Abstract
Testosterone is well known to regulate sexual behavior in males, but this is dependent upon prior sexual experience. Aging is associated with decreased libido and changes in testosterone, but the role of experience in these age-related processes has not been systematically studied. We examined effects of age and sexual experience on serum hormones (total testosterone, free testosterone, estradiol, LH) and on numbers of androgen receptor (AR) and estrogen receptor α (ERα) immunoreactive cells in the hypothalamus. Extensive sexual experience was given to male rats at 4 months of age. Rats were euthanized at either 4 months (young) or 12 months (middle-aged (MA)). Comparable sexually naïve male rats were handled and placed into the testing arena but did not receive any sexual experience. Thus, we had four groups: young-naïve, young-experienced, MA-naïve and MA-experienced. Serum hormone levels were assayed, and numbers of AR and ERα cells were quantified stereologically in the medial preoptic nucleus (MPN) and the anteroventral periventricular nucleus (AVPV). Sexually experienced males had significantly elevated serum testosterone and free testosterone in both age groups. Both total and free testosterone were higher, and estradiol lower, in middle-aged than young rats. Experience did not alter either AR or ERα expression in the preoptic brain regions studied. Aging was associated with increased expression of AR, but no change in ERα. These results show that sexual experience can induce short-term and long-term alterations in serum hormones but these effects are not manifested upon their receptors in the hypothalamus.
Keywords: androgen receptor, estrogen receptor α, sexual experience, aging, anteroventral periventricular nucleus (AVPV), medial preoptic nucleus (MPN)
Introduction
Both the appetitive (motivational) and consummatory (copulatory) aspects of male sexual behavior are related to the actions of steroid hormones upon their receptors located in a network of interconnected limbic brain regions, principally the preoptic area (Davidson, 1966; Dominguez and Hull, 2005). Most work in this arena has focused on testosterone’s actions on the androgen receptor (AR), because castration is associated with the reduction and eventual disappearance of appetitive and consummatory sexual behaviors, which can be restored by testosterone treatment (Chambers et al., 1991; Davidson, 1966; McGinnis et al., 1989). Moreover, castration decreases AR expression in the preoptic area, and testosterone treatment restores it (Handa et al., 1996; Krey and McGinnis, 1990). Estrogen receptors (ER) also play a role in this process, as exogenous estradiol given to castrated rats can restore sexual behavior, although supraphysiological levels are necessary to provide a complete restoration (Davidson, 1969; Sodersten, 1973). Aromatase inhibitors block testosterone induction of copulation, again highlighting the role of estradiol in this process (Clancy et al., 2000; Morali et al., 1977; Vagell and McGinnis, 1997; Zumpe et al., 1993).
Some attention has focused upon the role of sexual experience in copulatory behavior. This is important because behavioral experience can change the brain, causing elevated metabolic capacity in the limbic system (Sakata et al., 2002) and plasticity of excitatory synapses in medial preoptic area (Malinina et al., 2006) that may modulate the ability of hormones to act upon preoptic brain regions to control behavior. A role for previous sexual experience has been shown for both motivational (Kelliher and Baum, 2002; Matuszczyk and Larsson, 1994) and consummatory components of behavior [cats (Rosenblatt and Aronson, 1958) and hamsters (Lisk and Heimann, 1980)]. Although conflicting results were found for the effect of experience in the timing of the loss of mating behaviors in rats (Bloch and Davidson, 1968; Rabedeau and Whalen, 1959; Retana-Marquez and Velazquez-Moctezuma, 1997), it was shown that male rat copulatory behavior is more efficient after sexual experience. Compared to naïve animals, sexually experienced rats could achieve an ejaculation with fewer mounts and intromissions; those males with prior sexual experience had lower mount, intromission and ejaculation latencies (Bialy et al., 2000; Dahlof and Larsson, 1978; Dewsbury, 1969; Frankel, 1981; Pfaus and Wilkins, 1995) and lower post-ejaculatory interval (Larsson, 1959). These effects are mediated by preoptic brain regions and their inputs, as supported by evidence that experienced rats showed greater sexual performance than non-experienced male rats after brain impairment caused by lesions of the medial preoptic area (de Jonge et al., 1989; Liu et al., 1997), medial amydala (de Jonge et al., 1992; Kondo, 1992) and the bed nucleus of stria terminalis (Claro et al., 1995).
During aging, there is a decline in male sexual performance (Chambers and Phoenix, 1983; Craigen and Bronson, 1982; Nicolosi et al., 2004; Smith et al., 1992), but the role of sexual experience in this process has not been systematically studied. This is important because experience may interact with aging to affect reproductive behavior and function, and cause structural and neurochemical changes in the nervous system. For example, levels of neuropeptide Y, a neurotransmitter involved in sexual behavior (Clark et al., 1985) and the control of gonadotropin-releasing hormone secretion (Kalra and Crowley, 1992), were significantly higher in middle-aged sexually experienced male rats than naïve middle-aged animals, an effect that was not found in young males (Clark, 1994). Middle-aged naive but not experienced male rats responded to a receptive female with significantly rising testosterone during mating while there was no difference between young naïve and young experienced males (Frankel, 1981). Further, compared to sexually naive males, experienced male rats have greater copulation-induced Fos-like immunoreactivity in the medial preoptic nucleus (Lumley and Hull, 1999). Beyond these and a few additional studies, little is known about how hormones, brain and sexual behavior are affected by sexual experience during aging.
Previously, we showed an age-related increase in AR, but little change in ERα, in two preoptic regions: the medial preoptic nucleus (MPN) and the anteroventral periventricular nucleus (AVPV; Wu et al., 2009). We hypothesized that sexual experience may increase sensitivity to steroid hormones by affecting their secretion or changing their actions upon their nuclear hormone receptors in hypothalamus. Using a male rat model, we compared effects of identical sexual experience on hormones and expression of AR and ERα in the preoptic area of young and middle-aged male rats.
Methods
Animals
At start of the experiments, 54 male Sprague-Dawley rats without sexual experience were purchased at 2–3 months from Harlan Sprague-Dawley Inc (Indianapolis, Indiana; Stock/Strain: Hsd:Sprague Dawley®™ SD®TM).
24 young female Sprague-Dawley rats (2–3 months) were purchased from Harlan Sprague-Dawley Inc. After ovariectomy, they were implanted with estradiol [one Silastic capsule (1.98-mm I.D. × 3.18-mm O.D. × 5-mm length; Dow Corning Corporation, Midland, MI) packed with 5% crystalline 17β-estradiol (Sigma-Aldrich, St. Louis, MO)]. These female rats were injected with 500 µg progesterone (Sigma-Aldrich, St. Louis, MO) in sesame oil (0.1 ml) three hours before the mating tests. Only females that showed lordosis after being mounted by male rats were used in mating trials.
Rats lived in an AAALAC-approved facility (two same-sex same-experience animals per cage, cage dimensions 47 × 20 × 25 cm) with Rat Sterilizable Diet (Harlan Teklad LM −485 7012, Madison, WI) and water available ad libitum. The light cycle was 12 h light, 12 h dark cycle (lights on 2300h), and temperature was kept at 21 ± 1°C. All animal procedures were approved by the UT-Austin Institutional Animal Care and Use Committee (Protocol number: 08030101) and studies were performed following The Guide for the Care and Use of Experimental Laboratory Animals.
Sexual Experience
Mating tests were performed under red dim lights, beginning 3 hours after lights out (1400h-1700h). Every female habituated to the chamber for 10 minutes first. Every male habituated to the chamber for 20 minutes before the female was introduced. 20-minute mating tests took place in a transparent Plexiglas chamber [38 cm (L) × 32 cm (W) × 46 cm (H)]. The test was terminated at 20 minutes. Mating tests were recorded using a Sony Handycam Hi-Fi Stereo Video 8 XR camcorder (Sony Corporation of America, New York, NY). As a control, the naïve group were put in clean test chambers without a female for 20 minutes.
