Summary
Osteonecrosis of the jaw secondary to bisphosphonate infusion (zoledronic acid-ZA) is assumed to be a bone disease. The purpose of this study was to investigate the effects of ZA on soft tissues using oral mucosal cells as an in vitro model of soft tissue cell death in the pathogenesis of bone necrosis.
Human gingival fibroblast and keratinocyte cell lines were exposed to different concentrations of ZA (0.25–3 μM), using 1μM as the expected baseline concentration. A dose response effect on apoptosis and cell proliferation (TUNEL and Annexin V or Coulter counter and MTS, respectively) was observed with increasing ZA concentrations; both reversed using siRNA against caspase 3 or 9. Gene expression analysis using RT2 Profiler PCR Arrays demonstrated the differential expression of multiple genes involved in apoptosis including: TNF, BCL-2, CASPASE, IAP, TRAF, and DEATH DOMAIN families. Western blot analysis confirmed the presence of activated forms of caspase 3 and 9 and underexpression of survivin protein expression.
The combined results from this study demonstrate that low concentrations of ZA rapidly and directly affect the oral mucosal tissues though the induction of a gene-regulated apoptotic process. These findings support the potential for soft tissue injury as an initiating/potentiating event for osteonecrosis.
Keywords: Zoledronic acid, Apoptosis, Fibroblasts, Epithelium, Bisphosphonate
Introduction
Bisphosphonates (BP) are synthetic analogues of the naturally occurring pyrophosphate with high affinity for calcium crystals, which allows this pyrophosphate to bind hydroxyapatite of bone and inhibit osteoclast-mediated bone resorption. (Mundy, 1987; Kanis, 1995; Rogers et al, 1997; Cremers et al, 2005) This provided the rationale for their use as a skeletal protector of cancer mediated cytokine induced hypercalcemia and in various malignancies, such as multiple myeloma (MM) and solid tumors with bone metastasis. (Mundy, 1987; Kanis, 1995; Rogers et al, 1997; Cremers et al, 2005) Zoledronic acid (ZA), a third generation nitrogen containing BP is the potent BP in clinical use. (Goffinet et al, 2006; Civitelli et al, 2007) ZA is known to inhibit cell signaling through the mevalonate pathway and block the prenylation of small signaling proteins (e.g. Ras, Rac and Rho) which are essential for normal cell function and survival. (Luckman et al, 1998; Goffinet et al, 2006; Weitzman et al, 2007) A recently reported adverse effect of ZA treatment is osteonecrosis of the jaw (ONJ). The reported incidence of osteonecrosis of the jaw ranges from 1.3% to 10%, with a higher frequency in the mandible than in the maxilla. (Bamias et al, 2005; Badros et al, 2006; Bagan et al, 2006; Woo et al, 2006; Khosla et al, 2007; Mavrokokki et al, 2007) The median time to ONJ was 3.5 years in MM patients. (Bamias et al, 2005; Badros et al, 2006; Bagan et al, 2006; Woo et al, 2006; Khosla et al, 2007; Mavrokokki et al, 2007) The pathophysiologic mechanisms of ONJ are not elucidated. (Fleisch, 2003) One hypothesis suggests that the microenvironment surrounding active osteoclasts is highly acidic inducing the release of the BP from the bone surface and creating high local BP concentrations, resulting in the inhibition of osteoclasts and leading to impaired bone repair and healing. (Rogers et al, 2000) ONJ is a mucosal dehiscence leading to a superficial mucosal ulcer, which progresses and results in detectable bone exposure and subsequent bone necrosis. (Bagan et al, 2006) To date, hypotheses of ONJ have focused on BP’s effect on the bone with a possible contibution of microbial infection as well as anti-angiogenic properties of BP.
We hypothize that the pathogenesis of ONJ is also directly related to BP effects on epithelial and fibroblast cells. Early studies of oral and IV BPs suggested their ability to induce esophageal, gastrointestinal and injection site ulcerations. (Graham, 2002) It has been suggested that mucosal erosions and ulcerations, are the result of direct contact with these agents. (Lanza, 2002; Parfitt & Driman, 2007; Reid et al, 2007) By selecting concentrations of ZA that are representative of clinical plasma levels after each infusion, this current study was designed to validate the hypothesis that the direct effects of low levels of ZA on mucosal cell apoptosis and normal mucosal cell turnover constitute an initiating and/or potentiating mechanism for ONJ. (Chen et al, 2002; Skerjanec et al, 2003; Smith, 2003; Coxon et al, 2004; Bilezikian, 2006; Wynn et al, 2007) Herein, we will assess the apoptotic effects of ZA on mucosal cells in an in vitro model using human gingival fibroblasts and oral mucosal cell lines.
