Abstract
Nicotine dependence may be expressed differently in teens than in adults. Thus, it may not be sufficient to build diagnostic and cessation treatment strategies for teens based on adult-derived clinical and research data. This is the first study to prospectively examine the development of withdrawal symptoms by level of nicotine dependence among adolescent smokers. Forty-seven adolescent smokers completed nicotine withdrawal symptoms measures during 10 weeks of cessation treatment. Nicotine dependence was assessed at baseline using the mFTQ. Change in withdrawal symptoms over time by level of nicotine dependence was examined via mixed model ANOVA. Nicotine withdrawal in daily adolescent smokers was strongly and prospectively associated with level of nicotine dependence. Craving was rated as the most problematic symptom at the baseline assessment. The results of this study may help guide the development of future research on diagnostic and cessation treatment strategies for teens.
Keywords: nicotine withdrawal, nicotine dependence, adolescent, smoking, mFTQ
Introduction
Cigarette smoking among adolescents remains a significant public health concern because teens that smoke on a regular basis generally continue smoking into adulthood. Indeed, it has been argued that regular smoking compared to nonsmoking during adolescence raises the risk for adult smoking by a factor of 16 (Chassin, Presson, Sherman, & Edwards, 1990). Although researchers and policy makers have called for the development of effective cessation treatments for teen smokers (U.S. Department of Health and Human Services, 1994), few controlled cessation trials have been conducted and the lack of effective approaches is a recognized problem (McDonald, Colwell, Backinger, Husten, & Maule, 2003).
Among adult smokers, cessation is typically accompanied by a “well-defined withdrawal syndrome” (DSM-IV-TR; American Psychiatric Association, 2000), reflecting an underlying state of nicotine dependence. The emergence of withdrawal symptoms and, in particular, the development of strong urges and cravings, increases the risk of relapse following cessation (Killen and Fortmann, 1997). Accordingly, cessation treatments for adults typically attempt to prevent or alleviate nicotine withdrawal symptoms. Several investigators have called for research into the nature of nicotine dependence among adolescent smokers in hopes of increasing the efficacy of teen smoking cessation programs (Colby, Tiffany, Shiffman, & Niaura, 2000; Shadel, Shiffman, Niaura, Nichter, & Abrams, 2000). Such research is warranted because nicotine dependence may be expressed differently in teens. Thus, it may not be sufficient to build cessation treatment strategies for teens based on adult-derived clinical and research data (Colby et al., 2000; Henningfield & Jude, 1999).
At present, our understanding of the relationships between nicotine dependence, nicotine withdrawal symptoms, and smoking behavior is limited due, in part, to the need for prospective research (Colby et al., 2000). To date, two short-term prospective studies designed specifically to assess nicotine withdrawal symptoms in teen smokers, have demonstrated that adolescent smokers exhibit signs and symptoms that are characteristically associated with nicotine deprivation in adult smokers (Jacobson, Krystal, Mencl, Westerveld, Frost & Pugh, 2005; Killen, Ammerman, Rojas, Varady, Haydel, & Robinson, 2001). However, in both studies, participants were required to remain abstinent for 24 hours or less. The time course of withdrawal symptoms may be different for adolescents who are trying to achieve and maintain long-term abstinence and who have varying levels of nicotine dependence.
Very few teen smoking cessation trials have examined withdrawal symptoms during the course of treatment. Two open label studies of the efficacy of nicotine replacement found that withdrawal scores decreased from baseline through end of treatment (Hurt, Croghan, Beede, Wolter, Croghan, & Patten, 2000; Smith, House, Croghan, Gauvin, Colligan, Offord, et al., 1996); however, none of the mean withdrawal scores exceeded 2.0, corresponding to a “mild” rating. Killen and colleagues (Killen, Robinson, Ammerman, Hayward, Rogers, Stone, et al., 2004) reported decreases in craving over the course of 10 weeks of treatment in randomized clinical trial that combined bupropion with nicotine patch therapy; however, only abstinent participants were included in the analysis. Hanson and colleagues (Hanson, Allen, Jensen, & Hatsukami, 2003) found a time trend toward lower craving scores, but not overall withdrawal symptom scores from a pre-quit visit through 2 weeks post-quit for adolescents on nicotine patch, but not for those given placebo. Neither the active or placebo group reported high withdrawal symptom scores over the course of treatment.
