Abstract
Purpose
This study aimed to assess whether a young age at the time of diagnosis with osteosarcoma has value to predict the prognosis.
Materials and Methods
Sixty-seven children with stage II osteosarcoma were stratified according to the age of 10. There were 32 preadolescents (≤10 years) and 35 adolescents (10<age≤15 years). The patients were analyzed for their clinical characteristics, the histologic response to preoperative chemotherapy, event-free survival (EFS) and the patterns of relapse.
Results
After a median follow-up of 54 months (range: 6~153 months), the 5-year EFS of the preadolescent and adolescent groups was 64.5±9.3% and 58.2±9.1%, respectively, and age did not have any statistical significance for survival (p=0.55). Cox regression analysis revealed that both the serum level of alkaline phosphatase and the histologic response to preoperative chemotherapy were significantly related to survival of the 67 patients. Those patients aged less than 7 years responded poorly to preoperative chemotherapy and their rate of amputation was 43%. However, their 5-year EFS was not statistically different from the older patients (57.1±18.7 vs 67.7±6.3%, respectively, p=0.58).
Conclusions
We could not find any statistical difference in the clinical characteristics and survival from osteosarcoma for the preadolescents and adolescents, so the current approach of having the same protocol for both groups of patients seems to be reasonable.
Keywords: Osteosarcoma, Age, Preadolescents, Prognosis
INTRODUCTION
Childhood cancer has a tendency to show a specific age distribution and this serves as a prognostic factor for some of these diseases (1). Acute lymphoblastic leukemia and neuroblastoma are good examples for which age carries prognostic significance and the therapeutic approach follows this factor (2~5). The incidence of osteosarcoma peaks at the onset of puberty and this disease is not common in the very young and preadolescent patients (6). However, there is controversy about the prognostic significance of age. Most of the previous series have arbitrarily set their cut-off points at between 10 to 15 years (7~9). McKenna et al reported that children 10 years of age and younger had a trend for earlier pulmonary metastases (10). Other published data has shown that the outcome of patients younger than 12 years is worse than that of older patients (11). On the contrary, different series have reported that the survival of patients 12 to 15 years old and younger is the same as adults (9,12). In addition to the confusing cutoff values, some of these studies included only a small number of children and the treatments were not homogeneous (7,12~14).
The high prevalence of osteosarcoma during the growth spurt period suggests that the osteosarcoma that develops in preadolescents might have different features for its pathogenesis and the clinical characteristics (14). We hypothesized that the prognosis of osteosarcoma in younger children might be worse than that of their older counterparts. While the previously reported series compared the survival data between children and adults, we limited our study population only to children 15 years old and younger. The reported age for pubertal changes is variable between genders and this is also influenced by the nutritional status. Therefore, we arbitrarily set the age at 10 and we divided the patients into two groups to include only the patients who are in their preadolescent period. We asked two questions: 1) is the survival of younger patients inferior to the survival of older patients? 2) Are there any differences in the clinicopathologic characteristics between these two groups?
MATERIALS AND METHODS
1) Patients selection
We retrospectively reviewed the medical records of 67 pediatric osteosarcoma patients who were treated at our institution from May 1992 to August 2005. They met the following criteria: (1) an age at diagnosis of 15 or less; (2) AJCC stage II (15) (3) the disease was located in the extremities; (4) there was no history of treatment except biopsy; (5) surgery and chemotherapy were done at our institution; (6) the event-free patients had more than 2-years follow up. These patients were categorized in two groups according to their ages at the time of diagnosis. There were 32 preadolescents (10 years and younger) and 35 adolescents (10~15 years old). The analyzed factors were the patients' characteristics, the treatment outcome and the pattern of relapse. Informed written consent was obtained from all the patients or their legal guardians.
