Abstract
The popular view that all lipid mediators are pro-inflammatory arises largely from the finding that non-steroidal anti-inflammatory drugs block the biosynthesis of prostaglandins. The resolution of inflammation was widely held to be a passive event until recently, with the characterization of novel biochemical pathways and lipid-derived mediators that are actively turned on in resolution possessing potent anti-inflammatory and pro-resolving actions. A lipid mediator informatics approach was employed to systematically identify new families of endogenous local-acting mediators from omega-3-polyunsaturated fatty acids (eicosapentaenoic acid and docosahexaenoic acid) in resolving exudates in addition to the lipoxins and aspirin-triggered lipoxins generated from arachidonic acid. These new chemical mediator families were coined resolvins and protectins, given their potent bioactions. In this annual review, we present recent advances on the biosynthesis and stereospecific actions of these new pro-resolving mediators, which have also proven to be organ protective and anti-fibrotic.
Keywords: anti-inflammation, leukocytes, lipoxins, neutrophils, macrophages, NSAID, omega-3-PUFA, protectins, and resolvins
Introduction
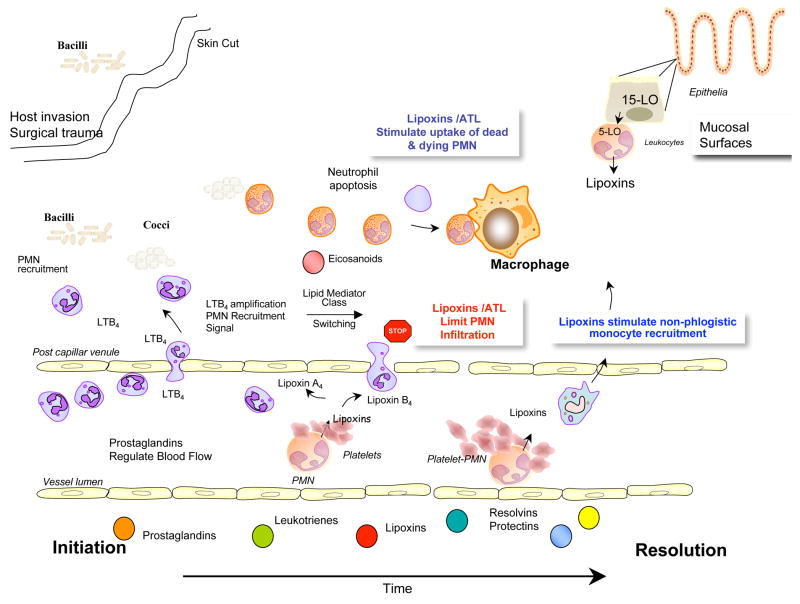
The inflammatory response is, in general, protective and ultimately rids tissues of both the cause and consequences of tissue injury that can accompany host defense (1). Acute inflammation, defined by its cardinal signs dolor, calor, and rubor may lead to chronic inflammation, scarring and eventual loss of function, if the tissue fails to completely resolve the inflammatory site (1). The polymorphonuclear neutrophils (PMN) of the first line of host defense, in this context, must also exit from the inflamed tissues (see Figure 1) in order to return to homeostasis and resolve (2). In recent years it has become widely appreciated that, in addition to the classic diseases associated with inflammation such as psoriasis, periodontal disease and arthritis, uncontrolled inflammation governs the pathogenesis of many other prevalent diseases including cardiovascular and cerebrovascular disease, cancer, obesity and Alzheimer’s disease (3). Of interest, another class of arachidonic acid-derived mediators, the lipoxins (LXs) and aspirin-triggered lipoxins (ATLs), were the first mediators recognized to have both endogenous anti-inflammatory and pro-resolving actions (4, 5) indicating that not all lipid mediators are “bad guys” in controlling inflammation and resolution (6).
Figure 1.
Hypothetical scheme emphasizing the role of lipid mediators in orchestrating the resolution of acute inflammation.