At ∼3 months of age, male rats were randomly assigned to one of two experimental groups: Naïve (n = 24) or Sexual experience (n = 30). For the experience group, male rats were initially given five mating tests every other day with different receptive females (Figure 1). Those males who mated in only one of the five tests or did not mate were excluded. The rest of the males in this group (n = 22) continued to receive sexual experience by staying with a receptive female overnight 15 times (every other day for 30 days) with females rotating among males. Ejaculations were confirmed by checking the females’ vaginas the next morning for the presence of sperm. All the males ejaculated for each of the 15 nights. Another 5 mating tests were performed for experienced rats to confirm mating ability (Figure 1). All the rats mated in 3 to 5 of the 5 mating tests.
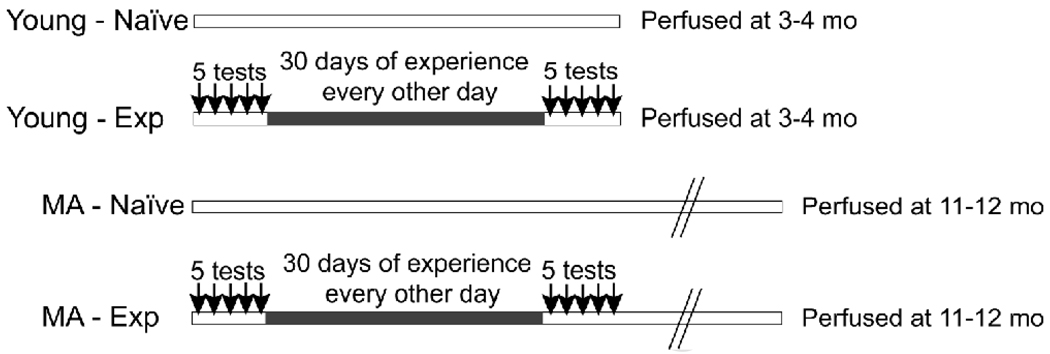
Figure 1.
The general experimental design is shown. Arrows indicate days of five consecutive mating tests in the experience groups given in two sessions, interspersed by a thirty day period in which males were placed with a receptive female every other day. Naïve rats were placed into the testing chambers for equivalent periods of time but did not have access to a female. Young rats were perfused one day after the last testing or handling period. Middle-aged rats were perfused 8 months after testing. MA: middle-aged; Exp: experienced; mo: months of age.
Both the naïve and experienced groups were then further randomly subdivided into two groups (Figure 1). Young rats were perfused at ∼4 months of age (1 day after the last mating test), and middle-aged rats at 11–12 months of age (approximately 8 months after the last mating test). This resulted in four experimental groups: young-naïve group (n = 12), young-experienced (n = 11), middle-aged-naïve (n = 12), middle-aged-experienced (n = 11).
Perfusion
Young-experienced rats were perfused the day after the last mating test. The young-naïve group was perfused at the same time relative to placement in the mating chamber without a female. Middle-aged rats (naïve or experienced) rats were perfused 8 months after testing or handling. For perfusions, rats were anesthetized with ketamine (40 mg) and xylazine (8 mg) (i.p. injection). Then, the thoracic cavity was opened, and a blood sample (6 ml) was drawn from the ventricle for subsequent hormone assays. Animals were subsequently perfused (48 ml/min) sequentially with 0.9% saline (24 ml), 0.9% saline with 10% heparin (24 ml), and 1% paraformaldehyde with 3.75% acrolein (48 ml), followed by 4% paraformaldehyde (480 ml; Wu et al., 2009). PBSA (phosphate-buffered saline A: 0.122 M PO4, 0.077 M NaCl, pH=7.3) was used to dissolve all fixatives. After perfusion, the brains were carefully removed and post-fixed for 3 hours in 4% paraformaldehyde and then transferred into PBSA with 0.05% sodium azide for storage at 4°C. A vibrating microtome (Leica VT 1000S, Leica Microsystems, Nussloch, Germany) was used to cut brain into sections (40 µm-thick) that were stored in PBSA with 0.05% sodium azide at 4°C until use within a 6-month period.
Immunohistochemistry
For AR immunohistochemistry, sections were incubated in the rabbit polyclonal AR antibody N-20 (1:1500; Santa Cruz Biotechnology, Inc.) developed in 1996 against a peptide mapping at the N-terminus (amino acids 299–315) of AR of human origin and which has been validated extensively by preabsorption of the antibody with the antigen, western blotting, and other controls (Chlenski et al., 2001; McKinnell et al., 2001), Wu & Gore, unpublished).
The ERα antibody was a purified rabbit polyclonal antibody C1355 (1:20,000, Upstate Biotechnology, Waltham, MA) generated against the final 15 C-terminal amino acids (TYYIPPEAEGFPNTI) of the rat ERα. It has also been very well validated for use in neural, reproductive and other tissues by preabsorption and western blotting controls (Chakraborty et al., 2003; Friend et al., 1997; Moffatt et al., 1998; Wu et al., 2009).
For immunohistochemistry, all the sections were processed in two runs. For each run, animals from each age and experience group were equally represented. In order to make the experimenter blind to age and experience group, sections IDs were then recoded. Sections containing the preoptic area were selected in a 1:2 series (AVPV region) or a 1:4 series (MPN region; see below for details) and rinsed in PBSB (phosphate-buffered saline B: 0.012 M, PO4, 0.154 M NaCl, pH=7.3) All steps, unless noted, occurred at room temperature (22°C) on a shaking platform. Procedures of immunohistochemistry, tissue handling and Nissl staining were done identically to our previously published work (Wu et al., 2009).
Stereological analysis
A stereological analysis was performed according to methods described in detail previously (Chakraborty et al., 2005; Wu et al., 2009). For the AVPV, a 1:2 series (5–6 sections) and for MPN a 1:4 series (8–9 sections) was used. A wet-mount of fresh tissue showed that average tissue thickness was 40.9 µm. The sections were carefully matched for rostral-caudal landmarks among all the animals, and the regions were identified in Nissl-stained sections by comparing anatomical landmarks to an atlas of the rat brain (Swanson, 1998). Quantitative analyses were done as previously described (Wu et al., 2009). For stereology, closed contours were drawn to surround the region of interest (AVPV and MPN) at low magnification (10X and 4X, respectively) using the laboratory Olympus BX-61 microscope. A buffer zone at the top and bottom of sections was set at 3 µm for all experimental stereology. For each rat, the regional volume of the AVPV and MPN was extrapolated based on the contours and tissue thickness (Volume = regional area x thickness). The Stereo Investigator® software (MicroBrightField, Williston, VT) randomly placed 135 µm × 80 urn grids (“disector frames”) within the AVPV contour and 120 µm × 180 µm grids within the MPN contour. Within these disector frames, the DAB-stained AR- or ERα- labeled nuclei were counted within a 50 µm × 50 µm counting frame (“optical disectors”). Based on these parameters, the number and density (# immunoreactive cells/volume of each nucleus) of AR-immunoreactive (AR-ir) or ERa-immunoreactive (ERα-ir) nuclei falling within the regions was quantified. The coefficients of error (Cruz-Orive/Geiser) and variation of the estimates were calculated as described by Schmitz and Hof (Schmitz and Hof, 2000). Photomicrographs were taken to produce the figures, and images were subjected to only minor adjustments of contrast using Adobe Photoshop 7.0 (Adobe, San Jose, CA). In order to avoid any bias, any adjustments were applied equally to tissues from rats of different ages.
Serum Hormone Concentrations
Blood samples collected at the time of perfusion were centrifuged (8000 rpm, 5 min) and the serum collected and stored at –80 °C for hormone assays.
