Abstract
AIM: To investigate the mucosal protective effect and the mechanisms of action of the anti-ulcer drug irsogladine maleate in gastric injury induced by indomethacin in rats.
METHODS: Gastric mucosal injury was induced in male Hos:Donryu rats by oral administration of indomethacin at a dose of 48 mg/kg. One hour before indomethacin treatment, animals were orally pretreated with irsogladine maleate at doses of 1 mg/kg, 3 mg/kg or 10 mg/kg. Four hours after indomethacin administration, the animals were sacrificed and their stomachs were rapidly removed and processed for the evaluation of gastric mucosal damage and the determination of the concentrations of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-8 and myeloperoxidase (MPO) in mucosal tissues.
RESULTS: Linear hemorrhagic mucosal lesions were observed primarily in the glandular stomach 4 h after oral administration of indomethacin. Pretreatment with irsogladine maleate markedly reduced the number and severity of these lesions in a dose-dependent manner. The mucosal concentrations of proinflammatory cytokines (TNF-α, IL-1β, and IL-8) and MPO, which indicates the degree of mucosal infiltration by neutrophils, increased concomitantly with the occurrence of gastric injury in the indomethacin-treated rats. Pretreatment with irsogladine maleate significantly decreased the levels of these inflammatory factors in gastric tissue elicited by indomethacin.
CONCLUSION: The mucosal protective effects afforded by irsogladine maleate on gastric injury induced by indomethacin are mediated by inhibition of mucosal proinflammatory cytokine production and neutrophil infiltration, leading to suppression of mucosal inflammation and subsequent tissue destruction.
Keywords: Irsogladine, Gastric injury, Indomethacin, Cytokine, Myeloperoxidase
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs), such as indomethacin and aspirin, are widely prescribed in clinical practice because they exhibit excellent efficacy in the management of pain, fever and inflammation through their suppression of the synthesis of prostaglandins (PGs) from arachidonic acids resulting from their inhibition of cyclooxygenase (COX)[1]. However, the use of NSAIDs is associated with significant risks of adverse gastrointestinal events, such as gastric mucosal erosion, ulceration, bleeding, and perforation[2,3]. Such side effects considerably limit the use of these drugs. In addition to the decrease in the intrinsic production of PGs in the gastric mucosa, other processes, such as increased mucosal production of proinflammatory cytokines, increased production of reactive oxygen species, and increased lipid peroxidation as well as increased mucosal infiltration of neutrophils, are closely associated with NSAID-induced gastric mucosal injury[4-6].
Although the anti-ulcer drug irsogladine maleate has been shown in animals to exhibit potent mucosal protective effects on various experimental gastrointestinal injuries induced by a variety of stimuli, including indomethacin[7-10], the exact mechanisms underlying the gastroprotective effect of irsogladine maleate remains to be fully elucidated. We have recently found that irsogladine maleate increases the intracellular levels of the second messenger cyclic adenosine monophosphate (cAMP) in rat glandular stomach and in human neutrophils through its previously unreported ability to inhibit cyclic nucleotide phosphodiesterase 4 (PDE4), and this inhibitory activity is probably involved in the antiulcer effects of the drug[11-13]. PDE4 inhibitors have been demonstrated to have multiple anti-inflammatory effects mediated by the suppression of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-8, as well as by inhibiting neutrophil infiltration by suppressing the expression of adhesion molecules[14-16].
Paying particular attention to the novel inhibitory action of irsogladine maleate on PDE4, in the present study we investigated the mucosal protective effect of irsogladine maleate and its underlying mechanisms of action by using a rat model of gastric injury elicited by indomethacin, focusing on the effect of the drug on cytokine production and neutrophil infiltration in the gastric mucosa. We found that irsogladine maleate markedly reduced gastric injury in indomethacin-treated rats with a concomitant decrease in levels of TNF-α, IL-1β, IL-8 and myeloperoxidase (MPO) in gastric mucosal tissue. The possible contribution of PDE4 inhibitory activity to the mucosal protective effect of irsogladine maleate was also discussed.
