Abstract
Objective To determine whether steroids plus antivirals provide a better degree of facial muscle recovery in patients with Bell’s palsy than steroids alone.
Design Meta-analysis.
Data sources PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials were searched for studies published in all languages from 1984 to January 2009. Additional studies were identified from cited references.
Selection criteria Randomised controlled trials that compared steroids with the combination of steroids and antivirals for the treatment of Bell’s palsy were included in this study. At least one month of follow-up and a primary end point of at least partial facial muscle recovery, as defined by a House-Brackmann grade of at least 2 (complete palsy is designated a grade of 6) or an equivalent score on an alternative recognised scoring system, were required.
Review methods Two authors independently reviewed studies for methodological quality, treatment regimens, duration of symptoms before treatment, length of follow-up, and outcomes. Odds ratios with 95% confidence intervals were calculated and pooled using a random effects model.
Results Six trials were included, a total of 1145 patients; 574 patients received steroids alone and 571 patients received steroids and antivirals. The pooled odds ratio for facial muscle recovery showed no benefit of steroids plus antivirals compared with steroids alone (odds ratio 1.50, 95% confidence interval 0.83 to 2.69; P=0.18). A one study removed analysis showed that the highest quality studies had the greatest effect on the lack of difference between study arms shown by the odds ratio. Subgroup analyses assessing causes of heterogeneity defined a priori (time from symptom onset to treatment, length of follow-up, and type of antiviral studied) showed no benefit of antivirals in addition to that provided by steroids.
Conclusions Antivirals did not provide an added benefit in achieving at least partial facial muscle recovery compared with steroids alone in patients with Bell’s palsy. This study does not, therefore, support the routine use of antivirals in Bell’s palsy. Future studies should use improved herpes virus diagnostics and newer antivirals to assess whether combination therapy benefits patients with more severe facial paralysis at study entry.
Introduction
Bell’s palsy is the abrupt paralysis of the facial nerve, resulting in an inability to control facial muscles on the affected side. A common condition, Bell’s palsy has an annual incidence of 11 to 40 cases per 100 000 population.1 Many patients recover without intervention; however, up to 30% have poor recovery of facial muscle control and experience facial disfigurement, psychological trauma, and facial pain.2 Two main types of pharmacological treatment have been used to improve outcomes from Bell’s palsy: steroids and antivirals.3 The rationale for these treatments is based on the presumed pathophysiology of Bell’s palsy, namely inflammation and viral infection.
For decades, surgeons have noted facial nerve swelling during decompression surgery.4 More recently, enhancement of the facial nerve on magnetic resonance imaging has been observed in Bell’s palsy, suggesting that inflammation is in part responsible for the associated paralysis.5 As a consequence, steroids have been used to treat Bell’s palsy and have been shown to significantly improve outcomes compared with placebo.6
The neuronal inflammation associated with Bell’s palsy is thought to be secondary to viral infection. Herpes simplex virus has been detected in the endoneurial fluid in patients with Bell’s palsy.7 On the basis of this evidence, some clinicians treat patients with antivirals, including aciclovir, famciclovir, and valaciclovir.8 The benefits of antivirals alone are not clear, thus the role of combination therapy with steroids plus antivirals has been investigated for the treatment of Bell’s palsy.6 9 10 11 12 13 14 15 16 17 Studies have produced somewhat conflicting results, however, and there is debate over the effectiveness of antivirals on top of steroids.18 The most recent guidelines from the American Academy of Neurology suggest that aciclovir combined with prednisone is “possibly effective” for Bell’s palsy.19 Despite a lack of clear evidence, many clinicians treat Bell’s palsy with combination therapy. Given the emergence of this clinical practice and the conflicting data on the benefits of antivirals over and above those of steroids, we performed a meta-analysis to determine whether steroid treatment plus antivirals provides a better degree of facial muscle recovery than does steroids alone.
Methods
Search strategy and selection criteria
This review was conducted to the suggested QUOROM guideline standards.20 We began our meta-analysis by performing a detailed literature search for articles published between 1984 (year that aciclovir was licensed for clinical use) and January 2009 using PubMed, Embase, Web of Science, and the Cochrane Central Register of Controlled Trials. In addition, we searched reference lists of systematic reviews for appropriate articles. Hand searching of conference abstract books was not performed. Our search terms included: “bell palsy,” “bell’s palsy,” “bell’s palsies,” “bells palsy,” “idiopathic facial paralyses,” “idiopathic facial paralysis,” “herpetic facial paralysis,” “anti-viral agents,” “acyclovir,” “valacyclovir,” “famcyclovir,” and “famciclovir.” Steroids were not included in the search terms as we were not interested in studies that only assessed the benefits of steroids alone. Two independent investigators performed the search.
