Abstract
AIM: To evaluate the ability of Curcuma longa (CL) and Tinospora cordifolia (TC) formulation to prevent anti-tuberculosis (TB) treatment (ATT) induced hepatotoxicity.
METHODS: Patients with active TB diagnosis were randomized to a drug control group and a trial group on drugs plus an herbal formulation. Isoniazid, rifampicin, pyrazinamide and ethambutol for first 2 mo followed by continuation phase therapy excluding Pyrazinamide for 4 mo comprised the anti-tuberculous treatment. Curcumin enriched (25%) CL and a hydro-ethanolic extract enriched (50%) TC 1 g each divided in two doses comprised the herbal adjuvant. Hemogram, bilirubin and liver enzymes were tested initially and monthly till the end of study to evaluate the result.
RESULTS: Incidence and severity of hepatotoxicity was significantly lower in trial group (incidence: 27/192 vs 2/316, P < 0.0001). Mean aspartate transaminase (AST) (195.93 ± 108.74 vs 85 ± 4.24, P < 0.0001), alanine transaminase (ALT) (75.74 ± 26.54 vs 41 ± 1.41, P < 0.0001) and serum bilirubin (5.4 ± 3.38 vs 1.5 ± 0.42, P < 0.0001). A lesser sputum positivity ratio at the end of 4 wk (10/67 vs 4/137, P = 0.0068) and decreased incidence of poorly resolved parenchymal lesion at the end of the treatment (9/152 vs 2/278, P = 0.0037) was observed. Improved patient compliance was indicated by nil drop-out in trial vs 10/192 in control group (P < 0.0001).
CONCLUSION: The herbal formulation prevented hepatotoxicity significantly and improved the disease outcome as well as patient compliance without any toxicity or side effects.
Keywords: Hepatoprotection, Anti-tuberculous treatment, Curcumin longa, Tinospora cordifolia
INTRODUCTION
During the first few years of introduction of Isoniazid (INH), it was considered so safe that in 1963, The American Thoracic Society recommended for all tuberculin-positive persons to receive a year of INH chemoprophylaxis regardless of age or duration of the tuberculin positivity[1]. A retrospective study reported a 1% incidence of clinical hepatitis and a 0.1 % death in patients taking INH chemoprophylaxis[2]. A large multicenter prospective surveillance study by United States Public Health Service revealed a 1% incidence of hepatitis and 0.06% deaths from hepatitis due to INH[3]. After introduction of Rifampicin (RMP), several reports suggested that hepatitis was more frequent and severe in patients receiving both INH and RMP than in those receiving INH alone[4]. For preventing acquired resistance and a successful treatment; it is recommended to start with a combination chemotherapy containing INH, RMP, and Pyrazinamide (PZA)-one more hepatotoxic agent- with or without ethambutol for the initial 2 mo followed by a continuation phase of 4-6 mo of INH + RMP[5]. It is well known that drug induced hepatotoxicity is a potentially serious adverse effect of anti-tuberculosis treatment (ATT) containing INH, RMP and PZA[6]. Preventive therapy of latent TB with 2-mo course of RMP and PZA has been associated with fatal and severe hepatotoxicity more often than does 6 mo of INH therapy or curative treatment of clinical TB[7-9]. Similar high risk was observed with PZA and ethambutol or a fluoroquinolone when given to contacts of multi-drug resistant TB patients[10]. Now integrating these observations with the fact that about one third of the world’s population has latent TB and roughly 9 million cases of active TB emerge annually resulting in 2-3 million deaths[11], exemplifies the magnitude and importance of the problem, especially when most new cases occur in the most populated nations like India and China[12]. Also, a higher risk of hepatotoxicity has been reported in Indian patients (up to 11.5%) than in their western counterpart (up to 4.3%)[4]. In a study of European patients, the incidence of ATT induced hepatotoxicity was found to be 18.2% in group having risk factors like, old age, extensive TB, malnutrition, alcoholism, HIV and chronic viral hepatitis B and C infections, as against 5.8% in group without risk factors indicating the significance of risk factors[13].
The only measure available for managing ATT induced hepatotoxicity in clinical cases is stopping the offending agents, once there is an evidence of liver damage and reintroducing the same after normalization of liver enzymes[14,15]. To reduce the incidence of hepatotoxicity in latent TB patients, recommendations for drugs and patient selection criteria have been revised several times by organizations like Center for Disease Control, American Thoracic Society, Joint Tuberculosis Committee of British Thoracic Society, and Hong Kong Tuberculosis Service etc., but until today no drug has been developed for prevention of hepatotoxicity.
The pathogenesis of hepatotoxicity is not entirely clear, but INH and RMP induced damage may involve oxidative stress[16], lipid peroxidation[17], choline deficiency leading to lowering of phospholipids protein synthesis with alteration in cell wall configuration[18], reduced glutathione level[19] and activation of CYP2E1[20]. It is well known that some non-toxic herbs are having opposite activities in the form of membrane stabilizing, anti-oxidative and CYP2E1 inhibitory effects[21]. A review of available literature suggests that reduction in lipid peroxide content in tissue and increase in superoxide dismutase, catalase, glutathione, glutathione-s-transferase and glutathione peroxidase activities should help to maintain liver cell integrity and control the increase in level of liver enzymes.
In a previous preclinical study we found Curcuma longa (CL) and Tinospora cordifolia (TC) to offer protection in guinea pig model of ATT induced hepatotoxicity[22]. Both these herbs have an excellent safe toxicological profile[23,24]. Phase-I clinical trials on curcumin showed that it is safe to humans up to 12 000 mg/d when taken orally[25,26]. Several animal experiments for various activities of TC did not reveal any toxicity at dosage as high as 400 mg/kg while no adverse reactions have been noted in international adverse reaction database in spite of several clinical trials and wide spread usage in Ayurvedic system of medicine[27]. So it was logical and ethical to conduct a randomized controlled clinical trial to evaluate the efficacy of CL and TC to control the hepatotoxic episodes in patients diagnosed to have TB and undergoing ATT. The primary aim of the present study was to estimate to what extent CL and TC addition in the standard regime affects hepatotoxicity profile and the secondary being whether the well known immunomodulatory and tonic activity of these herbs have any impact on the outcome of TB itself. To the best of our knowledge, such a clinical trial using hepatoprotective herbs as an adjuvant medicine to prevent ATT induced hepatotoxicity has not been performed anywhere.
