Abstract
Maximal inspiratory pressure (PIMAX), the maximum negative pressure generated during temporary occlusion of the airway, is commonly used to measure inspiratory muscle strength in mechanically ventilated infants and children. There are, however, no guidelines as to how the PIMAX measurement should be made. We compared the maximum inspiratory pressure generated during airway occlusion (PIMAXOCC) to that when a unidirectional valve (PIMAXUNI), which allowed expiration, but not inspiration was used.
Twenty two mechanically ventilated children (mean (SD) age 4.8 (4.5) years) were studied. Three sets of end expiratory occlusions were performed for each method in random order. The expired volume during PIMAXUNI was assessed and related to the functional residual capacity (FRC) measured using a helium dilution technique.
The mean (SD) PIMAXUNI (45.5 (15.2) cmH2O) was significantly greater than mean (SD) PIMAXOCC (30.9 (9.0) cmH2O) (p<0.0001). The mean (SD) expired volume during PIMAXUNI, was 98 ml (62.3), a mean reduction in FRC of 33.1% (SD 13.9). There were no significant differences between techniques in the baseline respiratory drive, the number of efforts required and the time to reach PIMAX. Regardless of technique, PIMAX was reached in 10 inspiratory efforts or 15sec of airway occlusion.
A unidirectional valve allowing expiration, but not inspiration yields greater PIMAX values in children. Occlusions should be maintained for 12 seconds or eight breaths (99% CI of mean).
Keywords: Child, Human, Intensive Care, Respiratory Function Tests, Respiratory Muscles
Introduction
Respiratory muscle strength in mechanically ventilated children may be reduced for a variety of reasons. Prolonged mechanical ventilation 1,2, injury to the phrenic nerve due to surgery or trauma 3, sepsis 4,5, critical illness 6,7, medications 7-10 and chronic illness 11-14 are recognised causes of respiratory muscle weakness and can delay weaning from ventilatory support. Accurate assessment of respiratory muscle strength, therefore, can provide important diagnostic and prognostic information.
Maximal inspiratory pressure (PIMAX), the maximum negative pressure generated during temporary occlusion of the airway, is commonly used to measure inspiratory muscle strength in mechanically ventilated children, being non invasive and simple to perform 15. A normal or near normal value for PIMAX is useful in ruling out weakness of the respiratory muscles and avoiding the necessity for more complex and/or invasive tests 15. Equally, serial measurements of PIMAX could be a useful monitoring tool to track respiratory muscle function. There are, however, no guidelines as to how the PIMAX measurement should be made in ventilated infants and children.
Studies in ventilated adult patients 16,17 have demonstrated higher values of PIMAX when a one-way valve was used, which allowed flow during expiration but not inspiration. PIMAX was achieved approximately 20 seconds or 10 efforts after initiating occlusion 17. Baumeister et al 18 observed that PIMAX in mechanically ventilated children was most negative 12 to 15 seconds after airway occlusion, using a one way valve. A direct comparison with airway occlusion during both inspiration and expiration was, however, not performed. In addition, the mechanisms underlying the greater pressures achieved with inspiratory occlusion compared to maintained inspiratory and expiratory occlusion are unclear, although enhanced respiratory drive and reduced lung volume may be important 16,17.
We hypothesised that PIMAX would be higher during airway occlusion when only inspiratory efforts were occluded (PIMAXUNI) compared to those where the occlusion was maintained throughout both inspiration and expiration (PIMAXOCC). The aim of this study was, therefore, to compare PIMAXOCC to PIMAXUNI in mechanically ventilated children with a broad range of ages and diagnoses. In addition, we wished to determine the relationship between any change in lung volume during PIMAXUNI and inspiratory pressure and also the influence of respiratory drive. Some of the results of these studies have been previously reported in the form of an abstract 19
Materials and methods
A prospective study was conducted in the Variety Club Children's Hospital paediatric intensive care unit at King's College Hospital, London. Children who were mechanically ventilated for more than 24 hours were eligible for entry into the study. Informed written consent was obtained from the parent(s) or carers. Measurements were performed when the children were deemed ready for extubation by the clinical team. Approval was obtained from the Research Ethics Committee of King's College Hospital NHS Trust.