Enzyme immunoassay (EIA) of serum total testosterone
Total testosterone concentrations were measured by a single enzyme immunoassay in two plates using the EIA kit DSL-10–4000 according to the manufacturer’s methods provided by Diagnostic Systems Laboratories, Inc. (Webster, TX). Duplicate samples were run at a volume of 50 µl each. The minimum detectable level of testosterone was 0.04 ng/ml per tube. Intra-assay variabilities of two plates were 7.61% and 7.48% respectively. Inter-assay variability was 0.79%.
Enzyme immunoassay (EIA) of serum free testosterone
Free testosterone concentrations were measured by two testosterone enzyme immunoassays using the EIA kit DSL-10–49000 according to manufacturer’s protocol (Diagnostic Systems Laboratories, Inc.). Duplicate samples were run at a volume of 50 µl each. The minimum detectable level of testosterone was 0.19 pg/ml per tube. Intra-assay variabilities were 2.38% and 4.59% respectively. Inter-assay variability was 9.98%.
Radioimmunoassay (RIA) of serum estradiol
Estradiol concentrations were measured by a single ultra-sensitive estradiol RIA using the DSL-4800 RIA kit according to the method described by Diagnostic Systems Laboratories, Inc. Duplicate samples were run at a volume of 200 µl each. The minimum detectable level of estradiol was 2.2 pg/ml per tube. Intra-assay variability was 1.86%.
RIA of serum luteinizing hormone (LH)
Serum LH concentrations were assayed by Dr. Michael Woller, University of Wisconsin-Whitewater. Duplicate 50 µl samples were run in a double-antibody RIA with the rat LH RP-3 standard and iodinate, and using the rabbit antibody from the National Hormone and Pituitary Program of the NIDDK (reagents kindly provided by Dr. A.F. Parlow, Torrance, CA). Primary antibody was precipitated using goat anti-rabbit gamma globulin secondary antibody. Assay sensitivity was 0.03 ng/tube at 85% binding, and intra-assay variability was 5.76%.
Statistical Analysis
Statistical analysis was done with each rat as the unit of analysis. Using JMP statistical software (version 7.0, SAS institute, Cary, NC), effects of age and experience were evaluated on the following endpoints each for the AVPV and MPN: AR-ir and ERα-ir cell numbers, regional volume, and immunoreactive cell density (calculated as cell numbers/volume). Effects of age and experience on serum hormone levels were similarly analyzed. First, datasets were tested for homogeneity of variance and normality. For datasets that met these criteria, comparisons were made by two-way ANOVA followed by post hoc analysis when indicated by a significant interaction. Otherwise data were square root transformed to normalize variation across a broad range of values and then analyzed by two-way ANOVA. Those datasets that were transformed were: AR-ir density in MPN; ERα-ir cell number in AVPV; ERα-ir cell density in MPN; MPN volume; serum hormones. The criterion for statistical significance was p < 0.05.
Results
AR Immunoreactivity in AVPV and MPN
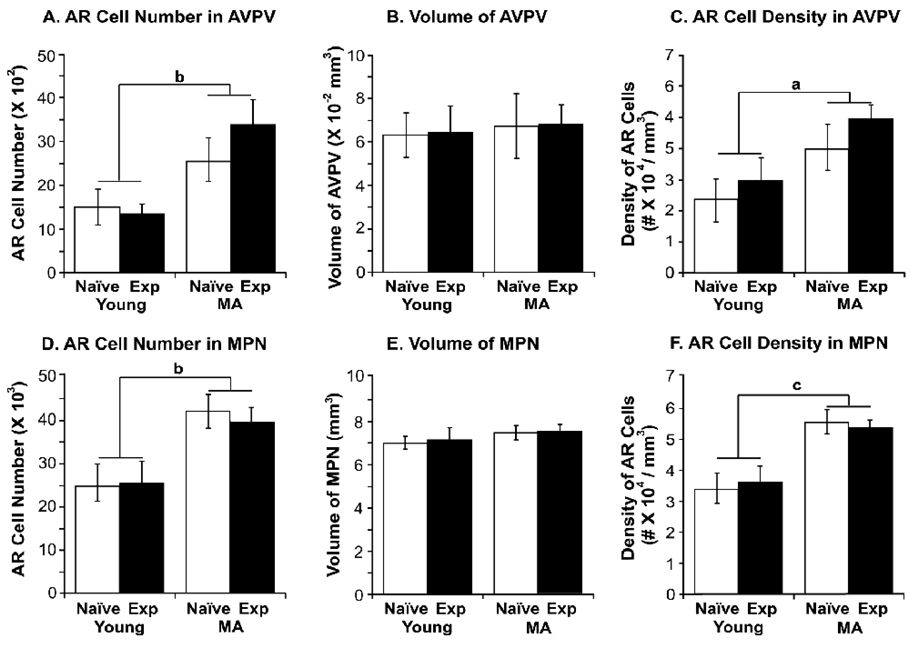
Representative photomicrographs of AR immunoreactivity in the AVPV and MPN are shown in young and middle-aged male rats with or without sexual experience, and demonstrate robust expression of AR-ir in these regions (Figure 2). Stereologic analyses of AR-ir cell numbers and densities in AVPV and MPN were performed (Figure 3). A significant main effect of age was detected on AR-ir cell numbers and densities in both of these preoptic regions (AVPV: AR-ir cell numbers P < 0.01, AR-ir cell densities P < 0.05; MPN: AR-ir cell numbers P < 0.01, AR-ir cell densities P < 0.001). Specifically, middle-aged rats had higher numbers and densities of AR-ir cells in AVPV and MPN compared to young rats. There was no significant effect of experience on AR cell number and density in AVPV and MPN (Figure 3).
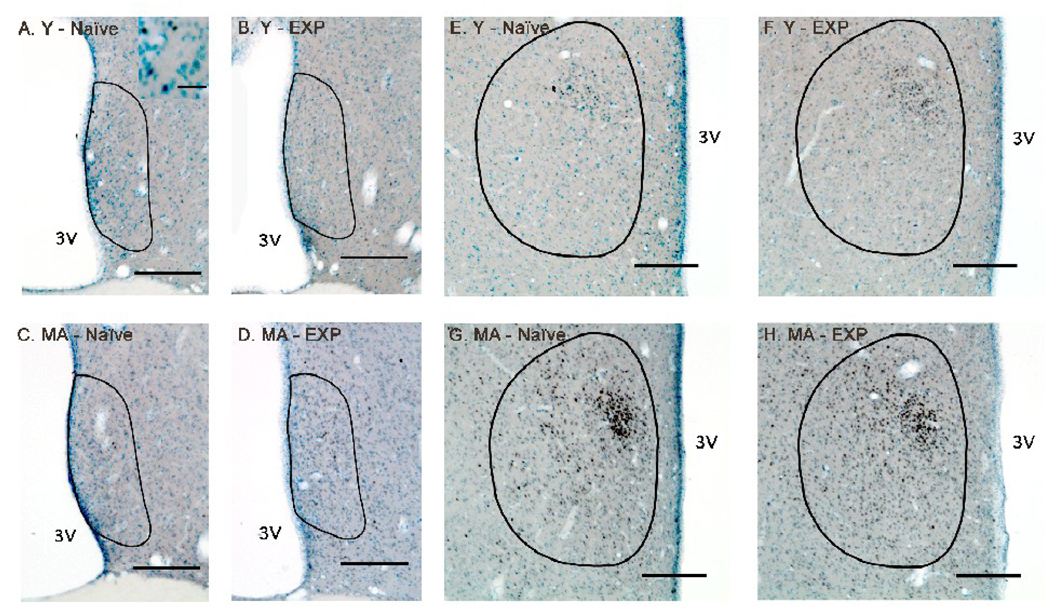
Figure 2.