2) Treatment and follow-up after completion of treatment
The procedures to define the extent of tumor included plain radiography and MRI of the primary tumor, 99mTc-methylene diphosphonate whole-body bone scanning, computed tomography scans of the chest and measurement of the alkaline phosphatase level. The reference value for alkaline phosphatase varied according to age; 145~420 U/L for the patients 1~9 years old; 130~560 u/L for the patients 10~11 years old; 130~495 U/L for the 12~15 year-old boys; 130~420 for the 12~13 year-old girls; 70~230 U/L for the 14~15 year-old girls. The diagnosis of osteosarcoma was confirmed through the histological slides of the tumor tissue, which was obtained from open or needle biopsy, as well as from resected specimens. The patients underwent two cycles of preoperative chemotherapy followed by four cycles of postoperative chemotherapy. Briefly, 12 g/m2 of methotrexate was administered twice, on days 1 and 7. On day 14, 100 mg/m2 cisplatin was given for 2 hours. Subsequently, 60 mg/m2 of doxorubicin was delivered for 48 hours. Definitive surgery was scheduled between weeks 10 and 12. A limb salvage operation was performed except in those cases when the tumor involved neurovascular structures or functional restoration was not expected. The post-operative chemotherapy protocol followed the identified histologic response to preoperative chemotherapy. The histological grading followed the criteria of Rosen et al. with less than 10% residual viable tumor indicating a good response, whereas more than 10% residual viable tumor indicated a poor response (16). The good responders received the same therapy as the preoperative chemotherapy, while the poor responders were switched to an ifosfamide-bleomycin-doxorubicin-cisplatin-based protocol. The dosages of cisplatin and doxorubicin were the same as the preoperative doses, and ifosfamide (14 g/m2) was infused continuously for 7 days along with the bleomycin (30 mg/m2). The scheduled duration of chemotherapy was 24 weeks for the good responders and 36 weeks for the poor responders.
The follow-up consisted of monthly plain radiographs of the primary site and the chest, and routine laboratory tests. Computed tomography of the chest and 99mTc-methylene diphosphonate whole-body bone scanning were done every 3 months for 2 years. After 3 years from the completion of therapy, these studies were repeated every 6 months.
3) Statistical analysis
The patient characteristics were compared by chi square tests. Disease progression, metastasis, local recurrence and death due to chemotherapy-related toxicities were considered as adverse events. Event-free survival was defined as the time from study entry until an adverse event or the last patient contact. The survival was calculated by the Kaplan-Meier method and then this was compared between the groups by the log-rank test. The prognostic significance of the various factors on survival was examined by Cox regression analysis.
RESULTS
1) Comparison of the clinicopathologic variables between the two groups
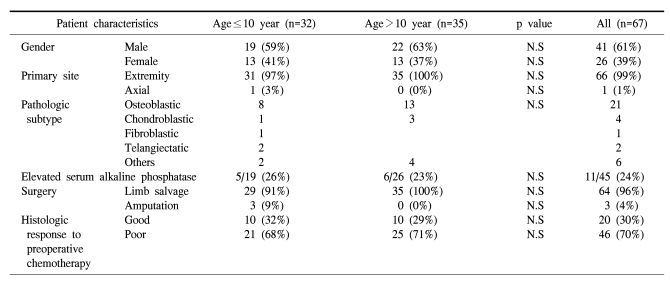
There were no differences in gender, tumor location and the serum levels of alkaline phosphatase between the two age groups. The distribution pattern of the histologic subtypes showed no differences between the two age groups. The dose intensity of chemotherapy in each group was the same; preadolescents did not require any delay or dose reduction of the chemotherapeutic agents. In the preadolescent group, 29 patients underwent limb salvage operation, and there were 3 cases of amputation. The rate of limb salvage was slightly higher in the control group (91% vs. 100%, respectively). The percent of good responders was 32% and 29%, respectively (Table 1).
Table 1.
Comparison of patient characteristics, type of treatment and histologic response to preoperative chemotherapy
2) Comparison of survival between the two groups
The median follow-up duration of the 67 patients was 54 months (range: 6~153 months). The median follow-up duration was not different between the two age groups (39 months for the preadolescents vs 33 months for the adolescents). The median follow-up duration was 54 months (range: 6~153 months). The final survival status of the preadolescent group was being continuously disease free for 23 patients, 1 patient was alive with disease and 8 patients died of disease.