In recent years, this laboratory identified novel enzymatic pathways activated during the resolution phase that are initiated from precursors EPA and DHA, major n-3 fatty acids also widely known as the omega-3 PUFA or simply fish oils. These new compounds are biosynthesized and possess potent actions in controlling the resolution of inflammatory exudates (7–9). The term resolvins, resolution phase interaction products, was introduced to signify that the new structures are endogenous, local-acting mediators possessing potent anti-inflammatory and immunoregulatory properties (8). At the cellular level, these include reducing neutrophil infiltration and regulating the cytokine-chemokine axis and reactive oxygen species, as well as lowering the magnitude of the inflammatory response (7, 8). The protectin family and specifically the term neuro-protectin D1 when generated in the neural tissue (10) were introduced, given the formation and potent anti-inflammatory (9) as well as protective actions demonstrated for the novel and potent DHA-derived 10,17-docasatriene in animal models of stroke (11) and Alzheimer’s disease (12). Both families of lipid mediators, the resolvins and protectins, are potent local-acting agonists of endogenous anti-inflammation that promote resolution (13). The connection of these new anti-inflammatory mediators (LX, Rv and PD; see Figure 1) to the control of an acute inflammatory response and its timely resolution are illustrated in Figure 1.
Since the early 20th century, omega-3 fatty acids (PUFA) were known to possess beneficial roles in health and organ function (14). At high concentrations in vitro, ω-3 PUFAs decrease production of pro-inflammatory prostaglandins, cytokines, and reactive oxygen species held to play critical roles in inflammatory diseases (15). Clinically relevant anti-inflammatory properties have been reported with high doses of omega-3 fatty acids in both rheumatoid arthritis (16) and periodontal disease (17), whereas the evidence available at this time remains inconclusive for other conditions such as asthma [reviewed in ref. (16)]. Moreover, cardiovascular disease was reduced with high-dose omega-3 in a multicenter clinical trial (18, 19), and blood levels of EPA and DHA were recently shown to reduce the risk of cardiovascular disease (20). Taken together, these findings raised the question of what mechanism(s) underlie the many beneficial actions of omega-3 PUFA.
Because the precursors to both resolvins and protectins are the essential omega-3 PUFAs (Figs. 1–3), their relation to dietary supplementation by omega-3 PUFAs also raises new and interesting questions, given the widely appreciated notion that omega-3 supplementation reduces inflammatory diseases and brings about an anti-inflammatory status. Resolvins and protectins are, thus, distinct chemical families that now join the lipoxins (5) as potent agonists of endogenous anti-inflammation and are pro-resolving chemical mediators of interest in human health and disease. It is important to note that the biosynthesis of resolvins and protectins is stereochemically controlled via enzymatic reactions that give rise to specific products that carry potent bioactions and require precise stereochemistry to activate specific receptors. Resolvins and protectins are, therefore, distinct from autooxidized DHA or EPA, which may also arise in tissues via interactions with reactive oxygen radicals (21). These compounds include complex mixtures, namely racemates of mono-, dihydroxy- and trihydroxy- products of PUFA (22) as well as specific isoprostanes and neuroisoprostanes that serve as biomarkers of oxidative stress (23) where uncontrolled inflammation and pathologic tissue damage persist. Recent new findings on resolvin and protectin biosynthesis and their actions are reviewed (vide infra).
Figure 3.
Biosynthesis of aspirin-triggered lipid mediators.
Lipid Mediators: Chemical Autacoids in Inflammation and Resolution
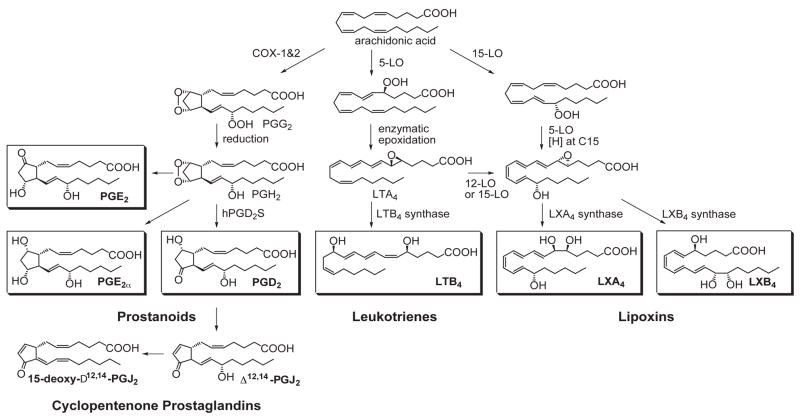
Lipid-derived mediators are well positioned to play key role(s) as signaling molecules in inflammation because they are small molecules, local acting, rapidly generated and locally inactivated. Acute inflammation is characterized by the rapid, time-dependent influx of PMN into the site, the first line of phagocytic host defense (see Figure 1). Pro-inflammatory prostaglandins and leukotriene B4 control local blood flow, vascular dilation and permeability changes needed at the site for leukocyte adhesion, diapedesis, and recruitment (1, 2). Microbial invaders, tissue injury, or surgical trauma activate the release and formation of arachidonate-derived eicosanoids. These chemical mediators are enzymatically generated via specific cyclooxygenase (COX) and lipoxygenase (LOX) pathways (24). As exudates form and pustules are walled off, prostaglandins initiate a number of responses relevant in inflammation (i.e., vasoconstriction, vascular permeability changes, pain, vasodilation and edema). Interestingly, both PGE2 and PGD2 also signal the end by activating the transcriptional regulation of 15-lipoxygenase (LO) in human neutrophils that in turn gives rise to the temporal dissociation of eicosanoids and production of lipoxins (2, 25). Hence, the prostaglandins and leukotrienes are rapidly generated, whereas the lipoxins, also produced from arachidonate, are generated later in the time course with the onset of the resolution phase (Figure 1).
