Introduction
The public health burden of pulmonary nontuberculous mycobacteria (P-NTM) disease cannot be accurately assessed in the absence of data on prevalence and treatment cost. These data are essential for allocation of research funding and creation of public health policy addressing control of this emerging disease. Population-based surveys conducted by the CDC during 1981-1983 estimated the prevalence of P-NTM disease at 1-2 cases per 100,000 persons in the US (1); a similar survey from 1993-96 reported an annual case rate of 7-8/100,000 (2). A more recent retrospective analysis from Canada found an annual increase in NTM isolate prevalence of 8.4% from 1997 through 2003 (3). Similar trends have been noted in other areas of the world (4-9).
Guidelines published by the American Thoracic Society and the Infectious Diseases Society of America (ATS/IDSA) for the diagnosis, treatment and prevention of P-NTM infections predict a good outcome for patients who tolerate therapy; however, they acknowledge the frequent occurrence of adverse drug reactions that make compliance with a multi-drug regimen challenging (10). The actual antibiotic prescription practices and associated costs have never been systematically analyzed. To better estimate costs of antibiotic treatment associated with NTM disease and identify factors associated with increased cost, we analyzed data from a cohort of patients enrolled in a NTM natural history study.
Methods
Study population
Subjects were adult HIV-seronegative patients enrolled in an IRB-approved natural history study of NTM at the National Institutes of Health (NIH). Patients had been recruited through listing in the NIH announcement of protocols, on clinicaltrials.gov, and by self or physician referral. To allow at least 18 months of follow-up, we included patients enrolled between January 1st 2004 and December 31st 2005. We included only subjects who met ATS/IDSA diagnostic criteria for P-NTM disease at initial diagnosis (10).
Data collection
Symptom, comorbidity, mycobacteriology, and treatment histories were abstracted from all available records. A detailed history of antibiotic usage from the time of diagnosis to the date of abstraction in August 2007 was reconstructed for each patient. Data included the antibiotic name, dosage, duration, and reasons for discontinuation or dose modification. Patients with insufficient information to reconstruct a complete treatment history or who were not treated were excluded.
Severity assessment
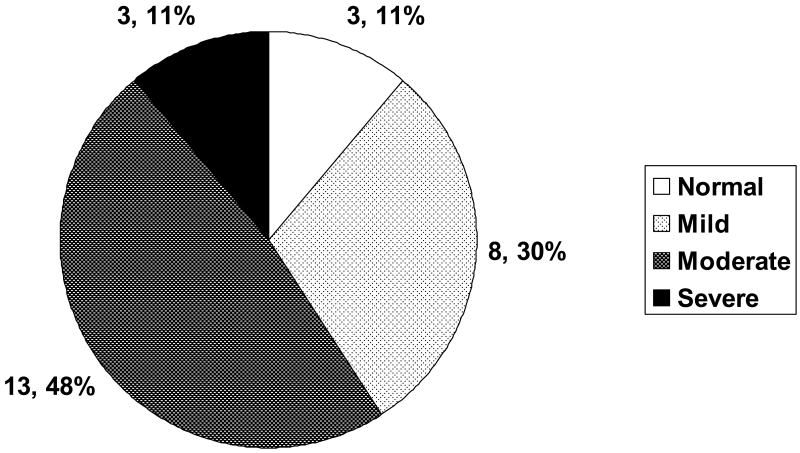
No measure has been validated for the assessment of severity in P-NTM infections. Chest computed tomography (CT) scans obtained at the time of initial evaluation at the NIH were independently reviewed and categorized retrospectively by two investigators (GB, KNO) as normal, mild, moderate or severe based on the numbers of lobes affected, and the presence of nodular-bronchiectasis or cavities (11) (Figure 1).
Figure 1.
Figure 1a: CT findings. Normal = no nodular bronchiectasis or cavitary disease; these subjects received on average, 5 months of treatment before enrollment at the NIH. Mild = nodular bronchiectasis limited to less than 3 lobes with no cavitary disease. Moderate = nodular bronchiectasis involving 3 or more lobes and/or cavitary disease limited to one lobe. Severe = multilobar cavitary disease regardless of presence or extent of nodular bronchiectasis. For the purposes of this categorization, the lingula was considered a separate lobe.