MATERIALS AND METHODS
Study population
All the cases between the ages of 15 to 85 years having evidence of pulmonary or extra-pulmonary TB requiring a full curative course of ATT and coming to the clinic run by Shree Gurudev Sarvajanik Charitable Trust (SGSCT) from April 2005 to March 2007 were screened for enrollment in the trial with reservations as shown in Table 1. As the six centers of the mobile clinic were attending patients on a weekly basis for any illness, catering more than 200 villages of the District Surat and adjoining state Maharashtra, the local primary care agencies were advised and allowed to give routine treatment for inter-current illnesses and patients were advised to inform about the episodes and treatment given.
Table 1.
Protocol for selection of patients for recruitment
| Protocol for selection of patients for recruitment |
| Criteria for selection |
| Evidence of active pulmonary or extra-pulmonary tuberculosis |
| Age between 15 and 85 years |
| Readiness to comply with randomization, treatment and follow-up protocols |
| Patients giving written informed consent |
| Absence of diseases warranting treatment with systemic steroids, antimetabolites or warfarin |
| Concomitant conditions allowed |
| Patients with relapse, multiple relapse, treatment failure of DOTS regimen |
| Patients with hypertension, diabetes mellitus, COPD (chronic obstructive pulmonary disease) with continuation of required medications |
| Patients with asthma on inhalational steroid |
| Skin conditions requiring topical small area steroid application |
| Patients with HIV positivity but without symptoms suggestive of AIDS |
| Hepatitis B/C virus carrier |
| Criteria for rejection |
| Presumptive diagnosis or lack of evidence of active tuberculosis |
| Age < 15 or > 85 |
| Patients taking other alternative therapies for tuberculosis |
| Patients declining to give consent or comply with protocol |
| Pregnant females |
| Heavy alcoholism history, > 80 g of alcohol/d for males and > 20 g of alcohol/d for females[50] |
| Concomitant conditions not allowed |
| Preexisting liver disease with AST, ALT raised > twice upper normal, evidence of portal hypertension. |
| Preexisting renal disease with S. Creatinine raised > twice upper normal |
| Sickle cell disease with history of crisis, anemia and jaundice |
| History of gout |
| Recent drop-outs from other TB centers due to complications and side effects |
| Patients on steroid and/or antimetabolite for other collagen, auto-immune or neoplastic diseases |
As shown in Figure 1, out of 578 patients screened 528 were found eligible. Periodic review was done by an independent review committee at 48, 100, 148 and 200 patients’ recruitment in each group. The interim analysis of first 400 patients performed in October, 2006 revealed zero incidence of hepatotoxicity in the trial as against 22 patients with any grade of hepatotoxicity and 16 patients with no improvement in functional status in control group. The trial was granted with an intention to treat and as the criteria for outcome assessment were objective and as the sample size exceeded the WHO criteria for adequacy of sample size with power > 80%, the control arm was truncated on ethical ground and later all the patients were recruited in trial arm till March, 2007; only to find out whether any case of hepatotoxicity was encountered.
Figure 1.
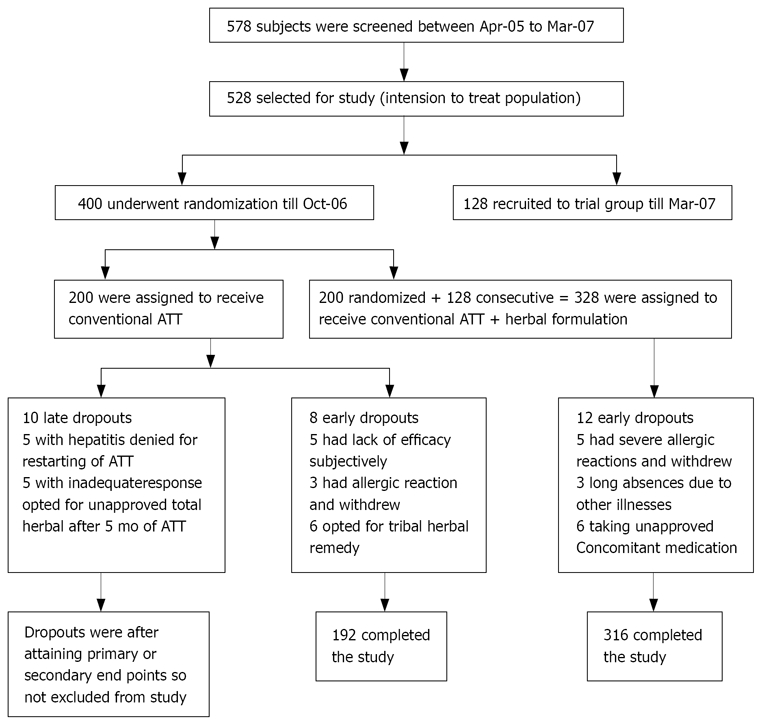
Study design and enrollment and follow-up of patients.
During their baseline visit, patients underwent a review of symptoms that included history of nausea, vomiting, jaundice, abdominal pain, weight loss, arthralgia, headache and neuropathy.
Herbal formulation
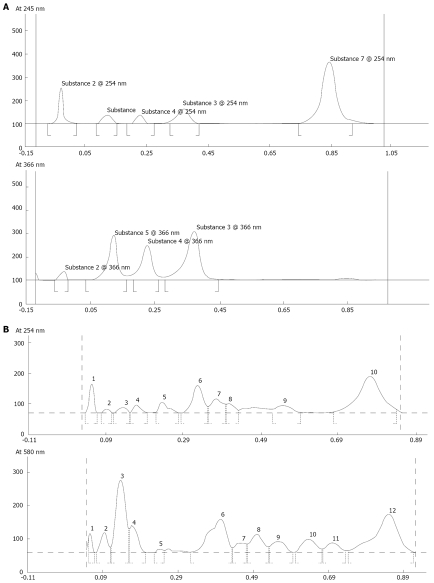
By high performance thin layer chromatography (HPTLC) analysis of different samples, we found that Salem types of turmeric powder contained highest 4% of curcuminoids that are the main active molecules. In Ayurvedic literature, the dose for turmeric has been described to be 6-12 g/d[28]. Our patient population being malnourished with an average wt of 38 kg, we preferred equivalent of 6 g/d by enriching the turmeric powder curcumin content to 25% so that 1 g dose would suffice. The dose of Tinospora cordifolia is also described to be 6-12 g/d[29] and again we preferred to make 1 g/d equivalent of 6 g/d by enriching the crude Tinospora cordifolia with 10:1 strength hydro-ethanolic extract to 50%. The phytochemical groups with active principles of both the herbs are shown in Table 2[30-32] and HPTLC fingerprinting of the formulations used were done for standardization purpose and have been shown in Figure 2.