Photomicrographs of androgen receptor (AR) immunoreactivity in the anteroventral periventricular nucleus (AVPV; (A – D) and medial preoptic nucleus (MPN; E – H) are shown for representative young-experienced (A, E), young-naïve (B, F), middle-aged-experienced (C, G) and middle-aged-naïve (D, H) male rats. The contours of the AVPV or MPN are drawn based on Nissl staining and according to Swanson’s rat brain atlas (1998). Scale bar = 200 µm (panels A–G). The inset in panel A shows a higher magnification view of some of the cells, demonstrating that immunoreactive AR cells were easily distinguishable from the Nissl label. Scale bar in panel A inset = 20 µm. Abbreviations: MA, middle-aged; EXP, experienced; 3V, third ventricle.
Figure 3.
Stereologic analysis results of AR-immunoreactive cell number, regional volume and cell density (number of AR-ir cells/regional volume) are shown for young-experienced, young-naïve, middle-aged-experienced and middle-aged-naïve rats in the AVPV (A, B, C) and MPN (D, E, F). Although there was no effect of experience, aging was associated with significantly higher numbers and density of AR cells in both AVPV and MPN. The bars represent the mean ± SEM. a, P < 0.05; b, P < 0.01; c, P < 0.001. N=6 rats per group.
ERα Immunoreactivity in AVPV and MPN
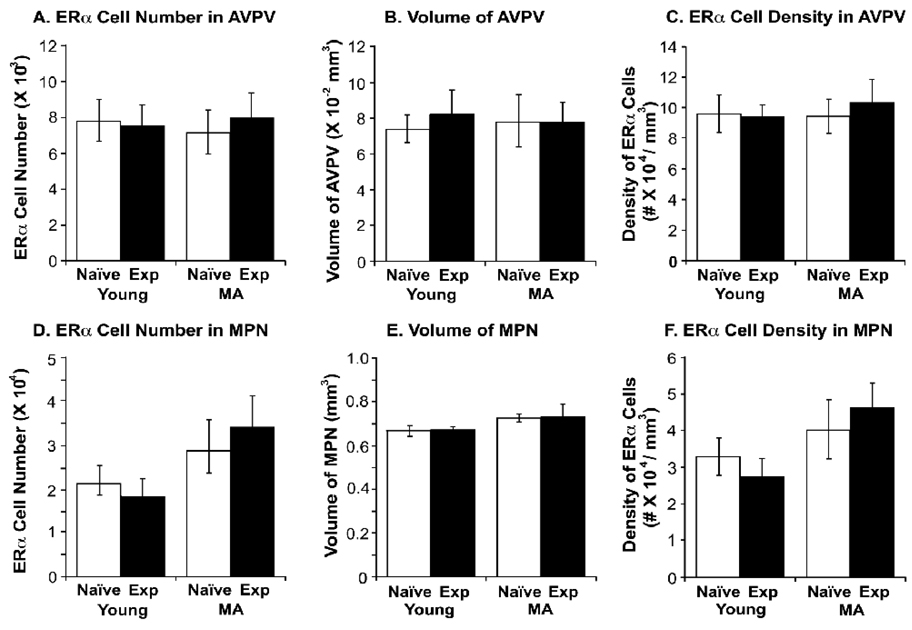
Representative photomicrographs of ERα immunoreactivity in the AVPV and MPN are shown in Figure 4 for young and middle-aged naïve and experienced rats. Although expression of ERα cells was abundant, stereologic analyses of ERα-ir cell numbers and densities showed no significant effect of either age or sexual experience (Figure 5).
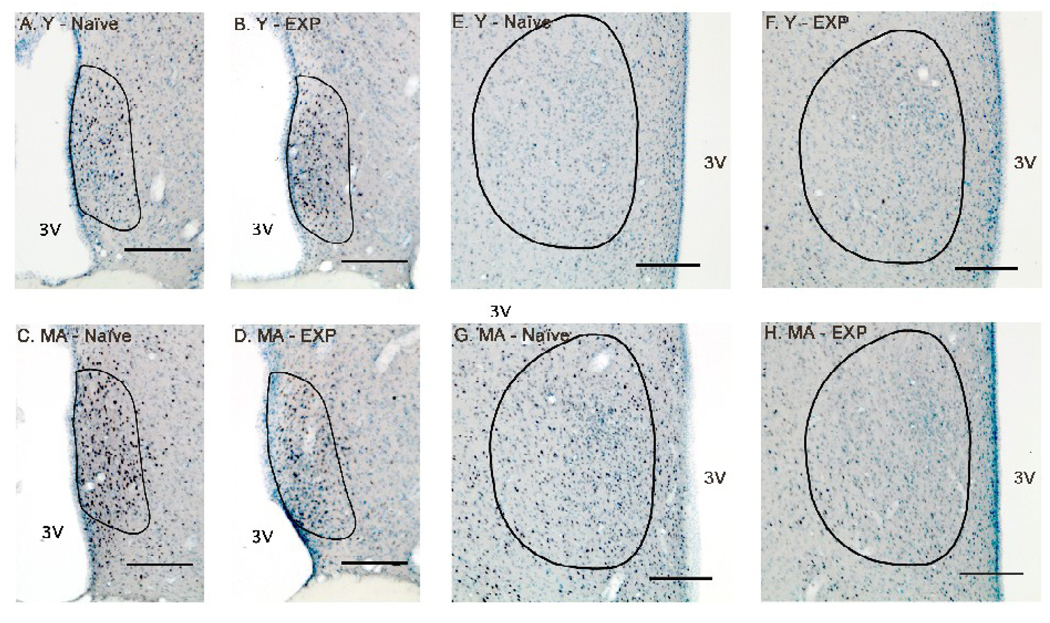
Figure 4.
Photomicrographs of ERα immunoreactivity in the AVPV (A – D) and MPN (E – H) are shown for representative young-experienced (A, E), young-naïve (B, F), middle-aged-experienced (C, G) and middle-aged-naïve (D, H) male rats. The contours of the AVPV or MPN are drawn based on Nissl staining and according to Swanson’s rat brain atlas (1998). Scale bar = 200 µm. Abbreviations: MA, middle-aged; EXP, experienced; 3V, third ventricle.
Figure 5.
Stereologic analysis results of ERα-immunoreactive cell, regional volume and cell density (#ERα-ir cells/regional volume) are shown for young-experienced (n=7), young-naïve (n=6), middle-aged-experienced (n=6) and middle-aged-naïve (n=6) rats in the AVPV (A, B, C) and MPN (D, E, F). There was no significant effect of age or experience on these endpoints.
