Abstract
AIM: To investigate the effects of a ghrelin receptor agonist GHRP-6 on delayed gastrointestinal transit in alloxan-induced diabetic mice.
METHODS: A diabetic mouse model was established by intraperitoneal injection with alloxan. Mice were randomized into two main groups: normal mice and diabetic mice treated with GHRP-6 at doses of 0, 20, 50, 100 and 200 μg/kg ip. Gastric emptying (GE), intestinal transit (IT), and colonic transit (CT) were studied in mice after they had a phenol red meal following injection of GHRP-6. Based on the most effective GHRP-6 dosage, atropine was given at 1 mg/kg for 15 min before the GHRP-6 injection for each measurement. The mice in each group were sacrificed 20 min later and the percentages of GE, IT, and CT were calculated.
RESULTS: Percentages of GE, IT, and CT were significantly decreased in diabetic mice as compared to control mice. In the diabetic mice, GHRP-6 improved both GE and IT, but not CT. The most effective dose of GHRP-6 was 200 μg/kg and atropine blocked the prokinetic effects of GHRP-6 on GE and IT.
CONCLUSION: GHRP-6 accelerates delayed GE and IT, but has no effect on CT in diabetic mice. GHRP-6 may exert its prokinetic effects via the cholinergic pathway in the enteric nervous system, and therefore, has therapeutic potential for diabetic patients with delayed upper gastrointestinal transit.
Keywords: GHRP-6, Diabetes mellitus, Gastric emptying, Intestinal transit, Colonic transit
INTRODUCTION
Ghrelin is a peptide synthesized by endocrine cells of the gastric mucosa, initially identified in rodents[1]. The major actions of this recently discovered peptide include stimulation of growth hormone (GH) release[1-3], regulation of appetite and nutrient ingestion[4-6], and improvement of digestive motility[7-9]. When injected into mice[7,10], rats[8], human[9], or dogs[11], ghrelin accelerates gastric emptying of a liquid or solid meal.
Delayed gastrointestinal transit is a well-known diabetic complication, and may lead to uncomfortable gastrointestinal symptoms, such as frequent vomiting, emaciation, and unpredictable changes in blood glucose, all of which impair the quality of life of diabetic patients[12,13]. Gastrointestinal transit of solid or nutrient liquid meals is abnormally slow in approximately 50% of diabetic patients[12]. Delayed gastrointestinal transit may be associated with cardiac autonomic neuropathy, blood glucose concentration, and gastrointestinal symptoms[12].
GHRP-6 is a peptide ghrelin receptor agonist, which has previously been reported to increase gastric emptying in normal rats[14]. Ghrelin has been shown to accelerate gastric emptying in animal models of postoperative ileus[15], septic ileus[10], burn-induced slow gastrointestinal transit[16] and diabetes mellitus[17,18]. In the present study, the prokinetic effect of GHRP-6 was investigated in a mouse model of diabetes mellitus.
MATERIALS AND METHODS
Chemicals
GHRP-6 was obtained from Tocris Cookson (Bristol, UK). Atropine sulphate, phenol red, and alloxan were purchased from Sigma (St Louis, MO).
Diabetic mouse model
C57 mice (18-22 g) were provided by the Experimental Animal Center of Shanghai Academia Sinica. All procedures for the animal experiments were approved by the Medical Ethical Committee of Shanghai Jiaotong University. The mice were housed in stainless steel cages at a controlled temperature (22 ± 2°C) with a 60%-65% relative humidity in a normal 12-h light and dark cycle. After exposure to a high-fat diet for 3 wk, the mice were fasted overnight with free access to water and injected intraperitoneally with alloxan (0.2 g/kg body weight) dissolved in a sterile normal saline solution. Seventy-two hours later, the fasting blood glucose level in the mice was determined using the glucose oxidase method with a glucose analyzer. Mice with a blood glucose level higher than 11.1 mmol/L were classified as diabetic mice (DB mice). The DB mice were continuously fed without control of blood glucose for 4 wk, and a model of DB mice was established for further investigations.
Animal grouping for gastrointestinal transit studies
Gastric emptying, intestinal and colonic transit studies were then performed. For each study, mice were divided into two groups: a normal (control) group and a diabetic group. Mice in the diabetic group were treated with different doses of GHRP-6 (0, 20, 50, 100 and 200 μg/kg) given in a random order, with a total of six mice in each subgroup. A dose-response curve for GHRP-6 was obtained for the experiment. Based on the most effective dose of GHRP-6, another group of 6 mice were given atropine (1 mg/kg) injections 15 min before GHRP-6 injection.