We included all randomised controlled trials that compared steroids with the combination of steroids and antivirals in patients with Bell’s palsy. No studies were excluded on the basis of language. Other inclusion criteria included: at least one month of follow-up after treatment initiation; and an assessment of facial muscle recovery, as determined by a recognised scoring system such as the House-Brackmann grade, Yanagihara score, or the facial paralysis recovery index.21 We excluded animal studies, review articles, meta-analyses, case series, studies involving children or pregnant women, studies lacking a control group, studies that did not report the proportion of patients with facial recovery, and studies comparing steroids with antivirals alone. Where duplicate papers using overlapping data sets were published, the study with the larger population was included.
Data extraction and quality assessment
The following variables were extracted from all studies: (a) year of publication; (b) geographic region of the study; (c) study design; (d) patient demographics; (e) number of patients in each treatment group; (f) type of antiviral used and dose; (g) type of steroid used and dose; (h) duration of symptoms before treatment initiation, (i) length of follow-up; (j) type of facial muscle recovery outcome scale used; (k) definition for facial recovery; and (l) proportion of patients with facial recovery at each follow-up time point. Two independent reviewers extracted the data from each study. There was one inter-reviewer disagreement during data extraction, which was resolved by a third independent reviewer. The Sullivan et al,6 the Minnerop et al,9 and the Adour et al22 studies did not report the necessary data regarding the groups of interest; the corresponding authors were contacted and kindly provided the necessary information.
Two independent investigators evaluated study quality. Given that all included studies were randomised controlled trials, the Jadad score was used to assess study quality.23 This scoring system evaluates the randomisation process (two questions), blinding (two questions), and the description of withdrawals and dropouts (one question). The included studies were then ranked from one to six, with one being the highest quality study. Studies with the same Jadad score were differentiated by the number of patients in the study, with the study containing a larger sample size receiving a higher rank.
Primary outcome
The primary outcome of this meta-analysis was the proportion of patients with at least partial facial muscle recovery from Bell’s palsy at the longest follow-up point and who attended a follow-up visit at least one month after initiation of treatment. Partial facial muscle recovery was defined as a House-Brackmann grade of at least 2 or an equivalent score on an alternative scoring system.21 Complete facial muscle recovery was defined as a House-Brackmann grade of 1. Despite their importance to this disease, secondary outcome measures such as facial pain or disfigurement were not consistently reported by investigators and were thus not analysed in this meta-analysis.
Statistical analysis
The Comprehensive Meta-Analysis software (CMA version 2; Biostat, Englewood, NJ) was used for all statistical analyses. We calculated a pooled odds ratio and 95% confidence interval for the proportion of patients with at least partial facial muscle recovery who were treated with steroids plus antivirals compared with those who received steroids alone. An odds ratio of greater than one favours steroids plus antivirals, and the odds ratio would be considered statistically significant at the P<0.05 level if the 95% confidence interval does not include the value 1. Despite communication with Adour et al, we were unable to determine which intervention arm three of the 20 patients lost to follow-up were randomised to.22 Therefore, patients lost to follow-up were excluded in order to ensure consistent analyses were performed across all studies. We also used intention to treat data for all studies except Adour et al22; for this study, we performed a sensitivity analysis under the extreme assumption that the three patients lost to follow-up had been randomised to the steroid arm or the steroid plus antiviral arm.
Given our expectation of heterogeneity between studies, we used a random effects model to assess the data and performed a standard quantitative test of heterogeneity: the I2 statistic, which describes the percentage of total variation across studies that is attributable to heterogeneity rather than chance alone. We also evaluated publication bias using a funnel plot24 and a trim and fill analysis,25 which is an algorithm that assesses the symmetry of a funnel plot via rank correlation and adds to the plot studies that appear to be missing. The odds ratio and 95% confidence interval were recalculated after the addition of potential missing studies.
Sources of possible heterogeneity between studies were determined a priori and were evaluated in our analysis. These included: study quality; time between symptom onset and treatment; length of follow-up; and type of antiviral studied. Type of scoring system used for facial muscle recovery was not thought to be a source of heterogeneity, as the three systems used in the included studies are comparable.21 We performed an analysis using a cumulative random effects model based on the defined sources of heterogeneity, starting with the highest quality study and continuing in decreasing order, and a one study removed model to determine the relative contribution of each study to the overall random effects estimate. Furthermore, subgroup analyses were performed for time from symptom onset to treatment (divided into ≤3 days and >3 days), length of follow-up (≤3 months and >3 months), and type of antiviral. Interactions between subgroups were also assessed using an analysis of variance (F test).