Serum Hormone Concentrations
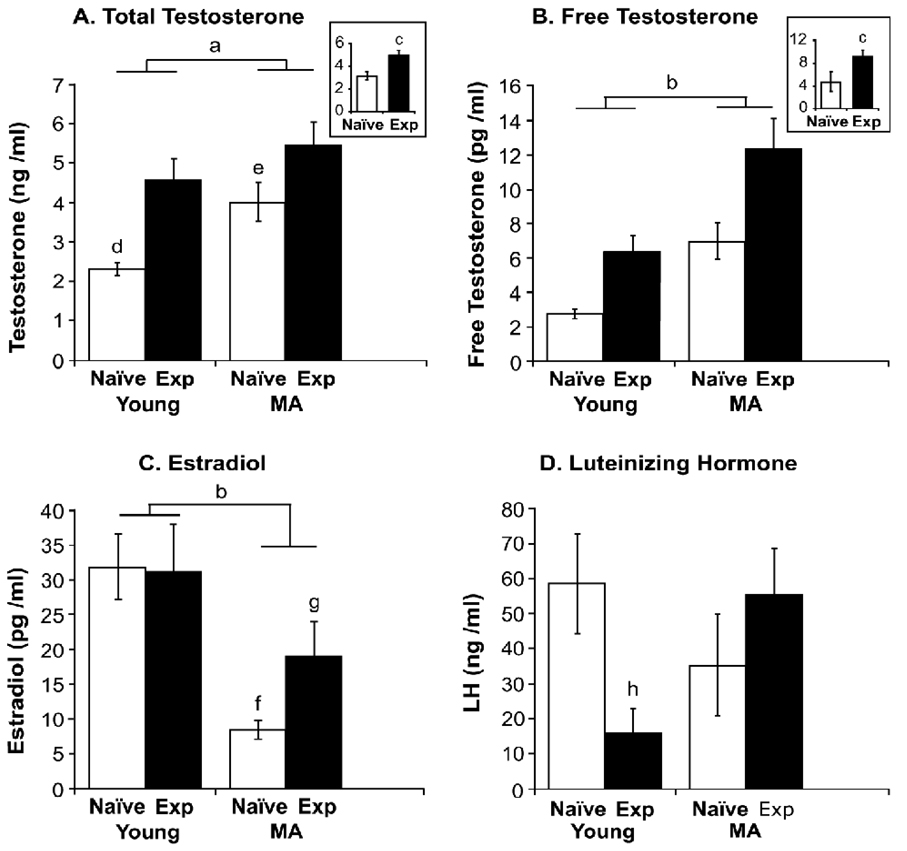
Serum total testosterone, free testosterone, estradiol and LH in were assayed in young and middle-aged male rats (Figure 6). A significant main effect of age on serum testosterone was detected (P < 0.001) with the middle-aged group having higher levels of testosterone than young rats. Experience also had a significant effect on serum testosterone (P < 0.0001) with experienced groups having higher serum testosterone. In addition, there was a significant interaction of age and experience (P < 0.05). Specifically, the young naïve group had lower testosterone than the other three groups (P < 0.01, P < 0.0001, P < 0.05 vs. young-experienced, MA-experinced, and MA-naïve, respectively). The MA-naïve rats also had lower testosterone than MA-experienced rats (P < 0.05).
Figure 6.
Serum total testosterone (A), free testosterone (B), estradiol (C) and LH (D) were assayed in young and middle-aged male rats with or without experience. For both total and free testosterone, significant main effects of age and experience were detected, with levels higher in middle-aged than young rats, and in experienced than naïve groups. Estradiol levels were significantly lower in middle-aged than young rats. The interaction of age and experience was also significant. For LH, a significant interaction of age and experience was found. The bars represent the mean ± SEM. a, P < 0.001 young vs. MA; b, P < 0.0001 young vs. MA; c, P < 0.0001 vs. naive group; d, P < 0.01, P < 0.05, P < 0.0001, vs. young-EXP, MA-naive and MA-EXP respectively; e, P < 0.05 vs. MA-EXP; f, P < 0.05, P < 0.01 vs. young-EXP, MA-EXP respectively; g, P < 0.05 vs. young-naïve; h, P < 0.05, P < 0.05 vs. young-naïve, MA-EXP respectively. Sample sizes for hormone assays were: young-naïve (n = 12), young-experienced (n = 11), middle-aged-naïve (n = 12), middle-aged-experienced (n = 11).
For serum free testosterone, a significant main effect of age was detected (P < 0.0001) with middle-aged rats having higher levels of free testosterone. Experience also had a significant effect, with the experienced groups having significant higher free testosterone than naïve groups (P < 0.0001). The interaction of age and experience was not significant.
The main effect of age on serum estradiol was significant (P < 0.001) with middle-aged rats having lower levels than young rats. Although there was not a significant effect of experience, the interaction of age and experience was significant (P < 0.05). Specifically, the MA-naïve group had significantly lower estradiol concentrations than young-experienced and young-naïve rats (P < 0.05, P < 0.01). The middle-aged-experienced group also had significantly lower estradiol than the young-naïve group (P < 0.05).
For serum LH, only the interaction of age and experience was significant (P < 0.05). The young-experienced group had significantly lower LH concentrations than the young-naïve and middle-aged experienced groups (P < 0.05 for both comparisons).
Discussion
The current study compared the effects of age and experience on the expression of steroid hormone receptors, AR and ERα, in preoptic areas. We observed an increase in expression of AR with aging, but no change in ERα, in agreement with a previous study (Wu et al., 2009). However, experience did not alter either AR or ERα expression in the preoptic brain regions studied. Serum hormones, by contrast, were affected by both age and sexual experience. With regard to age, we found that both total and free testosterone were higher, and estradiol lower, in middle-aged than young rats. This finding contrasts with the literature (Goya et al., 1990; Gruenewald et al., 2000; Luine et al., 2007; Roselli et al., 1986; Wu et al., 2009), with differences potentially due to the nature and timing of sexual experience of the animals. In addition, experience significantly elevated serum testosterone and free testosterone in both young and middle-aged groups, a finding that is consistent with previous reports [rat: (Edinger and Frye, 2007); lizard: (Crews et al., 1997)]. An interaction of age and experience was observed for testosterone, estradiol and LH. Together, these results suggest that the extensive sexual experience given in the current study has long-lasting effects on serum hormones but not on their nuclear receptors in the preoptic area.
In this study, young and middle-aged rats had the same extensive experience at the age of 3–4 months. These animals differed in the lag between experience and euthanasia, as well as their chronological age. When we originally designed the experiment, we also considered keeping the level of experience constant, but delaying the behavioral tests until middle-aged rats were 12 months of age. However, we could not pursue this route because rats that are not given any sexual experience until that age are very inconsistent in their mating behavior, often not mating at all [Wu and Gore, unpublished observations; (Frankel, 1981)]. Therefore, present observations must be interpreted in light of the matched experienced, but differences in age and time after experience.
Hormones, Aging and Experience
An unexpected finding was that there was an age-related increase of total and free testosterone in the rats. This is contrary to our previous report (Wu et al., 2009) and that of others (Bernardi et al., 1998; Luine et al., 2007; Roselli et al., 1986; Taylor et al., 1996; Gruenewald et al., 1990; Goya et al., 1990) that serum testosterone decreases with aging. There are a number of potential explanations for this surprising finding. The significant age-related increase in testosterone may be partially attributable to differences in circadian patterns of circulating testosterone in middle-aged rats (Bremner et al., 1983; Clark, 1994; Fentie et al., 2004; Kalra and Kalra, 1977), which are blunted compared to young rats (Fentie et al., 2004; Simpkins et al., 1981). However, we collected blood samples at a consistent time, at the end of the lights-on phase of our light cycle, when the peak of testosterone is anticipated to occur (Fentie et al., 2004). Therefore, the age-related increase of testosterone is more likely to be related to the timing of sexual experience relative to when animals were killed. Although sexual experiences were matched, young rats were euthanized one day after the last mating (or control) trial, whereas middle-aged rats were euthanized eight months later. In addition, some differences between our and published studies may be due to strain of rat, the timing and amount of sexual experience relative to euthanasia, and the age of the animals. Notably, our rats in this study were middle-aged, whereas most other studies utilized older males, although this cannot explain all of the differences. For example, Herrera-Pérez et al. (2008) used Wistar males (sexual experience not specified) to show decreases in testosterone and estradiol in middle-aged (12–14 mo) compared to young rats. Thus, that study (Herrera-Pérez et al., 2008) was similar to ours in that both showed an age-related decrease in estradiol; however, the studies differed in the testosterone results, possibly attributable to rat strain or sexual experience. The issue of strain also cannot entirely explain the difference in results, as our own lab showed previously using a similar cohort of Sprague-Dawley rats that testosterone concentrations are lower in middle-aged than young rats (Wu et al., 2009). That earlier experiment, however, did not perfectly control the timing of amount of sexual experience as did the current study. Recently, we have replicated the current experiment in animals with very carefully controlled experience, and our preliminary results also show that total testosterone is about two-fold higher in middle-aged than young male Sprague-Dawley rats (Wu & Gore, unpublished). Finally, the increase in testosterone cannot be explained by an aberration of the hormone assays, as the total and free testosterone assays were run separately and independently, and both measures showed an increase from young to middle-aged rats. Clearly, future experimentation is necessary to resolve the reasons for these differences in serum testosterone with aging and to tease apart several important issues such as the importance of the timing of the last sexual activity relative to euthanasia.