Smith and colleagues (Smith, Cavallo, McFetridge, Liss, & Krishnan-Sarin, 2008) assessed tobacco withdrawal in adolescent smokers given behavioral smoking cessation therapy by an overall symptoms score and by each individual symptom. They observed increases in craving, restlessness and total withdrawal symptoms score from baseline to quit-day assessment (approximately 14 hours following the participant’s last reported cigarette). In addition, girls reported an initial peak in these symptoms that declined over time, whereas boys experienced little change in symptomatology over the course of the treatment. As with other studies, the symptoms were relatively minor, with craving as the most severe. One limitation of this study is that the inclusion of only participants who maintained abstinence over the 4 weeks of treatment resulted in a sample size of 23 teens and no further data was reported on the 45% that dropped out of the study.
Several factors limit the conclusions that can be drawn from the above investigations. First, only one study provided data on the full spectrum of nicotine withdrawal symptoms (Smith et al., 2008). The other studies focused the analyses on craving (Killen et al., 2004) or total withdrawal symptoms (Hanson et al., 2003; Hurt et al., 2000). Shiffman and colleagues argued against solely reporting an aggregated score of individual withdrawal and craving symptoms (Shiffman, West, & Gilbert, 2004) given evidence from factor analytic studies in adult smokers that reveal distinct factors of nicotine withdrawal (Etter & Hughes, 2006; Welsch, Smith, Wetter, Jorenby, Fiore, & Baker, 1999) and differences in the trajectories of individual withdrawal symptoms (Hughes, Higgins, & Bickel, 1994). Second, most of these trials included pharmacotherapy, thus preventing examination of the natural history of withdrawal symptoms in teen smokers. Third, evidence from the adult smoking cessation literature indicates that withdrawal symptoms severity is associated with level of nicotine dependence usually indexed by Fagerström score (Killen, Fortmann, Newman, & Varady, 1991). However, none of these studies with adolescents attempted to link baseline nicotine dependence level with level and severity of developing withdrawal symptoms over the course of treatment. Findings confirming such linkages would increase confidence in the validity and utility of nicotine dependence constructs and measures in adolescent smokers.
The current study adds to the existing adolescent smoking cessation literature by examining the development of nicotine withdrawal symptoms over time by baseline level of nicotine dependence. The results of this study might inform future research on diagnostic and cessation treatment strategies for teens.
Methods
Recruitment of Participants
Adolescent smokers were recruited from continuation high schools in the San Francisco Bay Area during the 2007-2008 academic school year. Students were recruited through brief classroom presentations and informational tables set up at breaks and lunchtime during the school day. In order to participate, teens had to meet the following criteria: 14-18 years of age; smoking at least 5 cigarettes per day; an interest in quitting smoking; no current use of antidepressants, antipsychotics or benzodiazepines; not currently seeing a psychologist or physician for major depression, panic disorder, social anxiety, or agoraphobia; and no current heavy (defined as more than 3 times per week within the past two weeks) alcohol or substance use. Parental consent was required for students under the age of 18 and was obtained through either written or verbal consent. The study was approved by the Stanford University Administrative Panel on Human Subjects in Medical Research.
Design
Data used in the current analyses are part of a larger, ongoing study assessing the effectiveness of extended treatment for smoking cessation in daily adolescent smokers. All eligible teens participated in 50-minute group cognitive-behavioral smoking cessation treatment sessions once a week for a total of ten weeks. A quit date was set for all participants for Week 2 of the intervention with Week 3 representing the first post-quit assessment. Questionnaires were completed and physiological measurements were collected at the beginning of each treatment session.