Respiratory flow was measured using a pneumotachograph (series 4500, Hans Rudolph Inc., Kansas City, Mo) and associated differential pressure transducer (MP45, Validyne Corporation, Northridge CA, USA) attached to a three-way slide valve (2820 Series, Hans Rudolph Inc., Kansas City, MO) (deadspace 2 ml) used to occlude the airway, and inserted between the endotracheal tube and ventilator circuit (Fig 1). Airway pressure was measured from a side port on the pneumotachograph using a differential pressure transducer (MP45, Validyne Corporation, Northridge CA, USA). The flow and pressure signals were amplified (CD 280, Validyne, Northridge, CA, USA) and recorded and displayed in real time on a computer running an application written using Labview software (National Instruments, Austin TX, USA) with 100 Hz analog to digital sampling (DAQ 16XE-50, National Instruments, Austin TX USA). Tidal volume was obtained by digital integration of the flow signal by the software.
Fig 1.
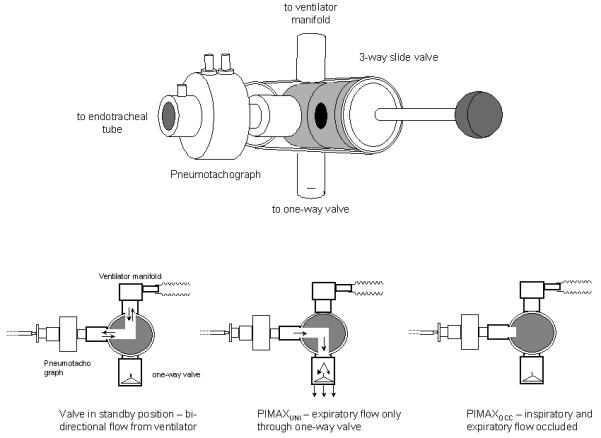
A diagram of the 3 way slide valve and its position in the ventilator circuit. Arrows show direction of airflow with the valve positioned at standby, for PIMAXOCC and PIMAXUNI
Lung volume was determined by measurement of functional residual capacity (FRC) using a helium (He) dilution system (EBS 2615, Equilibrated Biosystems, Melville, NY, U.S.A.) with a circuit specifically designed for paediatric use (total volume 95 ml). The circuit contained a one litre rebreathing bag, enclosed in an airtight cylinder, which was filled with a mixture of He and oxygen (O2). The rebreathing bag was inserted into the ventilator circuit immediately above the endotracheal tube via a three-way valve. Actuation of the valve, at end-expiration, connected the patient to the rebreathing bag and diverted flow from the ventilator to the airtight cylinder, thus maintaining mechanical ventilation. The change in He concentration against time was displayed in real time on a flat panel display. Rebreathing of the He/O2 gas mixture was continued until equilibration of He within the system, indicated by no change in the He concentration over a 15 second period. The FRC was corrected for oxygen consumption, assumed to be 7 ml/kg/minute 20 and to body temperature under atmospheric pressure and water vapour saturated conditions. The FRC was measured twice and expressed as the mean of paired measurements within 10% of each other.
Protocol
All measurements were performed with the patients supine, with stable blood gases and blood pressure. The medical team responsible for routine clinical care determined the ventilator settings. Patients were sedated but not receiving muscle relaxants and all were intubated using cuffed endotracheal tubes. Endotracheal tube suction was undertaken 10 to 15 minutes prior to each study. Patients were closely monitored throughout and airway occlusion discontinued if desaturation or bradycardia occurred. FRC was measured prior to PIMAX assessment. The patients were switched over to continuous positive airway pressure (5 cmH2O) during PIMAX measurements.