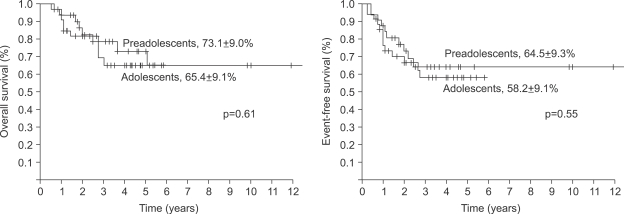
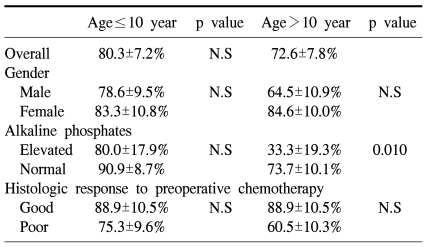
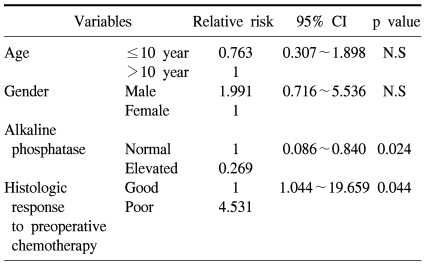
The 5-year overall survival of all the patients was 69.2±6.4% and there was no difference between the two groups (73.1±9.0% for the preadolescents vs 65.4±9.1% for the adolescents, p=0.61, Fig. 1). The 5-year EFS for each group was 64.5±9.3% and 58.2±9.1%, respectively, and there were no significant differences between the two groups (Fig. 1, p=0.55). The presence of the following factors favored better survival in all 67 cases; normal serum alkaline phosphatase levels and a good histologic response to preoperative chemotherapy. Yet the abovementioned results were not reproducible when we analyzed these factors in the individual subgroups. While the serum alkaline phosphatase level had an impact on event free survival in the control group, there was haphazard correlation in the preadolescent age groups (Table 2). The Cox regression analysis also revealed that the serum level of alkaline phosphatase and the histologic response to preoperative chemotherapy were independently related to event free survival. The patient's age at the time of diagnosis did not have any impact on EFS (Table 3).
Fig. 1.
Comparison of overall (OS) and event-free free survival (EFS) of patients according ages. There were no survival difference between preadolescents and adolescents.
Table 2.
Event free survival at 2 year in patients with age≤10 years and >10 years
Table 3.
Prognostic factors for event-free survival in whole patients
3) Pattern of relapse and outcome in the two groups
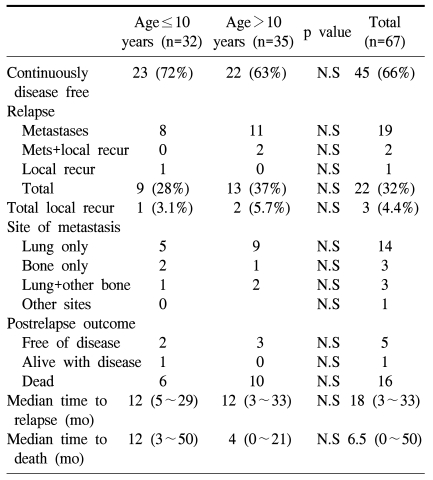
There were 9 cases of relapse (28%) in the preadolescent group; 8 had lung metastasis and 1 had local recurrence. The adolescent group showed a 37% rate of relapse and a 5.7% rate of local recurrence. The median time to relapse was 14 months in the preadolescent group and 12 months in the adolescent group. The final outcomes of the 9 relapsed cases in the preadolescent group were no evidence of disease for 2, alive with disease for 1 and 6 patients died of disease. The median survival of the 6 expired patients was 12 months from the manifestation of relapse (Table 4).
Table 4.
