 10.1016/s0378-4320(01)00190-7. [DOI] [PubMed] [Google Scholar]
- Inokuchi J. Insulin resistance as a membrane microdomain disorder. Biol Pharm Bull. 2006;29:1532–1537. doi: 10.1248/bpb.29.1532. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Otsu K, Oshikawa J. Caveolin; different roles for insulin signal? Cell Signal. 2005;17:1175–1182. doi: 10.1016/j.cellsig.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocrine Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Kahn CR, Accili D. Insulin receptor knockout mice. Annu. Rev. Physiol. 2003;65:313–332. doi: 10.1146/annurev.physiol.65.092101.142540. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab. 2008;93:2089–2096. doi: 10.1210/jc.2007-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Tian D, Shaul YD, Lev S. Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J Biol Chem. 2000;275:32736–32746. doi: 10.1074/jbc.M004200200. [DOI] [PubMed] [Google Scholar]
- Liu F, Austin DA, Dipaolo D, Mellon PL, Olefsky JM, Webster NJG. GnRH activates ERK1/2 leading to the induction of c-fos and LHβ protein expression in LβT2 cells. Mol. Endocrinol. 2002a;16:419–434. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- Liu F, Austin DA, Webster NJ. Gonadotropin-releasing hormone-desensitized LbetaT2 gonadotrope cells are refractory to acute protein kinase C, cyclic AMP, and calcium-dependent signaling. Endocrinology. 2003;144:4354–4365. doi: 10.1210/en.2003-0204. [DOI] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002b;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both Gq/11 and Gs proteins in gonadotropin-releasing hormone receptor-mediated signaling in Lbeta T2 cells. J. Biol. Chem. 2002c;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E, Brown DA. Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Mahesh VB. The dynamic interaction between steroids and gonadotropins in the mammalian ovulatory cycle. Neurosci. Biobehav. Rev. 1985;9:245–260. doi: 10.1016/0149-7634(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Mason AJ, Hayflick JS, Zoeller RT, Young WS, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986a;234:1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Mason AJ, Pitts SL, Nikolics K, Szonyi E, Wilcox JN, Seeburg PH, Stewart TA. The hypogonadal mouse: Reproductive functions restored by gene therapy. Science. 1986b;234:1372–1378. doi: 10.1126/science.3097822. [DOI] [PubMed] [Google Scholar]
- Maudsley S, Naor Z, Bonfil D, Davidson L, Karali D, Pawson AJ, Larder R, Pope C, Nelson N, Millar RP, Brown P. Proline-rich tyrosine kinase 2 mediates gonadotropin-releasing hormone signaling to a specific extracellularly regulated kinase-sensitive transcriptional locus in the luteinizing hormone beta-subunit gene. Mol Endocrinol. 2007;21:1216–1233. doi: 10.1210/me.2006-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Bartucci M, Surmacz E. Estrogen receptor-alpha regulates the degradation of insulin receptor substrates 1 and 2 in breast cancer cells. Oncogene. 2003;22:4007–4016. doi: 10.1038/sj.onc.1206436. [DOI] [PubMed] [Google Scholar]
- Morrow IC, Rea S, Martin S, Prior IA, Prohaska R, Hancock JF, James DE, Parton RG. Flotillin-1/reggie-2 traffics to surface raft domains via a novel golgi-independent pathway. Identification of a novel membrane targeting domain and a role for palmitoylation. J Biol Chem. 2002;277:48834–48841. doi: 10.1074/jbc.M209082200. [DOI] [PubMed] [Google Scholar]
- Muller G, Frick W. Signalling via caveolin: involvement in the cross-talk between phosphoinositolglycans and insulin. Cell Mol Life Sci. 1999;56:945–970. doi: 10.1007/s000180050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z, Benard O, Seger R. Activation of MAPK cascades by G-protein-coupled receptors: the case of gonadotropin-releasing hormone receptor. Trends Endocrinol. Metab. 2000;11:91–99. doi: 10.1016/s1043-2760(99)00232-5. [DOI] [PubMed] [Google Scholar]
