Abstract
Purpose
To assess the characteristics of bone metastasis from hepatocellular carcinoma and the radiation field arrangement based on imaging studies.
Materials and Methods
Fifty-three patients (84 lesions) with bone metastasis from a primary hepatocellular carcinoma completed palliative radiation therapy. All patients underwent one of following imaging studies prior to the initiation of radiation therapy: a bone scan, computed tomography or magnetic resonance imaging. The median radiation dose was 30 Gy (7~40 Gy). We evaluated retrospectively the presence of soft tissue formation and the adjustment of the radiation field based on the imaging studies.
Results
Soft tissue formation at the site of bony disease was identified from either a CT/MRI scan (41 lesions) or from a symptomatic palpable mass (5 lesions). The adjustment of the radiation field size based on a bone scan was necessary for 31 of 41 soft tissue forming lesions (75.6%), after a review of the CT/MRI scan. The median survival from the initial indication of a hepatoma diagnosis was 8 months (2 to 71 months), with a 2-year survival rate of 38.6%. The median survival from the detection of a bone metastasis was 5 months (1 to 38 months) and the 1-year overall survival rate was 8.7%.
Conclusion
It was again identified that bone metastasis from a primary hepatocellular carcinoma is accompanied by soft tissue formation. From this finding, an adjustment of the radiation field size based on imaging studies is required. It is advisable to obtain a CT or MRI scan of suspected bone metastasis for better tumor volume coverage prior to the initiation of radiation therapy.
Keywords: Bone metastasis, hepatocellular carcinoma, soft tissue formation, radiation therapy
INTRODUCTION
Hepatocellular carcinoma (HCC) ranks third as the most common carcinoma, constituting approximately 11.3% of all tumors. Annually, approximately 11,000 new patients are diagnosed with HCC in Korea. In 2004, HCC accounted for 22.6% of all cancer-related deaths, followed in frequency by lung cancer and gastric cancer. The overall 5-year survival rate, according to sex, was 11.8% for men and 13.3% for women (1).
Due to developments in surgical techniques and the introduction of effective local therapeutic modalities, such as transarterial chemoembolization (TACE) or radiofrequency ablation, the overall survival rate of HCC patients has increased, even in the cases of unresectable disease (2). However, there is also a higher chance for an extrahepatic metastasis, regardless of primary site control (3,4). The most favorable sites of extrahepatic metastasis from an HCC were the lung and central nervous system (CNS). In contrast to gastric cancer, lung cancer or breast cancer, the incidence of bone metastasis from HCC was rare, and the incidence has been reported as ranging from 3% to 20% (3~7).
In the cases of an extrahepatic metastasis, anthracycline-based chemotherapy is effective in a limited role as many patients have accompanying chronic liver disease or liver cirrhosis. The reported response rate is about 20%, while the median survival is approximately 4 months (8).
In general, radiation therapy is a preferred treatment modality for symptomatic palliation of a bone metastasis from many carcinomas. However, there have been few published reports about the role of radiation therapy for a bone metastasis from a primary HCC (9,10). Some studies with a limited number of patients have described the characteristics of bone metastasis from HCC, such as soft tissue formation (7,9,11~13).
The purpose of this study is to describe the characteristics of bone metastasis from HCC and to evaluate the effectiveness of radiation therapy.
MATERIALS AND METHODS
1) Patient characteristics
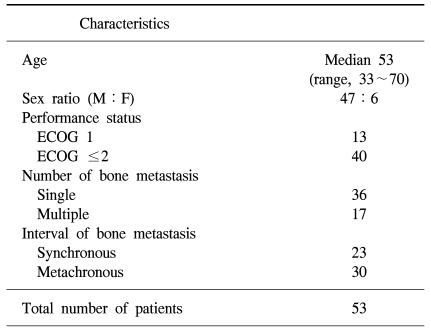
Between October 1994 and December 2004, a total 60 patients were referred for palliative radiation therapy for bone metastasis from HCC. Fifty-three patients completed planned radiation therapy for 84 lesions. The remaining seven patients stopped treatment because of either their own refusal or deterioration of performance status regardless of treatment. The median age of all patients was 53 years-old (range 33 to 70 years). With respect to performance status, 13 patients were scored as ECOG 1, 28 patients were scored as ECOG 2, and the remaining 12 patients were scored as ECOG 3. Thirty-seven patients had a single metastatic site, while 16 patients had multiple lesions. Twenty-three patients presented with synchronous bony metastasis with a diagnosis of primary HCC. The median interval between the day of the initial HCC diagnosis and that of the metachronous bone metastasis was 8.5 months, ranging from 2 months to 70 months (Table 1).