Table 2.
Chemical constituents of formulation
| Chemical constituents of formulation with active principles | |
| Chemical constituents of curcuma longa with active principles | |
| Curcuminoids | Desmethoxycurcumin |
| Bisdesmethoxycurcumin | |
| Curcumin | |
| Turmerones | Ar-turmerone |
| α-turmerone | |
| β-turmerone | |
| Curcumenes | γ-Curcumene, ar-Curcumene, Dehydrocurcumene |
| zingiberene | |
| β-bisabolene | |
| β-sesquiphellandrene | |
| Miscellaneous | Terpinolene, P-Cymene, 1-8-Cineole, Curlone |
| Chemical constituents of Tinospora cordifolia with active principles | |
| Alkaloids | Berberine, Palmatine,Tembetarine, |
| Magnoflorine, Choline,Tinosporin, | |
| Isocolumbin, Tetrahydropalmatine | |
| Glycosides | 18-norclerodane glycoside, |
| Furanoid diterpene glucoside, | |
| Tinocordiside,Tinocordifolioside, | |
| Syringin, Syringin-apiosylglycoside, | |
| Palmatosides C, PalmatosidesP | |
| Cordifolioside A, Cordifolioside B | |
| Cordifoliside- A, -B, -C, -D and -E | |
| Diterpenoid Lactones | Furanolactone, Clerodane derivatives, |
| Tinosporon, Tinosporides, Jateorine, | |
| Columbin | |
| Steroids | β-sitosterol, δ-sitosterol, 20β-hydroxyecdysone |
| Ecdysterone, Makisterone A, Giloinsterol. | |
| Sesquiterpenoid | Tinocordifolin |
| Aliphatic compounds | Octacosanol, Heptacosanol, Nonacosan-15-one |
| Miscellaneous compounds | 3, (α, 4-dihydroxy-3-methoxy-benzyl)-4-(4- hydroxy-3-methoxy-benzyl)- tetrahydrofuran, |
| Jatrorrhizine, Tinosporidine, cordifol, cordifelone | |
| N-trans-feruloyl tyramine as diacetate, Giloin, Giloinin | |
| Tinosporic acid | |
Figure 2.
A: HPTLC finger print of formulation of Curcuma longa. Sample was extracted in MeOH and Solvent system for Mobile phase used was Chloroform: MeOH (99.5: 0.5). Note: Before loading the material on TLC plate, it was impregnated with di NaHPO4 (anhydrous)-1.25 g dissolved in 25 mL double distilled water (DDW). B: HPTLC finger print of formulation of Tinospora cordifolia. Sample was extracted in-MeOH and NH4OH: MeOH (9:1) and solvent system for mobile phase used was -Chloroform: MeOH (8:2).
Study design
Ninety percent of all the patients were tribal, either working as laborers or owner of small piece of agricultural land, while 10% were belonging to suburban area and belonging to other casts and occupations. Hence patients were explained in their local language about the protocol and those agreeing to comply were assigned the group by permuted block randomization for a balanced distribution. Because the evaluation criteria were fully objective and as the biochemical assessments were done in laboratories at a distance by unknown technicians, patients were not blinded conventionally about the therapy. Being a joint project it was approved by both the institutional ethical committee and informed consents were obtained from all the patients.
All of the eligible patients in the control group were started with an intensive phase ATT comprising of INH 300 mg/d, RMP 450 mg/d and PZA 20 mg/kg body wt to the closest 250 mg in two divided doses and Ethambutol 800 mg HS for initial 2 mo and than three drugs excluding PZA for 4 mo. Similar ATT medications supplemented with curcumin enriched (25%) CL and a hydro-ethanolic extract enriched (50%) TC 1 g/d each in two divided doses started from day one in trial group. Other supplemental medications like vitamins, iron, H2 blockers, proton pump inhibitors, anti-emetics, gut motility enhancers and calcium were also being given on an individual basis to patients in any group as deemed necessary by the physician. Two weeks quota of medications was given for self administration and patients were advised to return to the clinic every two weeks or earlier if any troublesome symptoms mentioned earlier were experienced. Blood sample for liver enzymes and bilirubin were taken every alternate visit and a prefilled requisition form was given with the instruction to get it done at nearby center having pathology laboratory facility if patient could not come at time of appearance of symptoms. At each visit the patients were evaluated for signs and symptoms of adverse events by trained staff and adherence to treatment schedule was confirmed for both the groups. Sputum examination was repeated at fourth week in all sputum AFB positive cases.
Hepatotoxicity gradation was based on the WHO classification and defined by liver enzyme level, however, aspartate transaminase (AST) level was preferred instead of alanine transaminase (ALT) level as it is well known that AST/ALT ratio > 2 is one important criteria for differential diagnosis between drug or chemical and viral hepatitis[33]. Grade I for any AST level of 51-125 U/L; grade II for any AST level of 126-250 U/L; Grade III for AST level of 251-500 U/L and grade IV for any level greater than 500 U/L or more than 250 U/L with symptoms of fulminant hepatitis. In case of grade I or II liver injury ATT was continued for 2 wk and enzymes were repeated. In grade III and IV hepatitis ATT was stopped till enzyme level came to normal. AST was checked weekly, once its rise beyond 2 fold was confirmed after initial 2 wk follow-up till it came to normal level. Patients were called for monthly follow-up visits after completion of scheduled treatment for 3 mo in both the groups.
Outcomes
Primary endpoints were to observe the development of hepatotoxicity in both the groups with assessment of severity by biochemical parameters and liver function tests if the hepatotoxicity exceeded Grade III parameters. Secondary outcome was to assess the impact of adjuvant medicine on the outcome of TB itself as defined by follow-up investigations, clinical cure and functional improvement[34] by the end of scheduled ATT and follow-up period. Completion of the regimen was defined as taking > 80% of scheduled medication for both the groups. Occasional breaks of one or two weeks due to some tribal festivals or an episodes of minor illness were not considered to be a break in the treatment.
Statistical analysis
A sample of approximately 500 patients would provide 99% power in two sided tests with an α value of 0.05 to detect a decrease in expected incidence of hepatotoxicity of any grade from 15% in control to < 3% in trial group.