To measure PIMAXOCC the slide valve was used to occlude the airway during inspiration and expiration. For PIMAXUNI the slide valve was positioned to direct expiratory flow through a unidirectional valve, allowing expiratory but not inspiratory flow (Fig 1). All occlusions were initiated at end expiration, judged using respiratory flow, and maintained for approximately 10 breaths or 20 seconds, whichever was longer. Three sets of occlusions were performed using each method in random order and the maximum peak pressure values for each technique noted. The intra-occasion coefficients of variation for both techniques were determined. The number of inspiratory efforts and the time to reach PIMAX from the start of occlusion were assessed. Baseline respiratory drive as assessed by the pressure generated in the first 100 ms (P0.1) of the first inspiratory effort during the occlusion and the volume of expired air during PIMAXUNI were also measured. P0.1 was measured to ensure that differences between techniques were not due to differences in the underlying baseline respiratory drive. Adequate time was allowed to elapse between occlusions to allow baseline conditions to be re-established.
Statistical analysis
Data from all occlusions and those from occlusions that resulted in the maximum inspiratory pressure were analysed for each technique. The data for P0.1 were demonstrated to be non-normally distributed (Shapiro-Wilk) and hence are expressed as median and range and differences assessed for statistical significance using the Wilcoxon signed rank test. All other variables are expressed as mean and standard deviation and differences assessed using paired t test. The agreement between the techniques was assessed using Bland and Altman analysis 21. Spearman's correlation analysis was used to assess the strength of relationships. Statistical analysis was performed using GraphPad Prism (version 3.03 for Windows, GraphPad Software, San Diego California USA)
Results
Twenty-two infants and children (mean (SD) age 4.8 (4.5) years (Table 1), with a mean (SD) duration of mechanical ventilation of 4.4 (4.7) days were studied. Occlusions were discontinued in two patients, because they coughed and desaturated; the measurements were successfully completed in both subjects on a later occasion. Both manoeuvres were well tolerated by the other patients. The mean FRC was 399.3 ml (331.6), 20.8 (3.0) ml/kg when corrected for body weight (Table 1).
Table 1.
Characteristics of the patients included in the study
| Age (yrs) |
Wt (Kg) |
Ht (cm) |
Length of ventilation (days) |
Diagnosis | FRC (ml) |
FRC per unit body weight (ml/kg) |
|
|---|---|---|---|---|---|---|---|
| 1 | 16 | 65 | 165 | 5 | Post op, GI bleeding | 1585.5 | 24.4 |
| 2 | 5.5 | 12.6 | 96 | 2 | Liver Transplant | 255.0 | 20.2 |
| 3 | 5.3 | 20 | 110 | 7 | Sepsis, Multi organ failure |
541.5 | 27.1 |
| 4 | 4 | 16 | 102 | 18 | Spinal Injury, Diaphragm weakness |
270.0 | 16.9 |
| 5 | 0.2 | 3.96 | 60 | 1 | Lower RespiratoryTract Infection |
80.5 | 20.3 |
| 6 | 1.5 | 14 | 82 | 5 | Adenovirus Lower RespiratoryTract Infection |
269.5 | 19.3 |
| 7 | 0.83 | 12.7 | 78.5 | 4 | Meningitis | 244.0 | 19.2 |
| 8 | 1.5 | 10.7 | 76 | 1 | Liver Transplant | 241.0 | 22.5 |
| 9 | 16 | 47 | 140 | 5 | Cystic Fibrosis, Respiratory Failure |
904.5 | 19.2 |
| 10 | 0.5 | 5.1 | 65 | 3 | Liver Failure | 116.3 | 22.8 |
| 11 | 9 | 32 | 137 | 2 | Head Injury | 743.5 | 23.2 |
| 12 | 1.8 | 10 | 75 | 3 | Sepsis, Liver Failure | 186.5 | 18.7 |
| 13 | 3.5 | 15 | 96 | 16 | Sepsis, ARDS | 264.0 | 17.6 |
| 14 | 2 | 10.5 | 78 | 3 | Liver Transplant | 200.0 | 19.1 |
| 15 | 7 | 20.5 | 120 | 2 | Aspiration Pneumonia | 396.0 | 19.3 |
| 16 | 8 | 18.7 | 118 | 3 | Cerebral Palsy, Respiratory Failure |
424.0 | 22.7 |