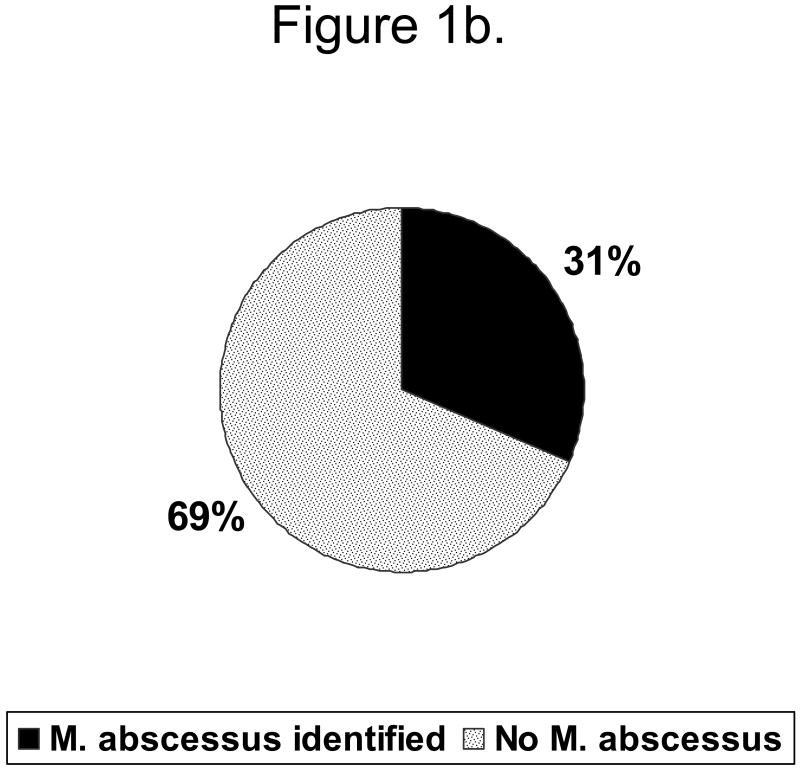
Figure 1b: Distribution of organism among persons with moderate\severe disease
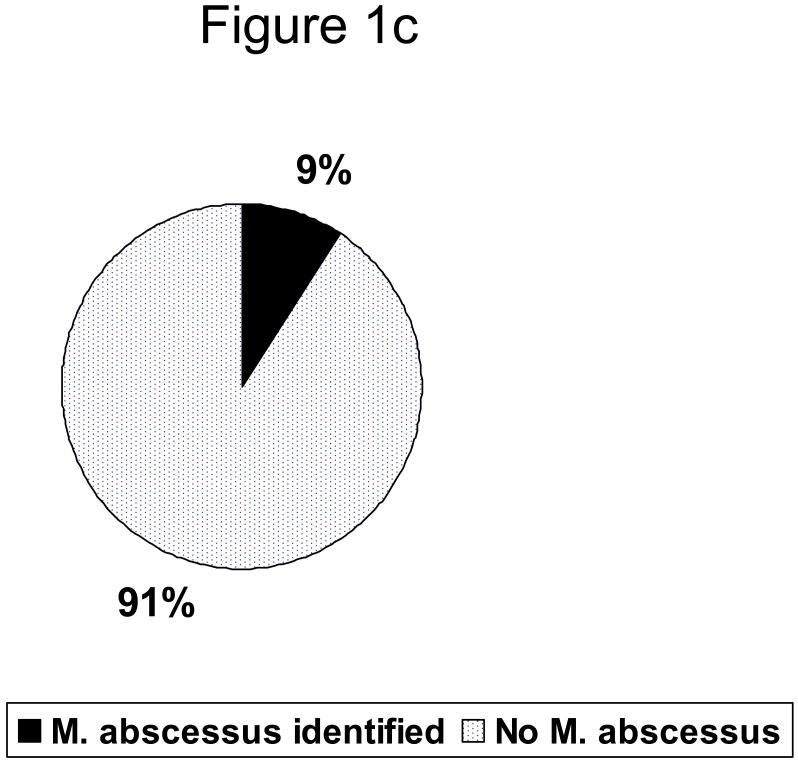
Figure 1c. : Distribution of organism among persons with mild\moderate disease
Cost data
Wholesale prices of antibiotics as reported by all available manufacturers were obtained from the “Red Book 2007: Pharmacy's Fundamental Reference”. For drugs with multiple manufacturers, we calculated an average cost. Costs of administration (e.g. nursing care, intravenous access devices) were not evaluated.