Materials and Methods
Cell Lines and Cell Cultures
All experiments were performed using an established human keratinocyte cell line (HaCat) and a human gingival fibroblast cell line (HGF) (donated by Dr. Silvio Gutkind; National Institutes of Health and John Sauk; University of Maryland, respectively), selected as cells to represent the oral mucosa. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum, 100 units of penicillin, 100 μg/ml streptomycin or 1:1 mix of Ham’s F12 and Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum, 100 units of penicillin, 100 μg/ml streptomycin and 0.4 g/ml of hydrocortisone (Sigma Chemical Company, St. Louis, MO), respectively. The cells were cultured at 37°C in a 5% CO2 air atmosphere until confluent and sub-cultured using a disaggregration assay with Trypsin (0.1%) and EDTA (0.01%) in PBS (pH 7.5). For all experiments, cells were grown in 6 or 24 well plates at 5x104 cells per well and grown to 80% confluence. Control cells (NM) for all experiments were treated with the infusion solution alone in normal media (non-calcium containing infusion solution). All experiments were performed in triplicate and repeated on two separate occasions. In additions to standard apoptosis and proliferation studies, siRNA was used to demonstrate specificity of the assays by reversing ZA effects.
Drug Treatments
ZA injectable (Zometa®, Novartis Pharmaceuticals Corp, East Hanover, NJ) was used for all experiments at concentrations: 0.25, 0.5, 1 or 3 μM up to 24 h (pre-concentration baseline plasma level is 1μM). The concentrations were selected because they are clinically relevant in patients receiving ZA as representative of the lower limits of estimated plasma concentrations following a 15 minute infusion. (Skerjanec et al, 2003; Smith, 2003; Coxon et al, 2004) Cells were visualized, photographed and assayed during the 24 h treatment.
Direct Microscopic Observation
HaCat and HGF cells either untreated (normal media with infusion solution) or exposed to ZA 0.25, 0.5, 1 or 3 μM diluted in non-calcium containing infusion solution were examined hourly for up to 24 h on a Zeiss Axiovert 135 microscope and images were captured with a digital Nikon capture system.
Terminal Deoxynucleotidyl Transferase–Mediated dUTP-Biotin End Labeling (TUNEL) Staining
Adherent HaCat and HGF cell lines (treated and untreated with ZA) were washed twice with PBS and fixed with a fixation solution of 4% paraformaldehyde in PBS (pH 7.4) for 1 h at 20°C. Cells were rinsed twice with PBS and then incubated with permeabilisation solution for two minutes on ice. The cells were rinsed in PBS and labeled using 50 μl of a 9:1 solution of Label and Enzyme solutions from the In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Sciences, Mannheim, Germany), with appropriate controls labeled only with the Label solution. The cells were incubated for 1 h at 37°C in a humidified atmosphere in the dark, rinsed in PBS and examined with a fluorescent microscope with a detection range of 515 to 565 nm.
Flow Cytometry and Annexin V Studies
Apoptosis was evaluated using annexin V–fluorescein isothiocyanate method. Cells were treated with either the infusion solution alone or ZA at 0.25, 0.5, 1 or 3 μM in normal infusion solution for 24 h or in combination with siRNA against Caspase 3 or 9, respectively (as described below) and washed with Hank’s balanced salt solution, followed by lysis using trypsin (0.1%) and EDTA (0.01%) in PBS at pH 7.5. The cells were washed with cold PBS, and resuspended in 1X binding buffer (BD-Pharmingen Biosciences, San Diego, CA). Five microliters of annexin and 5 ml of propidium iodide were added to the cells, vortexed, and incubated for 15 minutes in the dark. Finally, 400 ml of 1X binding buffer was added, and samples were evaluated by Beckman Coulter Epics Elite ESP flow cytometer.
Cell Proliferation
Cells were plated in 24 or 96-well plates using a density of 5 X 104 cells/well or 2500 cells/well, respectively and allowed to grow to 80% confluence then treated with serum-free medium for 12 h, or left in normal media. Subsequently, either infusion solution alone or ZA at 0.25, 0.5, 1 or 3 μM in infusion solution was added to normal growth medium and incubated for 24 h. Further, Caspase 3 or 9 Silencer® select siRNA or scrambled control siRNA (Ambion, Austin, TX) were added at 5nM to 25μl of optimem media (Invitrogen, Carlsbad, CA), with the addition of 4μl plus regent (Invitrogen, Carlsbad, CA), and allowed to incubate for 15 minutes, while 1μl lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) was added to 25μl of optimem media and incubated for 15 minutes. The siRNA was combined with the lipofectamine 2000 reagent and incubated for an additional 15 minutes at room temperature. The combined mix was then added to the cells which were incubated at 37°C at 5% CO2 for 3 hours followed by the addition of normal medium or the various treatments for 24 hours. Transfection efficiency was evaluated by immunofluorescence analysis. Further analysis included cell proliferation assays. The cells were washed, and removed enzymatically and counted using a Coulter counter (Model ZI; Coulter, Miami, FL) or subjected to Cell Titer 96® AQueous Non-Radioactive MTS Cell Proliferation Assay (Promega; Madison, WI), which was read at a wavelength of 570nm, using a 96-well Dynatech MR4000 Microplate Palate Reader (Dynatech; Chantilly VA). The percentage of cell growth was determined by setting as 100% the growth of control cells treated only with infusion solution in normal media. All analyses were performed in triplicate and repeated on separate occasions.