| 17 | 7.5 | 20 | 115 | 3 | Pneumonia, Respiratory Failure |
415.0 | 20.8 |
| 18 | 1.3 | 14 | 80 | 2 | Liver Failure, encephalopathy |
301.0 | 21.5 |
| 19 | 1.25 | 13.5 | 76 | 3 | Liver Transplant | 259.5 | 19.2 |
| 20 | 4.2 | 16 | 100 | 12 | Multiple Fractures, Lung contusion, ARDS |
242.0 | 15.1 |
| 21 | 2.8 | 12 | 82 | 3 | Meningococcemia | 244.5 | 20.4 |
| 22 | 5.5 | 22 | 115 | 2 | Post operative evacuation of subdural hematoma |
601.0 | 27.3 |
Occlusions resulting in the maximum inspiratory pressure
The mean (SD) PIMAXUNI was 45.5 (15.2) cmH2O which was significantly greater than the mean PIMAXOCC which was 30.9 (9.0) cmH2O (p<0.0001), Table 2). PIMAXUNI was greater than PIMAXOCC in all but two patients. Bland and Altman analysis demonstrated a mean (SD) difference of −14.7 (13.1) cmH2O between PIMAXOCC and PIMAXUNI (Fig 2). There was a significant relationship (r2 = 0.29, p<0.01) between the mean of PIMAXOCC and PIMAXUNI and the difference between PIMAXOCC and PIMAXUNI, indicating the difference between measurements increased as PIMAX increased. The mean (SD) total expired volume through the one-way valve during PIMAXUNI was 98 ml (62.3) equivalent to a mean (SD) reduction in FRC of 33.1% (13.9). There was, however, no significant relationship between the difference between PIMAXOCC and PIMAXUNI and the % change in FRC during PIMAXUNI (r2 = 0.06). As airways obstruction can affect the accuracy of FRC measured by He dilution, we performed a sub-analysis in a subset of patients (n=16) without obvious obstructive lung disease. No significant relationship between the difference between PIMAXOCC and PIMAXUNI and the % change in FRC during PIMAXUNI (r2=0.03) was observed. There were no statistically significant differences in the number of efforts required and the time to reach PIMAX (Table 2) between the two techniques. There were no statistically significant differences in baseline respiratory drive during assessment of the two techniques or the peak pressure generated during the first occluded inspiratory effort (Table 2). The mean (SD) within occasion coefficients of variation for PIMAX were similar for both techniques (PIMAXOCC 15.6% (12.7) and PIMAXUNI 15.3 % (8.4)
Table 2.
Occlusions resulting in the maximum inspiratory pressure. Mean (SD) PIMAX results for both techniques, number of efforts and time to reach PIMAX, the pressure generated for the first inspiratory effort during the occlusion and the median (range) respiratory drive as indicated by the P0.1.
| PIMAXOCC | PIMAXUNI | P value | |
|---|---|---|---|
| PIMAX (cmH2O) | 30.5 (9.0) | 45.5 (15.2) | <0.001 |
| No. of efforts | 6.6 (2.8) | 7.2 (2.9) | 0.2 |
| Time to PIMAX (sec) | 11.3 (3.1) | 10.9 (2.9) | 1 |
| P0.1 (cmH2O) | 2.2 (1.0-5.4) | 2.4 (0.9-6.1) | 0.9 |
| 1st inspiratory effort (cmH2O) | 15.8 (6.8) | 16.5 (11.0) | 0.63 |
Fig 2.
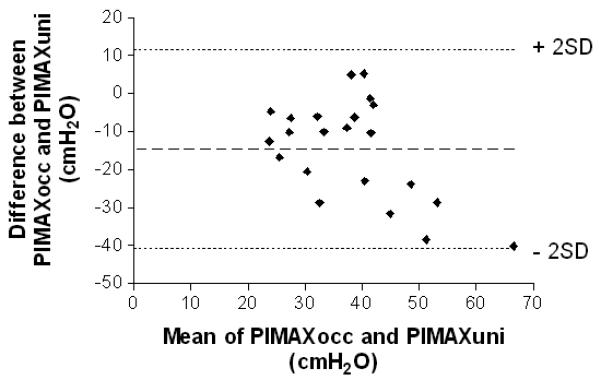
Bland and Altman plot of the agreement between PIMAXOCC and PIMAXUNI with the mean of PIMAXOCC and PIMAXUNI plotted on the horizontal axis and the difference between PIMAXOCC and PIMAXUNI plotted on the vertical axis. Dashed lines represent the mean difference and 2SD
All occlusions
When all sets of occlusions were analysed, including those that resulted in the maximum inspiratory pressure, a similar pattern between techniques was observed for the number of efforts required and the time to reach the maximum inspiratory pressure for individual occlusions, the pressure generated on the first effort of the occlusion and the baseline P0.1 (Table 3). Based on these data, the 99% CI of the mean indicated that a period of occlusion of 12 seconds or 8 inspiratory efforts, whichever longer, would be sufficient in mechanically ventilated infants and children for maximal inspiratory pressure measurement.