Data analysis
For each antibiotic we calculated the frequency of use, frequency of adverse reactions requiring discontinuation or dose reduction, mean duration of treatment, and mean cost per patient-month of treatment. Duration of treatment was calculated from the date of initial prescription until the end of treatment, discontinuation, or the date of data abstraction (if treatment was still ongoing). For each subject we calculated the total duration of treatment (including time before and after enrollment at the NIH) and the number and type of antibiotics used. The total antibiotic treatment burden for each subject was then calculated as the sum of the number of days on each drug (drug-days). The monthly burden was defined as the total burden divided by the number of months of follow-up. The total antibiotic cost was defined as the total number of drug-days for each drug, multiplied by the cost for the corresponding drugs, and summed for all prescribed drugs combined. The monthly cost was defined as the total cost divided by the number of months of follow-up on treatment. We defined “high treatment cost” as a monthly medication cost above the 75th quartile for our data set. For analysis of the association of organism with cost, any patient with M. abscessus was grouped in the M. abscessus category, regardless of other organisms found. We calculated odds ratios (OR) for the association between high treatment cost and the CT severity score and mycobacterial species. To assess the statistical significance of the ORs, we used the Fisher's exact test, with statistical significance assessed at p<0.05. Statistical analyses were conducted using SAS V8.02 (SAS Institute Inc, North Carolina).
Results
A total of 33 adult patients with P-NTM disease were enrolled in the natural history protocol during the period of interest. Of these, 27 were included in this analysis. Two patients were excluded due to insufficient documentation of treatment prior to enrollment, three were lost to follow-up within 18 months of enrollment, and one patient was never treated. The median age at enrollment was 59 years (range 26-82), and 24 (89%) subjects were female. Patients were followed a median of 53 months (range 19-151) from initial diagnosis and 28 months (19-40) from enrollment at NIH to data abstraction (Table 1). Of these 27 patients, 3 died during follow-up. Of the remaining 24, 13 were still receiving antibiotic treatment.
Table 1.
Patient Characteristics
| Sex | n (%) |
|---|---|
| Female | 24 (89) |
| Median age (range) | 59 (26-82) |
| Months of follow-up |
Median (range) |
| From initiation of treatment | 53 (19-151) |
| From enrollment at NIH | 28 (19-40) |
| Clinical findings | n (%) |
| Cough | 21 (78) |
| Hemoptysis | 12 (44) |
| Fatigue | 10 (37) |
| Weight Loss | 9 (33) |
| Fever | 8 (30) |
| Night Sweats | 7 (26) |
| Comorbidities & Past Medical History | n (%) |
| None | 18 (67) |
| Immunosuppression | 5 (19) |
| COPD | 2 (7) |
| Asthma | 1 (4) |
| History of tuberculosis | 1 (4) |
| AAT deficiency | 1 (4) |
| CFTR mutations | 1 (4) |
| Mycobacterium Species | n (%) |
| M. avium complex | 24 (89) |
| M. abscessus | 6 (22) |
| Other * | 5 (19) |
| Number of Species Isolated | n (%) |
| Single Species | 18 (67) |
Refers to the following species isolated only in combination with multiple isolates of M. avium complex: M. xenopi, M. fortuitum, M. gordonae, M. scrofulaceum.