- Navratil AM, Bliss SP, Berghorn KA, Haughian JM, Farmerie TA, Graham JK, Clay CM, Roberson MS. Constitutive localization of the gonadotropin-releasing hormone (GnRH) receptor to low density membrane microdomains is necessary for GnRH signaling to ERK. J Biol Chem. 2003;278:31593–31602. doi: 10.1074/jbc.M304273200. [DOI] [PubMed] [Google Scholar]
- Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LbetaT2. Mol. Endocrinol. 2004;18:1301–1312. doi: 10.1210/me.2003-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom FH, Chen H, Cong LN, Li Y, Quon MJ. Caveolin-1 interacts with the insulin receptor and can differentially modulate insulin signaling in transfected Cos-7 cells and rat adipose cells. Mol Endocrinol. 1999;13:2013–2024. doi: 10.1210/mend.13.12.0392. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Remacle-Bonnet M, Garrouste F, Baillat G, Andre F, Marvaldi J, Pommier G. Membrane rafts segregate pro- from anti-apoptotic insulin-like growth factor-I receptor signaling in colon carcinoma cells stimulated by members of the tumor necrosis factor superfamily. Am J Pathol. 2005;167:761–773. doi: 10.1016/S0002-9440(10)62049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RG, Diaugustine RP, Petrusz P, Clark GC, Sebastian J. Estradiol stimulates tyrosine phosphorylation of the insulin-like growth factor-1 receptor and insulin receptor substrate-1 in the uterus. Proc Natl Acad Sci U S A. 1996;93:12002–12007. doi: 10.1073/pnas.93.21.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A, Froment P, Perrot V, Quon MJ, Leroith D, Dupont J. The luteinizing hormone-releasing hormone inhibits the anti-apoptotic activity of insulin-like growth factor-1 in pituitary alphaT3 cells by protein kinase Calpha-mediated negative regulation of Akt. J Biol Chem. 2004;279:52500–52516. doi: 10.1074/jbc.M404571200. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- Schneider JE. Energy balance and reproduction. Physiol. Behav. 2004;81:289–317. doi: 10.1016/j.physbeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Soldani R, Cagnacci A, Yen SS. Insulin, insulin-like growth factor I (IGF-I) and IGF-II enhance basal and gonadotrophin-releasing hormone-stimulated luteinizing hormone release from rat anterior pituitary cells in vitro. Eur. J. Endocrinol. 1994;131:641–645. doi: 10.1530/eje.0.1310641. [DOI] [PubMed] [Google Scholar]
- Song KS, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Sosnowski R, Mellon PL, Lawson MA. Activation of translation in pituitary gonadotrope cells by gonadotropin-releasing hormone. Mol. Endocrinol. 2000;14:1811–1819. doi: 10.1210/mend.14.11.0550. [DOI] [PubMed] [Google Scholar]
- Stanislaus D, Janovick JA, Brothers S, Conn PM. Regulation of G(q/11)alpha by the gonadotropin-releasing hormone receptor. Mol. Endocrinol. 1997;11:738–746. doi: 10.1210/mend.11.6.0005. [DOI] [PubMed] [Google Scholar]
- Todd BJ, Ladyman SR, Grattan DR. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J. Neuroendocrinol. 2003;15:61–68. doi: 10.1046/j.1365-2826.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- Unger JW, Lange W. Insulin receptors in the pituitary gland: morphological evidence for influence on opioid peptide-synthesizing cells. Cell Tissue Res. 1997;288:471–483. doi: 10.1007/s004410050833. [DOI] [PubMed] [Google Scholar]
- Valdes CT, Elkind-Hirsch KE, Rogers DG, Adelman JP. The hypothalamic-pituitary axis of streptozotocin-induced diabetic female rats is not normalized by estradiol replacement. Endocrinology. 1991;128:433–440. doi: 10.1210/endo-128-1-433. [DOI] [PubMed] [Google Scholar]
- Withers DJ. Insulin receptor substrate proteins and neuroendocrine function. Biochem. Soc. Trans. 2001;29:525–529. doi: 10.1042/bst0290525. [DOI] [PubMed] [Google Scholar]
- Xia YX, Weiss JM, Polack S, Diedrich K, Ortmann O. Interactions of insulin-like growth factor-I, insulin and estradiol with GnRH-stimulated luteinizing hormone release from female rat gonadotrophs. Eur. J. Endocrinol. 2001;144:73–79. doi: 10.1530/eje.0.1440073. [DOI] [PubMed] [Google Scholar]