Nominal and ordinal data in patient characteristics were described with percentage, median and inter quartile range (IQR) between 25% and 75%, while measurement data by mean ± SD. Significance for each characteristic was tested to ensure basic homogeneity of both the groups. Normality test was performed as the patients were representative of a tribal ethnicity and belonged to a disease that may have specific propensity for a subgroup, so the distribution may not be Gaussian despite a large sample. Those parameters failing the normality test were further analyzed by Wilcoxon’s rank sum test and those passing it were tested by unpaired T test with Welch’s modification where variance was unequal. Student’s T test and χ2 test with Yate’s correction were done as needed. P ≤ 0.05 was considered significant. Number needed to treat (NNT) was decided for the adjuvant as test therapy. Finally more common outcomes including completion of treatment and response to treatment were analyzed by Fisher Exact test. Analyses were done by using KyPlot Version 2 beta 15, GraphPad InStat Version 3.06 and GraphPad StatMate 2 version 2.00.
RESULTS
Clinical and demographic characteristics at baseline
As shown in Figure 1, 528 patients were enrolled at six centers of SGSCT mobile clinic. A high proportion of patients were of tribal ethnicity from Chaudhari, Gamit, Vasava and Bhil community known to carry sickle cell trait in up to 30% of population[35] and disease in 1.5%[36], as reported by different governmental agencies. Eight patients from control and 12 patients from trial group were excluded from study due to violation of the protocol, hence control had 192 and trial had 316 patients for final analysis. As seen in Table 3 the median age and IQR for both the groups were quite similar. Also the two groups were similar in sex, body weight, habits, disease profile, baseline hematologic means and liver function except that trial group contained significantly higher number of patients with Cavitary Koch’s than the control group.
Table 3.
Baseline demographic and disease characteristics in the intention-to-treat population
| Characteristics of patients | Control | Trial | P |
| Sex | |||
| Male (%) | 104 (54) | 169 (53) | 0.934 |
| Female (%) | 88 (46) | 147 (47) | 0.927 |
| Age-yr median(IQR 25th & 5th) | 35 (23.75-45) | 35 (27-45) | 0.229 |
| Weight (kg, mean ± SD) | 37.61 ± 5.94 | 38.32 ± 6.08 | 0.199 |
| Ethnicity (%)1 | |||
| Tribals | 171(89) | 287(91) | 0.883 |
| Others | 21(11) | 29(9) | 0.559 |
| Habits (%) | |||
| Smoking | 58 (30.2) | 103 (32.6) | 0.685 |
| Alcohol | 23 (12) | 33 (10.4) | 0.633 |
| Both | 26 (13.5) | 53 (16.7) | 0.403 |
| Socio-Economic status (%) | |||
| Upper-Middle | 20 (10.4) | 40 (12.6) | 0.499 |
| Middle | 30 (15.6) | 52 (16.4) | 0.833 |
| Lower | 142 (74) | 224 (71) | 0.763 |
| Pulmonary (%)2 | |||
| Open cases (sputum +ve) | 67 (35) | 137 (43.4) | 0.214 |
| Parenchymal | 152 (79.2) | 258 (81.6) | 0.822 |
| Cavitary | 18 (9.4) | 66 (21) | 0.003 |
| Effusion | 9 (4.6) | 20 (6.3) | 0.464 |
| Fibrosis | 27 (14) | 68 (21.5) | 0.081 |
| Extra pulmonary (%)2 | |||
| Cervical lymph node | 18 (9.4) | 31 (9.8) | 0.883 |
| Abdominal | 4 (2.1) | 6 (2) | 0.886 |
| Laryngeal | 7 (3.6) | 11 (3.5) | 0.925 |
| Central nervous system | 6 (3.1) | 6 (2) | 0.389 |
| Bones/Joints | 10 (5.2) | 19 (6) | 0.72 |
| Skin | 0 | 3 (1) | 0.598 |
| Genito-Urinary | 2 (1.04) | 1 (0.3) | 0.304 |
| Type of case (%) | |||
| New | 140 (73) | 195 (61.7) | 0.245 |
| Relapse | 43 (22.4) | 97 (30.7) | 0.122 |
| Chronic | 9 (4.7) | 24 (7.6) | 0.225 |
| Hematology | |||
| Hb (%) | 8.84 ± 1.93 | 8.95 ± 1.95 | 0.808 |
| Total leucocyte count/mm3 | 10 179 ± 4 680 | 9575 ± 4075 | 0.478 |
| ESR | 66.79 ± 30.64 | 70.49 ± 27.91 | 0.517 |
| Liver Function (mean, 95%CI) | |||
| Serum bilirubin | 1.07 (0.7-1.3) | 1.09 (0.8-1.4) | 0.952 |
| AST | 34.35 (17-63) | 32.4 (11-61) | 0.658 |
| ALT | 27.45 (9-57) | 31.15 (6-52) | 0.655 |
| ALP | 100 (50-260) | 107 (50-260) | 0.092 |
Unless stated otherwise, P values were calculated with the use of a two sided t-test with Welch’s modification (when unequal variance) for continuous data and a χ2 test for categorical data.
Ethnic group was assessed by investigation on screening. Tribal consists of Chaudhari, Gamit, Vasava, Bhil, Valvi, Nayka and Halpati.
Patients may belong to more than one category. ALP: Alkaline phosphatase.
Hepatotoxicity
Tables 4 and 5 depict the effects of herbal adjuvant on primary outcome in the form of significantly decreased incidence of hepatotoxicity (2/316 vs 27/192) in the trial group as compared to control group. Mean onset of asymptomatic hepatitis was delayed in trial group and none in the trial group suffered from symptomatic hepatitis as against 63% of control group hepatitis patients. Grades II and III hepatotoxicity were observed in control group only. Serum AST, ALT and bilirubin level were raised in control group much significantly when compared with trial group hepatotoxicity cases.
Table 4.