RT2 Profiler tm PCR Array (PAHS-012A)
Cells were incubated with either normal media (plus infusion solution) or ZA at the concentration of 0.5, 1 or 3 μM for 24 h and washed twice with PBS, followed by lysis using trypsin (0.1%) and EDTA (0.01%) in phosphorylated buffered saline (PBS) pH 7.5. Total RNA was isolated using TRIZOL Reagent (BRL/Life Technologies) and integrity and concentration determined spectrophotometrically. A total of 1μg RNA was used with RT2 First Strand kit (C-03) (Superarray, Fredrick, MD) with the addition of 2 μl of 2GE (5x gDNA elimination buffer). The RNA was incubated at 42°C for 5 minutes and placed on ice of 1 minute. RT cocktail of 4 μl of BC3 (5x RT buffer 3), 1 μl of P2 (primer & external control mix), 2 μl RE3 (RT enzyme mix), and 3 μl of RNase free water was added to each sample. The mixture was centrifuged, incubated at 42°C for 15 minutes and then heated at 95°C for 5 minutes and placed on ice.
PCR was performed using 25 μl of the following mixture: 1275 μl of 2x SuperArray RT2 qPCR Master Mix, 102 μl diluted first strand cDNA synthesis reaction and 1173 μl of double distilled water in the wells of a 96-well microtiter plate. The amplification process included 1 cycle for 10 mins at 95°C, 40 cycles for 15 secs at 95°C, followed by 40 cycles for 1 min at 60°C. Thermal cycling and fluorescence detection were performed using an ABI 5700 Prism (PE Applied Biosystems). The signals of the target cDNAs were normalized by comparison with the housekeeping genes (GAPDH, Actin, reverse transcription control, and positive PCR control) supplied within the 96-well microtiter plate. The normalized amount of each target mRNA present in each cell line was calculated by designating cells growing in normal medium (infusion solution) without drug addition as a calibrator using a comparative Ct method following PE Biosystems protocols. Final calculations were made using a Web-Based PCR Array Data Analysis tool. (Superarray, Fredrick, MD)
Western Blot Analysis
Cell were treated with either normal medium with infusion solution or ZA at 0.25, 0.5, 1 or 3 μM for 24 h. Cells were washed twice with ice-cold PBS, followed by lysis with radioimmunoprecipation assay buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% deoxycholic acid, sodium salt, 0.1% sodium dodecyl sulfate, 100 mg/ml phenylmethysulfonyl fluoride, 1 mg/ml aprotinin, 1 mM dichlorodiphenyltrichloroethane, and 1 mM sodium orthovanadate) for 10 minutes at 4°C. The wells were scraped and recovered cell products were centrifuged at 40,000g for 15 minutes at 4°C. Recovered proteins were measured and equalized using Bio-Rad Protein Assay (Bio-Rad Laboratories, Richmond, CA) per manufacturer’s instructions. Western blot analysis was performed using a survivin polyclonal antibody (Abcam, Cambridge, UK), or caspase 3 or caspase 9 monolonal antibodies (Cell Signaling, Beverly, MA). Actin antibody (Sigma, St. Louis, MO) was used as a loading control.
Statistical Analysis
For all measurements, as needed, a Student’s t test or t-test was employed to assess the statistical significance of treated groups versus control groups and applied to standard error measurements. A statistically significant difference was considered to be present at P < 0.05.
Results
ZA-induced apoptosis and cell-growth inhibition
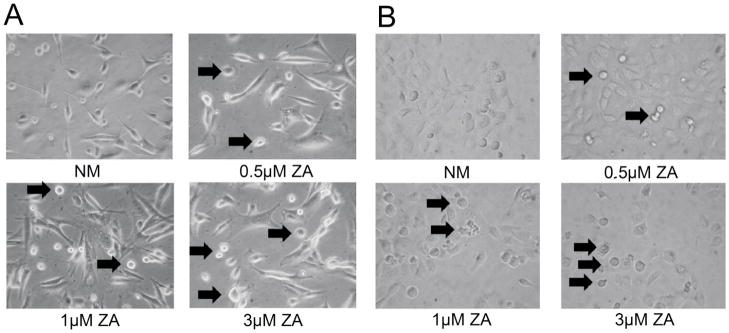
Physical signs of apoptosis in individual HGF and HaCat cells exposed to 0.25, 0.5, 1 or 3 μM of ZA were determined microscopically. Initial cell rounding and damage to the cells treated with 3 μM was visualized as early as 6 h of incubation, whereas damage to the 1 μM and 0.5 μM treated cells was observed at 10 and 14 h, respectively. At 24 h, both HGF and HaCat cells appeared to “ball-up”, fragment and begin to show dead floating cells, indicating apoptosis in a dose dependent manner. (Fig 1A, B)
Fig 1. Microscopic Apoptosis.