Table 1.
Patient characteristics
Of the 84 lesions treated, 48 lesions were examined with CT/MRI. Among them, 41 lesions were also examined with a bone scan. Thirty-one lesions were examined with a bone scan only. The remaining 5 lesions, despite of a lack of evaluation with either a CT/MRI or bone scan, revealed abnormal findings on a plain x-ray as symptomatic palpable masses.
2) Radiation therapy
Radiation therapy was delivered with a linear accelerator, utilizing 6 or 15 MV photon beams or electrons, according to the depth of the lesion. The treatment volume covered the gross tumor volume (GTV), which was identified with imaging data, adding a margin of 1.5 to 2 cm in all directions. If vertebral bodies were targeted for treatment, the treatment volume included the vertebral bodies, plus one additional segment in both the cephalad and caudal directions. The median total dose was 30 Gy, ranging from 7 Gy to 40 Gy. The daily fraction size was 2.5 or 3 Gy, with the exception of a single fraction treatment of 7 Gy.
We focused on the comparison of the size of the radiation field as determined with a bone scan only to the field size determined by both a bone scan and CT or MRI. We evaluated the need of adjustment of the radiation field according to the imaging modality.
After completion of radiation therapy, all patients received follow-up by either a hospital visit or phone call. The median period of follow-up was 6 months. Patient data was analyzed using the Statistical Package for the Social Science (SPSS, Chicago, IL USA) software package. The Kaplan-Meier method was also used. The end-point was the day of the patient death, both from the day of the initial diagnosis of primary disease and from the day of the initial diagnosis of a bone metastasis.
RESULTS
1) Disease characteristics
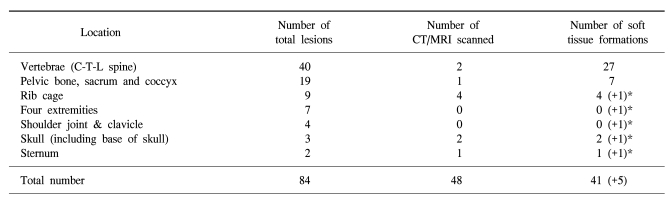
Total 84 metastatic sites received radiation treatment. The C-T-L spines were the most common site (40 lesions), followed by the pelvic bone, including the hip joints (19 lesions), ribs (9 lesions), upper and lower extremities (7 lesions), shoulder joint (4 lesions), skull (3 lesions), and sternum (2 lesions).
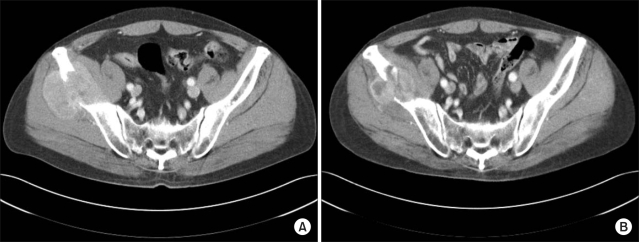
Among the 48 lesions evaluated by either CT or MRI, 41 lesions showed bone destruction combined with soft tissue formation (Fig. l). There were additional 5 lesions with soft tissue formation, which were not examined with CT or MRI, but presented with clinically palpable soft tissue at the site of bone metastasis as observed on plain x-rays.
Fig. 1.
(A) The pre-treatment CT scan shows osteolysis of the right iliac wing by soft tissue formation. (B) The mass size decreases 2 months after completion of radiation therapy with persistent bony destruction.
Metastatic lesions at the vertebrae were the most frequent ones with both bone destruction and soft tissue formation (27/29). Three soft tissue lesions were expressed as a cold spot (or as a photon defect) rather than with hot uptake as seen on the bone scan. However, there was no way to detect existing soft tissue formation around metastatic bone in lesions evaluated with a bone scan only (Table 2).
Table 2.