MATERIALS AND METHODS
Animals
Male Hos: Donryu rats (Japan SLC, Hamamatsu, Japan) at 6 weeks of age were used following quarantine and acclimation for a week after delivery to the laboratory. The rats were housed in rooms at 20-26°C with a relative humidity of 35%-75% in a 12 h light-dark cycle (lights on, 8:00-20:00) and a ventilation frequency of at least 15 times per hour. The rats were fed standard laboratory chow (F-2; Funabashi Farm, Funabashi, Chiba, Japan) with free access to tap water. All the animals were fasted for 18 h before the experiments. However, free access to tap water was allowed 1 h before the experiments. All experimental procedures were approved by the Committee for the Institutional Care and Use of Animals of Nippon Shinyaku Co.
Preparation of drugs
Irsogladine maleate, synthesized at Nippon Shinyaku Co., was suspended in 5 g/L methylcellulose solution. Indomethacin, purchased from Sigma Chemicals (St. Louis, MO, USA), was dissolved in 50 g/L NaHCO3 solution.
Production of indomethacin-induced gastric mucosal injury in rats
Gastric mucosal injury was produced by intragastric gavage of indomethacin at a dose of 48 mg/kg. Irsogladine maleate was given orally 1 h before indomethacin treatment. Four hours after indomethacin treatment, the animals were sacrificed with an overdose of diethyl ether and their stomachs were rapidly removed and processed for the evaluation of gastric mucosal damage and determination of TNF-α, IL-1β, IL-8 and MPO in the mucosal tissues.
Evaluation of gastric mucosal injury
The stomach was distended with 10 mL of 8 g/L formaldehyde and immersed in the formaldehyde solution for 10 min. The lightly fixed stomach was then opened along the line of the greater curvature and spread out on a board. The hemorrhagic lesions in the mucosal layer were examined under a dissecting microscope (Olympus SZ40, Olympus Optical Co., Tokyo, Japan) at × 10 magnification by an observer who was unaware of the treatments. The gastric ulcer index was calculated for each rat as the sum of the lengths in millimeters of the hemorrhagic lesions (seen as red streaks).
Determination of the concentrations of TNF-α, IL-1β and IL-8 in gastric mucosa
To evaluate the effect of irsogladine maleate on the mucosal production of cytokines elicited by treatment with indomethacin, the concentrations of TNF-α, IL-1β, and IL-8 in gastric mucosa were determined. Briefly, the gastric mucosa was rapidly scraped from the gastric wall of each rat and immediately frozen on dry ice and kept at -70°C until analysis. Mucosal samples were homogenized with a Physcotron NS-310E mechanical microhomogenizer (Niti-on, Tokyo, Japan) in 1 mL of PBS containing complete protease inhibitor cocktail (Roche, Indianapolis, IN, USA). The homogenate was centrifuged for 30 min at 15 000 r/min (4°C) in a Hitachi Himac CF 15D2 centrifuge (Hitachi High-Technologies, Tokyo, Japan) and the supernatant was retained for determination of proinflammatory cytokines. TNF-α and IL-1β were determined by enzyme-linked immunosorbent assay (ELISA) with commercial kits from R&D Systems (Minneapolis, MN, USA). IL-8 was determined with a commercial ELISA kit from Panapharm Laboratories (Kumamoto, Japan). The total protein in the supernatant was determined with the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA), and the concentrations of TNF-α, IL-1β and IL-8 were expressed as ng per gram of total protein.
Measurement of mucosal MPO levels
To estimate the degree of mucosal infiltration by neutrophils elicited by treatment with indomethacin, the MPO levels were measured in the gastric mucosa. Briefly, gastric mucosal samples were homogenized with a microhomogenizer in 1 mL of 10 mmol/L Tris (pH 7.4) containing 200 mmol/L NaCl, 5 mmol/L EDTA, 100 mL/L glycine, 1 mmol/L phenylmethanesufonyl fluoride, 1 μg/mL leupeptin, and 28 μg/mL aprotinin. The homogenate was centrifuged and the supernatant was retained for determination of MPO with an ELISA kit from Hycult Biotechnology (Uden, Netherlands) according to the manufacturer’s instructions. The total protein in the supernatant was also determined as described above and the concentration of MPO was expressed as ng per gram of total protein.