Measures
Nicotine withdrawal symptoms
Subjective withdrawal symptoms were measured at the beginning of each weekly intervention session (weeks 0-9). Symptoms were rated on a Likert scale from 1 to 10 with a higher number indicating a stronger experience of the symptom. The symptoms assessed were based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000) criteria for a diagnosis of Nicotine Withdrawal. The following symptoms were assessed: anger, anxiety, craving, depression, difficulty concentrating, frustration, hunger, and restlessness. Although DSM-IV-TR treats craving as an associated feature of nicotine withdrawal rather than a specific withdrawal symptom, we also assessed craving because of its demonstrated importance as a factor in maintaining smoking (Killen & Fortmann, 1997).
The following instructions were provided for completing the ratings: “When people don’t smoke for a period of time, they often suffer from cravings or other withdrawal symptoms. We’d like to know if you are experiencing any symptoms now and how upsetting or troubling these symptoms have been. Below we’ve listed many of the most common withdrawal symptoms. Read each symptom; circle the number that best describes your experience with that symptom.”
Modified Fagerström Tolerance Questionnaire (mFTQ; Rojas, Killen, Haydel, & Robinson, 1998)
The mFTQ was administered at the baseline assessment (Week 0). This questionnaire is a modified version of the instrument first developed by Fagerström (1978) as a self-report assessment of level of nicotine dependence. The modified questionnaire consists of the following five questions: “When you are in a place where smoking is forbidden, is it difficult for you not to smoke?”, “Do you smoke more in the morning than during the rest of the day?”, “Do you smoke even when you are really sick (for example, coughing or vomiting a lot)?”, “How deeply do you inhale the smoke?”, and “How soon after you wake up in the morning do you smoke your first cigarette?” Scores on the mFTQ range from a minimum of 5 to a maximum total of 24. Test-retest reliability (r=0.68) for the total mFTQ score was established previously for a sample of adolescent smokers (Rojas et al., 1998).
Cigarettes per day
Participants reported how many cigarettes, on average, they smoked per day.
Data Analyses
Baseline descriptive characteristics, including baseline cigarettes per day and baseline mFTQ, were computed by gender, percent change in smoking, and total sample (n = 66). Only those participants who had reduced their cigarettes per day (CPD) by at least 50% compared to the amount smoked at screening were included in the analyses assessing withdrawal symptoms by level of nicotine dependence (n = 47). An average of the CPD for Week 3 (the week after their quit day) through Week 9 was used to determine the reduction in smoking. Spearman correlations were computed to determine the relationship between baseline mFTQ, baseline CPD, and all withdrawal symptoms. Paired t-tests were used to compare the level of baseline craving scores with other withdrawal symptom scores; effect sizes were calculated using Cohen’s d formulas (Cohen, 1988).
To assess withdrawal symptoms by level of nicotine dependence, tertiles were created based on mFTQ scores; mFTQ scores ranged from 6 to 23, with those scoring below 13 assigned to the low nicotine dependence strata (n = 14), those scoring 13-15 in the medium nicotine dependence strata (n = 15), and those scoring 16 or higher (n = 18) assigned to the high nicotine dependence strata. Mixed model analysis of variance was used to compare the weekly mean scores on each separate withdrawal symptom over time and by mFTQ strata (high, medium, and low); only the symptom of craving was assessed using an average of 2 items (cravings, urges). The analyses were conducted using SAS PROC MIXED with compound symmetry covariance structure. We chose this method as it is designed to deal with missing data points within the analyses. When significant group differences were found, post-hoc analyses were conducted using Scheffé’s test.
Results
Descriptive Data
Of 190 teens who signed assent forms during school recruitment, an ethnically/racially-diverse sample of 66 remained eligible and interested after the initial teen and parent screening telephone calls. The majority of the teens were excluded because they did not smoke enough cigarettes. Descriptive characteristics by gender, percent change in smoking, and total sample are reported in Table 1. Of the 66 eligible teen smokers, 47 (71%) reduced their cigarette consumption by at least 50% and are included in the primary analyses. There were no statistically significant differences in age, reported cigarettes per day at screening, or baseline mFTQ scores between those who reduced their cigarette consumption by at least 50% and those who did not (see Table 1).
Table 1.