We also observed an age-related decline in estradiol concentrations. Again, this result is different from our previous work (Wu et al., 2009) and that of other labs (Fujita et al. 1990, Luine et al., 2007, Herath et al., 2001) that have reported increases in estradiol with age. We point out that Herrera-Pérez et al. (2008) showed a decline in estradiol between young and middle-aged Wistar males, consistent with our current finding in Sprague-Dawley rats. It is also interesting to note an experience-related difference of estradiol in the middle-aged but not the young group in the present study, with levels of estradiol being significantly lower in naïve than experienced rats at this age. To our knowledge, this has not been reported previously. Again, we believe that our results on estradiol differ from earlier studies due to the extensive and carefully matched sexual experience in our animals.
For LH, there were no overall effects of age or experience, but an interaction was found. Young-experienced rats had lower LH levels than young-naïve or middle-aged experienced rats. It is possible that young rats are more sensitive to negative feedback effects of testosterone than middle-aged rats, and that this is further altered by experience. Previously, middle-aged mammals have been reported to have diminished LH regulation by testosterone [Human: (Mitchell et al., 1995); Rat: (Bonavera et al., 1997; Chambers et al., 1991; Haji et al., 1981)]. Additional differences in LH may also be attributable to the pulsatile nature of this hormone’s release. There can be considerable individual variability in LH levels depending upon when during a pulse the sample is collected. This may also explain the relatively high variability in LH levels compared to variability in steroid hormones.
AR and ERα Expression: Effects of Age and Experience
The increase in expression of AR with aging we observed is in agreement with our previous study (Wu et al., 2009). However, our finding of a lack of effect of experience on AR-ir cell numbers differs from other reports showing that mating was associated with decreased AR density in a time-dependent manner (Fernandez-Guasti et al., 2003; Romano-Torres et al., 2007). However, in those reports, only young sexually active males were used. Therefore, some differences in results may be attributable to the extent of experience. Another important factor is again the timing of euthanasia relative to the last sexual experience. Whereas our young rats were mated one day prior to death, our middle-aged rats were last mated 8 months prior to death. This could certainly account for some differences in results. Again, though, there are differences between studies even when just comparing the young rats. Our current study shows that experience did not alter AR immunoreactive cell numbers in the MPN of young rats, whereas Fernandez-Guasti et al. (2003) showed that one day after mating, the density of AR immunolabeling was decreased in this region. These two studies differ in rat strain, in prior sexual experience, and in method of quantifying ARs, so further work is necessary to ascertain the reasons for these differences.
We also did not observe any age- and experience-related difference in the expression of ERα. This lack of change with aging is consistent with our previous observation in experienced rats (Wu et al., 2009). With respect to experience, Phillips-Farfán et al. (Phillips-Farfan et al., 2007) showed that mating resulted in an up-regulation of ERα, a finding that differs from our current result. However, the nature of experience was different between the two studies. Evidence that ERα expression may not be strongly related to masculine sexual behavior is confirmed by the demonstration that blockade of ERα with RU 58668, an antiestrogen, did not inhibit the restoration of copulatory behavior or partner preference in testosterone-treated gonadectomized male rats (Vagell and McGinnis, 1998).
Neural Mechanisms of Experience-facilitated Behavior
Sex steroid hormones affect sexual behaviors in males. At the same time, each sexual experience can alter hormones level by rapidly increasing testosterone level [rats: (Frankel, 1981); rhesus monkeys: (Herndon et al., 1981)] although whether acute experience-induced increases in testosterone can be accumulated is unknown. Therefore, the interactions between experience and effects of hormones on the brain provide a “chicken and egg” dilemma in understanding how these factors modulate one another, and whether one (or the other) provides the causal influence.
Most studies on sexual experience have compared naïve and experienced groups. However, exactly how much experience an animal has gained makes a difference. For example, rats showed significantly increasing pre-contact vocalizations while they were getting more sexual experience (Bialy et al., 2000). In addition, rats allowed to mate 16 times had significant higher metabolic capacity in sexual behavior related brain areas than naïve males or those who only mated 3 times (Sakata et al., 2002). These data indicate that experience changes brain activity. In fact, repetitive electrical stimulation can evoke short-term plastic changes in MPN neurons in vitro (Malinina et al., 2006), and more neurons are activated in the MPN after ejaculation in males with 7 sexual experiences than those one with only one experience (Lumley and Hull, 1999). We hypothesize that as experience is acquired, communication of neurons and the synthesis of key proteins in the hypothalamus is altered. That is, experience may increase neural plasticity, which may explain why sexually experienced rats had smaller impairments caused by lesions of the medial preoptic area (de Jonge et al., 1989; Liu et al., 1997), medial amygdala (de Jonge et al., 1992; Kondo, 1992) and the bed nucleus of the stria terminalis (Claro et al., 1995). However, our current results suggest that this plasticity does not appear to involve numbers of AR- or ERα-immunoreactive cells in the preoptic area, as these did not change with experience.
Hull, Dominguez and colleagues (Dominguez et al., 2006) have proposed that testosterone stimulates masculine sexual behavior through a complex network of neurons in the preoptic area, including neuronal nitric oxide, dopamine, and glutamate. One study showed that previous but not acute sexual experience increased the number of neuronal nitric oxide synthase (nNOS)-ir cells (nNOS is the enzyme involved in the synthesis of nitric oxide) and nNOS protein concentrations in the MPN, although testosterone level was not measured in that study (Dominguez et al., 2006). Based on the fact that the presence of a receptive female significantly enhances testosterone levels in experienced but not naïve males (Bonilla-Jaime et al., 2006) and 50–60 % nNOS colocalized with AR and 53–77% with ERα in AVPV and MPN (Sato et al., 2005), the upstream mechanism may be related to increased secretion of testosterone and/or increased sensitivity to testosterone. Although we observed an effect of experience on increased serum testosterone in both young and middle-aged male rats, an earlier study (Smith et al., 1977) showed that for intact male rats who could complete a copulatory series, there was no correlation between the behavior measures and testosterone levels. A second possibility is that experience modulates sensitivity to testosterone by increasing hormone receptor expression. Although, we did not observe any experience-induced changes in either AR or ERα cell numbers in AVPV or MPN, this does not exclude the possibility that there are post-translational properties of these receptors (e.g., phosphorylation) that may alter their function, something that could not be distinguished by the current immunohistochemical approach. In addition, or alternatively, it is likely that other regions of the brain than the AVPV or MPN may change their expression of these molecules, but this was beyond the scope of the current study. It will also be interesting to look at neurotransmitters and their receptors involved in masculine sexual behavior (e.g., nitric oxide, dopamine, glutamate systems) to ascertain effects of experience and aging on these steroid-sensitive circuits, something we intend to do in future studies.
Conclusion
We report that sexual experience increases testosterone and free testosterone in both young and middle-aged males. Sexual experience also interacts with aging to alter testosterone, estradiol and LH. However, we did not find experience-related difference in expression of AR or ERα immunoreactive cell numbers in two preoptic regions, the AVPV or MPN. This investigation is important because sexual experience can induce short-term and long-term alterations in hormones. It also suggests that males with matched sexual experience in young adulthood may differ in the time from last experience and in chronological age, but have a similar phenotype of expression of steroid hormone receptors in specific preoptic brain regions.