Lipoxins
Lipoxins were the first family of mediators identified in vivo with anti-inflammatory and pro-resolving actions (Box 1). Lipoxins are trihydroxytetraene-containing products of arachidonic acid (Fig. 2). Their biosynthesis and actions were recently reviewed in detail (for in-depth reviews, see ref. 5) and are discussed here in view of their role(s) in anti-inflammation and resolution. Eicosanoid class switching refers to changes in production within the arachidonate-derived family, for example, prostaglandin and leukotriene, to lipoxin, which initiate and/or are coincident with termination. In this case, PMNs switch from leukotriene B4 to lipoxin production (2). Lipoxins, specifically LXA4 and LXB4, as well as their aspirin-triggered forms (vide infra), stop further PMN entry into the exudates as well as counter-regulate the main signs of inflammation (Table 1). As new PMNs parachute into exudates, older and apoptotic PMN must be removed from the site in a timely fashion for inflammation to resolve (Figure 1). Once PMNs enter an exudate, they interact with other cells (such as other leukocytes, platelets, endothelial, mucosal epithelial, fibroblasts) in their immediate vicinity and are able to perform transcellular biosynthesis to produce LX and eventually new mediators. The process of transcellular biosynthesis is defined as the generation of new bioactive compounds that neither cell type can produce on its own. For example, human platelets on their own do not produce LX. When platelets adhere to PMN (Fig. 1, right), the platelet-PMN aggregates become a major intravascular source of LX that in turn halts further PMN diapedesis and recruitment (reviewed in ref. 26). When PMNs interact with mucosal epithelial cells in the lung, oral or gastrointestinal mucosa (Fig. 1, upper right), these PMNs generate LX from precursor 15-HETE donated by interactions with mucosal epithelial cells (27–29). These results also clearly demonstrated that PMNs switch their phenotype in that they change the profile of lipid mediators that they produce depending on their local environment (2, 7). Hence, they switch their lipid mediator phenotype compared to, for example, peripheral blood PMN, which generate LTB4 as their main product when activated (2, 30). During the course of inflammation and complete resolution, as discussed below, mediator switching also occurs between families of lipid mediators, namely from eicosanoids to resolvins of the E and D series as well as protectins (7, 8, 31). The progression is dependent on the availability of substrate within the evolving exudates.
Box 1 Mediators in Resolution
Lipoxins
Aspirin-triggered lipoxins
PGE2, PGD2
Glucocorticoids
Resolvins
Protectins
Annexin 1
Cyclopentenone Prostaglandins
Figure 2.
Key eicosanoids that play pivotal roles in initiating inflammation and its resolution.
Table 1.