Table 3.
All occlusions. Mean (SD) number of efforts and time to reach maximum occlusion pressure, the pressure generated for the first inspiratory effort during the occlusion and the median (range) respiratory drive as indicated by the P0.1 for all sets of occlusions for both techniques (n=66).
| PIMAXOCC | PIMAXUNI | P value | |
|---|---|---|---|
| No. of efforts | 6.2 (2.6) | 7.2 (3.1) | 0.08 |
|
Time to max occlusion pressure (sec) |
11.2 (3.2) | 10.7 (3.4) | 0.4 |
| P0.1 (cmH2O) | 2.0 (0.9-6.5) | 2.5 (0.7-6.2) | 0.3 |
| 1st inspiratory effort (cmH2O) | 13.2 (6.5) | 14.6 (8.8) | 0.31 |
Discussion
We have demonstrated that in mechanically ventilated children, greater maximal inspiratory pressures were obtained using a one-way valve that occludes only during inspiration compared to occlusion during inspiration and expiration. As muscle length and geometry can influence the pressure development by the diaphragm 22, the timing of the occlusion could affect PIMAX. In the current study, care was taken to ensure that the occlusions for both techniques were performed at end-expiration. This was achieved by close examination of respiratory flow from the pneumotachograph inserted into the ventilator circuit. Accurate timing of the occlusion was also supported by the fact that there was no statistically significant difference between techniques for the first inspiratory effort during the occlusion. To further reduce variability, occlusions with both techniques were performed with the patient in the same supine position to eliminate differences in body position and therefore ribcage configuration. The coefficients of variation for both techniques were comparable to those obtained in healthy, non ventilated children performing the equivalent volitional technique 23. The level of respiratory drive would also directly affect PIMAX; measurements of P0.1, a reliable index of respiratory drive in paediatric subjects 24, demonstrated no significant difference in baseline respiratory drive at the start of the occlusion between measurement techniques. All the children studied were intubated using cuffed endotracheal tubes and therefore pressure loss due to leak was not a factor. A number of the children with obstructive lung disease had low FRCs measured using He dilution spirometry. There are few techniques currently available to measure lung volume in children receiving mechanical ventilation and He dilution using a “bag in bottle” system provides a relatively accurate means of measurement while ensuring mechanical ventilation is maintained at predetermined levels. He dilution, however can only measure the volume of the lung in communication with the central airways and hence in severe obstruction may underestimate lung volume. A sub-analysis performed in patients without obvious obstructive lung disease, however, failed to demonstrate a relationship between the reduction in lung volume and PIMAX.
The children in our study group had a wide range of ages and a variety of diagnoses, including primary lung disease and patients with primary muscle/nerve abnormalities. We felt it was important to use a heterogeneous group of patients and hence to make a robust comparison of the performance of PIMAXUNI and PIMAXOCC across a range of pathophysiological conditions. The children were all mechanically ventilated at the time of study and hence their respiratory muscle strength may have been reduced. The main goal of the study was to compare the performance of PIMAXOCC and PIMAXUNI and investigate underlying mechanisms, but not to generate normative data for comparative purposes. A similar study in ventilated adult patients 16 found that PIMAXUNI exceeded PIMAXOCC only 75% of the time. PIMAXUNI was greater than PIMAXOCC in all but two patients (Table 1). Neither patient was markedly different from the rest of the cohort in terms of their age, lung volume, or duration of ventilation. The difference between PIMAXOCC and PIMAXUNI in the two patients was relatively small compared to the overall difference for the cohort (mean (SD) −14.7 (13) cmH2O), we therefore, feel our data supports the assertion that a unidirectional valve yields greater values of PIMAX in mechanically ventilated infants and children.