Baseline descriptive characteristics by gender, percent change in smoking, and total sample (N = 66)
| Percent change in smoking ≥ 50 % | Percent change in smoking < 50 % | ||||
|---|---|---|---|---|---|
| Males (N = 35) |
Females (N = 12) |
Males (N = 14) |
Females (N = 5) |
Total (N = 66) |
|
| Race/Ethnicity (N) | |||||
| Hispanic or Latino: | |||||
| American Indian | 2 | 0 | 1 | 0 | 3 |
| Asian | 0 | 0 | 0 | 0 | 0 |
| Native Hawaiian/Pacific Islander | 1 | 0 | 1 | 0 | 2 |
| Caucasian | 2 | 2 | 1 | 1 | 6 |
| Other | 9 | 0 | 1 | 0 | 10 |
| More than 1 race | 5 | 1 | 2 | 0 | 8 |
| Total | 19 | 3 | 6 | 1 | 29 |
| Not Hispanic/Latino: | |||||
| American Indian | 0 | 0 | 0 | 0 | 0 |
| Asian | 1 | 0 | 1 | 0 | 2 |
| Native Hawaiian/Pacific Islander | 3 | 1 | 3 | 0 | 7 |
| Caucasian | 7 | 6 | 3 | 3 | 19 |
| Other | 0 | 0 | 0 | 0 | 0 |
| More than 1 race | 5 | 2 | 1 | 1 | 9 |
| Total | 16 | 9 | 8 | 4 | 37 |
| M (and SD) | |||||
| Age | 16.9 (.8) | 16.8 (.6) | 16.9 (.6) | 17.0 (0) | 16.9 (.7) |
| Baseline Cigarettes Per Day | 12.4 (9.9) | 10.9 (5.3) | 9.5 (5.4) | 7.6 (3.3) | 11.2 (8.0) |
| Baseline mFTQ (range 5-24) | 14.1 (3.9) | 15.6 (2.8) | 14.4 (3.6) | 12.2 (2.8) | 14.3 (3.6) |
Notes: mFTQ = modified Fagerström Tolerance Questionnaire. There were no statistically significant differences in age, baseline cigarettes per day, or baseline mFTQ by percent change in smoking.
Over the course of the 10-week treatment program, 32 of the 47 participants (68%) attended at least 7 out of 10 sessions. Of the 47 teens who reduced smoking by at least 50%, 19 (40%) had at least one week of abstinence. At the quit date assessment (Week 3), 2 teens reported abstaining from smoking over the past week and remained abstinent throughout the course of the intervention. Six others quit between Weeks 4 and 8 and remained abstinent throughout the rest of the intervention weeks. Seven participants reported only one full week of abstinence over the course of the intervention and 4 participants had weeks of quitting intermixed with weeks of smoking.
Correlations between baseline withdrawal symptoms, mFTQ, and cigarettes per day
Although mFTQ and reported cigarettes per day were correlated at baseline (r = 0.31, p < .05), mFTQ score was more strongly correlated with withdrawal symptoms than reported cigarettes per day. Thus, baseline mFTQ was correlated with anxiety (r = 0.44, p < .01), cravings (r = 0.66, p < .001) and frustration (r = 0.40, p < .01), while baseline cigarettes per day was correlated only with baseline withdrawal symptom of depression (r = -0.32, p < .05). At baseline, the mean craving score (M = 5.7, SD = 2.7) was significantly higher than any other withdrawal symptom (Cohen’s d effect sizes ranged from .5 to 1.49).