COPD: chronic obstructive pulmonary disease; AAT: alpha-1 antitrypsin; CFTR: cystic fibrosis transmembrane conductance regulator
Clinical
Detailed documentation of clinical presentation at the time of initial diagnosis was not available for all patients. At the time of enrollment to the NIH natural history protocol, five patients (19%) reported no symptoms, 16 (59%) had between one and three symptoms, and six (22%) reported more than three symptoms, the most common being cough (78%), hemoptysis (44%), and fatigue (37%) (Table 1). Sixty-seven percent of subjects had no comorbid conditions. Seven (26%) had at least one comorbidity and three (11%) had two conditions. Immunosuppressive conditions were the most common: chronic steroid use (> 5 mg of prednisone or equivalent daily) and/or past medical history of cancer (breast n=2, colon n=1) were reported in five patients. Alpha-1 antitrypsin (A1AT) deficiency or mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene were found in four patients; no patients had a diagnosis of cystic fibrosis. Three patients had COPD or asthma and one subject had a history of pulmonary tuberculosis treated with a full course of therapy in the distant past.
Three subjects (11%) had a normal CT scan at the time of NIH enrollment; these patients had an average of five months of treatment prior to being seen at NIH. Eight (30%) subjects were classified as having mild disease and the remainder (60%) had evidence of either cavitations or nodular bronchiectasis involving three or more lobes and was classified as moderate to severe disease (Figure 1). The agreement between the two reviewers was 85%, with a Kappa of 69%. Using the results of the scoring from the second reviewer would not have changed these findings, as the total number of patients in the severe/moderate category would have increased by only one.
Mycobacteriology and Clinical/Radiographic findings
A single NTM species was isolated from 18 (67%) subjects (Table 1). Mycobacterium avium complex (MAC) was most common, comprising 56% of infections as a single pathogen, and 89% of infections either alone or in combination with other species. M. abscessus was the second most common, identified in 22%. Other organisms (M. xenopi, M. fortuitum, M. gordonae, and M. scrofulaceum) were recovered from sputum and bronchoalveolar lavage samples only in the background of multiple prior MAC cultures. Three patients had positive cultures for both MAC and M. abscessus. Overall 16 (59%) patients had moderate\severe disease (Fig 1a). Of these 16 with moderate severe disease, 5 (31%) had M. abscessus (fig. 1b), Among the 11 persons with mild disease 1 (9%) had M. abscessus identified (1c).
Antibiotic treatment for NTM and associated costs
Overall, 25 different antibiotics were prescribed (Table 2). Subjects received a median of 5 (range 1-10) antibiotics. The four most commonly prescribed antibiotics were ethambutol (85%), rifampin (64%), clarithromycin (64%) and azithromycin (25%). These relatively inexpensive drugs are readily available and easily administered. Courses of treatment with these four most commonly prescribed drugs were on average (760 days) longer than treatment with the four drugs (linezolid, ertapenem, cefoxitin, and imipenem) with an average monthly cost > $1,000 (174 days). Five of 6 patients whose treatment cost was over the 75th quartile received at least one (median 2, range 1-2) of these most expensive drugs. These drugs were part of the initial antibiotic regimen for only one patient. Only one patient whose treatment cost was above the 75th quartile was never treated with costly drugs; his treatment was more expensive because of lengthy duration. Overall, the proportion of patients with drug interruptions due to side effects ranged from 17% for ciprofloxacin to 75% for levofloxacin. For the most commonly prescribed drugs, the frequency of these reported interruptions ranged from 18% for azithromycin to 50% for ethambutol (Table 2).
Table 2.