Statistical analysis
The results are presented as mean ± standard error of the mean (SE). The statistical significance of differences between normal and control groups was evaluated by the Aspin-Welch test, and the statistical significance of differences among the control and irsogladine maleate-treated groups was evaluated by the Williams test. P < 0.05 was considered statistically significant. All tests were two-tailed, and performed with SAS version 8.2 (SAS Institute, Cary, NC, USA).
RESULTS
Effect of irsogladine maleate on gastric mucosal injury induced by indomethacin
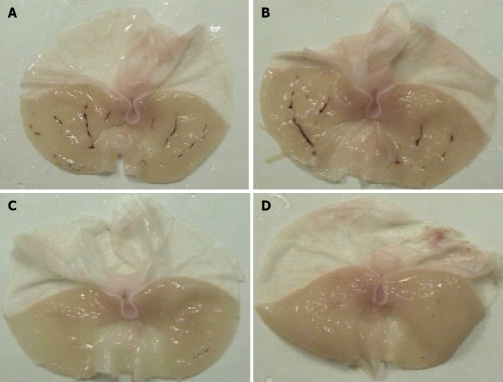
Four hours after oral administration of indomethacin, at a dose 48 mg/kg, gross linear hemorrhagic mucosal lesions were clearly observed in the glandular mucosa, and pretreatment with irsogladine maleate markedly reduced the number and severity of these lesions (Figure 1). Gastric mucosal injury was quantified under a dissecting microscope, and a gastric ulcer index of 39.1 ± 7.7 mm was calculated (Figure 2). Pretreatment with irsogladine maleate reduced the gastric ulcer index in a dose-dependent manner to 27.1 ± 6.2 mm at the dose of 1 mg/kg, 18.3 ± 7.2 mm at the dose 3 mg/kg, and 5.7 ± 4.0 mm at the dose of 10 mg/kg. In particular, irsogladine maleate at the dose of 3 mg/kg or 10 mg/kg significantly reduced the gastric ulcer index.
Figure 1.
Protective effect of irsogladine maleate on gastric mucosal lesions induced by indomethacin in rats. Stomachs from rats not pretreated with irsogladine maleate (A) and pretreated with irsogladine maleate at the doses of 1 mg/kg (B), 3 mg/kg (C) or 10 mg/kg (D), respectively, 1 h before administration of indomethacin. Nine rats were used in each group.
Figure 2.
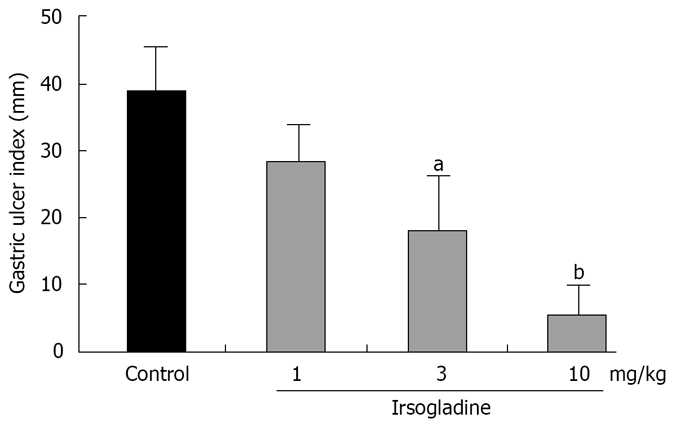
Protective effect of irsogladine maleate on gastric mucosal lesions induced by indomethacin in rats. Each value represents the mean ± SE for 9 rats. aP < 0.05 and bP < 0.01 vs control group.