Results
Studies included
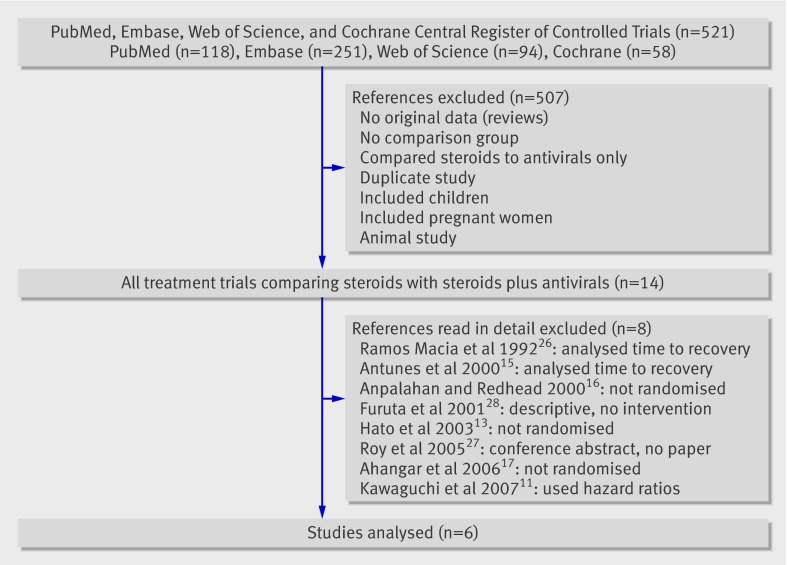
The results of our search strategy are shown in fig 1. A total of 521 studies were extracted from the search. Fourteen studies met our inclusion criteria and underwent thorough review. Of these, eight were removed for the following reasons: they were not a randomised study (n=3)13 16 17; the proportion of patients with facial muscle recovery was not documented (n=3)11 15 26; they comprised a conference abstract only (n=1)27; or they studied a poorly defined intervention (n=1).28 Of the six remaining studies, four were double blind, one was a single blind, and one was a non-blinded randomised controlled trial.
Fig 1 Flow diagram of the study selection process. The initial study numbers from each database do not represent the number of unique articles. Duplicate articles from different databases were removed in the first exclusion stage
The study demographics and clinical characteristics of the six included studies are shown in tables 1 and 2, respectively. A total of 574 patients who received steroids alone and 571 patients who received steroids and antivirals were included. Three studies used aciclovir as the antiviral,6 10 22 two studies used valaciclovir,12 14 and one study used famciclovir.9 Most of the included studies were of high quality (Jadad score ≥3) except for Minnerop et al,9 which was not blinded. Studies varied with respect to the duration of symptoms before treatment initiation and length of follow-up. Two studies subdivided patients into those who presented early for treatment (≤3 days of symptom onset)6 10 12 14 22 and those who presented later (>3 days).10 12 Length of follow-up varied between studies and ranged from 4 months to more than 12 months; some trials had multiple follow-up times.
Table 1.
Study demographics and therapeutic treatments prescribed in the six studies included in the meta-analysis
| Year published | Country | Study type | Number of patients on steroids only at follow-up | Number of patients on steroids plus antivirals at follow-up | Type of steroid | Steroid dose | Type of antiviral | Antiviral dose | |
|---|---|---|---|---|---|---|---|---|---|
| Adour et al22 | 1996 | United States | RCT (double blind) | 46 | 53 | Prednisolone oral | 1 mg/kg/day for 5 days followed by taper to 10 mg/kg/day over 5 days | Aciclovir | 2000 mg/day for 10 days |
| Hato et al12 | 2007 | Japan | RCT (single blind) | 107 | 114 | Prednisolone oral | 60 mg/day for 5 days, 30 mg/day for 3 days, 10 mg/day for 2 days | Valaciclovir | 1000 mg/day for 5 days |
| Sullivan et al6 | 2007 | United Kingdom | RCT (double blind) | 127 | 124 | Prednisolone oral | 50 mg/day for 10 days | Aciclovir | 2000 mg/day for 10 days |
| Engstrom et al14 | 2008 | Sweden | RCT (double blind) | 180 | 186 | Prednisolone oral | 60 mg/day for 5 days, 50 mg/day for 1 day, 40 mg/day for 1 day, 30 mg/day for 1 day, 20 mg/day for 1 day, 10 mg/day for 1 day | Valaciclovir | 3000 mg/day for 7 days |
| Minnerop et al9 | 2008 | Germany | RCT (not blinded) | 67 | 50 | Prednisone oral | 1 mg/kg/day for 4 days, taper over 8 days | Famciclovir | 250 mg/day for 7 days |
| Yeo et al10 | 2008 | South Korea | RCT (double blind) | 47 | 44 | Prednisolone oral | 1 mg/kg/day for 4 days, 60 mg/kg/day for 2 days, 40 mg/kg/day for 2 days, 20 mg/kg/day for 2 days | Aciclovir | 2400 mg/day for 5 days |
RCT, randomised controlled trial.
Table 2.