Location of soft tissue formation
*An additional soft tissue lesion that was not evaluated with a CT/MRI scan but presented with a symptomatic palpable mass.
2) Adjustment of the radiation field
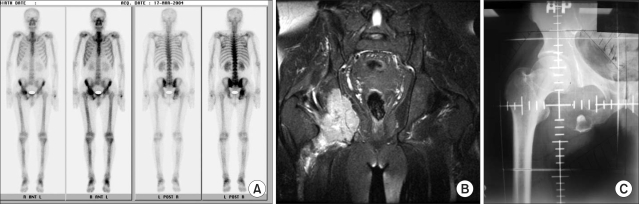
Forty-one lesions were examined with both a bone scan and by CT/MRI. We compared the radiation field size based on a bone scan image to the field size based on a CT/MRI image. Thirty-one lesions required an adjustment of the radiation field size when the radiation field size was determined with CT/MRI findings rather than with bone scan findings (Fig. 2). These lesions showed characteristic destruction of the bony framework and infiltration into the surrounding muscle or fat tissue extensively. The extent of infiltration into the surrounding muscle or fat tissue determined the size of modification of the radiation field. The amount of radiation field adjustment was an increment of an average 2.5 cm in length (range, 1~7 cm).
Fig. 2.
A bone scan (A) and pelvic MRI (B) of a 46-year-old male patient. Note the osteolytic lesion with expansile soft tissue formation on the right iliac bone. (C) The difference of radiation field based on the bone scan and MRI of the pelvis.
3) Survival
At the time of analysis, we identified 50 patient deaths. Three patients were lost to follow-up. The median survival period of all patients from the day of the initial HCC diagnosis was 8 months (ranging from 2 to 71 months). The survival rate was 53.9%, 38.6% and 30.1% at 1, 2 and 3 years, respectively. From the time of the initial diagnosis of bone metastasis, the overall survival rate was 8.7% at 1 year, with a median survival period of 5 months (ranging from 1 to 38 months). Only one patient survived for more than 2 years. This male patient was diagnosed with a bone metastasis 6 years after the initial diagnosis of primary HCC. The median survival period of patients diagnosed with primary HCC and bone metastasis synchronously and metachronously were nearly the same-5 months and 4.5 months, respectively (ranging from 2 to 38 months, and 1 to 21 months, respectively). We evaluated the influence of prognostic factors by use of the log-rank test. By univariate analysis, the presence of metastasis to other solid organs was the only significant prognostic factor. The 1-year survival rate of those patients that had another solid organ metastasis at the time of the diagnosis of a bone metastasis was 0%, with a median survival period of 3 months, but the 1-year survival rate of patients that had only a bone metastasis was 14.5% with a median survival period of 8 months (p=0.03). However, age, sex, performance status, primary disease status and multiplicity of bony metastasis did not have an impact on patient prognosis.
DISCUSSION
A bone metastasis from a primary HCC is not common, with the incidence ranging from 3% to 20% (4~7). Therefore, there are few studies concerning the role of radiation therapy for bone metastasis from HCC.
As mentioned above, the most impressive characteristic of a bone metastasis from HCC was soft tissue formation that lyses the bone framework. Many established studies have been reported with a small number of cases. A retrospective study like the current study is rare.
Fukutomi et al(7) reported the characteristics of bone metastasis from HCC. In that study, 24 cases comprising a total of 29 lesions (83%) examined with MRI showed osteolysis by expansile soft tissue formation. In another investigation, Sato et al. (11) found 5 osteolytic lesions by soft tissue formation on ultrasonography. With a radiation dose of 30 Gy, there was more than a 50% reduction of gross tumor volume in all cases. In our study, all 41 lesions evaluated with a CT/MRI scan revealed expansile soft tissue formation. In addition, another five lesions presented with a palpable mass at the site of the metastasis.
Seong et al(9) investigated the effectiveness of palliative radiation therapy for bone metastasis from HCC. In that study, 51 patients received radiation therapy for 77 bony metastatic lesions, with a median total dose of 30 Gy. There was pain relief in 56 lesions (73%). The median survival period from the occurrence of a bone metastasis was 5 months, with a 1-year survival rate of 15%.