Lipoxins Counter-Regulate Cardinal Signs of Inflammation: Reduce Inflammation and Promote Resolution
| Lipoxin and Aspirin-Triggered Lipoxin Actions | Reference |
|---|---|
| • Regulate leukocyte traffic | Colgan et al., 1993 (121) |
| - Stop PMN and eosinophil infiltration | Lee et al., 1989(122) |
| - Stimulate non-phlogistic monocyte recruitment | Maddox and Serhan, 1996 (113) |
| - Stimulate macrophage uptake of apoptotic PMN | Godson et al., 2000 (65) |
| • Redirect chemokine-cytokine axis | |
| - Block EL-8, EL-1 gene expression | Gewirtz et al., 1998 (123) |
| - Block TNFα actions and release | Takano et al., 1997(124) |
| - Stimulate TGFβ | Bannenberg et al., 2005 (31) |
| • Reduce edema | Bandeira-Melo et al., 2000 (60) |
| - Regulate actions of histamine | Pouliot et al., 2000 (50) |
| Menezes-de-Lima et al., 2006 (125) | |
| • Block pain signals | Serhan et al., 2001 (64) |
| LX/LT regulate neuronal stem cells, proliferation and differentiation | Svensson et al., 2007 (63) |
| Wada et al., 2006 (126) | |
The Link Between COX-2 and Pro-Resolving Pathways
Prostaglandins such as PGE2 (that can be produced by both COX-1 and COX-2) are generated in the initial phase of inflammation (Figs. 1 and 2) as well as have a dual role in stimulating resolution (32). Signaling pathways leading to prostaglandin E2 and D2 actively switch on the transcription of enzymes (15-LOX type 1) required for the generation of lipoxins (2) as well as PUFA-derived resolvins and protectins (31, 33). Selective COX-2 inhibition, by blocking production of PGE2 and PGD2, delays the onset of resolution (34). Hence, COX-2 has a role in both the initiation of acute inflammatory response and the resolution phase (35). Lipoxins promote resolution by stopping entry of PMN to sites of inflammation and decreasing reperfusion or re-flow tissue injury (reviewed in ref. 13). They also reduce vascular permeability and promote non-phlogistic recruitment of monocytes and stimulate clearance of apoptotic neutrophils via macrophages (Fig. 1). Hence, a key event in resolution is the temporal “switch” in the lipid mediator class from pro- to anti-inflammatory eicosanoids, which has direct implications for the treatment of inflammatory diseases. Drugs that disrupt this switch may have unwanted side effects in resolution (32, 36).
COX-2 also plays a key role in the biosynthesis of prostaglandin D2 (Fig. 2), which is precursor to the cyclopentenones. These include prostaglandin J2, which is an extracellular activator of PPARγ. This property has led to the proposal that cyclopentenone prostaglandins can be anti-inflammatory and enhance the resolution of inflammation via regulation of NFκB (reviewed in refs. 32, 34). These studies also bring to light the dual role of cyclooxygenase-2 in initiation versus termination of acute inflammation and the induction of proresolving lipid mediators. Continued study of the mechanism of action of cyclopentenones in resolution as well as other regulators of the transcriptosome may provide additional new leads for therapeutics in treating the resolution phase.
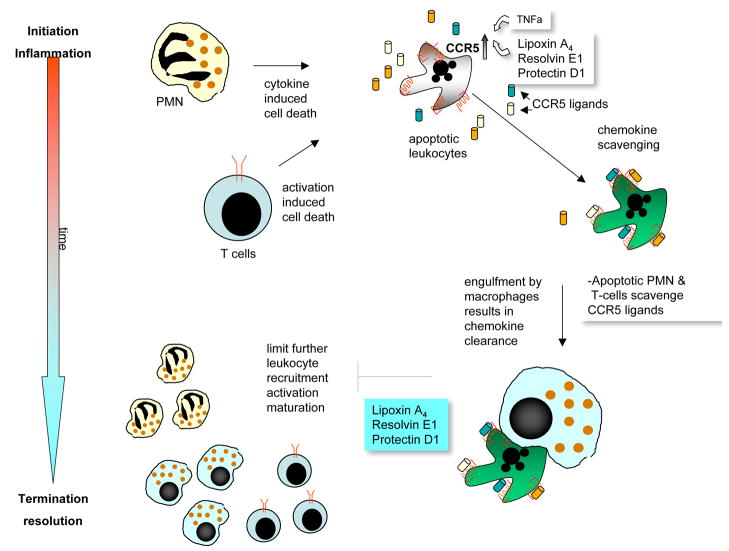
Resolution of acute inflammation in murine models involves the appearance in exudates of EPA and DHA, which follow the appearance of unesterified arachidonate (31). Precursors are transformed via enzymatic mechanisms to bioactive compounds such as lipoxins, resolvins and protectins that regulate the duration and magnitude of inflammation (shorten the period of neutrophil infiltration and initiate clearance of apoptotic PMN). LX, Rv and PD1 also increase the expression of CCR5 receptors on T cells and aging PMN, which help clear local chemokine depots from the inflammatory site (Fig. 10) (37). Apoptotic neutrophils are then phagocytized by macrophages, leading to neutrophil clearance and release of anti-inflammatory and reparative cytokines such as transforming growth factor-beta1 (31, 38).