Timing of treatment, outcome definitions, and quality of the six studies included in the meta-analysis
| Timing of treatment | Outcome definition | Quality | ||||
|---|---|---|---|---|---|---|
| Duration of symptoms before treatment | Maximum length of follow-up | Outcome scales used in study | Definition of positive outcome for meta-analysis | Equivalent outcome on House-Brackmann grade | Jadad score | |
| Adour et al22 | ≤3 days | 4 months | Facial paralysis recovery index | Facial paralysis recovery index score >7 | Recovery to grade 3 or higher | 4 |
| Hato et al12 | ≤3 days or 4-7 days | 6 months | Yanagihara score | Recovery to 36 points or higher | Recovery to grade 1 or high grade 2 | 3 |
| Sullivan et al6 | ≤72 hours | 9 months | House-Brackmann grade | Recovery to grade 1 | Recovery to grade 1 | 5 |
| Engstrom et al14 | ≤72 hours | 12 months | Sunnybrook system and House-Brackmann grade | Complete recovery: Sunnybrook score 100 or House-Brackmann grade 1 | Recovery to grade 1 | 5 |
| Minnerop et al9 | <5 days | >12 months | House-Brackmann grade | Recovery to grade 2 | Recovery to grade 2 or higher | 1 |
| Yeo et al10 | ≤3 days or >3 days | 6 months | House-Brackmann grade | Recovery to grade 2 or higher | Recovery to grade 2 or higher | 3 |
Outcomes for meta-analysis
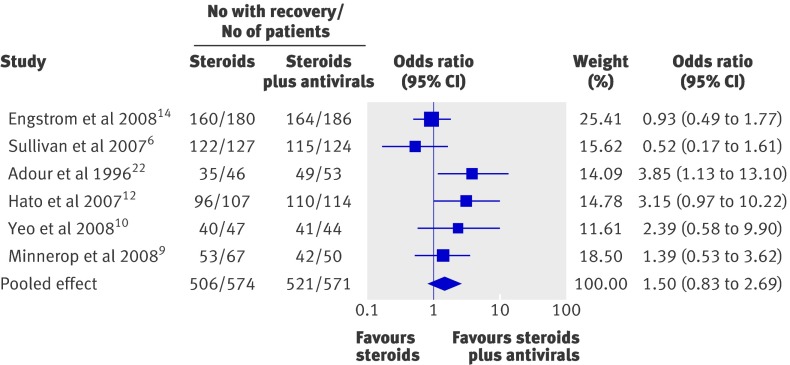
The proportion of patients with at least partial facial muscle recovery at the longest follow-up time in both the steroids only group and the steroids plus antivirals group is shown in fig 2. A high proportion of patients with Bell’s palsy achieved at least partial facial recovery when given steroids or steroids and antivirals (89.7% overall using per protocol data). The pooled proportion of patients with facial muscle recovery was 88.2% (506/574) among those who received steroids alone compared with 91.2% (521/571) in those who received steroids and antivirals. The odds ratio favoured combination therapy in four studies,9 10 12 22 but the confidence intervals crossed 1 in three of these (fig 2). The two highest quality studies had odds ratios that were less than one, favouring steroids alone, with confidence intervals also crossing 1.6 14
Fig 2 Forest plot of the six included studies ordered according to Jadad score (highest quality to lowest) showing the odds ratio estimates and their 95% confidence intervals. The pooled estimate is based on a random effects model
After performing an analysis using a random effects model that included all six studies, the degree of facial muscle recovery was not significantly better in patients who received steroids plus antivirals than in patients who received steroids alone (odds ratio 1.50, 95% confidence interval 0.83 to 2.69; P=0.18). The I2 statistic for this model was 47.1%, suggesting heterogeneity between studies. No significant difference in results was observed when a fixed effects model was used or when intention to treat data from each study were used (data not shown). This held true whether the three unaccounted lost to follow-up patients from the Adour et al study22 were added to the steroid arm (1.30, 0.99 to 1.71; P=0.063) or the steroid plus antiviral arm (1.25, 0.98 to 1.60; P=0.072).