Antibiotic Usage and Cost
| Antibiotic | N | Interruptions due to side effects (%) * | Average duration of treatment (Drug-days) | Average cost per patient ($US) | Average monthly cost ($US per month of treatment) |
|---|---|---|---|---|---|
| Ethambutol | 24 | 12 (50) | 739 | 2,897 | 119 |
| Rifampin | 18 | 8 (45) | 845 | 2,852 | 103 |
| Clarithromycin | 18 | 6 (33) | 660 | 5,792 | 267 |
| Azithromycin | 17 | 3 (18) | 793 | 6,251 | 240 |
| Moxifloxacin | 10 | 3 (30) | 779 | 8,676 | 339 |
| Amikacin+ | 10 | 5 (50) | 334 | 1,767 | 161 |
| Rifabutin | 7 | 5 (71) | 124 | 986 | 242 |
| Ciprofloxacin | 6 | 1 (17) | 369 | 3,463 | 286 |
| Other ** | 5 | 1 (20) | 241 | 4,405 | 556 |
| Levofloxacin | 4 | 3 (75) | 123 | 1,714 | 424 |
| Clofazimine | 4 | 1 (25) | 291 | 196 | 21 |
| Imipenem+ | 3 | - | 330 | 35,338 | 3,259 |
| Isoniazid | 3 | - | 148 | 28 | 6 |
| Linezolid | 3 | 2 (67) | 315 | 13,445 | 1,299 |
| Cefoxitin+ | 2 | 2 (100) | 97 | 10,849 | 3,403 |
| TMP/SMX | 2 | - | 186 | 56 | 9 |
| Streptomycin+ | 2 | - | 228 | 542 | 72 |
| Amox /Clav | 2 | - | 118 | 938 | 242 |
| Ertapenem+ | 1 | - | 6 | 359 | 1,821 |
Number of patients that had to interrupt or decrease the dose of antibiotic due to side effects attributed to the drug
Other: Ofloxacin, Pyrazinamide, Clindamycin, Doxycycline, Aztreonam+, Ceftazidime+
$US: US dollars; TMP/SMX: Trimetropin/Sulfamethoxazole; Amox/Clav: Amoxicillin/Clavulanate
Drug administered intravenously or intramuscularly (streptomycin)
The median burden of treatment for the entire cohort was 2,683 (range 84 – 7,689) drug-days (Figure 2). After adjustment for variable follow up, the monthly treatment burden was 68 (1 – 109) drug-days, roughly equivalent to treatment with two drugs per day over the entire follow up period. The median overall medication cost per patient was $19,876 ($398 - $70,917) with a median monthly treatment cost of $481 ($3 - $2,075). However, the cost varied greatly by infecting organism. Patients infected with MAC had an overall median medication cost of $14,730 compared with an overall median cost of $47,240 for those infected with M. abscessus. Patients with more severe disease had an overall cost of $28,738 compared with an overall median cost of $10,706 for those who had mild disease.
Figure 2.
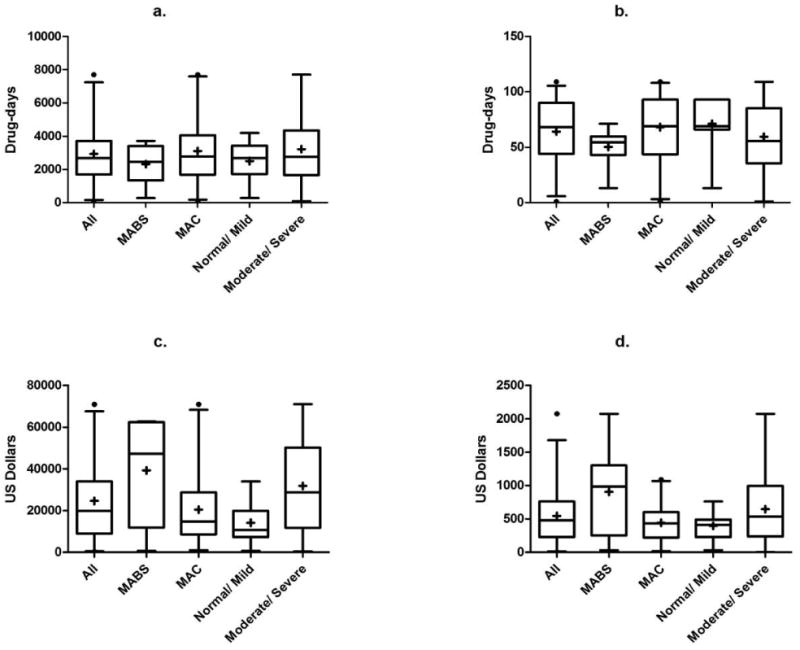
Burden of treatment and medication costs. Box and whisker plots where the plus signs and horizontal lines within the boxes represent the mean and median values, the top and bottom of boxes represent the 75th and 25th percentiles, the vertical lines represent the 95th and 5th percentiles, and the circles represent outliers. Data are categorized for all subjects, those with Mycobacterium abscessus (MABS), and those with Mycobacterium avium complex (MAC) and represent: a. Total burden of treatment per patient in drug-days (sum of the number of days on each drug); b. Monthly burden of treatment (total burden divided by the number of months of follow-up); c. Total medication costs per patient; d. Monthly medication costs per patient.