Withdrawal symptoms over time by mFTQ score
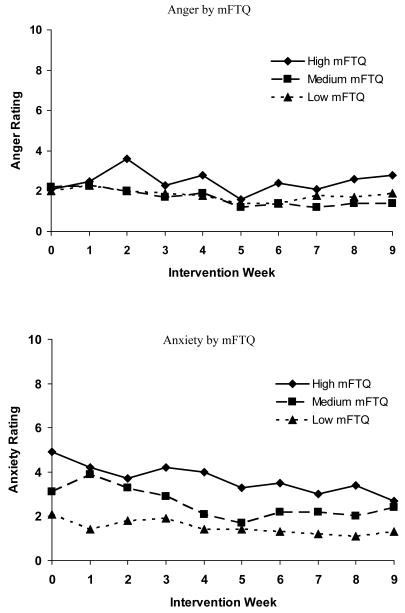
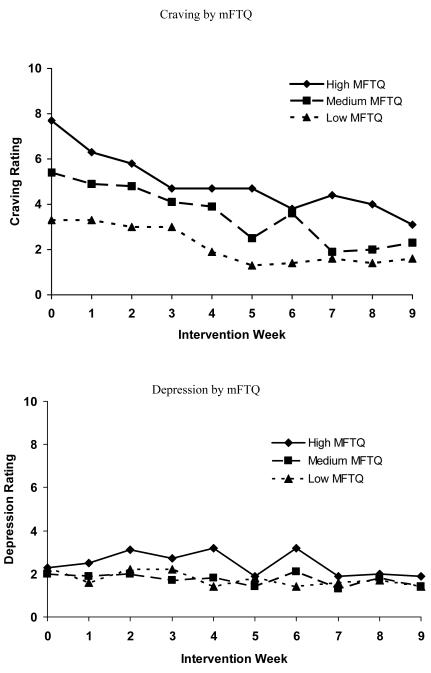
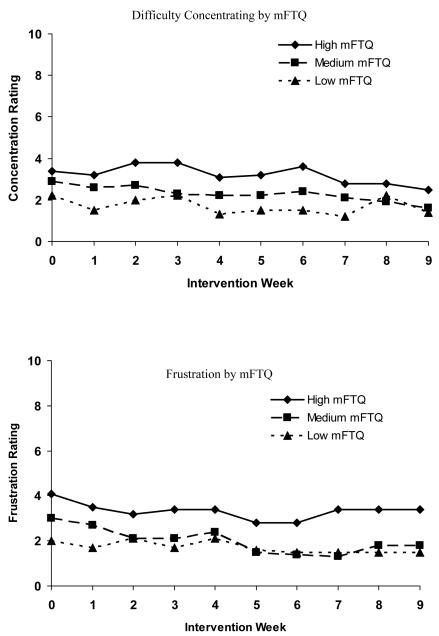
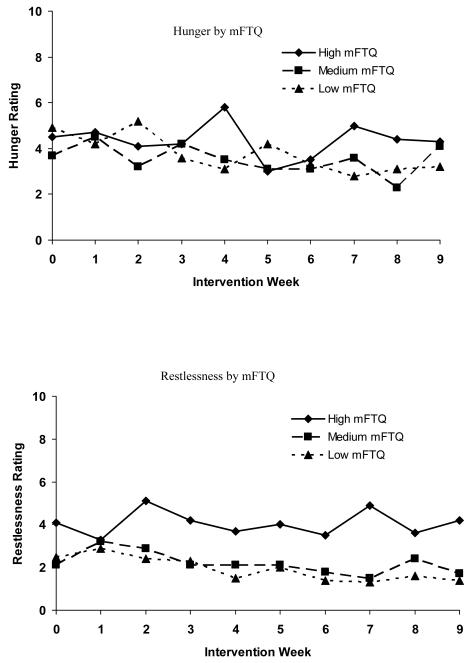
Figure 1 shows the relationship of baseline mFTQ and each withdrawal symptom over time. Baseline mFTQ levels were associated with the following symptoms: anger F(2, 352) = 7.0, p < .001, anxiety F(2, 352) = 38.9, p < .0001, craving, F(2, 352) = 43.8, p < .0001, depression, F(2, 352) = 6.9, p < .0012, difficulty concentrating F(2, 352) = 18.4, p < .0001, frustration F(2, 352) = 24.7, p < .0001, and restlessness F(2, 352) = 35.0, p < .0001; there was no significant relationship between mFTQ score and hunger over time. Post-hoc analyses revealed that for all symptoms, differences between the low and high mFTQ groups and the medium and high mFTQ groups met criteria for statistical significance. For all symptoms except for anxiety and craving, differences between medium and low mFTQ groups were not statistically significant. With respect to craving and anxiety, comparisons among all three mFTQ strata revealed differences that met criteria for statistical significance.