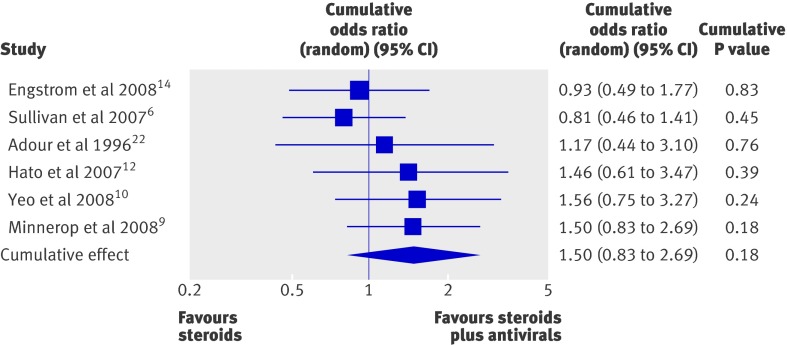
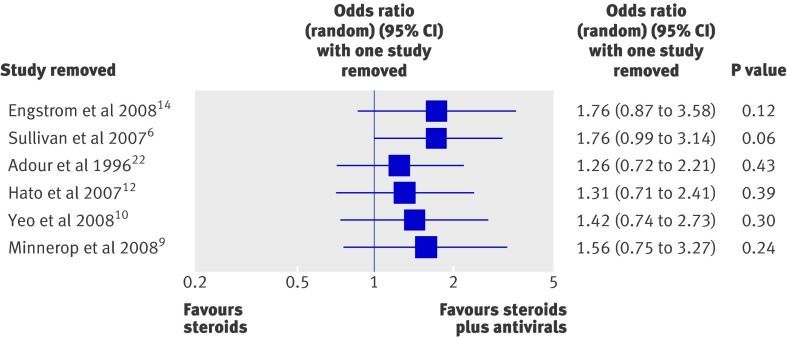
To assess the effect of study quality on our results, we performed a cumulative forest plot analysis based on quality rank (highest to lowest; fig 3 ). This revealed that the lower quality studies were responsible for drawing the pooled odds ratio towards favouring steroids plus antivirals. Despite this relationship, the confidence interval for each cumulative odds ratio always crossed 1. In addition, we performed a one study removed analysis to assess the influence of any one particular study on the meta-analysis results (fig 4). This showed that the random effects estimate was not greatly influenced by any one particular study except for the two highest quality studies, Sullivan et al6 and Engstrom et al.14 As expected, a more pronounced effect of steroids plus antivirals compared with steroids alone was observed after either of these studies was removed.
Fig 3 Cumulative forest plot of the six included studies, ordered according to study quality (highest quality to lowest)
Fig 4 Forest plot of a one study removed analysis showing the odds ratio estimate and 95% confidence interval if the study specified in the “Study removed” column is not included
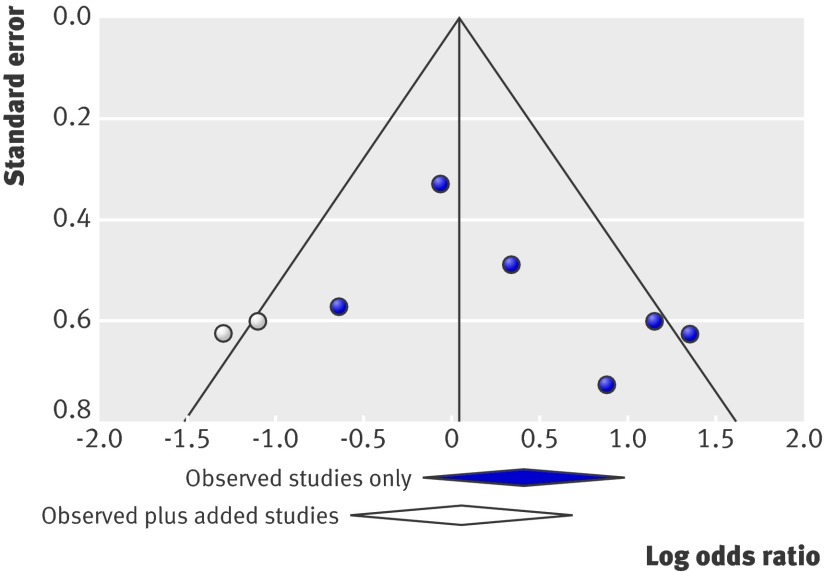
A funnel plot suggested publication bias (fig 5). When studies calculated using a trim and fill algorithm were added,25 the effect of steroids plus antivirals compared with steroids alone was even less.
Fig 5 Funnel plot of the six included studies (solid circles) and two added studies (open circles) based on a trim and fill algorithm. The pooled odds ratio and 95% confidence interval based on the six included studies (solid diamond) and following the addition of the extra studies (open diamond) are shown
Subgroup analyses
We performed subgroup analyses related to the sources of heterogeneity we determined a priori. We observed no benefit of adding antivirals to steroids in patients who received treatment within three days (odds ratio 1.51, 95% confidence interval 0.68 to 3.34; P=0.31)6 10 12 14 22 or after three days (2.15, 0.48 to 9.60; P=0.32)10 12 of symptom onset. When studies were stratified by length of follow-up (≤3 months v >3 months), no difference in outcome was observed between the shorter follow-up period (0.93, 0.63 to 1.36; P=0.70)6 10 14 and longer follow-up period (1.50, 0.83 to 2.69; P=0.18).6 9 10 12 14 22 Type of antiviral had no effect on the overall results (data not shown). The F test for interaction was not significant for any subgroup.