Acknowledgments
We thank Ha Ton for helping with the ERα stereological analysis, Dr. Michael Woller (University of Wisconsin-Whitewater) for performing the LH RIA in his laboratory, Dr. A.F. Parlow for LH assay reagents, and NIH grants AG028051 and AG16765 (ACG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernardi F, Salvestroni C, Casarosa E, Nappi RE, Lanzone A, Luisi S, Purdy RH, Petraglia F, Genazzani AR. Aging is associated with changes in allopregnanolone concentrations in brain, endocrine glands and serum in male rats. Eur. J. Endocrinol. 1998;138:316–321. doi: 10.1530/eje.0.1380316. [DOI] [PubMed] [Google Scholar]
- Bialy M, Rydz M, Kaczmarek L. Precontact 50-kHz vocalizations in male rats during acquisition of sexual experience. Behav. Neurosci. 2000;114:983–990. doi: 10.1037//0735-7044.114.5.983. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Davidson JM. Effects of a drenalectomy and experience on postcastration sex behavior in the male rat. Physiol. Behav. 1968;3:461–465. [Google Scholar]
- Bonavera JJ, Swerdloff RS, Leung A, Lue YH, Baravarian S, Superlano L, Sinha-Hikim AP, Wang C. In the male brown-Norway (BN) male rat, reproductive aging is associated with decreased LH-pulse amplitude and area. J. Androl. 1997;18:359–365. [PubMed] [Google Scholar]
- Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm. Behav. 2006;49:376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J. Clin. Endocrinol. Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J. Comp. Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Rajendren G, Gore AC. Expression of estrogen receptor a in the anteroventral periventricular nucleus of hypogonadal mice. Exp. Biol. Med. (Maywood) 2005;230:249–256. doi: 10.1177/153537020523000106. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Phoenix CH. Sexual behavior in response to testosterone in old long-term-castrated rhesus males. Neurobiol. Aging. 1983;4:223–227. doi: 10.1016/0197-4580(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Thornton JE, Roselli CE. Age-related deficits in brain androgen binding and metabolism, testosterone, and sexual behavior of male rats. Neurobiol. Aging. 1991;12:123–130. doi: 10.1016/0197-4580(91)90050-t. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Nakashiro K, Ketels KV, Korovaitseva GI, Oyasu R. Androgen receptor expression in androgen-independent prostate cancer cell lines. Prostate. 2001;47:66–75. doi: 10.1002/pros.1048. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, Michael RP. Estrogen in the medial preoptic area of male rats facilitates copulatory behavior. Horm. Behav. 2000;38:86–93. doi: 10.1006/hbeh.2000.1602. [DOI] [PubMed] [Google Scholar]
- Clark JT. Aging-induced decrements in neuropeptide Y: the retention of ejaculatory behavior is associated with site-selective differences. Neurobiol. Aging. 1994;15:191–196. doi: 10.1016/0197-4580(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Kalra SP. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology. 1985;117:2435–2442. doi: 10.1210/endo-117-6-2435. [DOI] [PubMed] [Google Scholar]
- Claro F, Segovia S, Guilamon A, Del Abril A. Lesions in the medial posterior region of the BST impair sexual behavior in sexually experienced and inexperienced male rats. Brain Res. Bull. 1995;36:1–10. doi: 10.1016/0361-9230(94)00118-k. [DOI] [PubMed] [Google Scholar]
- Craigen W, Bronson FH. Deterioration of the capacity for sexual arousal in aged male mice. Biol. Reprod. 1982;26:869–874. doi: 10.1095/biolreprod26.5.869. [DOI] [PubMed] [Google Scholar]
- Crews D, Coomber P, Gonzalez-Lima F. Effects of age and sociosexual experience on the morphology and metabolic capacity of brain nuclei in the leopard gecko (Eublepharis macularius), a lizard with temperature-dependent sex determination. Brain Res. 1997;758:169–179. doi: 10.1016/s0006-8993(97)00222-9. [DOI] [PubMed] [Google Scholar]
- Dahlof LG, Larsson K. Copulatory performances of penile desensitized male rats as a function of prior social and sexual experience. Behav. Biol. 1978;24:492–497. doi: 10.1016/s0091-6773(78)90863-5. [DOI] [PubMed] [Google Scholar]
- Davidson JM. Activation of the male rat's sexual behavior by intracerebral implantation of androgen. Endocrinology. 1966;79:783–794. doi: 10.1210/endo-79-4-783. [DOI] [PubMed] [Google Scholar]
- Davidson JM. Effects of estrogen on the sexual behavior of male rats. Endocrinology. 1969;84:1365–1372. doi: 10.1210/endo-84-6-1365. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, Louwerse AL, Ooms MP, Evers P, Endert E, van de Poll NE. Lesions of the SDN-POA inhibit sexual behavior of male Wistar rats. Brain Res. Bull. 1989;23:483–492. doi: 10.1016/0361-9230(89)90194-9. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, Oldenburger WP, Louwerse AL, Van de Poll NE. Changes in male copulatory behavior after sexual exciting stimuli: effects of medial amygdala lesions. Physiol. Behav. 1992;52:327–332. doi: 10.1016/0031-9384(92)90279-b. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Copulatory behaviour of rats (Rattus norvegicus) as a function of prior copulatory experience. Anim. Behav. 1969;17:217–223. doi: 10.1016/0003-3472(69)90004-9. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Brann JH, Gil M, Hull EM. Sexual experience increases nitric oxide synthase in the medial preoptic area of male rats. Behav. Neurosci. 2006;120:1389–1394. doi: 10.1037/0735-7044.120.6.1389. [DOI] [PubMed] [Google Scholar]
- Dominguez JM, Hull EM. Dopamine, the medial preoptic area, and male sexual behavior. Physiol. Behav. 2005;86:356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Sexual experience of male rats influences anxiety-like behavior and androgen levels. Physiol. Behav. 2007;92:443–453. doi: 10.1016/j.physbeh.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Fentie IH, Greenwood MM, Wyss JM, Clark JT. Age-related decreases in gonadal hormones in Long-Evans rats: relationship to rise in arterial pressure. Endocrine. 2004;25:15–22. doi: 10.1385/ENDO:25:1:15. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Swaab D, Rodriguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinology. 2003;28:501–512. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Frankel AI. Hormone release during computer-monitored sexual behavior in mature and aged male rats. Horm. Behav. 1981;15:312–320. doi: 10.1016/0018-506x(81)90020-9. [DOI] [PubMed] [Google Scholar]
- Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol. Cell Endocrinol. 1997;131:147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Goya RG, Lu JK, Meites J. Gonadal function in aging rats and its relation to pituitary and mammary pathology. Mech. Ageing Dev. 1990;56:77–88. doi: 10.1016/0047-6374(90)90116-w. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in hypothalamic gonadotropin-releasing hormone (GnRH) gene expression, but not pituitary responsiveness to GnRH, in the male Brown Norway rat. J. Androl. 2000;21:72–84. [PubMed] [Google Scholar]
- Haji M, Kato KI, Nawata H, Ibayashi H. Age-related changes in the concentrations of cytosol receptors for sex steroid hormones in the hypothalamus and pituitary gland of the rat. Brain Res. 1981;204:373–386. doi: 10.1016/0006-8993(81)90596-5. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, DonCarlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Brain Res. Mol. Brain Res. 1996;39:57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Perachio AA, Turner JJ, Collins DC. Fluctuations in testosterone levels of male rhesus monkeys during copulatory activity. Physiol. Behav. 1981;26:525–528. doi: 10.1016/0031-9384(81)90182-7. [DOI] [PubMed] [Google Scholar]