Figure 1.
Withdrawal symptoms over time by mFTQ (modified Fagerström Tolerance Questionnaire) strata (N = 47). Week 3 represents the first data collection time point since the set quit day.
Withdrawal symptoms over time
There was a reduction in teen smokers’ cravings to smoke F(9, 352) = 9.5, p < .0001 and levels of anxiety F(9, 352) = 2.4, p < .01 over the course of treatment; none of the other symptoms were significantly different over time. There were no statistically significant strata X time interactions.
Discussion
Several results merit attention. First, nicotine dependence, as measured by the mFTQ, was associated with the severity of all but one nicotine withdrawal symptom. Those with the highest scores on the mFTQ at baseline experienced more severe withdrawal symptoms during treatment compared to those with low to moderate nicotine dependence. For cravings and anxiety, there were statistically significant differences between all three levels of nicotine dependence and symptom severity. This is the first prospective study with adolescents to demonstrate the relationship between baseline nicotine dependence (mFTQ) and withdrawal symptom severity over the course of treatment. The finding that withdrawal symptoms differed by level of nicotine dependence increases confidence in the utility of the mFTQ and withdrawal symptoms questionnaires in adolescents. Knowing that withdrawal symptoms severity varies by level of nicotine dependence may aid in individualizing treatment for adolescents. The mFTQ could be a valuable tool in identifying those teens that are likely to experience more significant withdrawal symptoms while quitting smoking. Given the variability in quantity of daily smoking among adolescents (Nichter, Nichter, Thompson, Shiffman, & Moscicki, 2002) and our finding that mFTQ score was more strongly correlated with symptoms of withdrawal than was level of cigarette consumption at baseline, mFTQ score may serve as a better indicator of whether a teen smoker would benefit from nicotine replacement therapy for withdrawal symptom reduction.
Second, the relationship was most robust between nicotine dependence and craving. Teens with the highest mFTQ scores reported more than twice the level of craving at baseline compared to those with low mFTQ scores and continued to report higher levels of craving over the course of treatment. Craving may be the most difficult problem for adolescent smokers attempting to quit. In general, adult smokers report substantial difficulty coping with strong urges and cravings for cigarettes but indicate very little difficulty managing other withdrawal symptoms (Killen & Fortmann, 1997; Hughes, Gust, Skoog, Keenan, & Fenwick, 1991; West & Schneider, 1987). In similar fashion, during the initial weeks of treatment, adolescent smokers were most troubled by cravings that emerged during attempted cessation. Craving severity levels decreased most dramatically over the course of the 10 week intervention compared to other withdrawal symptoms, and craving was the only one of two symptoms to show a significant hierarchical relationship with all mFTQ strata. The high level of cravings, and relatively low levels of other nicotine withdrawal symptoms, reported in our sample are consistent with previous examinations of symptom severity in both adult (Hughes, Higgins & Hatsukami, 1990; Killen & Fortmann, 1997) and adolescent smokers deprived of nicotine (Killen et al., 2001; Smith et al., 2008) and suggest that cessation treatments might profit from the use of strategies to help adolescents cope with cravings associated with onset of cessation.
Third, ratings of the severity of craving and anxiety decreased over time, but there were no significant changes in the other symptoms over time. This lack of change over time could be due to the fact that the other nicotine withdrawal symptoms were low, even during the initial weeks of treatment. Others have reported a decrease in craving and/or withdrawal symptoms over time in smoking cessation trials targeting adolescent smokers (Killen et al., 2004; Hurt et al., 2000; Hanson et al., 2003; Smith et al., 1996); however, these studies utilized pharmacotherapy which may prevent or alleviate development of symptoms. Our results suggest that skills-based interventions may have a similar effect in decreasing some, but not all, symptoms of withdrawal. Results from other non-pharmocological interventions have been mixed. Hanson et al. (2003) did not find a time trend for reduced craving scores or overall withdrawal symptom scores for participants in the placebo patch condition. Smith and colleagues (2008) found that over the course of their 4-week intervention, females’ overall withdrawal symptoms and craving scores peaked at day 7 and declined over the subsequent weeks, whereas males reported more consistent levels of symptoms across weeks. They did not find a decrease over time in any symptoms, other than craving, when assessed individually. The fact that the majority of our teens were males may account for the differences between our study and the Smith study (2008). Further research is warranted to explore the effects of both skills-based and pharmacotherapy treatment on craving and individual withdrawal symptoms in adolescents over time, as well as whether gender differences exist in the time course of withdrawal symptoms.