Figure 10.
Interactions between lipid mediators and peptide mediators during resolution: Resolvins enhance the scavenging and clearance of chemokines via macrophages.
A set of ‘resolution indices’ was recently introduced as a quantitative means for assessing key resolution parameters and the impact of specific agents within active resolution. These parameters and indices are listed in Table 3A. Temporal and differential changes in self-limited experimental murine peritonitis were investigated as a model system to identify anti-inflammatory and pro-resolving circuits (31). With these resolution indices defined, specific lipid mediators (e.g., RvE1, ATLa and PD1) were pinpointed to promote resolution via specific and separate mechanisms (Table 3B). When grossly viewed as the same outcome, namely anti-inflammation, each of these mediators can be considered anti-inflammatory (31). It is now clear, however, that anti-inflammation and pro-resolution are not the same processes. These measurable indices are a useful tool applicable for evaluating the molecular basis of novel therapeutic interventions in disease models where inflammation-resolution is a component as well as identifying when agents are resolution-toxic (4).
Table 3.
| Table 3A. Kinetics of Resolution: Defining the Key Events | ||||
|---|---|---|---|---|
| Resolution Index | Definition | |||
| Ψmax | Maximal PMN numbers | |||
| R50 | 50% of Ψmax | |||
| T50 | Time point when PMN numbers reduce to 50% of Ψmax | |||
| Tmax | Time point when PMN numbers reach maximum | |||
| Ri | Resolution interval; time interval from the maximum PMN point (Ψmax) to the 50% reduction point (R50) [i.e., T50-Tmax] | |||
| IpMN=monocyte | Point of intersection when the increase in mononuclear cells intersects the decrease in PMN [i.e., PMN number = mononuclear cell number] | |||
| Table 3B. Specific Impact of Novel Lipid Mediators in Resolution Indices | ||||
|---|---|---|---|---|
| Lipid mediator | Ψmax PMN number | Tmax h | T50 h | Ri h |
| Acute inflammation | 16.5 × 106 | 11 | 24 | 13 |
| + ATLa | 13.2 × 106 | 12 | 24 | 12 |
| + RvE1 | 12.0 × 106 | 8 | 20 | 12 |
| + NPD1/PD1 | 10.0 × 106 | 5 | 11 | 6 |
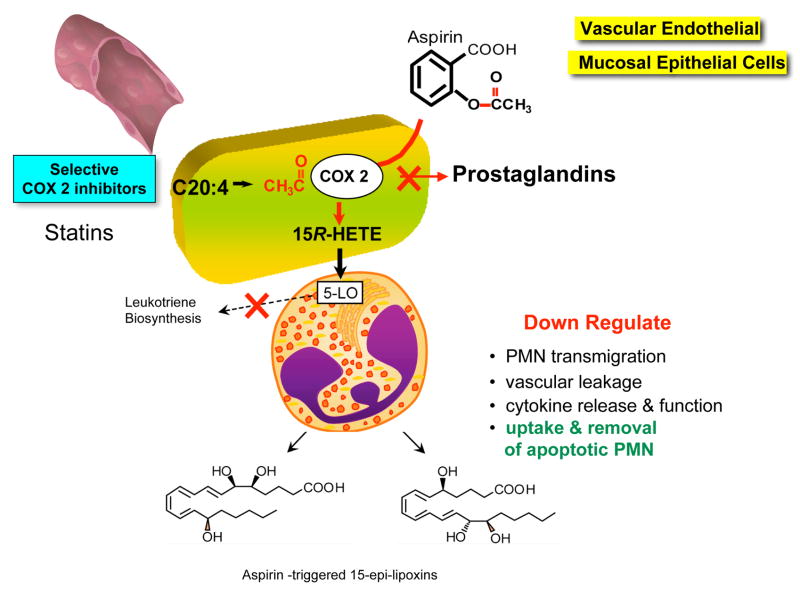
Aspirin triggers endogenous anti-inflammatory mediators and jump-starts resolution Aspirin-triggered lipoxins
Hippocrates first advocated the use of willow bark in the treatment of pain of childbirth and fever; it was later found to contain antipyretic substances (reviewed in refs. 39, 40). Salicylates were isolated years later, and in 1899 in Berlin aspirin was launched on the trademark list in the German Patent Office; Felix Hoffman working at Bayer added the acetyl group to the structure with the goal of enhancing its uptake and actions. Today, aspirin remains one of the most widely used non-steroidal anti-inflammatory drugs and is present in many over-the-counter drugs. It is estimated that 1012 aspirin tablets have been consumed. Aspirin’s many clinical benefits still appear to be unfolding in the results of many clinical studies. Although it is clear that aspirin inhibits prostaglandin and thromboxane