Gastric emptying
After a 12 h fast, the diabetic mice were injected with different doses of GHRP-6 (0, 20, 50, 100 and 200 μg/kg). After GHRP-6 injection, the mice received a gavage feeding (5 mg/kg body weight) of a phenol red test meal (0.5 g/L in 0.9% NaCl with 1.5% methylcellulose). Twenty minutes later, the mice were sacrificed. Their stomachs were clamped with a string above the lower oesophageal sphincter and a string beneath the pylorus to prevent leakage of phenol red. Gastric emptying was determined spectrophotometrically, according to the method previously described with certain slight modifications. The stomach of each individual mouse was resected just above the lower oesophageal sphincter and pyloric sphincter. Phenol red remained partly in the lumen of the stomach. The stomach and its contents were put into 5 mL of 0.1 mol/L NaOH. The stomach was minced. The samples containing the total amount of phenol red present in the stomach were further diluted to 10 mL with 0.1 mol/L NaOH and left at room temperature for 1 h. Five milliliters of the supernatant was then centrifuged at 800 g for 20 min. The absorbance was read at a wavelength of 546 nm on a spectrophotometer (Shanghai Yixian Company, China), and the phenol red content in the stomach was calculated. Percentage of gastric emptying of the phenol red was calculated as [(infusion amount-remains)/infusion amount] × 100%.
Intestinal and colonic transit
After an overnight fast, mice were given general anesthesia (2%-3% isoflurane inhalation) and underwent abdominal surgery. A small polyethylene tube was placed in the duodenum (or colon) via the stomach (or cecum), 0.5 cm distal to the pylorus (or ileocolic junction), fixed with sutures to the gut wall, and then tunneled through the abdominal wall subcutaneously to exit from the skin at the nape of the neck. Midline incisions were sutured, and mice were left to recover in separate cages. Food and water were provided abundantly. Three days later, after a 12 h fast, the mice were given different doses of GHRP-6 (0, 20, 50, 100 and 200 μg/kg) intraperitoneally. After GHRP-6 injection, a phenol red test meal at 5 mg/kg (0.5 g/L in 0.9% NaCl with 1.5% methylcellulose) was injected into the duodenum (or colon) via the implanted polyethylene tube. After 20 min, the mice were sacrificed. The distance of phenol red transit and the full length of the intestine or colon were calculated. Small intestine or colonic transit was assessed using the percentage ratio of phenol red transit over the full intestinal or colonic length.
Statistical analysis
Statistical analysis of the data was conducted using one-way ANOVA for multiple comparisons. Data are expressed as mean ± SE. P < 0.05 was considered statistically significant.
RESULTS
Gastric emptying and intestinal and colonic transit in diabetic mice
Significantly delayed gastric emptying, intestinal and colonic transits were found in diabetic mice. Gastric emptying was significantly decreased in diabetic mice compared to normal mice (22.9% ± 1.4% vs 28.1% ± 1.3%, P = 0.0216).
Also, intestinal transit was significantly decreased in diabetic mice compared to normal mice (33.5% ± 1.2% vs 43.2% ± 1.9%, P = 0.0132). In addition, colonic transit was decreased in diabetic mice compared to normal mice (29.5% ± 1.9% vs 36.3% ± 1.6%, P = 0.0148).
Effect of GHRP-6 on delayed gastric emptying in diabetic mice
GHRP-6 significantly accelerated gastric emptying in diabetic mice. The percentage of gastric emptying was 23.6% ± 1.9%, 26.9% ± 1.3%, 31.3% ± 0.9%, and 34.6% ± 1.5%, respectively, in the mice treated with 20, 50, 100, and 200 μg/kg GHRP-6. Except for the lowest dose, all of these doses normalized the rate of gastric emptying in diabetic mice (P = 0.0421, P = 0.0324 and P = 0.0103, respectively; Figure 1). We considered that the dosage of 200 μg/kg could most effectively increase the rate of gastric emptying.
Figure 1.