As in other studies (e.g., Hanson et al., 2003; Killen et al., 2006; Smith et al., 1996), teen smokers in this trial reported withdrawal symptoms prior to quitting. Thus, it could be argued that such “symptoms” represent typical feelings and/or emotions that adolescents experience in daily life and not tobacco withdrawal symptoms per se. However, our finding that withdrawal symptoms varied by level of nicotine dependence strongly supports our conclusion that teens were reporting true nicotine withdrawal symptoms. Smith and colleagues (1996) suggest that reports of withdrawal symptoms prior to quitting may reflect teens’ limited access to cigarettes during school hours. Additionally, many teens in our trial cut back on their cigarette consumption prior to their quit attempt. Results from controlled laboratory studies indicate that withdrawal symptoms are evident among adolescents who are abstinent for relatively short periods of time. In one laboratory study with an 8 hour baseline smoking control phase that included both an active patch group and a placebo control group, the withdrawal symptoms of craving, anxiety, and restlessness were significantly higher than baseline during an 8 hour period of nicotine deprivation for both groups (Killen et al., 2001).
Several limitations of our study should be noted. Cut-points for nicotine dependence were based on the scores of this specific sample, creating somewhat arbitrary distinctions between low vs. moderate vs. high nicotine dependence. There are not standard criteria for cut-points on the mFTQ for adolescent smokers to distinguish between low, medium, and high levels of dependence. Given the small sample size, it was necessary to divide the sample in this manner to ensure an adequate number of participants in each group. Further research should be conducted with a larger sample size to replicate these findings.
As is common with most smoking cessation interventions, few participants were continuously abstinent over the course of treatment. However, withdrawal from nicotine dependence can occur among smokers who are not completely abstinent (DSM-IV-TR, 2000). Indeed, Shiffman and colleagues (2004) suggested that analyses of nicotine withdrawal should be conducted in both abstinent subjects and in smokers intending to quit or nearly abstinent. Given that 73% of our sample reduced their cigarette consumption by at least 50% and all the teens in the program reported a willingness to quit smoking, we are confident that this sample of teens were actively trying to achieve abstinence; however, we did not have enough teens who maintained consistent abstinence over the course of the program to conduct analyses with an abstinent-only subgroup. Future studies with larger sample sizes are warranted to further explore the relationship between withdrawal symptoms and smoking cessation among adolescents.