formation by acetylating and blocking the catalytic activity of COX-1 and hence is a major mechanism in anti-inflammatory and anti-thrombotic therapy (41, 42), aspirin’s well-appreciated ability to limit leukocyte traffic into sites of inflammation, the key mechanism in reducing leukocyte infiltration during inflammation, remained to be established. In this regard, aspirin turns on the production of the body’s own endogenous anti-inflammatory lipid mediators, namely aspirin-triggered lipoxins. This novel class of lipid mediator actively dampens host inflammation (Tables 1 and 2). As depicted in Figure 3, this action of aspirin involves cell-cell interaction between COX-2-bearing cells (vascular endothelial cells or epithelial cells) and leukocytes (7). By acetylating COX-2, aspirin re-directs COX-2’s catalytic activity away from generating the intermediate for prostaglandins and thromboxanes towards producing 15R-HETE. The COX-2 enzyme with this modification remains catalytically active. This product of vascular endothelial cells and a wide range of other cells is converted to 15-epi-LXA4 by leukocyte 5-lipoxygenase, termed aspirin-triggered 15-epi-lipoxin A4 (ATL). ATL shares actions with LXA4 as well as appears to be longer acting, resisting rapid dehydrogenation in vivo (Table 2). It is important to note that the role of PMN is well appreciated in the pathogenesis of rheumatoid arthritis (43), a notion that was recently reaffirmed (44). Aspirin also increases the hepatic biosynthesis of 15-epi-LX via enhancing p450 production of 15R-HETE in a COX-2-independent pathway in rats (45), which is also likely to contribute to ATL plasma levels in healthy individuals taking low-dose aspirin (46).
Table 2.
Lipoxin and Aspirin-Triggered Lipoxin Deficiencies in Human Disease: Lipoxin Analog Pro-Resolving Treatments in Animal Disease Models
| Human Disease | Reference | Animal Model | Reference |
|---|---|---|---|
| Cardiovascular disease LX/LT | Brezinski et al. (51) | Rabbit and mouse | Jain et al. (127) Shen et al. (96) |
| Asthma | Mouse Tg hALX | Levy et al. (52) | |
| Aspirin-sensitive asthma | Levy et al. (52) | ||
| Cystic fibrosis (classic non-resolving) | Karp et al. (54) | Mouse | Karp et al. (54) |
| Rheumatoid arthritis | Thomas et al. (128) | ||
| Gastrointestinal disease | Mangino et al. (55) | Mouse | Fiorucci et al. (112) Wallace et al. (129) |
| Renal ischemia reperfusion injury | -- | Mouse | Kieran et al. (130) |
| Glomerulonephritis | Gene therapy approach | Munger et al. (131) | |
| Periodontal disease | Pouliot et al. (50) | Rabbit TgLO | Serhan et al. (95) |
EPA and DHA are also converted via aspirin-acetylated COX-2 to generate bioactive epimers (7–9, 11) of resolvins and protectins (Figs. 3, 4 and 6; vide infra). Thus, aspirin is unique among anti-inflammatory drugs in that it blocks pro-inflammatory prostaglandins as well as ‘jump-starts resolution’ by generating endogenous epimers of resolution mediators that share characteristic features with their counterparts in terms of dampening inflammation and PMN-mediated injury, major culprits in many human diseases.
Figure 4.