Effect of irsogladine maleate on TNF-α levels in gastric mucosa
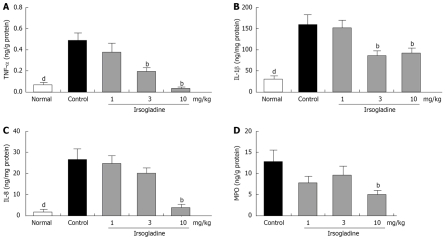
Intragastric administration of indomethacin induced a marked increase in mucosal TNF-α concentrations. The TNF-α level in the gastric mucosa of normal animals was 0.070 ± 0.014 ng/g protein, which was significantly increased to 0.490 ± 0.072 ng/g protein after intragastric administration of indomethacin (Figure 3A). Pretreatment with irsogladine maleate reduced the concentration of mucosal TNF-α in a dose-dependent manner to 0.354 ± 0.086 ng/g protein at the dose of 1 mg/kg, 0.186 ± 0.042 ng/g protein at the dose of 3 mg/kg, and 0.045 ± 0.005 ng/g protein at the dose of 10 mg/kg, respectively. In particular, irsogladine maleate at the dose of 3 mg/kg or more significantly reduced the TNF-α levels in gastric mucosa.
Figure 3.
Effect of irsogladine maleate on gastric mucosal TNF-α (A), IL-1β (B), IL-8 (C) and MPO (D) induced by indomethacin in rats. Each value represents the mean ± SE for 9 rats, bP < 0.01 vs control group, dP < 0.01 vs normal group.
Effect of irsogladine maleate on IL-1β levels in gastric mucosa
Intragastric administration of indomethacin induced a marked increase in mucosal IL-1β concentrations. The IL-1β level in the gastric mucosa of normal animals was 36.1 ± 8.1 ng/g protein, which was significantly increased to 160.2 ± 19.8 ng/g protein after intragastric administration of indomethacin (Figure 3B). Pretreatment with irsogladine maleate reduced the concentration of mucosal IL-1β to 154.0 ± 15.7 ng/g protein at the dose of 1 mg/kg, 85.4 ± 15.4 ng/g protein at the dose of 3 mg/kg, and 93.0 ± 17.4 ng/g protein at the dose of 10 mg/kg, respectively. In particular, irsogladine maleate at the dose of 3 mg/kg or more significantly reduced the IL-1β levels in gastric muccosa.
Effect of irsogladine maleate on IL-8 levels in gastric mucosa
Intragastric administration of indomethacin induced a marked increase in mucosal IL-8 concentrations. The IL-8 level in gastric mucosa of normal animals was 1.1 ± 0.5 ng/g protein, which was significantly increased to 27.0 ± 6.6 ng/g protein after intragastric administration of indomethacin (Figure 3C). Pretreatment with irsogladine maleate reduced the concentration of mucosal IL-8 in a dose-dependent manner to 24.8 ± 6.0 ng/g protein at the dose of 1 mg/kg, 19.9 ± 5.9 ng/g protein at the dose of 3 mg/kg, and 2.8 ± 0.6 ng/g protein at the dose of 10 mg/kg, respectively. In particular, irsogladine maleate at the dose of 10 mg/kg significantly reduced the IL-8 levels in gastric mucosa.
Effect of irsogladine maleate on MPO levels in gastric mucosa
The MPO level in the gastric mucosa of indomethacin-treated animals was 12.7 ± 4.3 ng/g protein (Figure 3D). Pretreatment with irsogladine maleate reduced the MPO concentration in gastric mucosa to 7.0 ± 1.6 ng/g at the dose of 1 mg/kg, 9.1 ± 2.2 ng/g at the dose of 3 mg/kg and 4.9 ± 0.8 ng/g at the dose of 10 mg/kg, respectively. In particular, irsogladine maleate at the dose of 10 mg/kg significantly reduced the MPO levels in gastric mucosa.