Discussion
Principal findings
Our meta-analysis of data from six randomised controlled trials shows that a high proportion of patients with Bell’s palsy achieve at least partial facial recovery when given steroids or steroids and antivirals (89.7% overall using per protocol data). Our pooled odds ratio for facial muscle recovery showed that antivirals provided no added benefit over steroids alone (odds ratio 1.50, 95% confidence interval 0.83 to 2.69; P=0.18). The two highest quality studies,6 14 which were double blind, had the greatest effect on the pooled odds ratio. Subgroup analysis of patients treated within three days of symptom onset showed similar findings, with no benefit seen with combination therapy. Likewise, subgroup analysis of length of follow-up and type of antiviral showed no benefit of antivirals in addition to steroids.
There are, however, more subtle differences between the included studies that need to be explored. Firstly, the severity of facial muscle paralysis at presentation differed between two of the more recent studies.6 12 The mean House-Brackmann grade for patients in the Sullivan et al study, 6 which showed no benefit of adding aciclovir to steroids, was 3.6, whereas the mean score was 4.3 in the Hato et al study,29 which reported a significant benefit of adding valaciclovir. In subgroup analyses, Hato et al showed that the benefit of valaciclovir was greater in patients with severe facial paralysis at presentation than in those with moderate paralysis.12 Furthermore, Minnerop et al performed a subgroup analysis of patients who presented with severe facial muscle paralysis (House-Brackmann grade of 5 or 6) and found significantly better facial muscle recovery in patients who received famciclovir plus steroids than in those on steroids alone (72% v 47%, respectively, achieved normal function).9 However, only 18 and 17 patients respectively were included in this analysis. These data suggest that antiviral therapy may benefit in particular those patients with more severe facial paralysis at presentation. On the other hand, one of the most recently published trials, by Engstrom et al,14 is in opposition to this argument. Patients in this trial had a median House-Brackmann grade of 4 at presentation, which is very similar to the level of palsy observed by Hato et al,12 and the authors convincingly showed no benefit of adding valaciclovir to steroids alone.
Secondly, the definition of the primary end point, facial muscle recovery, was slightly different in the six studies. The two highest quality studies used complete facial recovery (House-Brackmann grade of 1) as their primary end point,6 14 whereas the remainder of the studies used partial facial recovery (House-Brackmann grade of ≥2). It is unclear how this disparity would change the final results, but if the increment of benefit of antivirals was small then using partial facial muscle recovery as the end point could show more favourable results for combination therapy. This issue also introduces the question of the best end point to use for a disease in which a very high proportion of patients recover with current standard therapy (steroids alone). Time to facial muscle recovery may be a more sensitive end point. This end point was analysed by Engstrom et al and no differences were observed between treatment arms.14 Moreover, three other prospective studies that were not included in our analysis owing to the fact that they used time to facial muscle recovery as the outcome measure showed no statistically significant difference in recovery rates with combination therapy.15 26 11 These studies were small, however, and may have been underpowered to show a significant difference in time to recovery.
Thirdly, investigators studying Bell’s palsy rarely perform thorough molecular diagnostic testing for viral aetiologies. Varicella zoster virus, for example, can cause facial muscle paralysis in the absence of vesicles (zoster sine herpete) and has been reported to be associated with 8% to 28% of Bell’s palsy cases.29 This virus is less sensitive to antivirals than are other viruses associated with Bell’s palsy, and the doses used in treatment trials are generally not high enough to treat a Varicella zoster virus infection. Thus, if patients with this type of infection were included in the trials studied in this meta-analysis, as may be the case for the Sullivan et al and Engstrom et al studies,6 14 the potential benefit of antiviral therapy may be diluted.
Finally, the type of antiviral used also varied between studies, and the newer agents (valaciclovir and famciclovir) have greater oral bioavailability. We observed no difference in results when the studies were grouped on the basis of antiviral used.
Strengths and weaknesses of study
A key strength of our meta-analysis is the inclusion of high quality randomised controlled trials. Non-randomised trials have been published on the topic of antivirals in Bell’s palsy13 16 17; however, one should be guarded in making final conclusions from these studies given the limitations of the study design. Despite other meta-analyses being performed on the treatment of Bell’s palsy,8 30 our meta-analysis is the first to define whether the addition of antivirals provides any benefit over treatment with steroids alone. We purposely used a random effects model owing to our concerns about study heterogeneity and performed several subgroup analyses in an attempt to characterise the potential causes of heterogeneity. Furthermore, we accounted for publication bias.