A) Microscpoic images of apoptosis in human HGF cells using either treatment with normal media (NM) or ZA at 0.5 μM, 1 μM or 3 μM showing the balling up and breakdown of cells, both indicators of apoptosis. (Arrows) (400x magnification) B) Microscpoic images of apoptosis in human HaCat cells using either treatment with normal media (NM) or ZA at 0.5 μM, 1 μM or 3 μM and showing balling and breakdown of cells. (Arrows) Cells were observed over 24 hours with images taken at 24 hours. (Original magnification 400x)
Confirmation of Apoptosis
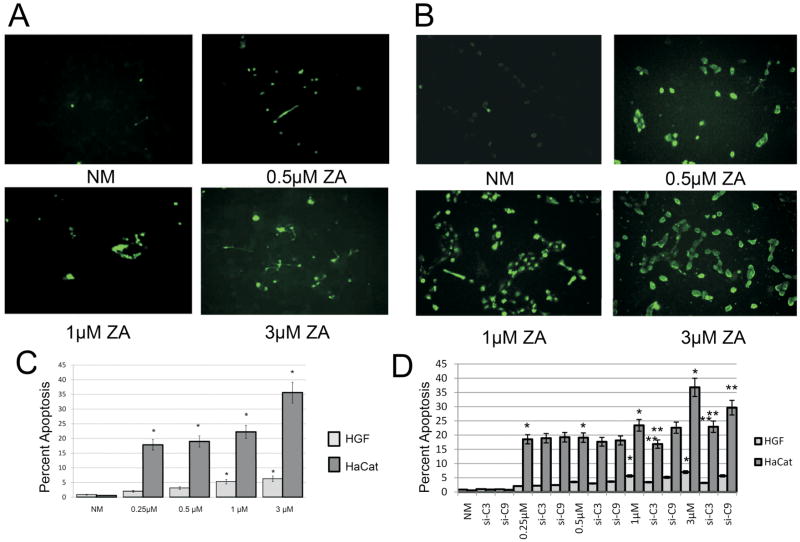
The dose dependent-increase in apoptosis in the ZA-treated (0.25, 0.5, 1 or 3μM) HGF and HaCat cells observed microscopically was corroborated with the results from TUNEL (Terminal Uridine Deoxynucleotidyl Transferase dUTP Nick End Labeling) and Annexin-V flow cytometry apoptosis assays. (Fig 2A, B) In these studies, HGF cells exposed to ZA (0.25, 0.5, 1 or 3μM) demonstrated an increase in apoptosis of 1.99, 3.11, 5.27 and 6.25 fold (p<0.05) (Fig 2C) whereas the HaCat cells demonstrated a 17.84, 18.98, 22.23 and 35.62 fold increase in apoptosis compared to normal media, respectively.(p<0.05) (Fig 2C) Furthermore, siRNA directed against caspase 3 or 9 significantly reversed the apoptotic effects seen with ZA treatments of 1μM (38.6 and 8.5%) and 3 μM (54.5 and 18.9%) respectively in HGF cells (p<0.05). (Fig 2D) HaCat cells demonstrated a similar significant reversal of apoptosis with siRNA treatment against caspase 3 showing a decrease in apoptosis at 1μM ZA (28.1%) and an effect of both caspase 3 and 9 at 3 μM ZA (37.7 and 19.4%). (p<0.05) (Fig 2D)
Fig 2. Apoptosis.
TUNEL assay showing a dose dependent increase in apoptosis in A) human HGF cells or B) human HaCat cells treated with increasing doses of ZA 0.5 μM, 1 μM or 3 μM and compared to control cells (NM) at 24 hours. (Original magnification 200x). C) Annexin V: Assessment of human HGF and HaCat cell apoptosis following 24h treatment with increasing doses of ZA 0.25 μM, 0.5 μM, 1 μM or 3 μM compared to control cells (NM). Results demonstrated dose dependent increase in apoptosis with ZA exposure. D) Annexin V: Assessment of human HGF and HaCat cell apoptosis following 24h treatment with increasing doses of ZA 0.25 μM, 0.5 μM, 1 μM or 3 μM or ZA with siRNA against caspase 3 or 9. Results demonstrate a significant reversal of apoptosis in ZA 1 μM and 3 μM treated cells when treated with either siRNA. Error bars indicate the standard errors of the means. *Statistically significant (p< 0.05) differences compared to NM cells. ** Statistically significant (p< 0.05) differences following siRNA treatment compared to ZA treatment on a dose dependent basis.