Effects of herbal formulation on incidence, onset, duration and severity of hepatitis
| ATT induced hepatitis outcome (Table 2) | Control (n = 192) | Trial (n = 316) | P |
| Patients with raised AST (%) | 27 (14) | 2 (0.63) | < 0.0001 |
| Patients with raised S bilirubin (%) | 17/27 (63) | 0 | < 0.0001 |
| Mean onset of hepatitis symptomatic | 49.12 ± 17.22 | - | < 0.0001 |
| Mean onset of hepatitis asymptomatic | 29.9 ± 17.63 | 39 ± 11.31 | 0.443 |
| Mean onset of any hepatitis | 42 ± 19.48 | 39 ± 11.31 | 0.776 |
| Mean duration of raised AST | 27.5 ± 7.25 | 21 | < 0.0001 |
| Hepatitis gradation | |||
| Grade-I | 10 | 2 | < 0.0001 |
| Grade-II | 9 | 0 | < 0.0001 |
| Grade-III | 8 | 0 | < 0.0001 |
| Grade-IV | 0 | 0 | |
| Reinstitution of ATT in patients (%) | 22/27 (81.5) | NA |
P calculated by χ2 (categorical data) and two-sided Student’s t-test with Welch’s procedure (continuous data with unequal variance). NA: Not applicable.
Table 5.
Comparison of liver enzymes and bilirubin in patients developing hepatitis
| Control (n = 27) | Trial (n = 2) | P | |
| AST | 195.93 ± 108.74 | 85 ± 4.24 | < 0.0001 |
| ALT | 75.74 ± 26.54 | 41 ± 1.41 | < 0.0001 |
| AST/ALT | 2.35 ± 0.74 | 2.08 ± 0.18 | 0.207 |
| ALP | 241.74 ± 140.96 | 147.5 ± 45.96 | 0.118 |
| Serum bilirubin | 5.4 ± 3.4 | 1.5 ± 0.42 | < 0.0001 |
Two-sided Student’s t-test with Welch’s procedure for continuous data with unequal variance. ALP: Alkaline phosphatase.
Disease outcome
As shown in Table 6, the number of sputum-positive cases after 1 mo of treatment in the trial group (2.9%) was significantly lower than control group (14.93%). Incidence of poorly resolved parenchymal lesion by roentgenogram and failure to improve functional status by ECOG score[34] being significantly lower and treatment dropouts nil in the trial group proved the efficacy of herbal adjuvant formulation.
Table 6.
Effects of herbal formulation addition on disease outcome
| Treatment outcome for ATT | Control | Trial | P |
| Number of sputum +ve case at start (%) | 67/192 (35) | 137/316 (43) | 0.03 |
| Number of sputum +ve case after 1-month of treatment (%) | 10/67 (14.93) | 4/137 (2.9) | 0.0068 |
| Poorly resolved parenchymal lesion (%) | 9/152 (5.92) | 2/258 (0.78) | 0.0037 |
| Failure to improve functional status (%) | 19/192 (10) | 6/316 (2) | 0.00013 |
| Treatment dropouts (%) | 10/192 (5.2) | 0 | 0.0001 |
| Weight (kg) | 39.17 ± 5.5 | 40.65 ± 6.22 | 0.0016 |
| Hb (%) | 10.92 ± 2.01 | 11.17 ± 1.97 | 0.176 |
| ESR mm in 1st hour | 45.96 ± 18.52 | 38.84 ± 22.37 | 0.0001 |
P calculated by χ2 (categorical data) and two-sided Student’s t-test with Welch’s procedure (continuous data with unequal variance).
Weight and hematologic outcome
As seen in Table 3 and Table 6, the mean weight in control increased to 39.17 ± 5.5 kg from 37.61 ± 5.94 kg before ATT (P = 0.0063) and in trial group mean weight was 40.65 ± 6.22 kg from 38.32 ± 6.08 kg before ATT and herb treatment (P < 0.0001). While comparing the weight gain, trial group showed significantly higher weight gain than control group. Mean Hb of control and trial groups increased significantly after therapy; however, the inter group difference was nonsignificant. Erythrocyte sedimentation rate (ESR) in both groups decreased significantly compared from start to end of the treatment period; however, inter group comparison that was nonsignificant at the start showed a significantly more decrease in trial group at completion.
Non-hepatotoxic adverse events (data not shown)
Non-hepatotoxic adverse events occurred occasionally in both the groups and included nausea, vomiting, skin rash, epigastric pain and discomfort, malaise, dizziness, arthralgia, peripheral neuropathy, anorexia and insomnia and sickle crisis. They were treated symptomatically with appropriate medications by the physician. Early dropouts occurred in both groups due to allergic reactions to ATT and they were excluded from the analysis as shown in Figure 1.
DISCUSSION
The present clinical trial was based on unpublished results of previous preclinical studies at our center. As the conventional ATT has been well established and the Ayurvedic herbs have excellent safety profile as described previously, a phase I trial omission was granted. As the efficacy of the herbs was shown in concurrent administration design in an animal model of ATT induced hepatotoxicity[22], it was considered scientific, as well as ethical, to try a similar model for a human clinical trial, hence herb treatment was started along with ATT at the same time and continued until completion of ATT.
Curcumin has anti-inflammatory, free radical scavenging and hepatoprotective activities tested in vitro. It suppresses production of superoxide by macrophages, exerts potent anti-inflammatory action that inhibits production of tumor necrosis factor alpha (TNF-α), interleukin (IL) 1-β and the activation of NF-κB in human monocytic derived cells[37,38]. Also, it has a strong antioxidant property and it inhibits lipid peroxidation in rat liver microsomes, erythrocyte membranes and brain homogenates, by maintaining the activity of SOD, catalase and glutathione peroxidase at a higher level[39]. These properties clearly explain the hepatoprotective activity of CL in preclinical studies and present trial as well. Curcumin has antiviral, antiprotozoal, antibacterial, anti inflammatory, and antioxidant actions, in addition to its hepatoprotective activity, making it a suitable adjuvant agent that actually enhanced efficacy of ATT in present study.
In preclinical studies, TC has been shown to induce enzymes of drug metabolism and antioxidant system and to inhibit lipid peroxidation in mice. Cytochrome P450, NADP-Cytochrome (P sub 450) reductase, glutathione-s-transferase, DT diaphorase, SOD and catalase are enhanced. These effects improve liver function, protect against toxic assaults and increase protein synthesis by liver[40]. These activities can account for its hepatoprotective potential. It is a known immunostimulant enhancing cell mediated as well as humoral immune response in mice. Treatment of mice with dried stem crude extract prevented cyclophosphamide induced myelosuppression as well as immunosuppression[41]. Improved humoral immunity, enhanced macrophage and Kupffer cell function, enhanced neutrophil function, antioxidant activity and excellent hepatoprotective potential might explain the result of present clinical trial. Since many years the concept of classical phytotherapy using herbal drug combinations with superior efficacy and lesser side effects in comparison with single isolated constituents of plant extracts has been repeatedly assessed clinically as well as pharmacologically. The exact mechanisms of action underlying these synergy effects are unknown. It could be explained by a multitarget action of compounds on a molecular level or partly by an improved resorption rate and a change of pharmacokinetics[42]. Hence, a formulation of CL and TC was used with an idea to enhance the potency due to synergistic action that was backed by promising results of a small preclinical pilot study (unpublished). As such, many standard Ayurvedic preparations also contain both of these herbs, albeit in a lower dose along with other multiple herbs.