Patterns of relapse and outcomes
4) Features of the patients aged 7 years old or younger
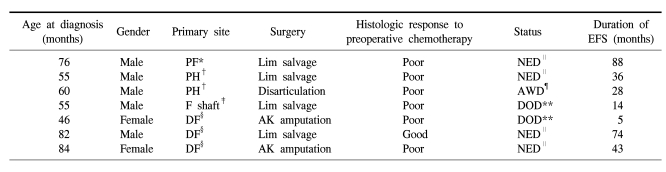
In the preadolescent group, 7 patients were under 7 years old and they showed two distinctive features from the other age groups. First, their histologic response to preoperative chemotherapy was much worse than that of the older children. While 30% of the all the patients showed a good response to preoperative chemotherapy, only 14% (1 case) showed a good response. Second, limb salvage was possible in 57% of the patients. The rate of amputation (43%) was much higher, when considering the limb salvage rate of 100% for the adolescent group (Table 5). However, the 5-year EFS of the patients younger than 7 years was not different from the survival of the patients older than 7 years (57.1±18.7 vs 67.7±6.3%, respectively, p=0.58).
Table 5.
Clinical characteristics of patients age ≤7 years
*proximal femur, †proximal humerus, ‡femur shaft, §distal femur, ∥no evidence of disease, ¶alive with disease, **died of disease.
DISCUSSION
In the clinical setting, an age-dependent occurrence for childhood malignancy has been reported (1,3). The age at diagnosis is generally considered as a prognostic factor, and administering risk-adapted therapy based on these factors seems to be a reasonable approach. For acute lymphoblastic leukemia, the improved survival by administering risk-adapted chemotherapy based on both age and the initial white blood cell count support the aforementioned concept (2,3). Our study evaluated whether the patient's age at the time of diagnosis could be used as a prognostic factor to design risk-adapted therapy.
Although our study included a relatively large number of children who were treated at a single institution with the same protocol, it has several limitations. First, the patients were not randomized or controlled with respect to the treatment protocols. Second, the data about the endocrinologic status of the patients was not sufficient, for example, there was no information concerning the Tanner stage, the bone age or the hormone levels. We could not define any specific age for the onset of puberty. Instead, we chose the age of 10 to purely select those patients who were in their preadolescent period and we presume that this approach might have served to offset the lack of endocrinologic data.
We could not observe any age-dependent associations between the clinical characteristics and survival. Our hypothesis was that preadolescents with osteosarcoma might have inferior outcomes as compared to adolescents. To avoid the masking effect of older patients and to clearly analyze the impact of patient age on survival, we included only those patients younger than 15 years and we then divided them into two groups by the age of 10. Because all these patients were treated and followed-up with same chemotherapy protocol and surgical principles, any bias according to the treatment options was eliminated. The prognostic significance of age for osteosarcoma patients is a matter of debate. From the results of the clinical trials done in the 1960s through early 1980s, osteosarcoma in patients aged younger than 10 or 12 years showed an unfavorable prognosis (7,10,11). However, the recent data concluded that there were no differences in the clinical characteristics and survival between the two age groups (9,12,14). The largest series of analysis to date reported on comparing preadolescents (≤12 years) and older patients (13~40 years), and there was no difference in survival, and the study had strong statistical power (17). The reason why what was once considered to be a poor prognostic factor lost its meaning in another study is not clear. Since prognostic factors are dependent on the treatment, as well as on the intrinsic biology of a tumor, a younger age might no longer be a poor prognostic factor thanks to the progress of the chemotherapeutic protocols, the supportive care and the surgical methodology.
Although interpretation of our data is limited due to the small number of cases, the disease pattern of the patients at 7 years and younger is noteworthy. Most of the patients in this age group showed a poor response to preoperative chemotherapy (6 of 7), and their rate of amputation was much higher (3 of 7) than that for the patients older than 7 years. The amputation rate of 43% during the modern chemotherapy era suggests resistant tumor behavior or a large tumor burden for their body size. Theoretically, a high rate of amputation and a poor response to preoperative chemotherapy should result in a poor outcome. However, the event free survival of these patients was not statistically different from that of the patients older than 7 years. This finding needs to be clarified in further studies. Until now, a poor response to preoperative chemotherapy has been considered to be the most powerful prognostic factor for localized disease. If osteosarcoma in preschool children responds poorly to chemotherapy, yet it has a lower potential to metastasize, then the histologic response to preoperative chemotherapy might lose its predictive power for survival in this age group.
CONCLUSION
The clinical characteristics and survival of children 10 years or younger with osteosarcoma were not different from those of their older counterparts. This justifies the current approach of having the same treatment protocol for both children and adults.