Cell Proliferation
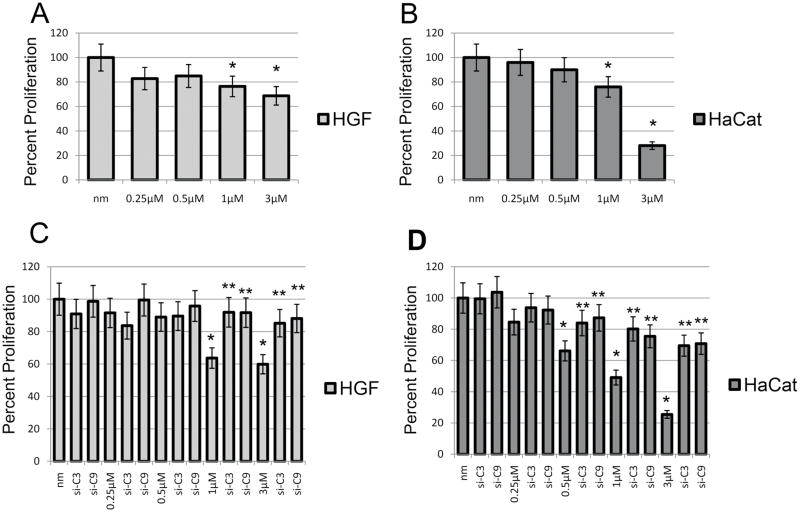
Cell proliferation results using Coulter counter indicated a 23.6 and 31.3% decrease in cell proliferation of HGF cells and a 24.0 and 72.0% decrease of HaCat cells following 24 h exposure to 1 and 3 μM dose of ZA, respectively compared to unexposed cells (p<0.05) (Fig 3A, B). Similar results were seen using an MTS assay, showing a 45.7 and 51.1% decrease in cell proliferation of HGF cells and a 45.2 and 83.9% decrease of HaCat cells following 24 h exposure to 1 and 3 μM dose of ZA, respectively compared to unexposed cells. (p<0.05) (Fig 3C, D) However, no significant differences in proliferation were observed with ZA dose of 0.25μM (17.2 and 4/5 and 14%) and 0.5 μM (15.1 and 10/12.2 and 13.5%) in HGF and HaCat cells, respectively (Coulter counter/MTS assay). Additionally, siRNA directed against caspase 3 or 9 reinstated the proliferation capabilities of HGF (Fig 3C) and HaCat (Fig 3D) cells using MTS assay. HGF cells demonstrated a reversal of ZA 1 μM (63.7%) and 3 μM (59.87%) induced decrease in cell proliferation using siRNA versus caspase 3 (91.9 and 85.2%), an average 24.85% reversal or caspase 9 (91.7 and 88.1%) an average 30.03% reversal, respectively. HaCat cells demonstrated a reversal of ZA 0.5 μM (66.15%), 1 μM (49.1%) and 3 μM (25.5%) induced decrease in cell proliferation using siRNA versus caspase 3 (84, 80.2 and 69.5%) or caspase 9 (87.3, 75.5 and 70.8%), respectively. These correlate with a restoration of cell proliferation versus ZA 0.5 μM, 1 μM or 3 μM with siRNA for caspase 3 of 17.85, 31.1 and 44%, or with caspase 9 of 21.15, 26.4 and 45.3%, respectively.
Fig 3. Cell Proliferation.
Assessment of human HGF and HaCat cell proliferation following 24h treatment compared to control cells. A,B) Decrease in tumor cell proliferation using Coulter counter and C,D) MTS assay in a dose dependent manner following exposure to 0.25 μM, 0.5 μM, 1 μM or 3 μM ZA compared to control cells (NM). C) MTS assay: Assessment of human HGF cell proliferation following 24h treatment with increasing doses of ZA 0.25 μM, 0.5 μM, 1 μM or 3 μM or ZA doses with siRNA against caspase 3 or 9. Results demonstrate a significant reversal of cell proliferation in ZA 1 μM and 3 μM treated cells along with treatment with either siRNA. D) MTS assay: Assessment of human HaCat cell proliferation following 24h treatment with increasing doses of ZA 0.25 μM, 0.5 μM, 1 μM or 3 μM or ZA doses with siRNA versus caspase 3 or 9. Results demonstrate a significant reversal of cell proliferation in ZA 0.5 μM, 1 μM and 3 μM treated cells along with treatment with either siRNA. Error bars indicate the standard errors of the means. The growth of control cells (NM) has been set to 100%. *Statistically significant (p< 0.05) differences compared to control cells. ** Statistically significant (p< 0.05) differences following siRNA treatment compared to ZA treatment on a dose dependent basis.