High monthly treatment cost (>75th quartile, i.e. $759) was not associated with age, number of comorbidities, or number of symptoms at the time of enrollment. However, subjects with high treatment costs were 12 (OR=12.0; 95%CI 1.5-97.2) times more likely to have M. abscessus (Table 3). Subjects with a high treatment cost were six fold more likely to have extensive disease based on CT findings, although this association was not statistically significant (95%CI 0.6-59.3). To assess the effects of each factor on cost, we constructed a multivariate logistic regression model including organism and CT disease severity. We found an adjusted nine fold increased likelihood of M. abscessus infection (adjusted OR 9.5; 95%CI 1.1, 82.4) and a non-significant four fold likelihood of moderate to severe disease (adjusted OR 4.2; 95%CI 0.4, 49.4) among those with high cost.
Table 3.
Association of severity and infecting organism with high treatment cost
| Variable | High cost/Total | OR | 95% CI | Adjusted OR | Adjusted 95% CI |
|---|---|---|---|---|---|
| Organism | |||||
| M abscessus | 4 / 6 | 12.0 * | 1.5 - 97.2 | 9.5 * | 1.1-82.4 |
| Non-M. abscessus | 3 / 21 | 1.0 | |||
| CT Findings | |||||
| Moderate\Severe | 6 / 16 | 6 | 0.6 – 59.3 | 4.2 | 0.4-49.4 |
| Normal\Mild | 1 / 11 | 1.0 |
p<0.05
Discussion
Cost is a critical factor in evaluating the public health burden of any condition. Our study represents the first systematic description of patterns of antibiotic prescription and treatment costs associated with P-NTM disease. We found the number and type of antibiotics prescribed were consistent with ATS/IDSA recommendations, and the cost of these antibiotics as assessed during a median follow-up period of over four years was approximately $20,000 with an average monthly cost over $500. Infecting organism was the factor most strongly associated with antibiotic cost; the rapid growing organisms (e.g. M. abscessus) have quite different and expensive treatment recommendations. The data also suggest that severity of disease is predictive of cost, though the association based on CT severity in this small sample was not statistically significant.
In a retrospective cohort of patients with P-NTM disease, Huang et al emphasized the chronic nature of the disease and found the recommended macrolide-based regimens have a significantly high rate of adverse reactions which compromise tolerability and ultimately result in limited therapeutic success (12). In our study population, ethambutol, rifampin, clarithromycin and azithromycin were the drugs most widely prescribed, in concordance with the ATS/IDSA recommendations for therapy of MAC (10). The frequency of adverse effects requiring a dose reduction or discontinuation of the drug was as high as 50% for the most commonly prescribed antibiotics, and up to 100% for less commonly used drugs (Table 2). The occurrence of adverse effects not requiring treatment changes was much higher. Gastrointestinal symptoms (epigastric discomfort, nausea and vomiting) were the most common complaints. Toxicity was higher than previously reported for these drugs used for other diseases or populations (13,14) and may reflect complex interactions of multiple concomitant drugs or a particular vulnerability of this population. It may also relate to severity of disease or lack of response to initial treatment as reasons for referral to the NIH protocol. The guidelines recommend treatment until 12 consecutive months of negative sputum cultures are obtained. Frequent interruptions and substitutions of antibiotic classes occurred at variable intervals. This made it difficult to identify discrete courses of treatment throughout the follow-up period. Examples of treatment regimens which demonstrate the number of drugs and the length of treatment with each are displayed in Figure 2.