Regarding the design of the trial, the strength lied in the base line characteristics which were remarkable similar and all the parameters for hepatotoxicity were objective and being tested by laboratory technicians at different places and totally unaware about the trial or outcome, so there could not be any chance for a bias in the detection and gradation of hepatotoxicity. In many complex clinical situations adaptive random allocation methods are implemented to maintain balance between stratification variables[43]; however, periodic evaluation revealed acceptably similar distribution among groups even with permuted block randomization. When one treatment or an intervention is expected to be superior; a Bayesian adaptive randomization (BAR) method is considered desirable to conventional randomization because it utilizes the accruing data in a “learn-as-you-go” fashion that arguably makes more sense than ignoring the trial’s data until it is completed[44]. BAR would allocate more numbers of patients to the potentially advantageous group without compromising the randomization, but making the trial design very complicated. Instead the control arm was truncated for ethical reasons with caution at periodic evaluations so that no dissimilarity in favor of the trial group should occur.
Though sickle cell disease patients were excluded from the study, persons having sickle cell traits are prone to have anemia, repeated chest infection and theoretically sickle cell crisis in extremes of situations with dehydration and acidosis. In the present trial two events of sickle cell crisis occurred in the control group. They might have phenotypic sickle cell disease[45].
The average weight of the study population was around 38 kg in both the groups confirming malnutrition in addition to effect of TB alone. The significance of this characteristic could not be overemphasized because a low weight would culminate in to a higher per kg dosage of the hepatotoxic ATT. Considering the average weight to be 38 kg, the per kg dose of INH, RFM, PZA and EMB would come to 7.9, 11.84, 26.31 and 21 mg, respectively. Comparing this dose with a previous study[46] in which the per kg doses for INH, RFM and PZA were 5, 10 and 25-30 mg, respectively, and the period for observation was 59 d with reported hepatotoxicity to be 11%; it is apparent that the chances of developing hepatotoxicity would be definitely more for the patients in present study. In addition to malnutrition as a known risk factor, a higher per kg dose could also play some role.
With all these background, the results of present trial carried utmost importance that hepatotoxicity did not occur in the trial group despite a slightly unfavorable profile in the form of significantly higher number of patients with Cavitary pulmonary TB. Only two instances of raised AST were detected during monthly surveillance of liver enzymes and ATT was not stopped as it was asymptomatic grade I hepatotoxicity. Extensive disease, relapse cases, multiple relapse cases, miliary TB cases and cases with malnutrition, HIV carrier, and hepatitis B and C carrier, sickle cell trait, other back ground illnesses like COPD, hyper-eosinophilic syndrome, MB leprosy, diabetes mellitus, asthma, rheumatoid arthritis were all recruited in the control as well as the trial group and yet no incidence of clinically significant hepatotoxicity occurred in trial group, suggesting that regular administration of adjuvant herbs had taken care of all the known, hypothesized and unknown risk factors predisposing to ATT induced hepatotoxicity in clinical cases of TB.
Though with a power of 99% and highly significant out-come, this was a pilot study and much has remained to be done to exploit the full potential of the hepatoprotective ability of these herbs in cost-effective manner with defined recommendation for different subclasses of patients including latent TB cases and different high risk groups of clinical cases. A separate study with a low risk control, a high risk control and a high risk trial would serve both the purposes of testing the efficacy of herbs in high risk group as well as the validity of the hypothesis that low risk group has lower incidence of hepatotoxicity in the same population having similar ethnicity. The answer to the question whether low risk patients should be subjected to adjuvant medication or not, could also be answered from the results of study with such a design.
As latent TB cases on different preventive regimes have been shown to be at a greater risk for developing hepatotoxicity[7-9], a separate well-designed trial for this class of patients could not be over-emphasized. The efficacy of these herbs may also be tested in patients showing liver enzyme increase detected by surveillance to decide whether ATT could be continued without risk of it to progress to higher grade hepatotoxicity as it is observed that sooner the detection, lesser the risk and later the appearance of hepatitis, greater the chances of fulminant failure[47].
The NNT analysis of the study with a single outcome of hepatoprotection would come to eight. This is because the herbs were given to all as an adjuvant medicine where the possibility of developing hepatitis itself was expected to be between 11% and 15%. Looking to the result on disease profile it is clear that the sputum conversion to negativity was significantly superior in herb treated group despite an unfavorable sample, resolution of parenchymal lesion was significantly higher, failure to improve functional status was significantly lower and dropout rate came to nil. If these treatment factors are added then NNT would surely come lower.
Weight and hemoglobin both increased and ESR reduced significantly on intra group pre- and post- treatment comparison in both the groups proving the efficacy of conventional ATT alone too. The inter group comparison post treatment showed a significant advantage in weight gain and reduction in ESR, but not in hemoglobin level in trial group suggesting the superiority of ATT plus adjuvant herbs over ATT alone. It is apparent that iron and vitamin supplements were being given to anemic patients as per diagnosis and role of ATT must be supportive only in rising the Hb. Tumor necrosis factor alpha (TNF) has been shown to cause hypoferremia and reduced intestinal iron absorption in mice and Curcumin inhibits TNF thereby having the potential to enhance iron absorption and help alleviate anemia[48,49]. Rise in Hb was not a direct function of both modalities and effects of curcumin on iron absorption in humans has not yet been tested; so the only valid comment would be that the theoretical advantage of CL was not observed practically in present study.
In conclusion CL and TC given as an adjuvant to standard ATT to any kind of TB patients prevented hepatotoxicity very significantly in terms of incidence, duration and severity and also helped improve outcome in terms of quicker and more efficient achievement of sputum negativity in open, potentially infectious cases; better response in parenchymal lesion resolution and helped improve patient compliance. Treated cases had better weight gain and significantly more reduction in ESR. A prospective trial for latent TB and MDR TB contacts can further exemplify its usefulness. Post-treatment design on detection of hepatotoxicity and concurrent administration design for different subgroups may clarify its efficacy in different clinical situations and provide a basis for evidence based recommendation for different sub groups of TB patients.