RT2 Profiler tm PCR Array
Gene expression analyses of 24h treated cells (ZA 0.5μM, 1μM, 3μM) demonstrated significant number of genes to be differentially regulated compared to control cells Most notable among the apoptotic genes are several families known to be members of the extrinsic (TNF, TRAF and DEATH DOMAIN) and intrinsic (BCL, IAP and CASPASE) apoptosis pathways. (Table I) Interestingly, differences in regulation of some genes were observed between HGF and HaCat cells. Among the most significantly overexpressed genes (near 2 fold greater expression) in HGF cells were TNF family members FAS; LTBR; TNFRSF10A and 11B; CD27; TNF; FASLG; BCL2 family members BAD; BCL2; BCL2L1, L10, L2; BID; CASPASE family members 3 and 5; IAP family member NAIP; TRAF family member 2 and DEATH DOMAIN family member TRADD. In HaCat cells the most overexpressed genes were TNF family members LTBR; TNFRSF1A and 21; BCL2 family members BAG1 and 3; CASPASE family members 1, 2, 3, and 8; TRAF family member 3; and DEATH DOMAIN family member FADD. The most underexpressed genes (near 2 fold lower expression) in HGF cells were TNF family member TNFRSF9; BCL2 family members BAX, BNIP3; CASPASE family members 1 and 2; IAP family members BIRC 2, 4 AND 6. In HaCat cells the most underexpressed genes were TNF family members TNFRSF25; CD27; CD40LG; FASLG; BCL2 family members BCL2; BCL2A1 and L10; CASPASE family members 14 and 5; IAP family members NAIP; BIRC 6 AND 8; and DEATH DOMAIN family member DAPK1.
Western Blot Analysis
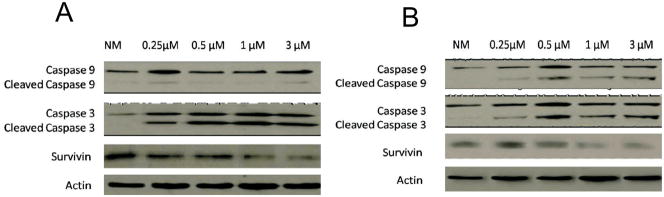
In order to confirm results from the apoptosis gene array, proteins were extracted from HGF and HaCat cells following 24h exposure to 0.25, 0.5, 1 or 3 μM ZA. Western Blot analyses of proteins following probing with antibodies to caspase 3 or caspase 9 and survivin demonstrated a significant increase in cleavage and activation of caspase 3 in HGF and HaCat cells, while caspase 9 was minimally activated in HGF cells and further activated in HaCat cells. (Fig 4A, B) A significant decrease in survivin expression was seen in both the HGF and HaCat cells. Differences in expression of these proteins were proportional to ZA dose.
Fig 4. Western blot analysis.
Assessment of (A) human HGF and (B) HaCat cell protein expression demonstrating in (A) an increase in caspase 3 and cleaved-caspase 3 expression in cells exposed to 0.25 μM, 0.5 μM, 1 μM or 3 μM ZA compared to control cells (NM) and a comparative decrease in survivin expression at 24h. (B) An increase in caspase 3 and 9 and their cleaved forms in cells exposed to 0.25 μM, 0.5 μM, 1 μM or 3 μM ZA compared to control cells (NM) and a comparative decrease in survivin expression at 24h. Actin was used as the loading control.
Discussion
In this study, we have shown for the first time proof of a potential mechanism of ONJ, whereby BP during circulation and/or following release from bone affects the soft tissues, inducing fibroblast and epithelial cell death by a combined intrinsic and extrinsic pathway of apoptosis. (Reid, et al, 2007) This leads to suppression of cell proliferation and mucosal thinning and eventually exposure of bone allowing for rapid microbial colonization. (Sedghizadeh, et al, 2008) The formation of a biofilm smear layer further prevents resolution of the inflammation, prevents healing in the oral cavity and leads to ONJ. (Sedghizadeh et al, 2008)
Microscopically, TUNEL and Annexin V studies demonstrated a ZA-induced dose dependent apoptotic process in both human epithelial (HaCat) and gingival fibroblast (HGF) cells. Additionally, proliferation studies demonstrated cell growth inhibition with 1 μM and 3 μM ZA. These data point to a direct effect of ZA on epithelium and fibroblasts in a temporal sequence that begins at very low concentrations of BP in the fibroblasts and then proceeds to the epithelial cells.
The cell treatments with siRNA versus caspase 3 or 9 showed a significant reversal of the apoptotic effects of ZA in both HGF and HaCat cells, demonstrating specificity toward the intrinsic and extrinsic apoptotic pathways for ZA induced cell death. However, despite significance shown in HGF cells, their low response in apoptosis makes the significance of this reversal less dramatic. Hence, studies using siRNA against caspase 3 or 9 on cell proliferation using an MTS assay were used to confirm these apoptotic findings. Here, siRNA versus caspase 3 or 9 showed a significant reversal of the cell proliferative effects of ZA in both HGF and HaCat cells, helping to lend credibility to the annexin V studies. These results lend specificity to the ZA induced effects on the human fibroblast and keratinocyte cell lines.