All our subjects reached PIMAX within 20 seconds or 10 inspiratory efforts of the airway occlusion regardless of the technique applied. Therefore we suggest that a period of occlusion of 12 seconds or 8 inspiratory efforts (99% CI of mean) whichever is longer, would be sufficient in mechanically ventilated infants and children for maximal inspiratory pressure measurement. This is less than that recommended in adults, in whom the occlusion has to be maintained for at least 20 to 25 seconds 17. The difference may be because PIMAX tests are often applied to conscious or semi-conscious adult patients in whom the degree of mental alertness will directly affect their cooperation with the test and the eventual result. Unlike adult patients, the majority of infants and children are at least lightly sedated and not fully conscious until just prior to extubation 25,26.
Previous studies in adult subjects 16,17 have suggested that the greater values obtained using the inspiratory only occlusion could be due to either a change in lung volume or increased respiratory drive during the manoeuvre. The unidirectional valve selectively permits exhalation, such that the inspiratory efforts occur from progressively lower lung volume, resulting in increased force generation due to improvements in the length-tension relationship of respiratory muscles 27,28. Measurement of mouth pressure accurately reflects both the pressure developed by the respiratory muscles, plus the passive elastic recoil pressure of the lung and chest wall. For inspiratory maneuvers against an occlusion performed at functional residual capacity (FRC), recoil pressure is zero so that mouth pressure directly reflects the pressure generated by the inspiratory muscles. However, for inspiratory efforts performed between FRC and residual volume (RV), the contribution of recoil pressure to PIMAX increases, such that in adult subjects the contribution of elastic recoil can be as much as 30% of PIMAX or more if inspiratory muscle strength is decreased. Therefore, the outward recoil pressure of the chest wall increasingly contributes to the pressure generated by respiratory muscles as lung volume reduces towards RV. In the current study, however, correlation analysis did not show a relationship between the difference in PIMAXOCC and PIMAXUNI and the percentage change in FRC during PIMAXUNI in either the group as a whole or only in patients without obvious obstructive lung disease in whom the FRC may have been underestimated. This suggests that the change in muscle length and chest wall geometry resulting from a decrease in lung volume may not fully explain the increased pressure development during PIMAXUNI. We did not formally assess the presence of intrinsic positive end expiratory pressure (PEEPi) and dynamic inflation in our patients and therefore we cannot discount that a possible cause for the lack of a significant relationship between reduction in lung volume and PIMAX was due to PEEPi and/or dynamic hyperinflation. Although it is acknowledged that there is a direct relationship between lung volume and respiratory muscle length and hence muscle strength 27,28 we were unable to demonstrate any relationship between PIMAXuni and a reduction in lung volume in mechanically ventilated children.
The increase in PaCO2 and decrease PaO2 that occur during an occlusion will lead to an increase in respiratory drive during PIMAXocc and PIMAXuni. Reducing lung volume below FRC, however, directly increases respiratory drive via a vagally mediated increase in respiratory frequency and tidal volume due to a decrease in the inspiratory and expiratory activity of slowly adapting stretch receptors 29. Hence, interaction between the mechanically and humorally mediated respiratory drive could lead to a greater increase in drive during a PIMAXUNI manoeuvre when the lung volume falls towards RV. In support of this, studies in healthy subjects have indicated that breath holding time is lung volume dependent and voluntary apnoea can be sustained longer at higher rather than lower lung volumes 30-33. A change in muscle length and chest wall geometry and enhancement of respiratory drive may, therefore, both contribute to the greater PIMAX observed when using a one-way valve.
Conclusions
Maximal inspiratory pressure measurements using a unidirectional valve, selectively permitting exhalation, yield greater values, in mechanically ventilated infants and children, than complete airway occlusion. We recommend a period of occlusion of 8 inspiratory efforts or 12 seconds as being adequate for measuring maximal inspiratory pressure in ventilated children.