Our meta-analysis has some limitations. The Adour et al study did not report intention to treat analyses22; therefore, we used the number of patients at final follow-up as our denominator, as these data were available for all studies. However, even when the Adour et al study was removed from the analysis of intention to treat data, the confidence interval of our pooled odds ratio still crossed 1 (odds ratio 1.20, 95% confidence interval 0.93 to 1.56; P=0.163). In addition, when the three unaccounted lost to follow-up patients from the Adour et al study were added to either arm, our primary finding did not notably change. Our subgroup analyses are limited by the small number of included studies and thus may lack statistical power. However, this was not the case in our subgroup analysis of studies that included patients who presented within three days of symptom onset. Finally, three prospective randomised studies were not included in this meta-analysis as they analysed time to recovery,11 15 26 and the number of patients in each arm was not reported in one such study.15 In addition, we were unable to obtain the necessary raw data from the investigators leading each of these studies. However, none of these three studies showed a significant benefit of combination therapy over steroids alone. In fact, in the largest of these, the mean time to recovery was shorter in patients on steroids alone (70.7 days) compared with those on combination therapy (76.4 days), although this difference was not significant (P=0.977).11
Conclusions and future research
In patients with Bell’s palsy, adding antivirals to steroids does not provide an added benefit in achieving at least partial facial muscle recovery compared with steroids alone; therefore, this meta-analysis does not support the routine addition of antivirals to steroids in Bell’s palsy. The benefit of antiviral therapy combined with steroids for patients with severe facial muscle paralysis at presentation who do not have Varicella zoster virus reactivation is, however, an ongoing question. Future prospective double blind studies that use modern diagnostics, such as polymerase chain reaction, for the detection of Herpes virus reactivation are needed to resolve this issue. In addition, such trials should study newer antivirals, such as valaciclovir or famciclovir, on the basis of their improved bioavailability over aciclovir.
What is already known on this topic
Bell’s palsy is the most common cause of facial muscle paralysis, and treatment with steroids has been shown to significantly improve recovery compared with placebo
Conflicting results exist on the benefit of antivirals in addition to steroids for the treatment of Bell’s palsy
What this study adds
Our meta-analysis suggests that treatment with antivirals plus steroids does not provide an added benefit in achieving at least partial facial muscle recovery in patients with Bell’s palsy compared with steroids alone
Future studies should determine the role of adding newer antivirals to steroids in patients with severe facial muscle paralysis at presentation
We thank Michael Stoto for his invaluable assistance with statistical analyses and for his thoughtful comments. We also thank Frank Sullivan, Martina Minnerop, and Kedar Adour for providing unpublished raw data; Roy Alcalay for obtaining an article; and Barbara Voetsch for translating an article.
Contributors: AYP was responsible for the conception of this study and ECQ, SSJ, RHM, AVC, and AYP were responsible for design. SSJ and AYP searched for and retrieved articles, and AVC and ECQ extracted the data. RHM acted as a third independent reviewer for disagreements in data extraction. Data analysis and interpretation were performed by ECQ, RHM, SSJ, AVC, MKB, and AYP. AYP wrote the manuscript, and ECQ, SSJ, RHM, AVC, and MKB revised it. ECQ and SSJ constructed the figures. ECQ, RHM, AVC, SSJ, and AYP are guarantors.
Funding: No specific funding was received for this study. However, ECQ was funded by an American National Cancer Institute Paul Calabresi Award for Clinical Oncology (K12CA090354), SSJ was funded by an American Academy of Neurology Clinical Research Training fellowship, RHM by an EA Baker Fellowship from the Canadian National Institute for the Blind, and AYP by a University of Queensland postgraduate fellowship. All researchers are independent of the funding body.
Competing interests: AYP has acted as an adviser for Abbott Molecular. All other authors have no competing interests.