Speculation could be made that this effect on mucosal cells is combined with BPs alteration of angiogenesis of new osseous tissue, inducing detrimental effects on the quality and quantity of bone perfusion, ultimately, leading to an altered response of osseous tissue to trauma, infection and wound healing. (Farrugia et al, 2006) Incorporation of the systemic alterations caused by the additional pharmacologic therapies that this type of patient must endure, would prevent exposed bone from fighting infections in the oral cavity, perpetuating the risk of ONJ. (Herbozo et al, 2007)
The possibility exists that direct effects of BPs on oral mucosal cells, function as a contributory and possibly even an initiating pathophysiologic mechanism, and this could explain why at least 5–15% of patients with ONJ have no history of prior surgery or trauma. In fact, many of the cases of ONJ occur at sites away from teeth on otherwise normal undisturbed tissues. (Badros et al, 2006) The fact that ONJ occurs as both a wound healing phenomenon, as well as a spontaneous occurrence, supports our suggestion that the pathogenesis is likely multifactorial.
Our gene expression analysis demonstrated the differential expression in genes of the extrinsic (TNF, TRAF and DEATH DOMAIN) and intrinsic (BCL, IAP and CASPASE) apoptotic pathways. Specifically, many of the anti-apoptotic family members including the IAP family, BCL2 and BNIP3 were found to be underexpressed, thereby corroborating the speculations of apoptotic properties to ZA. These results support the notion relating the complex signaling pathway interaction of the BCL2 family along with mitochondrial breakdown and death domain receptor activation, where both terminate in CASPASE activation and IAP inhibition. Interestingly, BPs are known to affect the isoprenoid biosynthetic pathway via an inhibition of farnesol pyrophosphate synthesis leading to impaired processing of both farnesylated and geranylgeranylated proteins. (Dunford et al, 2006) The potential upstream mechanism of BP induced impairment of protein prenylation of the Ras/Rho superfamily of small GTPases would lead to the gene regulation of apoptosis seen here and needs further study.
Our results were confirmed to have an active affect on these terminal effectors of apoptosis, including the cleavage and activation caspase 3 and 9, as well as the underexpression of survivin, an inhibitor of apoptosis. The data on survivin underexpression also support speculation regarding the poorly understood anti-metastatic effects of BP. Further studies are needed to fully elucidate these biochemical mechanisms and to determine if such effects are clinically relevant to development of ONJ and possible antitumor potential of bisphosphonates. Additionally, the effects on RNA expression in inducing apoptosis provide a new currently available model on which to base further studies.
Conclusions
Currently, only preventive strategies aimed at avoiding invasive oral interventions such as dental surgery and subsequent infection are the standard of care for patients with ONJ. Additionally, it is recommended that until healing from an invasive dental surgical treatment takes place, temporary discontinuation of BP therapy may be considered (up to 3 months) (Weitzman et al, 2007; Jeffcoat & Watts, 2008), however there is no evidence that this favorably affects outcomes. However, these recommendations flow from a clinical paradyme and fail to follow a causative mechanism. We provide for the first time studies showing that the direct contact of clinically relevant concentrations of ZA with epithelial or fibroblast cells induces apoptosis initially, with the potential result being ONJ, secondary to the clinical manifestation of exposure of the bone to the unique infectious microenvironment of the oral cavity..It is our expectation that expanding this in vitro model, to study the release of ZA secondary to calcium chelation in a mandibular bone model using primary cell lines from MM patients, including its effects on these overlaid cell lines, as well as in vivo studies to determine oral ZA levels in oral fluids will ultimately confirm this hypothesis.
Acknowledgments
Grant: Supported by K12 RR023250.