Acknowledgements
“The authors acknowledge financial support from the Wellcome trust (GH) and the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London.”
Support: Dr Harikumar was supported by the Wellcome Trust
Footnotes
Data presented at the European Respiratory Society Conference Copenhagen 2005
References
- 1.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;170(6):626–632. doi: 10.1164/rccm.200401-042OC. [DOI] [PubMed] [Google Scholar]
- 2.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169(3):336–341. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 3.Manczur TI, Greenough A, Rafferty GF, Dimitriou G, Baker AJ, Mieli-Vergani G, Rela SM, Heaton N. Diaphragmatic dysfunction after pediatric orthotopic liver transplantation. Transplantation. 2002;73(2):228–232. doi: 10.1097/00007890-200201270-00013. [DOI] [PubMed] [Google Scholar]
- 4.Leon A, Boczkowski J, Dureuil B, Desmonts JM, Aubier M. Effects of endotoxic shock on diaphragmatic function in mechanically ventilated rats. J Appl Physiol. 1992;72(4):1466–1472. doi: 10.1152/jappl.1992.72.4.1466. [DOI] [PubMed] [Google Scholar]
- 5.Lin MC, Ebihara S, El Dwairi Q, Hussain SN, Yang L, Gottfried SB, Comtois A, Petrof BJ. Diaphragm sarcolemmal injury is induced by sepsis and alleviated by nitric oxide synthase inhibition. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1656–1663. doi: 10.1164/ajrccm.158.5.9803112. [DOI] [PubMed] [Google Scholar]
- 6.Bolton CF. Neuromuscular manifestations of critical illness. Muscle Nerve. 2005;32(2):140–163. doi: 10.1002/mus.20304. [DOI] [PubMed] [Google Scholar]
- 7.Polkey MI, Moxham J. Clinical aspects of respiratory muscle dysfunction in the critically ill. Chest. 2001;119(3):926–939. doi: 10.1378/chest.119.3.926. [DOI] [PubMed] [Google Scholar]
- 8.Deem S, Lee CM, Curtis JR. Acquired neuromuscular disorders in the intensive care unit. Am J Respir Crit Care Med. 2003;168(7):735–739. doi: 10.1164/rccm.200302-191UP. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher CG. Respiratory steroid myopathy. Am J Respir Crit Care Med. 1994;150(1):4–6. doi: 10.1164/ajrccm.150.1.8025769. [DOI] [PubMed] [Google Scholar]
- 10.van Balkom RH, van der Heijden HF, van Moerkerk HT, Veerkamp JH, Fransen JA, Ginsel LA, Folgering HT, van Herwaarden CL, Dekhuijzen PN. Effects of different treatment regimens of methylprednisolone on rat diaphragm contractility, immunohistochemistry and biochemistry. Eur Respir J. 1996;9(6):1217–1223. doi: 10.1183/09031936.96.09061217. [DOI] [PubMed] [Google Scholar]
- 11.Hart N, Kearney MT, Pride NB, Green M, Lofaso F, Shah AM, Moxham J, Polkey MI. Inspiratory muscle load and capacity in chronic heart failure. Thorax. 2004;59(6):477–482. doi: 10.1136/thx.2003.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knook LM, de Kleer IM, van der Ent CK, van der Net JJ, Prakken BJ, Kuis W. Lung function abnormalities and respiratory muscle weakness in children with juvenile chronic arthritis. Eur Respir J. 1999;14(3):529–533. doi: 10.1034/j.1399-3003.1999.14c09.x. [DOI] [PubMed] [Google Scholar]
- 13.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 14.Siafakas NM, Salesiotou V, Filaditaki V, Tzanakis N, Thalassinos N, Bouros D. Respiratory muscle strength in hypothyroidism. Chest. 1992;102(1):189–194. doi: 10.1378/chest.102.1.189. [DOI] [PubMed] [Google Scholar]
- 15.Gaultier C, Allen J, England S. Tests of respiratory muscle function in children: ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:571–578. [Google Scholar]