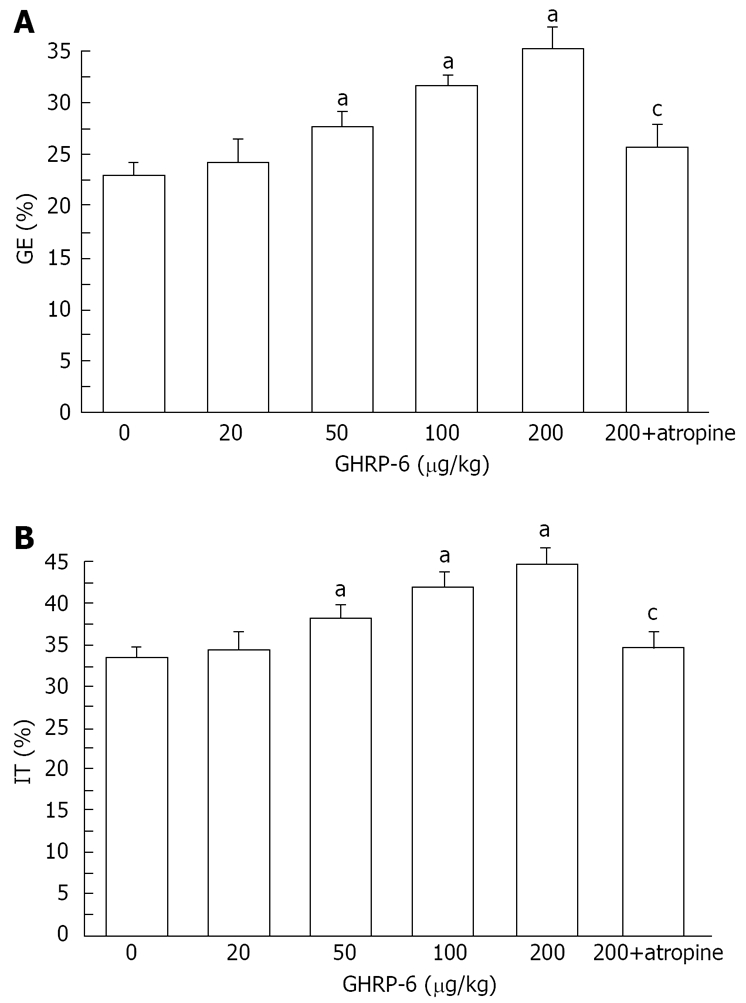
GHRP-6 significantly increases gastric emptying (A) and intestinal transit (B) in diabetic mice. aP < 0.05 vs control; cP < 0.05 vs 200 μg/kg GHRP-6 dose.
Effect of GHRP-6 on delayed small intestinal transit in diabetic mice
GHRP-6 significantly accelerated intestinal transit in diabetic mice. The percentage of intestinal transit was 34.2% ± 1.9%, 39.1% ± 1.5%, 42.6% ± 1.7%, and 44.5% ± 1.8%, respectively, in the mice treated with 20, 50, 100, and 200 μg/kg GHRP-6. All of these doses, except for 20 μg/kg, normalized the delayed intestinal transit (P = 0.0321, P = 0.0289 and P = 0.0184, respectively; Figure 1). As above, the 200 μg/kg GHRP-6 dose was considered most effective in accelerating intestinal transit.
Effect of GHRP-6 on delayed colonic transit in diabetic mice
GHRP-6 had no effect on delayed colonic transit. The percentage of colonic transit was 30.8% ± 1.4%, 29.5% ± 1.7%, 30.3% ± 1.9%, and 31.2% ± 2.3%, respectively, in the mice treated with 20, 50, 100, and 200 μg/kg GHRP-6. None of these doses was able to accelerate intestinal transit.
Effect of atropine on delayed gastric emptying and intestinal transit in diabetic mice
Atropine blocked the positive effects of 200 μg/kg GHRP-6 on gastric emptying and intestinal transit. The percentage of gastric emptying was significantly decreased from 34.6% ± 1.5% to 24.6% ± 1.8% (P = 0.0131, Figure 1). The small intestine transit was significantly decreased from 44.5% ± 1.8% to 35.4% ± 1.8% (P = 0.0145, Figure 1).