DISCUSSION
Though several studies have been published on the mechanisms underlying the mucosal protective effects of irsogladine maleate, an anti-ulcer drug often prescribed in Japan, on various gastrointestinal mucosal injuries[9-13], the exact mechanisms by which this drug exerts its mucosal protective effects are not completely clear. In the present study, we investigated the mechanisms underlying the gastroprotective effect of irsogladine maleate by using a rat experimental model of gastric injury elicited by indomethacin, focusing on the involvement of mucosal proinflammatory cytokine production and neutrophil infiltration into the gastric mucosa. We found that the concentrations of TNF-α, IL-1β, IL-8 and MPO in gastric mucosa of the rats increased concomitantly with the occurrence of gastric injury induced by a single intragastric dose of indomethacin. Pretreatment with irsogladine maleate markedly reduced the gastric injury with a concomitant decrease in the levels of these inflammatory factors.
Although the mechanisms of NSAID-induced gastric injury are not well understood, it is well accepted that both COX-dependent and independent mechanisms are involved in the pathogenesis of indomethacin-induced gastric injury. Mucosal proinflammatory cytokines, such as TNF-α, IL-1β and IL-8, are considered key inducers of gastric injury induced by NSAIDs like indomethacin[5,13]. For example, it is recognized that TNF-α may strongly promote inflammation and subsequent tissue destruction by recruiting leukocytes, particularly neutrophils and monocytes, through the induction of adhesion molecules on both leukocytes and vascular endothelial cells[17,18]. It is also known that TNF-α can induce the production of superoxide and proinflammatory cytokines by inflammatory cells[18,19]. In addition, intravenous administration of TNF-α induces gastric injury characterized by marked neutrophil infiltration into the gastric mucosa of rats[20], while TNF-α levels in the gastric mucosa correlate well with the severity of gastric injury in rats[21-23]. It was reported that IL-1β and IL-8, as well as TNF-α, can promote inflammatory response and are involved in NSAID-induced gastric mucosal injury in humans and animals[17,24-26].
In an attempt to clarify the mechanisms underlying the protective effects of irsogladine maleate on gastric mucosa, we evaluated it effects on the production of TNF-α, IL-1β, and IL-8 in gastric mucosa. We found that these cytokines were involved in the inflammatory response in a rat gastric mucosal injury model induced by indomethacin, and the concentrations of TNF-α, IL-1β and IL-8 in gastric mucosa increased concomitantly with the occurrence of gastric mucosal lesions, suggesting that the increased levels of these cytokines are closely associated with the occurrence and development of gastric mucosal lesions. Irsogladine maleate suppressed gastric mucosal injury at the dose of 3 mg/kg or higher. The doses of irsogladine maleate showing effective mucosal protection in this study are similar to those showing protection in previous studies of a variety of animal models of gastric injury[7-9,13]. Irsogladine maleate also significantly suppressed the increased levels of TNF-α, IL-1β and IL-8 in gastric mucosa, indicating that the suppression of proinflammatory cytokine production in gastric mucosa by irsogladine maleate significantly contributes to its protective effect on gastric mucosa.
In addition to proinflammatory cytokines, the accumulation and activation of neutrophils, which may lead to microcirculation disturbance and production of free radicals in gastric mucosa, are also important events in gastric injury induced by NSAIDs[6,27]. Studies on experimental models of gastric injury induced by NSAIDs have demonstrated that infiltration of neutrophils into the injured mucosa occurs in rats as early as 15-30 min after indomethacin administration[28], and gastric injury can be ameliorated in animals by eliminating the neutrophils through the administration of antineutrophil antibodies[6]. Neutrophil infiltration into gastric mucosa is, therefore, considered a critical step in the development of gastric injury induced by NSAIDs. In the present study, irsogladine maleate significantly reduced the MPO levels in gastric mucosa, indicating a reduction in mucosal infiltration by neutrophils. The suppression of neutrophil infiltration into the gastric mucosa may, therefore, contribute to the protective effect of irsogladine maleate on gastric mucosa.