References
- 1.Grovas A, Fremgen A, Rauck A, Ruymann FB, Hutchinson CL, Winchester DP, et al. The national cancer data base report on patterns of childhood cancers in the united states. Cancer. 1997;80:2321–2332. doi: 10.1002/(sici)1097-0142(19971215)80:12<2321::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.van den Berg H, van der Lelie J. Acute lymphoblastic leukaemia in puberty and adolescence. Ann Oncol. 2000;11:1375–1379. doi: 10.1023/a:1026538713236. [DOI] [PubMed] [Google Scholar]
- 3.Silverman LB, Sallan SE. Newly diagnosed childhood acute lymphoblastic leukemia: update on prognostic factors and treatment. Curr Opin Hematol. 2003;10:290–296. doi: 10.1097/00062752-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 4.London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the children's oncology group. J Clin Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 5.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 6.Buckley JD, Pendergrass TW, Buckley CM, Pritchard DJ, Nesbit ME, Provisor AJ, et al. Epidemiology of osteosarcoma and ewing's sarcoma in childhood: a study of 305 cases by the children's cancer group. Cancer. 1998;83:1440–1448. doi: 10.1002/(sici)1097-0142(19981001)83:7<1440::aid-cncr23>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Scranton PE, DeCicco FA, Totten RS, Yunis EJ. Prognostic factors in osteosarcoma. A review of 20 year's experience at the university of pittsburgh health center hospitals. Cancer. 1975;36:2179–2191. doi: 10.1002/cncr.2820360936. [DOI] [PubMed] [Google Scholar]
- 8.Winkler K, Beron G, Delling G, Heise U, Kabisch H, Purfurst C, et al. Neoadjuvant chemotherapy of osteosarcoma: Results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol. 1988;6:329–337. doi: 10.1200/JCO.1988.6.2.329. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, et al. Long-term results of the co-operative german-austrian-swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. 1998;9:893–899. doi: 10.1023/a:1008391103132. [DOI] [PubMed] [Google Scholar]
- 10.McKenna RJ, Schwinn CP, Higinbotham NL. Osteogenic sarcoma in children. Calif Med. 1965;103:165–170. [PMC free article] [PubMed] [Google Scholar]
- 11.Winkler K, Beron G, Kotz R, Salzer-Kuntschik M, Beck J, Beck W, et al. Neoadjuvant chemotherapy for osteogenic sarcoma: Results of a cooperative German/Austrian study. J Clin Oncol. 1984;2:617–624. doi: 10.1200/JCO.1984.2.6.617. [DOI] [PubMed] [Google Scholar]
- 12.Hudson M, Jaffe MR, Jaffe N, Ayala A, Raymond AK, Carrasco H, et al. Pediatric osteosarcoma: therapeutic strategies, results, and prognostic factors derived from a 10-year experience. J Clin Oncol. 1990;8:1988–1997. doi: 10.1200/JCO.1990.8.12.1988. [DOI] [PubMed] [Google Scholar]
- 13.Kozakewich H, Perez-Atayde AR, Goorin AM, Wilkinson RH, Gebhardt MC, Vawter GF. Osteosarcoma in young children. Cancer. 1991;67:638–642. doi: 10.1002/1097-0142(19910201)67:3<638::aid-cncr2820670319>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Rytting M, Pearson P, Raymond AK, Ayala A, Murray J, Yasko AW, et al. Osteosarcoma in preadolescent patients. Clin Orthop Relat Res. 2000;373:39–50. doi: 10.1097/00003086-200004000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Greene FL. The American joint committee on cancer: Updating the strategies in cancer staging. Bull Am Coll Surg. 2002;87:13–15. [PubMed] [Google Scholar]
- 16.Rosen G, Marcove RC, Huvos AG, Caparros BI, Lane JM, Nirenberg A, et al. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106(Suppl):55–67. doi: 10.1007/BF00625054. [DOI] [PubMed] [Google Scholar]
- 17.Bacci G, Longhi A, Bertoni F, Bacchini P, Ruggeri P, Versari M, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27:129–134. doi: 10.1097/01.mph.0000155860.38641.83. [DOI] [PubMed] [Google Scholar]