References
- Badros A, Weikel D, Salama A, Goloubeva O, Schneider A, Rapoport A, Fenton R, Gahres N, Sausville E, Ord R, Meiller T. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. Journal of Clinical Oncology. 2006;24:945–952. doi: 10.1200/JCO.2005.04.2465. [DOI] [PubMed] [Google Scholar]
- Bagan JV, Jimenez Y, Murillo J, Hernandez S, Poveda R, Sanchis JM, Diaz JM, Scully C. Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions. Study of 20 cases. Oral Oncology. 2006;42:327–329. doi: 10.1016/j.oraloncology.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. Journal of Clinical Oncology. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- Bilezikian JP. Osteonecrosis of the jaw--do bisphosphonates pose a risk? The New England Journal of Medicine. 2006;355:2278–2281. doi: 10.1056/NEJMp068157. [DOI] [PubMed] [Google Scholar]
- Chen T, Berenson J, Vescio R, Swift R, Goodin S, LoRusso P, Ma P, Ravera C, Deckert F, Schran H, Seaman J, Skerjanec A. Pharmacokinetics and pharmacodynamics of zoledronic acid in patients with cancer and bone metastases. Journal of Clinical Pharmacology. 2002;42:1228–1236. doi: 10.1177/009127002762491316. [DOI] [PubMed] [Google Scholar]
- Civitelli R, Napoli N, Armamento-Villareal R. Use of intravenous bisphosphonates in osteoporosis. Current Osteoporosis Reports. 2007;5:8–13. doi: 10.1007/BF02938617. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Oades GM, Kirby RS, Colston KW. Zoledronic acid induces apoptosis and inhibits adhesion to mineralized matrix in prostate cancer cells via inhibition of protein prenylation. BJU International. 2004;94:164–170. doi: 10.1111/j.1464-4096.2004.04831.x. [DOI] [PubMed] [Google Scholar]
- Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clinical Pharmacokinetics. 2005;44:551–570. doi: 10.2165/00003088-200544060-00001. [DOI] [PubMed] [Google Scholar]
- Dunford JE, Rogers MJ, Ebetino FH, Phipps RJ, Coxon FP. Inhibition of protein prenylation by bisphosphonates causes sustained activation of Rac, Cdc42, and Rho GTPases. Journal of Bone and Mineral Research. 2006;21:684–694. doi: 10.1359/jbmr.060118. [DOI] [PubMed] [Google Scholar]
- Farrugia MC, Summerlin DJ, Krowiak E, Huntley T, Freeman S, Borrowdale R, Tomich C. Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. The Laryngoscope. 2006;116:115–120. doi: 10.1097/01.mlg.0000187398.51857.3c. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Bisphosphonates in osteoporosis. European Spine journal. 2003;12:142S–S146. doi: 10.1007/s00586-003-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet M, Thoulouzan M, Pradines A, Lajoie-Mazenc I, Weinbaum C, Faye JC, Seronie-Vivien S. Zoledronic acid treatment impairs protein geranylgeranylation for biological effects in prostatic cells. BMC Cancer. 2006;6:60. doi: 10.1186/1471-2407-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DY. What the gastroenterologist should know about the gastrointestinal safety profiles of bisphosphonates. Digestive Diseases and Sciences. 2002;47:1665–1678. doi: 10.1023/a:1016495221567. [DOI] [PubMed] [Google Scholar]
- Herbozo PJ, Briones DL, Ferres AJ, Torrealba RL. Severe spontaneous cases of bisphosphonate-related osteonecrosis of the jaws. Journal of Oral and Maxillofacial Surgery. 2007;65:1650–1654. doi: 10.1016/j.joms.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Jeffcoat M, Watts NB. Osteonecrosis of the jaw: balancing the benefits and risks of oral bisphosphonate treatment for osteoporosis. General Dentistry. 2008;56:96–102. [PubMed] [Google Scholar]
- Kanis JA. Bone and cancer: pathophysiology and treatment of metastases. Bone. 1995;17:101S–105S. doi: 10.1016/8756-3282(95)00194-i. [DOI] [PubMed] [Google Scholar]
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E American Society for Bone and Mineral Research. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. Journal of Bone and Mineral Research. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- Lanza FL. Gastrointestinal adverse effects of bisphosphonates: etiology, incidence and prevention. Treatments in Endocrinology. 2002;1:37–43. doi: 10.2165/00024677-200201010-00004. [DOI] [PubMed] [Google Scholar]
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. Journal of Bone and Mineral Research. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in Australia. Journal of Oral and Maxillofacial Surgery. 2007;65:415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Bone resorption and turnover in health and disease. Bone. 1987;8:S9–16. [PubMed] [Google Scholar]
- Parfitt JR, Driman DK. Pathological effects of drugs on the gastrointestinal tract: a review. Human Pathology. 2007;38:527–536. doi: 10.1016/j.humpath.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Reid IR, Bolland MJ, Grey AB. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone. 2007;41:318–320. doi: 10.1016/j.bone.2007.04.196. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Watts DJ, Russell RG. Overview of bisphosphonates. Cancer. 1997;80:1652–1660. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1652::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sedghizadeh PP, Kumar SK, Gorur A, Schaudinn C, Shuler CF, Costerton JW. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. Journal of Oral and Maxillofacial Surgery. 2008;66:767–775. doi: 10.1016/j.joms.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Skerjanec A, Berenson J, Hsu C, Major P, Miller WH, Jr, Ravera C, Schran H, Seaman J, Waldmeier F. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. Journal of Clinical Pharmacology. 2003;43:154–162. doi: 10.1177/0091270002239824. [DOI] [PubMed] [Google Scholar]
- Smith MR. Antitumor activity of bisphosphonates. Clinical Cancer Research. 2003;9:5433–5434. [PubMed] [Google Scholar]
- Weitzman R, Sauter N, Eriksen EF, Tarassoff PG, Lacerna LV, Dias R, Altmeyer A, Csermak-Renner K, McGrath L, Lantwicki L, Hohneker JA. Critical review: updated recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in cancer patients May 2006. Critical Reviews in Oncology Hematology. 2007;62:148–152. doi: 10.1016/j.critrevonc.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- Wynn RL, Meiller TF, Crossley HL. Drug Information Handbook for Dentistry. 13. Ohio: Lexi-Comp; 2007. pp. 1685–1688. [Google Scholar]