PDE4 inhibitors may exert their anti-inflammatory action on various inflammatory diseases by suppressing the production of proinflammatory cytokines and the expression of adhesion molecules by increasing the levels of intracellular cAMP[14-16]. In addition, the PDE4 inhibitor rolipram can ameliorate indomethacin-induced gastric injury in rats by inhibiting the production of TNF-α[29]. We and others have recently reported that irsogladine maleate prevents ischemia-reperfusion-induced gastric injury and indomethacin-induced small intestinal lesions in rats, probably by inhibiting PDE4[10,13]. It is, therefore, likely that irsogladine maleate exerts its protective effect on indomethacin-induced gastric injury by suppressing mucosal TNF-α, IL-1β and IL-8 production by neutrophils and/or macrophages through its inhibition of PDE4 in these inflammatory cells. The suppression of these proinflammatory cytokines by irsogladine maleate may in turn decrease the expression of adhesion molecules in both vascular endothelial cells and neutrophils, resulting in a reduction in mucosal infiltration by neutrophils with consequent prevention of tissue destruction. Irsogladine maleate also inhibits the in vitro production and release of superoxide from human neutrophils by inhibiting PDE4[12]. Therefore, it is likely that the protective effects of irsogladine maleate on gastric mucosa arise not only from its suppression of neutrophil infiltration through its inhibition of proinflammatory cytokine production and adhesion-molecule expression, but also from its direct suppression of neutrophil activation through its inhibition of PDE4.
In conclusion, irsogladine maleate exerts its mucosal protective effects on gastric injury by suppressing the acute inflammatory response and subsequent tissue destruction in gastric mucosa through its suppression of mucosal proinflammatory cytokine production and neutrophil infiltration as well as by directly inhibiting neutrophil activation. It is also likely that the suppression of proinflammatory cytokines or neutrophils by irsogladine maleate arises from its ability to inhibit PDE4.
COMMENTS
Background
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely prescribed for their efficacy in the management of pain, fever and inflammation. However, NSAIDs are associated with significant risks of adverse gastrointestinal events, which considerably limit the use of these drugs. Although irsogladine maleate exhibits potent mucosal protective effects against indomethacin-induced gastrointestinal injuries in animals, its mechanisms of action are not fully understood.
Research frontiers
We investigated the mechanisms underlying the protective effect of irsogladine maleate on gastric mucosa by using a rat experimental model of gastric injury elicited by indomethacin, focusing on the involvement of mucosal proinflammatory cytokine production and neutrophil infiltration into the gastric mucosa.
Innovations and breakthroughs
The present study reported the effect of irsogladine maleate on mucosal proinflammatory cytokine production and neutrophil infiltration into the gastric mucosa in indomethacin-induced gastric injury.
Applications
A single intragastric dose of irsogladine maleate could markedly reduce gastric injury with a concomitant decrease in the levels of inflammatory factors in indomethacin-treated rats, suggesting that irsogladine maleate can be applied to the treatment of patients with NSAID-induced gastric injury.
Peer review
In this study, the authors examined the effects of irsogladine maleate on gastric mucosal injury and proinflammatory cytokine production and neutrophil infiltration (measured by MPO) using a rat model of gastric injury elicited by indomethacin. The authors found that irsogladine maleate could markedly reduce gastric injury in indomethacin-treated rats with a concomitant decrease in the gastric mucosal levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-8 and myeloperoxidase (MPO). The results of these straightforward experiments demonstrate that the protective effects of irsogladine are mediated by reducing neutrophil induction and inhibition of mucosal cytokine expression. This paper is well written and the data are novel.
Acknowledgments
The authors thank Dr. Gerald E. Smyth for his careful reading of the manuscript and Mr. Masaru Tamura for his assistance with the statistical analysis of the data.