Kaizu et al(10) reported the influence of the dose-response relationship on palliation. Patients that received radiation with time, dose and a fractionation factor (TDF) in excess of 77 (i.e., a daily dose of 2 Gy for a total of 48 Gy, or a daily dose of 3 Gy for a total of 39 Gy) showed better palliative responses than patients that received radiation with a TDF less than 77. The response rate was 83.8%, and the median survival period from the time of the initial occurrence of a bone metastasis was 6 months.
In our study, bone destruction with soft tissue formation presented in 85.4% of all cases that were examined by CT or MRI. These bone destructive lesions tended to infiltrate the surrounding muscle or fat lesions. A bone scan, which indicated abnormal findings within the bony structure, did not reveal the entire extent of the mass lesion in many cases. Determination of the radiation field based on a bone scan only could not cover the entire mass lesion adequately. This study showed that approximately three-quarters of all cases need an adjustment of the radiation field based on the findings of a CT/MRI scan as compared with cases that were based on a bone scan only. The median survival period from the occurrence of a bone metastasis in our study was 5 months, which was comparable with those of published results.
CONCLUSIONS
This study confirmed the characteristic finding of soft tissue formation at a bone metastasis from a primary heapatocellular carcinoma. There is a high possibility that a bone metastasis from a primary hepatocellular carcinoma is expressed as a large soft tissue mass lesion. The radiation field based on only a bone scan has a risk of a marginal miss. From this review, we suggest to obtain a CT scan or MRI scan of a suspected bone metastasis to define the radiation field adequately.
References
- 1.Park JW, Kim CM. Epidemiology of hepatocellular carcinoma in Korea. Korean J Hepatol. 2004;11:303–310. [PubMed] [Google Scholar]
- 2.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation, versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 3.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment: study of 850 patients. Cancer. 1985;56:918–928. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.The Liver Cancer Study Group of Japan. Primary liver cancer in Japan: Sixth report. Cancer. 1987;60:1400–1411. doi: 10.1002/1097-0142(19870915)60:6<1400::aid-cncr2820600639>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki N, Yoshino M, Yoshida T, Hirohashi S, Kishi K, Shimosato Y. Bone metastasis in hepatocellular carcinoma. Cancer. 1985;55:1991–1994. doi: 10.1002/1097-0142(19850501)55:9<1991::aid-cncr2820550927>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.Lee YT, Geer DA. Primary liver cancer: pattern of metastasis. J Surg Oncol. 1987;36:26–31. doi: 10.1002/jso.2930360107. [DOI] [PubMed] [Google Scholar]
- 7.Fukutomi M, Yokota M, Chuman H, Harada H, Zaitsu Y, Funakoshi A, et al. Increased incidence of bone metastasis in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2001;13:1083–1088. doi: 10.1097/00042737-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Johnson PJ. Is there a role for systemic therapy in hepatocellular carcinoma, and if so, can we assess response? Vol. 38. Am Soc Clin Oncol; 2002. pp. 310–315. Ed Book. [Google Scholar]
- 9.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastasis from hepatocellular carcinoma. Liver Int. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaizu YT, Karasawa K, Tanaka Y, Matuda T, Kurosaki H, Tanaka S, et al. Radiotherapy for osseous metastasis from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998;93:2167–2171. doi: 10.1111/j.1572-0241.1998.00614.x. [DOI] [PubMed] [Google Scholar]
- 11.Sato M, Ishida H, Konno K, Hamashima Y, Naganuma H, Komatsuda T, et al. Peripheral bone metastasis from hepatocellular carcinoma: sonographic findings. Abdom Imaging. 2001;26:524–528. doi: 10.1007/s002610000198. [DOI] [PubMed] [Google Scholar]
- 12.Goilimbu C, Firooznia H, Rafii M. Hepatocellular carcinoma with skeletal metastasis. Radiology. 1985;154:617–618. doi: 10.1148/radiology.154.3.2982174. [DOI] [PubMed] [Google Scholar]
- 13.Kuhlman JE, Fishman EK, Leichner PK, Magid D, Order SE, Siegelman SS. Skeletal metastasis from hepatoma: frequency, distribution and radiographic features. Radiology. 1986;160:175–178. doi: 10.1148/radiology.160.1.3012630. [DOI] [PubMed] [Google Scholar]