Our study likely underestimates the true cost of treating NTM pulmonary infections. The direct medication cost did not include the cost of administering intravenous antibiotics such as amikacin. We also did not include the cost of outpatient visits or hospitalizations (productivity time lost, etc). A recent analysis of the prevalence and economic burden of bronchiectasis, (commonly associated with NTM) utilizing a health-care claims database, estimated that drug costs (majority were antibiotics) accounted for only 18% of the total healthcare costs (15). Our study population is likely to be biased by recruitment to a tertiary research referral center; if they are more likely to have severe disease, we may have overestimated cost. Severity was likely not significantly strongly associated with cost in this study for several reasons. First, the small sample size reduced the ability to detect a statistically significant association. Our measure of severity, CT scans, was assessed at enrollment to the NIH natural history study when most patients had already started treatment (median 23 months), and therefore their lesions may have appeared to be milder. Prior surgeries were not considered in the severity categorization; therefore, a patient with localized cavitary disease from M abscessus infection who underwent surgical resection prior to enrollment and had nodular bronchiectasis confined to one or two remaining lobes would have been classified as having mild disease. Lastly, the opportunity for therapeutic intervention is never defined solely on CT changes but considered within a broader clinical picture; a more comprehensive scoring system that includes clinical, radiographic and analytical data is likely to be more accurate in determining true disease severity and predict cost of disease.
The overall median monthly cost of $481 corresponds to an annual cost of $5,772 ($5,196 for those infected with MAC and $11,796 for those infected with M. abscessus). Comparisons of these costs to those for treating other similar diseases are limited by differences in study settings and methods. A prospective cohort study of the cost-effectiveness of treating multi drug-resistant tuberculosis (MDR-TB) in the Philippines found an average per patient cost of $1,557 assessed in 2002 for patients who had been followed over a three year period (16). Pulmonary infections with NTM are analogous to a chronic disease requiring non-discrete courses of medical treatment during prolonged periods of time. Treatment of P-NTM infections may be more comparable to treatment of HIV/AIDS, a chronic condition of infectious origin. Data from the HIV Research Network were used to estimate the annual cost of anti-retroviral therapy (ART) at $18,396 across their entire population of patients (17). A similar study estimated annual average costs of ART at $12,665 per patient based on National AIDS surveillance data during 2002-03 (18). Detailed follow-up data on persons living with HIV/AIDS have allowed estimation of the lifetime cost of treatment. If we assume a relatively high survival with lifetime treatment, the overall cost may be similarly increased. With a median annual cost of $5,772 and a median cumulative cost of nearly $ 20,000 over a 4 year treatment course, the cost of treating P-NTM infections may be comparable to the cost of treating MDR-TB and HIV/AIDS. With a characteristically chronic course and an apparent increasing prevalence, our findings emphasize the potential impact of P-NTM disease in public health and underscore the relevance of P-NTM infections to all pulmonary and infectious disease practitioners.
In summary, P-NTM represent an underappreciated disease burden in the US population, with an associated cost comparable to that for other chronic diseases of infectious origin such as HIV/AIDS. Further data on P-NTM prevalence in varied populations are needed to better estimate the true burden of treating NTM in the US.
Figure 3.
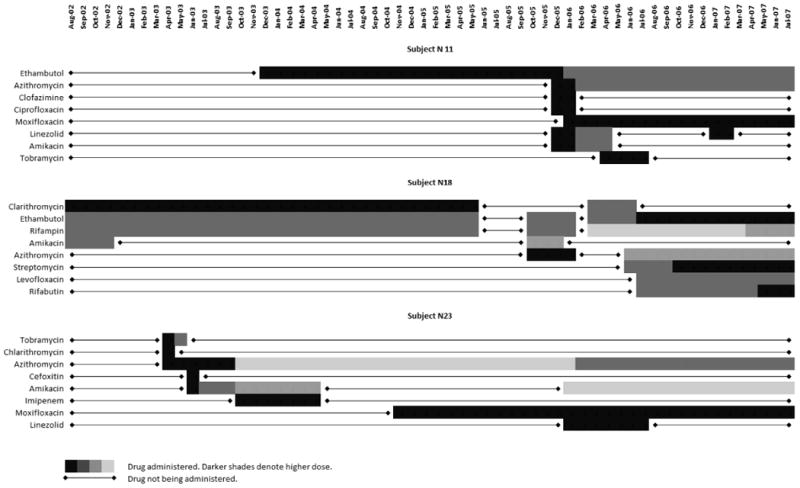
Three examples of NTM treatment courses. Frequent interruptions, substitutions, and dosage changes made identification of discrete antibiotic courses difficult.