- Herrera-Pérez JJ, Martinez-Mota L, Fernandez-Guasti A. Aging increases the susceptibility to develop anhedonia in male rats. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2008;32:1798–1803. doi: 10.1016/j.pnpbp.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Kalra PS, Kalra SP. Circadian periodicities of serum androgens, progesterone, gonadotropins and luteinizing hormone-releasing hormone in male rats: the effects of hypothalamic deafferentation, castration and adrenalectomy. Endocrinology. 1977;101:1821–1827. doi: 10.1210/endo-101-6-1821. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Crowley WR. Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol. 1992;13:1–46. [PubMed] [Google Scholar]
- Kelliher K, Baum M. Effect of sex steroids and coital experience on ferrets' preference for the smell, sight and sound of conspecifics. Physiol. Behav. 2002;76:1–7. doi: 10.1016/s0031-9384(02)00691-1. [DOI] [PubMed] [Google Scholar]
- Kondo Y. Lesions of the medial amygdala produce severe impairment of copulatory behavior in sexually inexperienced male rats. Physiol. Behav. 1992;51:939–943. doi: 10.1016/0031-9384(92)90074-c. [DOI] [PubMed] [Google Scholar]
- Krey LC, McGinnis MY. Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: relationships with gonadotropin secretion. J. Steroid Biochem. 1990;35:403–408. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- Larsson K. Experience and maturation in the development of sexual behaviour in male puberty rat. Behaviour. 1959;14:101–107. [Google Scholar]
- Lisk RD, Heimann J. The effects of sexual experience and frequency of testing on retention of copulatory behavior following castration in the male hamster. Behav. Neural. Biol. 1980;28:156–171. doi: 10.1016/s0163-1047(80)91503-4. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J. Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, Maclusky NJ. Chronic stress and neural function: accounting for sex and age. J. Neuroendocrinol. 2007;19:743–751. doi: 10.1111/j.1365-2826.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- Lumley LA, Hull EM. Effects of a D1 antagonist and of sexual experience on copulation-induced Fos-like immunoreactivity in the medial preoptic nucleus. Brain Res. 1999;829:55–68. doi: 10.1016/s0006-8993(99)01338-4. [DOI] [PubMed] [Google Scholar]
- Malinina E, Druzin M, Johansson S. Short-term plasticity in excitatory synapses of the rat medial preoptic nucleus. Brain Res. 2006;1110:128–135. doi: 10.1016/j.brainres.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Matuszczyk JV, Larsson K. Experience modulates the influence of gonadal hormones on sexual orientation of male rats. Physiol. Behav. 1994;55:527–531. doi: 10.1016/0031-9384(94)90112-0. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Mirth MC, Zebrowski AF, Dreifuss RM. Critical exposure time for androgen activation of male sexual behavior in rats. Physiol. Behav. 1989;46:159–165. doi: 10.1016/0031-9384(89)90249-7. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Saunders PT, Fraser HM, Kelnar CJ, Kivlin C, Morris KD, Sharpe RM. Comparison of androgen receptor and oestrogen receptor beta immunoexpression in the testes of the common marmoset (Callithrix jacchus) from birth to adulthood: low androgen receptor immunoexpression in Sertoli cells during the neonatal increase in testosterone concentrations. Reproduction. 2001;122:419–429. doi: 10.1530/rep.0.1220419. [DOI] [PubMed] [Google Scholar]
- Mitchell R, Hollis S, Rothwell C, Robertson WR. Age related changes in the pituitary-testicular axis in normal men; lower serum testosterone results from decreased bioactive LH drive. Clin. Endocrinol (Oxf) 1995;42:501–507. doi: 10.1111/j.1365-2265.1995.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Moffatt CA, Rissman EF, Shupnik MA, Blaustein JD. Induction of progestin receptors by estradiol in the forebrain of estrogen receptor-alpha gene-disrupted mice. J. Neurosci. 1998;18:9556–9563. doi: 10.1523/JNEUROSCI.18-22-09556.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morali G, Larsson K, Beyer C. Inhibition of testosterone-induced sexual behavior in the castrated male rat by aromatase blockers. Horm. Behav. 1977;9:203–213. doi: 10.1016/0018-506x(77)90056-3. [DOI] [PubMed] [Google Scholar]
- Nicolosi A, Laumann EO, Glasser DB, Moreira ED, Jr, Paik A, Gingell C. Sexual behavior and sexual dysfunctions after age 40: the global study of sexual attitudes and behaviors. Urology. 2004;64:991–997. doi: 10.1016/j.urology.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Wilkins MF. A novel environment disrupts copulation in sexually naive but not experienced male rats: reversal with naloxone. Physiol. Behav. 1995;57:1045–1049. doi: 10.1016/0031-9384(94)00394-k. [DOI] [PubMed] [Google Scholar]
- Phillips-Farfan BV, Lemus AE, Fernandez-Guasti A. Increased estrogen receptor alpha immunoreactivity in the forebrain of sexually satiated rats. Horm. Behav. 2007;51:328–334. doi: 10.1016/j.yhbeh.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Rabedeau RG, Whalen RE. Effects of copulatory experience on mating behavior in the male rat. J. Comp. Physiol. Psychol. 1959;52:482–484. doi: 10.1037/h0041003. [DOI] [PubMed] [Google Scholar]
- Retana-Marquez S, Velazquez-Moctezuma J. Cholinergic-androgenic interaction in the regulation of male sexual behavior in rats. Pharmacol. Biochem. Behav. 1997;56:373–378. doi: 10.1016/s0091-3057(96)00229-8. [DOI] [PubMed] [Google Scholar]
- Romano-Torres M, Phillips-Farfan BV, Chavira R, Rodriguez-Manzo G, Fernandez-Guasti A. Relationship between sexual satiety and brain androgen receptors. Neuroendocrinology. 2007;85:16–26. doi: 10.1159/000099250. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Kaler LW, Resko JA. Hypothalamic aromatase activity in young and old male rats. Neurobiol. Aging. 1986;7:121–125. doi: 10.1016/0197-4580(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Aronson LR. The decline of sexual behavior in male cats after castration with special reference to the role of prior sexual experience. Behaviour. 1958;12:285–338. [Google Scholar]
- Sakata JT, Gonzalez-Lima F, Gupta A, Crews D. Repeated interactions with females elevate metabolic capacity in the limbic system of male rats. Brain Res. 2002;936:27–37. doi: 10.1016/s0006-8993(02)02491-5. [DOI] [PubMed] [Google Scholar]
- Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: co-localization and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J. Chem. Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Kalra PS, Kalra SP. Alterations in daily rhythms of testosterone and progesterone in old male rats. Exp. Aging Res. 1981;7:25–32. doi: 10.1080/03610738108259783. [DOI] [PubMed] [Google Scholar]
- Smith ER, Damassa DA, Davidson JM. Plasma testosterone and sexual behavior following intracerebral implantation of testosterone propionate in the castrated male rat. Horm. Behav. 1977;8:77–87. doi: 10.1016/0018-506x(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Smith ER, Stefanick ML, Clark JT, Davidson JM. Hormones and sexual behavior in relationship to aging in male rats. Horm. Behav. 1992;26:110–135. doi: 10.1016/0018-506x(92)90035-t. [DOI] [PubMed] [Google Scholar]
- Sodersten P. Estrogen-activated sexual behavior in male rats. Horm. Behav. 1973;4:247–256. doi: 10.1016/0018-506x(73)90009-3. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier. 1998 [Google Scholar]
- Taylor G, Bardgett M, Farr S, Humphrey W, Womack S, Weiss J. Aging of the brain-testicular axis: reproductive systems of healthy old male rats with or without endocrine stimulation. Proc. Soc. Exp. Biol. Med. 1996;211:269–275. doi: 10.3181/00379727-211-43953. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of aromatization in the restoration of male rat reproductive behavior. J. Neuroendocrinol. 1997;9:415–421. doi: 10.1046/j.1365-2826.1997.00598.x. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats. Horm. Behav. 1998;33:163–179. doi: 10.1006/hbeh.1998.1445. [DOI] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J. Comp. Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumpe D, Bonsall RW, Michael RP. Effects of the nonsteroidal aromatase inhibitor, fadrozole, on the sexual behavior of male cynomolgus monkeys (Macaca fascicularis) Horm. Behav. 1993;27:200–215. doi: 10.1006/hbeh.1993.1015. [DOI] [PubMed] [Google Scholar]