All authors had full access to all of the data in this study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Cite this as: BMJ 2009;339:b3354
References
- 1.De Diego-Sastre JI, Prim-Espada MP, Fernandez-Garcia F. The epidemiology of Bell’s palsy [Spanish]. Rev Neurol 2005;41:287-90. [PubMed] [Google Scholar]
- 2.Adour KK, Wingerd J. Idiopathic facial paralysis (Bell’s palsy): factors affecting severity and outcome in 446 patients. Neurology 1974;24:1112-6. [DOI] [PubMed] [Google Scholar]
- 3.Gilden DH. Clinical practice. Bell’s Palsy. N Engl J Med 2004;351:1323-31. [DOI] [PubMed] [Google Scholar]
- 4.Cawthorne T. The surgery of otosclerosis. Proc R Soc Med 1950;43:491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim IS, Shin SH, Kim J, Lee WS, Lee HK. Correlation between MRI and operative findings in Bell’s palsy and Ramsay Hunt syndrome. Yonsei Med J 2007;48:963-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med 2007;357:1598-607. [DOI] [PubMed] [Google Scholar]
- 7.Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med 1996;12427-30. [DOI] [PubMed]
- 8.Allen D, Dunn L. Aciclovir or valaciclovir for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev 2004; Issue 1. Art. No.: CD001869. Doi:10.1002/14651858.CD001869.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Minnerop M, Herbst M, Fimmers R, Matz B, Klockgether T, Wullner U. Bell’s palsy: Combined treatment of famciclovir and prednisone is superior to prednisone alone. J Neurol 2008;255:1726-30. [DOI] [PubMed] [Google Scholar]
- 10.Yeo SG, Lee YC, Park DC, Cha CI. Acyclovir plus steroid vs steroid alone in the treatment of Bell’s palsy. Am J Otolaryngol 2008;29:163-6. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi K, Inamura H, Abe Y, Koshu H, Takashita E, Muraki Y, et al. Reactivation of herpes simplex virus type 1 and varicella-zoster virus and therapeutic effects of combination therapy with prednisolone and valacyclovir in patients with Bell’s palsy. Laryngoscope 2007;117:147-56. [DOI] [PubMed] [Google Scholar]
- 12.Hato N, Yamada H, Kohno H, Matsumoto S, Honda N, Gyo K, et al. Valacyclovir and prednisolone treatment for Bell’s palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol 2007;28:408-13. [DOI] [PubMed] [Google Scholar]
- 13.Hato N, Matsumoto S, Kisaki H, Takahashi H, Wakisaka H, Honda N, et al. Efficacy of early treatment of Bell’s palsy with oral acyclovir and prednisolone. Otol Neurotol 2003;24:948-51. [DOI] [PubMed] [Google Scholar]
- 14.Engstrom M, Berg T, Stjernquist-Desatnik A, Axelsson S, Pitkaranta A, Hultcrantz M, et al. Prednisolone and valaciclovir in Bell’s palsy: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet Neurol 2008;7:993-1000. [DOI] [PubMed] [Google Scholar]
- 15.Antunes ML, Kukuda Y, Gurgel Testa JR. Tratamento clinico da paralisia de Bell: estudo comparativo com o uso de Valaciclovir mais Deflazacort versus Deflazacort versus placebo [Portuguese]. Acta Awho 2000;19:68-75. [Google Scholar]
- 16.Anpalahan V, Redhead J. Acyclovir and prednisolone combination treatment in Bell’s palsy. Australian J Otolaryngol 2000;3:476-8. [Google Scholar]
- 17.Ahangar AA, Hosseini S, Saghebi R. Comparison of the efficacy of prednisolone versus prednisolone and acyclovir in the treatment of Bell’s palsy. Neurosciences 2006;11:256-9. [PubMed] [Google Scholar]
- 18.Davenport RJ, Sullivan F, Smith B, Morrison J, McKinstry B. Treatment for Bell’s palsy. Lancet 2008;372:1219-20. [DOI] [PubMed] [Google Scholar]
- 19.Grogan PM, Gronseth GS. Practice parameter: Steroids, acyclovir, and surgery for Bell’s palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:830-6. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900. [DOI] [PubMed] [Google Scholar]
- 21.Alberton DL, Zed PJ. Bell’s palsy: a review of treatment using antiviral agents. Ann Pharmacother 2006;40:1838-42. [DOI] [PubMed] [Google Scholar]
- 22.Adour KK, Ruboyianes JM, Von Doersten PG, Byl FM, Trent CS, Quesenberry CP Jr, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomized, controlled trial. Ann Otol Rhinol Laryngol 1996;105:371-8. [DOI] [PubMed] [Google Scholar]
- 23.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [DOI] [PubMed] [Google Scholar]
- 26.Ramos Macias A, de Miguel Martinez I, Martin Sanches AM, Gomez Gonzalez JL, Martin Galan A. Incorporacion del aciclovir en el tratamiento de la paralisis periferica. Un estudio en 45 casos [Spanish]. Acta Otorrinolaring Esp 1992;43:117-20. [PubMed] [Google Scholar]
- 27.Roy A, Jose J, Kamath V, Matthew T. Efficacy of aciclovir and methylprednisolone versus methylprednisolone alone in the treatment of Bell’s palsy. J Neurol Sci 2005;238(Suppl 1):S207. [Google Scholar]
- 28.Furuta Y, Ohtani F, Chida E, Mesuda Y, Fukuda S, Inuyama Y. Herpes simplex virus type 1 reactivation and antiviral therapy in patients with acute peripheral facial palsy. Auris Nasus Larynx 2001;28(Suppl):S13-7. [DOI] [PubMed] [Google Scholar]
- 29.Hato N, Murakami S, Gyo K. Steroid and antiviral treatment for Bell’s palsy. Lancet 2008;371:1818-20. [DOI] [PubMed] [Google Scholar]
- 30.Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst Rev 2005; Issue 4. Art. No.: CD001942. Doi:10.1002/14651858.CD001942.pub3. [DOI] [PubMed] [Google Scholar]