DISCUSSION
In the present study, gastric emptying, intestinal and colonic transit were significantly delayed in the mice with alloxan-induced diabetes, which is consistent with previously reported findings[19,20]. Gastrointestinal motility disturbances including esophageal motor dysfunction, gastroparesis, constipation and diarrhea, are common in patients with diabetes mellitus. It has been reported that gastrointestinal transit is significantly slower in the diabetic animal model of human diabetes[21-23]. Inhibition of gastrointestinal motility has also been reported in humans with diabetes mellitus[24,25]. The pathogenesis of slow gastrointestinal transit in diabetes mellitus patients is not clear, but several mechanisms have been proposed[12]. Among them, autonomic neuropathy, a complication of long-standing diabetes mellitus, has been widely accepted as the culprit. This may lead to the absence of postprandial gastrointestinal response, a reflex that should present in healthy people[24]. Recent studies have shown that an acute change in blood glucose concentration also has a major effect on gastrointestinal motor function in healthy subjects[25]. In particular, acute hyperglycemia inhibits both the gastrointestinal and ascending components of peristaltic reflex. Poor glycemic control has the potential to cause delayed gastrointestinal transit in diabetic patients[26]. In our study, different doses of GHRP-6 were able to accelerate gastric emptying and intestinal transit in the diabetic mice, but had no effect on colonic transit, which is consistent with the results seen in animal models of postoperative ileus[15] and in burn-induced gastrointestinal delayed transit[16]. We believe that this result might be related to the distribution of ghrelin receptors in the gut[27,28]. The most effective dose of GHRP-6 for accelerating upper gastrointestinal transit was 200 μg/kg per mouse. Atropine blocked the GHRP-6 effect on gastric emptying and intestinal transit.
GHRP-6, possessing prokinetic characteristics, increases gastric emptying in healthy mice[29]. The mechanisms underlying the action of GHRP-6 seem to be neuron-dependent. In vitro, isolated strips of muscle fail to contract significantly when exposed to GHRP-6. In vivo the gastrokinetic effect of GHRP-6 in rats is abolished by atropine or vagotomy[14,29]. In our study, atropine blocked the effect of 200 μg/kg GHRP-6 on gastric emptying and intestinal transit, suggesting that the prokinetic effect of GHRP-6 is mediated via the cholinergic pathway in the enteric nervous system.
It is reasonable to assume that GHRP-6 can be used as a potential drug for the treatment of diabetic patents with delayed gastrointestinal transit. Clinically, improvement in gastrointestinal transit facilitates enteral resuscitation, corrects blood glucose concentrations and reduces gastrointestinal symptoms in diabetic patients. In conclusion, GHRP-6 accelerates gastric emptying and intestinal transit in diabetic mice, an action that may be mediated via the cholinergic pathway in the enteric nerve system. GHRP-6 may have a therapeutic potential for diabetic patients with delayed upper gastrointestinal transit. The physiological role of GHRP-6 in the gastrointestinal tract remains to be identified, and its pharmacotherapeutic potential deserves to be further explored in diabetic patients suffering from delayed upper gastrointestinal transit.
COMMENTS
Background
Delayed gastrointestinal transit is common in patients with chronic diabetes and is always associated with impairments in quality of life and diabetic control. GHRP-6 is a potent prokinetic peptide. The effect of GHRP-6 on delayed gastrointestinal transit was studied in diabetic mice.
Research frontiers
The effects of ghrelin on gastrointestinal motor activity and roles in motility regulation have been extensively studied. This study is the first to investigate the effects of ghrelin agonist GHRP-6 on diabetic mice with delayed gastrointestinal transit.
Innovations and breakthroughs
GHRP-6 has been shown to accelerate gastric activity in postoperative and septic ileus animal models. However, it has not been studied in a diabetic animal model.
Applications
According to animal experiments, GHRP-6 may be used as a potential therapeutic agent for the treatment of delayed gastrointestinal transit in diabetes.
Peer review
This paper shows that GHRP-6 has an effect on gastric emptying and intestinal transit in diabetic mice, which may be mediated through the cholinergic pathways in the enteric nerve system. These results show that GHRP-6 can be used as a potential therapeutic drug for the treatment of delayed gastrointestinal transit in diabetes.