Acknowledgments
This research was supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
Conflict of Interest: None of the authors have any conflicts of interest related to this article or the research described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Brien RJ, Lawrence JG, Snider DE. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am Rev Respir Dis. 1987;135:1007–1014. doi: 10.1164/arrd.1987.135.5.1007. [DOI] [PubMed] [Google Scholar]
- 2.Butler W, Crawford J, Shutt K. Nontuberculous mycobacteria reported to the Public Health Laboratory Information System by state public health laboratories, United States, 1993-1996. Atlanta: Centers for Disease Control and Prevention; 1999. [Google Scholar]
- 3.Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario. Thorax. 2007;62:661–666. doi: 10.1136/thx.2006.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates MD, Pozniak A, Uttley AH, Clarke R, Grange JM. Isolation of environmental mycobacteria from clinical specimens in South-East England: 1973-1993. Int J Tuberc Lung Dis. 1997;1:75–80. [PubMed] [Google Scholar]
- 5.Henry MT, Inamdar L, O'Riordain D, Schweiger M, Watson JP. Nontuberculous mycobacteria in non-HIV patients: epidemiology, treatment and response. Eur Respir J. 2004;23:741–746. doi: 10.1183/09031936.04.00114004. [DOI] [PubMed] [Google Scholar]
- 6.Martin-Casabona N, Bahrmand AR, Bennedsen J, et al. Non-tuberculous mycobacteria: patterns of isolation. A multi-country retrospective survey. Int J Tuberc Lung Dis. 2004;8:1186–1193. [PubMed] [Google Scholar]
- 7.Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005;20:913–925. doi: 10.3346/jkms.2005.20.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maugein J, Dallioux M, Carbonelle B, Vincent V, Grosset J, French Mycobacteria Study Group Sentinel-site surveillance of Mycobacterium avium complex pulmonary disease. Eur Respir J. 2005;26:1092–1096. doi: 10.1183/09031936.05.00148604. [DOI] [PubMed] [Google Scholar]
- 9.Koh WJ, Kwon OJ, Jeon K, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006;129:341–348. doi: 10.1378/chest.129.2.341. [DOI] [PubMed] [Google Scholar]
- 10.Griffith DE, Aksamit T, Brown-Elliot BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobaterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 11.Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral Bronchiectasis and Bronchiolitis at Thin-Section CT: Diagnostic Implications in Nontuberculosis Mycobacterial Pulmonary Infection. Radiology. 2005;235:282–288. doi: 10.1148/radiol.2351040371. [DOI] [PubMed] [Google Scholar]
- 12.Huang JH, Kao PN, Adi V, et al. Mycobacterium avium-intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease. Chest. 1999;115:1033–1040. doi: 10.1378/chest.115.4.1033. [DOI] [PubMed] [Google Scholar]
- 13.Jose J, Rao PG. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharmacol Res. 2006;54:226–233. doi: 10.1016/j.phrs.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Mandell LA, Ball P, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis. 2001;32:S72–S79. doi: 10.1086/319379. [DOI] [PubMed] [Google Scholar]
- 15.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;12:205–209. [Google Scholar]
- 16.Tupasi TE, Gupta R, Quelapio MID, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the phillippines. PLOS Medicine. 2006;3:e352. doi: 10.1371/journal.pmed.0030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson AB, Farnham PG, Dean HD, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43:451–457. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]