Footnotes
Peer reviewers: Dr. Bernardino Rampone, Department of General Surgery and Surgical Oncology, University of Siena, viale Bracci, Siena 53100, Italy; Shingo Tsuji, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine(A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan; Samuel B Ho, MD, Chief, Gastroenterology Section (111D), VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, United states; Yogesh K Chawla, Dr, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Zhong XY L- Editor Wang XL E- Editor Zhang WB
References
- 1.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. N Engl J Med. 1999;340:1888–1899. doi: 10.1056/NEJM199906173402407. [DOI] [PubMed] [Google Scholar]
- 3.Singh G, Ramey DR, Morfeld D, Shi H, Hatoum HT, Fries JF. Gastrointestinal tract complications of nonsteroidal anti-inflammatory drug treatment in rheumatoid arthritis. A prospective observational cohort study. Arch Intern Med. 1996;156:1530–1536. [PubMed] [Google Scholar]
- 4.Yoshikawa T, Naito Y, Kishi A, Tomii T, Kaneko T, Iinuma S, Ichikawa H, Yasuda M, Takahashi S, Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993;34:732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okada A, Kinoshita Y, Waki S, Fukui H, Maekawa T, Matsushima Y, Kawanami C, Kishi K, Nakata H, Wang HY, et al. Rat gastric mucosal cells express ICAM-1 and proinflammatory cytokines during indomethacin-induced mucosal injury. J Lab Clin Med. 1998;131:538–547. doi: 10.1016/s0022-2143(98)90062-2. [DOI] [PubMed] [Google Scholar]
- 6.Wallace JL, Keenan CM, Granger DN. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990;259:G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- 7.Ueda F, Aratani S, Mimura K, Kimura K, Nomura A, Enomoto H. Effect of 2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate (MN-1695) on gastric ulcers and gastric secretion in experimental animals. Arzneimittelforschung. 1984;34:474–477. [PubMed] [Google Scholar]
- 8.Ueda F, Aratani S, Mimura K, Kimura K, Nomura A, Enomoto H. Effect of 2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate (MN-1695) on gastric mucosal damage induced by various necrotizing agents in rats. Arzneimittelforschung. 1984;34:478–484. [PubMed] [Google Scholar]
- 9.Kyoi T, Oka M, Noda K, Ukai Y. Irsogladine prevents monochloramine-induced gastric mucosal lesions by improving the decrease in mucosal blood flow due to the disturbance of nitric oxide synthesis in rats. J Pharmacol Sci. 2003;93:314–320. doi: 10.1254/jphs.93.314. [DOI] [PubMed] [Google Scholar]
- 10.Kamei K, Kubo Y, Kato N, Hatazawa R, Amagase K, Takeuchi K. Prophylactic effect of irsogladine maleate against indomethacin-induced small intestinal lesions in rats. Dig Dis Sci. 2008;53:2657–2666. doi: 10.1007/s10620-008-0199-9. [DOI] [PubMed] [Google Scholar]
- 11.Kyoi T, Oka M, Noda K, Ukai Y. Phosphodiesterase inhibition by a gastroprotective agent irsogladine: preferential blockade of cAMP hydrolysis. Life Sci. 2004;75:1833–1842. doi: 10.1016/j.lfs.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Kyoi T, Noda K, Oka M, Ukai Y. Irsogladine, an anti-ulcer drug, suppresses superoxide production by inhibiting phosphodiesterase type 4 in human neutrophils. Life Sci. 2004;76:71–83. doi: 10.1016/j.lfs.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Kyoi T, Kitazawa S, Tajima K, Zhang X, Ukai Y. Phosphodiesterase type IV inhibitors prevent ischemia-reperfusion-induced gastric injury in rats. J Pharmacol Sci. 2004;95:321–328. doi: 10.1254/jphs.fpj04009x. [DOI] [PubMed] [Google Scholar]
- 14.Klemm P, Harris HJ, Perretti M. Effect of rolipram in a murine model of acute inflammation: comparison with the corticoid dexamethasone. Eur J Pharmacol. 1995;281:69–74. doi: 10.1016/0014-2999(95)00232-a. [DOI] [PubMed] [Google Scholar]