COMMENTS
Background
Isoniazid, rifampicin and pyrazinamide, three major drugs of intensive phase of conventional anti-tuberculosis (TB) treatment (ATT), are hepatotoxic and the incidence varies between 4%-11%. Healthy contacts of TB patients (latent TB cases) are more prone to develop hepatotoxicity by presently recommended regimes. There is no established drug therapy to cure or prevent hepatotoxicity except stopping the treatment for a while and restoring it gradually at a later date when liver enzymes come to a normal level. Stopping the therapy may increase morbidity or prolong disability, while shifting to suboptimal non-hepatotoxic regime for time being poses the risk of emergence of resistant strains.
Research frontiers
Search for non-toxic and highly effective new compounds for treating tuberculosis or an effective vaccine conferring sustained protective immunity have largely failed. In literature there are many published preclinical studies showing herbs to prevent CCl4, and paracetamol induced liver damage in rodents. Recently, four Ayurvedic herbs were shown to prevent ATT induced hepato-toxicity and immunosuppression in Guinea pigs. (http://www.wjgnet.com/1007-9327/13/3199.asp). But no clinical trial of hepatoprotective compound has ever been carried out in patients taking conventional ATT.
Innovations and breakthroughs
The present study is the first of its kind and the results are encouraging for the prevention of hepato-toxicity and improving the disease outcome by greater sputum clearance in Cavitary lesions and better resolution of parenchymal lesions. Thus, addition of two simple herbs makes the conventional ATT much safer and efficacious.
Applications
Incorporation of such a formulation in current conventional ATT at a mass level may contribute a lot to minimize the risk of liver injury, improve patient compliance and disease outcome that might ultimately help to control emergence of MDR strains of AFB. If similar protection is observed in latent tuberculosis by a prospective trial, it would be a boon for the latent TB cases subjected to a serious risk by preventive ATT.
Peer review
The manuscript is well written. This is an interesting trial. The study is nicely designed and the analytical data appear to be technically and scientifically sound. The outcome is remarkable and clearly shows beneficial effect of the herbal formulation in hepatic injury by ATT.
Acknowledgments
Dr. Manish Patel, Dr. Jyoti Patel, of Shivjyoti Hospital for helping in selection and follow-up studies of the patients. Dr. Pragna Kalarthi, Dr. Ramiben Chaudhari, Dr. Nilesh Nayak for helping at various stages of clinical trial in patient assessment and follow-up. All the peripheral centers that co-operated for bio-chemical tests, roentgenogram and hemogram on no-profit basis.
Footnotes
Supported by (in part) The Mukul Trust Bardoli, India
Peer reviewers: Anna S Gukovskaya, Professor, VA Greater Los Angeles Health Care System, University of California, Los Angeles, 11301 Wilshire Blvd, Los Angeles, CA 91301, United States; James Neuberger, Professor, Liver Unit, Queen Elizabeth Hospital, Birmingham B15 2TH, United Kingdom; Dr. Yukihiro Shimizu, Kyoto Katsura Hospital, 17 Yamada-Hirao, Nishikyo, Kyoto 615-8256, Japan; Shivendra Shukla, Professor, Department of Medical Pharmacology and Physiology, University of Missouri School of Medicine, 1 Hospital Drive, M530 Medical Sciences Bldg, Columbia, MO 65212, United States
S- Editor Zhong XY L- Editor Alpini GD E- Editor Lin YP
References
- 1.Runyon EH. Preventive Treatment in Tuberculosis: A Statement by the Committee on Therapy. American Thoracic Society. Am Rev Respir Dis. 1965;91:297–298. doi: 10.1164/arrd.1965.91.2.297. [DOI] [PubMed] [Google Scholar]
- 2.Garibaldi RA, Drusin RE, Ferebee SH, Gregg MB. Isoniazid-associated hepatitis. Report of an outbreak. Am Rev Respir Dis. 1972;106:357–365. doi: 10.1164/arrd.1972.106.3.357. [DOI] [PubMed] [Google Scholar]
- 3.Kopanoff DE, Snider DE Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 4.Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–471. doi: 10.1378/chest.99.2.465. [DOI] [PubMed] [Google Scholar]
- 5.Chan ED, Iseman MD. Current medical treatment for tuberculosis. BMJ. 2002;325:1282–1286. doi: 10.1136/bmj.325.7375.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahashur AA, Prabhudesai PP. Hepatitis and antitubercular therapy. J Assoc Physicians India. 1991;39:595–596. [PubMed] [Google Scholar]
- 7.Fatal and severe hepatitis associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection--New York and Georgia, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:289–291. [PubMed] [Google Scholar]
- 8.Update: Fatal and severe liver injuries associated with rifampin and pyrazinamide for latent tuberculosis infection, and revisions in American Thoracic Society/CDC recommendations--United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50:733–735. [PubMed] [Google Scholar]
- 9.Update: Fatal and severe liver injuries associated with rifampin and pyrazinamide treatment for latent tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2002;51:998–999. [PubMed] [Google Scholar]
- 10.Younossian AB, Rochat T, Ketterer JP, Wacker J, Janssens JP. High hepatotoxicity of pyrazinamide and ethambutol for treatment of latent tuberculosis. Eur Respir J. 2005;26:462–464. doi: 10.1183/09031936.05.00006205. [DOI] [PubMed] [Google Scholar]
- 11.Raviglione MC, Snider DE Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 12.World Health Organization. Global tuberculosis control: surveillance, planning, financing. WHO report 2002. Communicable Diseases. 2002:237. [Google Scholar]
- 13.Fernandez-Villar A, Sopena B, Fernandez-Villar J, Vazquez-Gallardo R, Ulloa F, Leiro V, Mosteiro M, Pineiro L. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2004;8:1499–1505. [PubMed] [Google Scholar]
- 14.Teleman MD, Chee CB, Earnest A, Wang YT. Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in Singapore. Int J Tuberc Lung Dis. 2002;6:699–705. [PubMed] [Google Scholar]
- 15.Wada M. [The adverse reactions of anti-tuberculosis drugs and its management] Nippon Rinsho. 1998;56:3091–3095. [PubMed] [Google Scholar]