Acknowledgements
This study was funded by NCI Grant #: R01 CA 118035.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Work was performed at the Stanford University School of Medicine, Stanford Prevention Research Center, Stanford, CA.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text revision Author; Washington, DC: 2000. [Google Scholar]
- Bryant FB, Yarnold PR. Principal components analysis and exploratory and confirmatory factor analysis. In: Grimm LG, Yarnold PR, editors. Reading and understanding multivariate analysis. American Psychological Association; Washington, DC: 1994. [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: Predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychology. 1990;9(6):701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Colby SM, Tiffany ST, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Drug and Alcohol Dependence. 2000;59:S83–S95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Etter J-F, Hughes JR. A comparison of the psychometric properties of three cigarette withdrawal scales. Addiction. 2006;101:362–372. doi: 10.1111/j.1360-0443.2005.01289.x. [DOI] [PubMed] [Google Scholar]
- Fagerström K-O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3(34):235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine & Tobacco Research. 2003;4:515–526. doi: 10.1080/1462220031000118559. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Jude NR. Prevention of nicotine addiction: neuropsychopharmacological issues. Nicotine & Tobacco Research. 1999;1(Suppl 1):S41–S48. doi: 10.1080/14622299050011581. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine & Tobacco Research. 2007;9(3):315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, et al., editors. Research advances in alcohol and drug problems. Vol. 10. Plenum Press; New York, NY: 1990. pp. 317–398. [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Archives of General Psychiatry. 1991;48(1):52–59. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: Similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Croghan GA, Beede SD, Wolter TD, Croghan IT, Patten CA. Nicotine Patch Therapy in 101 Adolescent Smokers. Archives in Pediatrics and Adolescent Medicine. 2000;154(1):31–37. [PubMed] [Google Scholar]
- Jacobson LK, Krystal JH, Mencl EW, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Experimental and Clinical Psychopharmacology. 2001;9(2):176–182. doi: 10.1037//1064-1297.9.2.176. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5(2):137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Newman B, Varady A. Prospective study of factors influencing the development of craving associated with smoking cessation. Psychopharmacology. 1991;105(2):191–196. doi: 10.1007/BF02244308. [DOI] [PubMed] [Google Scholar]
- Killen JD, Robinson TN, Ammerman S, Hayward C, Rogers J, Stone C, Samuels D, Levin SK, Green S, Schatzberg AF. Randomized clinical trial of the efficacy of buproprion combined with nicotine patch in the treatment of adolescent smokers. Journal of Consulting and Clinical Psychology. 2004;72(40):729–735. doi: 10.1037/0022-006X.72.4.729. [DOI] [PubMed] [Google Scholar]
- McDonald P, Colwell B, Backinger CL, Husten C, Maule CO. Better practices for youth tobacco cessation: Evidence of a review panel. American Journal of Health Behavior. 2003;27(Suppl 2):S144–S158. doi: 10.5993/ajhb.27.1.s2.5. [DOI] [PubMed] [Google Scholar]
- Nichter M, Nichter M, Thompson PJ, Shiffman S, Moscicki A-B. Using qualitative research to inform survey development on nicotine dependence among adolescents. Drug and Alcohol Dependence. 2002;68(Suppl 1):S41–S56. doi: 10.1016/s0376-8716(02)00214-4. [DOI] [PubMed] [Google Scholar]
- Rojas NL, Killen JD, Haydel FK, Robinson TN. Nicotine dependence among adolescent smokers. Archives of Pediatrics and Adolescent Medicine. 1998;152(2):151–156. doi: 10.1001/archpedi.152.2.151. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Shiffman S, Niaura R, Nichter M, Abrams DB. Current models of nicotine dependence: what is known and what is needed to advance understanding of tobacco etiology among youth. Drug and Alcohol Dependence. 2000;59(1):9–22. doi: 10.1016/s0376-8716(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, West RJ, Gilbert DG, the SRNT Work Group on the Assessment of Craving and Withdrawal in Clinical Trials Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine & Tobacco Research. 2004;6(4):599–614. doi: 10.1080/14622200410001734067. [DOI] [PubMed] [Google Scholar]
- Smith AE, Cavallo DA, McFetridge A, Liss T, Krishnan-Sarin S. Preliminary examination of tobacco withdrawal in adolescent smokers during smoking cessation treatment. Nicotine & Tobacco Research. 2008;10(7):1253–1259. doi: 10.1080/14622200802219357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TA, House RF, Croghan IT, Gauvin TR, Colligan RC, Offord KP, Gomez-Dahl LC, Hurt RD. Nicotine patch therapy in adolescent smokers. Pediatrics. 1996;98(4):659–667. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services . Preventing tobacco use among young people: A report of the surgeon general. USDHHS, PHS, CDC, Office on Smoking and Health; Atlanta, Georgia: 1994. [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Experimental and Clinical Psychopharmacology. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- West R, Schneider N. Craving for cigarettes. British Journal of Addiction. 1987;82(4):407–15. doi: 10.1111/j.1360-0443.1987.tb01496.x. [DOI] [PubMed] [Google Scholar]