- 15.Kasyapa CS, Stentz CL, Davey MP, Carr DW. Regulation of IL-15-stimulated TNF-alpha production by rolipram. J Immunol. 1999;163:2836–2843. [PubMed] [Google Scholar]
- 16.Sanz MJ, Cortijo J, Taha MA, Cerda-Nicolas M, Schatton E, Burgbacher B, Klar J, Tenor H, Schudt C, Issekutz AC, et al. Roflumilast inhibits leukocyte-endothelial cell interactions, expression of adhesion molecules and microvascular permeability. Br J Pharmacol. 2007;152:481–492. doi: 10.1038/sj.bjp.0707428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, Higuchi K, Hamaguchi M, Shiba M, Tominaga K, Fujiwara Y, Matsumoto T, Arakawa T. Monocyte chemotactic protein-1 regulates leukocyte recruitment during gastric ulcer recurrence induced by tumor necrosis factor-alpha. Am J Physiol Gastrointest Liver Physiol. 2004;287:G919–G928. doi: 10.1152/ajpgi.00372.2003. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Takano H, Naito Y, Oyamada H, Ueda S, Kondo M. Augmentative effects of tumor necrosis factor-alpha (human, natural type) on polymorphonuclear leukocyte-derived superoxide generation induced by various stimulants. Int J Immunopharmacol. 1992;14:1391–1398. doi: 10.1016/0192-0561(92)90010-i. [DOI] [PubMed] [Google Scholar]
- 19.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ Res. 2000;86:205–213. doi: 10.1161/01.res.86.2.205. [DOI] [PubMed] [Google Scholar]
- 20.Kahky MP, Daniel CO, Cruz AB, Gaskill HV 3rd. Portal infusion of tumor necrosis factor increases mortality in rats. J Surg Res. 1990;49:138–145. doi: 10.1016/0022-4804(90)90252-w. [DOI] [PubMed] [Google Scholar]
- 21.Toroudi HP, Rahgozar M, Bakhtiarian A, Djahanguiri B. Potassium channel modulators and indomethacin-induced gastric ulceration in rats. Scand J Gastroenterol. 1999;34:962–966. doi: 10.1080/003655299750025048. [DOI] [PubMed] [Google Scholar]
- 22.Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM, Morelli A. Pentoxifylline prevents indomethacin induced acute gastric mucosal damage in rats: role of tumour necrosis factor alpha. Gut. 1994;35:909–915. doi: 10.1136/gut.35.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santucci L, Fiorucci S, Di Matteo FM, Morelli A. Role of tumor necrosis factor alpha release and leukocyte margination in indomethacin-induced gastric injury in rats. Gastroenterology. 1995;108:393–401. doi: 10.1016/0016-5085(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 24.Hamlet A, Lindholm C, Nilsson O, Olbe L. Aspirin-induced gastritis, like Helicobacter pylori-induced gastritis disinhibits acid secretion in humans: relation to cytokine expression. Scand J Gastroenterol. 1998;33:346–356. doi: 10.1080/00365529850170964. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka J, Yuda Y, Yamakawa T. Mechanism of superoxide generation system in indomethacin-induced gastric mucosal injury in rats. Biol Pharm Bull. 2001;24:155–158. doi: 10.1248/bpb.24.155. [DOI] [PubMed] [Google Scholar]
- 26.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Horikawa Y, Matsuhashi T, Hatakeyama N, Oyake J, Ohba R, et al. Attenuation of gastric mucosal inflammation induced by aspirin through activation of A2A adenosine receptor in rats. World J Gastroenterol. 2006;12:568–573. doi: 10.3748/wjg.v12.i4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology. 1997;112:1000–1016. doi: 10.1053/gast.1997.v112.pm9041264. [DOI] [PubMed] [Google Scholar]
- 28.Anthony A, Sim R, Dhillon AP, Pounder RE, Wakefield AJ. Gastric mucosal contraction and vascular injury induced by indomethacin precede neutrophil infiltration in the rat. Gut. 1996;39:363–368. doi: 10.1136/gut.39.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura C, Otaka M, Odashima M, Jin M, Konishi N, Horikawa Y, Matsuhashi T, Watanabe S. Rolipram, a specific type IV phosphodiesterase inhibitor, ameliorates indomethacin-induced gastric mucosal injury in rats. Pathophysiology. 2003;9:195–200. doi: 10.1016/s0928-4680(03)00005-1. [DOI] [PubMed] [Google Scholar]