- 16.Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC, Nain CK, Singh K. Isoniazid- and rifampicin-induced oxidative hepatic injury--protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19:517–522. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- 17.Richards VE, Chau B, White MR, McQueen CA. Hepatic gene expression and lipid homeostasis in C57BL/6 mice exposed to hydrazine or acetylhydrazine. Toxicol Sci. 2004;82:318–332. doi: 10.1093/toxsci/kfh232. [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan S. Isoniazid and rifampicin treatment on phospholipids and their subfractions in liver tissue of rabbits. Drug Chem Toxicol. 2005;28:273–280. doi: 10.1081/dct-200064463. [DOI] [PubMed] [Google Scholar]
- 19.Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006;45:117–126. doi: 10.1016/j.jhep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Yue J, Peng RX, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid induced hepatotoxicity in rats. Acta Pharmacol Sin. 2004;5:699–704. [PubMed] [Google Scholar]
- 21.Tasduq SA, Kaisar P, Gupta DK, Kapahi BK, Maheshwari HS, Jyotsna S, Johri RK. Protective effect of a 50% hydroalcoholic fruit extract of Emblica officinalis against anti-tuberculosis drugs induced liver toxicity. Phytother Res. 2005;19:193–197. doi: 10.1002/ptr.1631. [DOI] [PubMed] [Google Scholar]
- 22.Adhvaryu MR, Reddy N, Parabia MH. Effects of four Indian medicinal herbs on Isoniazid-, Rifampicin- and Pyrazinamide-induced hepatic injury and immunosuppression in guinea pigs. World J Gastroenterol. 2007;13:3199–3205. doi: 10.3748/wjg.v13.i23.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Toxicology Program. NTP Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (CAS No. 8024-37-1) (Major Component 79%-85% Curcumin, CAS No. 458-37-7) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1993;427:1–275. [PubMed] [Google Scholar]
- 24.Kapil A, Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J Ethnopharmacol. 1997;58:89–95. doi: 10.1016/s0378-8741(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 25.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 26.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]
- 27.Adverse Reactions. In: Evaluation of a new class I substance- Tinospora cordifolia (Guduchi) IJEACCM New Zealand. 2006:43. [Google Scholar]
- 28.Pade SD. [Translated book from Marathi to Gujarati] Aryabhishak, 17th ed. Sastu Sahitya Vardhak Karyalaya, Mumbai. 1983. pp. 440–441. [Google Scholar]
- 29.Pade SD. [Translated book from Marathi to Gujarati] Aryabhishak, 17th ed. Sastu Sahitya Vardhak Karyalaya, Mumbai. 1983. pp. 202–205. [Google Scholar]
- 30.Matsuo T, Toyota A, Kanamori H, Nakamura K, Katsuki S, Sekita S, Satake M. Constituents of Representative Curcuma And Estimation of Curcuma species in Health Foods. Bulletin of the Hiroshima Prefectural Institute of Public Health and Environment. 2002;10:7–13. [Google Scholar]
- 31.Leela NK, Tava A, Shafi PM, John SP, Chempakam B. Chemical composition of essential oils of turmeric ( Curcuma longa L) Acta Pharm. 2002;52:137–141. [Google Scholar]
- 32.Leela NK, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. Chemistry and medicinal properties of Tinospora cordifolia (Guduchi) Indian J Phamacol. 2003;35:83–91. [Google Scholar]
- 33.Rej R. Aminotransferases in disease. Clin Lab Med. 1989;9:667–687. [PubMed] [Google Scholar]
- 34.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 35.Ahmed SK. A word of advice for those with sickle cell anaemia. 2008;5 [Google Scholar]
- 36.State govt constitutes group to study sickle cell disease. 1998 [Google Scholar]
- 37.Song EK, Cho H, Kim JS, Kim NY, An NH, Kim JA, Lee SH, Kim YC. Diarylheptanoids with free radical scavenging and hepatoprotective activity in vitro from Curcuma longa. Planta Med. 2001;67:876–877. doi: 10.1055/s-2001-18860. [DOI] [PubMed] [Google Scholar]
- 38.Lukita-Atmadja W, Ito Y, Baker GL, McCuskey RS. Effect of curcuminoids as anti-inflammatory agents on the hepatic microvascular response to endotoxin. Shock. 2002;17:399–403. doi: 10.1097/00024382-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Reddy AC, Lokesh BR. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol Cell Biochem. 1992;111:117–124. doi: 10.1007/BF00229582. [DOI] [PubMed] [Google Scholar]
- 40.Singh RP, Banerjee S, Kumar PV, Raveesha KA, Rao AR. Tinospora cordifolia induces enzymes of carcinogen/drug metabolism and antioxidant system, and inhibits lipid peroxidation in mice. Phytomedicine. 2006;13:74–84. doi: 10.1016/j.phymed.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Desai VR, Kamat J P, Sainis K B. An immuno-modulator from Tinospora cordifolia with antioxidant activity in cell free systems. Proc Indian Acad Sci (chem. Sci) 2002;114:713–719. [Google Scholar]
- 42.Wagner H. Multitarget therapy--the future of treatment for more than just functional dyspepsia. Phytomedicine. 2006;13 Suppl 5:122–129. doi: 10.1016/j.phymed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Med J Aust. 2002;177:565–567. doi: 10.5694/j.1326-5377.2002.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 44.Thall PF, Wathen JK. Practical Bayesian adaptive randomisation in clinical trials. Eur J Cancer. 2007;43:859–866. doi: 10.1016/j.ejca.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen-Solal M, Prehu C, Wajcman H, Poyart C, Bardakdjian-Michau J, Kister J, Prome D, Valentin C, Bachir D, Galacteros F. A new sickle cell disease phenotype associating Hb S trait, severe pyruvate kinase deficiency (PK Conakry), and an alpha2 globin gene variant (Hb Conakry) Br J Haematol. 1998;103:950–956. doi: 10.1046/j.1365-2141.1998.01094.x. [DOI] [PubMed] [Google Scholar]
- 46.Schaberg T, Rebhan K, Lode H. Risk factors for side-effects of isoniazid, rifampin and pyrazinamide in patients hospitalized for pulmonary tuberculosis. Eur Respir J. 1996;9:2026–2030. doi: 10.1183/09031936.96.09102026. [DOI] [PubMed] [Google Scholar]
- 47.Singh J, Garg PK, Tandon RK. Hepatotoxicity due to antituberculosis therapy. Clinical profile and reintroduction of therapy. J Clin Gastroenterol. 1996;22:211–214. doi: 10.1097/00004836-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397:61–67. doi: 10.1042/BJ20060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 50.Fairbanks K. Alcoholic liver disease. Gastroenterology & Hepatology. 2004 [Google Scholar]