Footnotes
Supported by National Natural Science Foundation of China, No. 30400429s
Peer reviewer: Michael Horowitz, Professor, Department of Medicine, University of Adelaide and Director, Endocrine and Metabolic Unit, Royal Adelaide Hospital, Level 6, Eleanor Harrald Bullding, North Terrace, Adelaide 5000, Australia
S- Editor Zhong XY L- Editor Li M E- Editor Yin DH
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Seoane LM, Tovar S, Baldelli R, Arvat E, Ghigo E, Casanueva FF, Dieguez C. Ghrelin elicits a marked stimulatory effect on GH secretion in freely-moving rats. Eur J Endocrinol. 2000;143:R7–R9. doi: 10.1530/eje.0.143r007. [DOI] [PubMed] [Google Scholar]
- 3.Tolle V, Zizzari P, Tomasetto C, Rio MC, Epelbaum J, Bluet-Pajot MT. In vivo and in vitro effects of ghrelin/motilin-related peptide on growth hormone secretion in the rat. Neuroendocrinology. 2001;73:54–61. doi: 10.1159/000054620. [DOI] [PubMed] [Google Scholar]
- 4.Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, et al. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- 5.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 6.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 7.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 8.Dornonville de la Cour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Cremonini F, Camilleri M, Roque M, McKinzie S, Burton D, Baxter K, Zinsmeister A. Obesity Does Not Increase Effects of Synthetic Ghrelin on Human Gastric Motor Functions. Gastroenterology. 2006;131:1431–1439. doi: 10.1053/j.gastro.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 10.De Winter BY, De Man JG, Seerden TC, Depoortere I, Herman AG, Peeters TL, Pelckmans PA. Effect of ghrelin and growth hormone-releasing peptide 6 on septic ileus in mice. Neurogastroenterol Motil. 2004;16:439–446. doi: 10.1111/j.1365-2982.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 11.Trudel L, Bouin M, Tomasetto C, Eberling P, St-Pierre S, Bannon P, L'Heureux MC, Poitras P. Two new peptides to improve post-operative gastric ileus in dog. Peptides. 2003;24:531–534. doi: 10.1016/s0196-9781(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz M, O'Donovan D, Jones KL, Feinle C, Rayner CK, Samsom M. Gastric emptying in diabetes: clinical significance and treatment. Diabet Med. 2002;19:177–194. doi: 10.1046/j.1464-5491.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 13.Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422–1428. doi: 10.1111/j.1572-0241.2001.03683.x. [DOI] [PubMed] [Google Scholar]
- 14.Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160–168. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- 16.Sallam HS, Oliveira HM, Gan HT, Herndon DN, Chen JD. Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R253–R257. doi: 10.1152/ajpregu.00100.2006. [DOI] [PubMed] [Google Scholar]
- 17.Qiu WC, Wang ZG, Wang WG, Yan J, Zheng Q. Gastric motor effects of ghrelin and growth hormone releasing peptide 6 in diabetic mice with gastroparesis. World J Gastroenterol. 2008;14:1419–1424. doi: 10.3748/wjg.14.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu WC, Wang ZG, Lv R, Wang WG, Han XD, Yan J, Wang Y, Zheng Q, Ai KX. Ghrelin improves delayed gastrointestinal transit in alloxan-induced diabetic mice. World J Gastroenterol. 2008;14:2572–2577. doi: 10.3748/wjg.14.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Salhy M. Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci. 2002;107:101–110. doi: 10.3109/2000-1967-133. [DOI] [PubMed] [Google Scholar]
- 20.Anjaneyulu M, Ramarao P. Studies on gastrointestinal tract functional changes in diabetic animals. Methods Find Exp Clin Pharmacol. 2002;24:71–75. doi: 10.1358/mf.2002.24.2.677129. [DOI] [PubMed] [Google Scholar]
- 21.El-Salhy M. Gastrointestinal transit in an animal model of human diabetes type 2: relationship to gut neuroendocrine peptide contents. Ups J Med Sci. 2002;107:101–110. doi: 10.3109/2000-1967-133. [DOI] [PubMed] [Google Scholar]
- 22.Anjaneyulu M, Ramarao P. Studies on gastrointestinal tract functional changes in diabetic animals. Methods Find Exp Clin Pharmacol. 2002;24:71–75. doi: 10.1358/mf.2002.24.2.677129. [DOI] [PubMed] [Google Scholar]
- 23.El-Salhy M. Gastrointestinal transit in relation to gut endocrine cells in animal models of human diabetes. Ups J Med Sci. 2002;107:23–33. doi: 10.3109/2000-1967-139. [DOI] [PubMed] [Google Scholar]
- 24.Triantafyllou K, Kalantzis C, Papadopoulos AA, Apostolopoulos P, Rokkas T, Kalantzis N, Ladas SD. Video-capsule endoscopy gastric and small bowel transit time and completeness of the examination in patients with diabetes mellitus. Dig Liver Dis. 2007;39:575–580. doi: 10.1016/j.dld.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Russo A, Sun WM, Sattawatthamrong Y, Fraser R, Horowitz M, Andrews JM, Read NW. Acute hyperglycaemia affects anorectal motor and sensory function in normal subjects. Gut. 1997;41:494–499. doi: 10.1136/gut.41.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung HK, Kim DY, Moon IH, Hong YS. Colonic transit time in diabetic patients--comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265–272. doi: 10.3349/ymj.2003.44.2.265. [DOI] [PubMed] [Google Scholar]
- 27.Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2001;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- 28.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 29.Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut. 2005;54:1078–1084. doi: 10.1136/gut.2005.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]