- 16.Caruso P, Friedrich C, Denari SD, Ruiz SA, Deheinzelin D. The unidirectional valve is the best method to determine maximal inspiratory pressure during weaning. Chest. 1999;115(4):1096–1101. doi: 10.1378/chest.115.4.1096. [DOI] [PubMed] [Google Scholar]
- 17.Marini JJ, Smith TC, Lamb V. Estimation of Inspiratory Muscle Strength in Mechanically Ventilated Patients : The Measurement of Maximal Inspiration Pressure. J Crit Care. 1986;1(1):32–38. [Google Scholar]
- 18.Baumeister BL, el-Khatib M, Smith PG, Blumer JL. Evaluation of predictors of weaning from mechanical ventilation in pediatric patients. Pediatr Pulmonol. 1997;24(5):344–352. doi: 10.1002/(sici)1099-0496(199711)24:5<344::aid-ppul7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Harikumar G, Greenough A, Moxham J, Rafferty GF. Maximal inspiratory pressure measurement in ventilated children – comparison of inspiratory occlusion to total respiratory occlusion. Eur Respir J. 2005;26(Suppl. 49):676s. [Google Scholar]
- 20.Hey EN. The relation between environmental temperature and oxygen consumption in the newborn baby. J Physiol (Lond) 1969;200(3):589–603. doi: 10.1113/jphysiol.1969.sp008710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 22.Grassino A, Goldman MD, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol. 1978;44(6):829–839. doi: 10.1152/jappl.1978.44.6.829. [DOI] [PubMed] [Google Scholar]
- 23.Stefanutti D, Fitting JW. Sniff nasal inspiratory pressure: Reference values in Caucasian children. Am J Respir Crit Care Med. 1999;159(1):107–111. doi: 10.1164/ajrccm.159.1.9804052. [DOI] [PubMed] [Google Scholar]
- 24.Gaultier C, Perret L, Boule M, Buvry A, Girard F. Occlusion pressure and breathing pattern in healthy children. Respir Physiol. 1981;46(1):71–80. doi: 10.1016/0034-5687(81)90069-4. [DOI] [PubMed] [Google Scholar]
- 25.Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288(20):2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- 26.Twite MD, Rashid A, Zuk J, Friesen RH. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5(6):521–532. doi: 10.1097/01.PCC.0000144710.13710.2E. [DOI] [PubMed] [Google Scholar]
- 27.Braun NM, Arora NS, Rochester DF. Force-length relationship of the normal human diaphragm. J Appl Physiol. 1982;53(2):405–412. doi: 10.1152/jappl.1982.53.2.405. [DOI] [PubMed] [Google Scholar]
- 28.Polkey MI, Hamnegard CH, Hughes PD, Rafferty GF, Green M, Moxham J. Influence of acute lung volume change on contractile properties of human diaphragm. J Appl Physiol. 1998;85(4):1322–1328. doi: 10.1152/jappl.1998.85.4.1322. [DOI] [PubMed] [Google Scholar]
- 29.Green JF, Kaufman MP. Pulmonary afferent control of breathing as end-expiratory lung volume decreases. J Appl Physiol. 1990;68:2186–2194. doi: 10.1152/jappl.1990.68.5.2186. [DOI] [PubMed] [Google Scholar]
- 30.Kelman GR, Wann KT. Mechanical and chemical control of breath holding. Quart J Exp Physiol. 1971;56:92–100. doi: 10.1113/expphysiol.1971.sp002111. [DOI] [PubMed] [Google Scholar]
- 31.Mithoeffer JC. Lung volume restriction as a ventilatory stimulus during breath holding. J Appl Physiol. 1959;14:701–705. doi: 10.1152/jappl.1959.14.5.701. [DOI] [PubMed] [Google Scholar]
- 32.Whitelaw WA, McBride B, Ford GT. Effect of lung volume on breath holding. J Appl Physiol. 1987;62(5):1962–1969. doi: 10.1152/jappl.1987.62.5.1962. [DOI] [PubMed] [Google Scholar]
- 33.Godfrey S, Campbell EJM. The control of breath holding. Respir Physiol. 1968;5:385–400. doi: 10.1016/0034-5687(68)90030-3. [DOI] [PubMed] [Google Scholar]