Biosynthesis of E-series resolvins.
Figure 6.
Biosynthesis of D-series resolvins and protectins.
LXA4 and Aspirin-Triggered 15-epi-lipoxin A4 in Animal Models and Human Diseases
LXA4 and ATL display counter-regulatory roles in animal models of disease (Table 2), possess local organ-specific functions, and modulate leukotriene formation and their activities. The protective actions of LXA4 and ATL are ligand-receptor dependent, since transgenic overexpression of human ALX, the G protein-coupled surface receptor for LXA4, leads to decreased PMN infiltration with endogenous LXA4 (47). The LXA4 receptor ALX is upregulated by glucocorticoids (Box 1 and ref. 48); its structure-activity relationship was recently reviewed in detail (49). Alterations in LX and ATL levels may be causally associated with the pathophysiology of several human diseases (13). LXA4 and ATL regulate TNF-α-directed neutrophil actions and stimulate IL-4 in exudates, and thus regulate endogenous mediators in the pathogenesis of inflammatory conditions such as periodontal disease (50) In many human diseases, LX production appears to be deficient compared to leukotrienes (Table 2). These include cardiovascular (51), asthma (52), kidney inflammation (53), cystic fibrosis (54), gastrointestinal (55) and periodontal disease (50), to name a few. Designed metabolically stable analogs of LXs and ATLs are useful tools in examining the role(s) and local actions of lipoxins in vivo (Table 2) (56–58). Administration of LX stable analogs in animal models protects from tissue damage and inflammation (5, 59) and enhances resolution (60). Identification of these anti-inflammatory properties of LXs and ATLs provided strong evidence for the existence of endogenous anti-inflammatory mediators derived from arachidonic acid, in addition to those listed in Box 1. Along with reducing PMN influx (61), redirecting chemokines and cytokines (62), and reducing pain (63, 64), LX and ATL have the ability to stimulate the removal of apoptotic PMN by macrophages in vitro (65) and at sites of inflammation in vivo (66). This pro-resolving agonist activity is shared by (Box 1) annexin 1(36) and glucocorticoids (67, 68), and accelerates the return of the tissue to homeostasis.
Vascular actions of lipoxins: endothelial cells and heme oxygenase-1
Lipoxins have a number of direct actions on endothelial cells that are protective and in line with their role in resolution. For example, lipoxins stimulate prostacyclin generation by endothelial cells (69) and stimulate NO production by vascular endothelial cells (70). Of interest, aspirin acetylation of COX-2 generates 15-epi-lipoxins that in turn stimulate the production of NO by eNOS. Aspirin, in either eNOS or iNOS knockouts, is not anti-inflammatory in IL-1β-induced murine peritonitis. Both aspirin and 15-epi-LXA4 had reduced effects on endothelial cell adherence from eNOS and iNOS knockouts compared to wild-type (70). This suggests that aspirin triggers the production of 15-epi-LXA4, which increases NO synthesis through both eNOS and iNOS. These findings suggest a tight regulation between the generation of 15-epi-LXA4 and the production of vascular-derived NO. Also, aspirin-triggered lipoxins and lipoxins block VEGF-stimulated angiogenesis and migration of endothelial cells (71). Aspirin induces heme oxygenase-1 (HO-1) expression in endothelial cells, which is increased by ATLa in a concentration- and time-dependent fashion in human endothelial cells (72). The induction of HO-1 by LX and ATL appears to regulate, in part, the organ protective actions observed with lipoxins. In this context, in a murine model of sepsis, treatment with ATLa spares lung tissues from inflammatory damage (73).
Statins and the biosynthesis of anti-inflammatory lipid mediators
The mechanisms of the well-recognized anti-inflammatory action of statins have been highly sought. Statins (e.g., atorvastatin) and pioglitazone regulate the production of S-nitrosylated COX-2 (74–77). The S-nitrosylated COX-2 produces 15R-HETE, which is converted by 5-lipoxygenase to 15-epi-LXA4 (Fig. 3). The finding that statins can regulate the production of 15-epi-lipoxin A4 suggests that the anti-inflammatory actions of statins are directly mediated by endogenous production of 15-epi-lipoxin A4 (75, 78). These widely used drugs aspirin and statins have in common a unique ability to trigger the endogenous production of 15-epi-LXA4. Further studies by Birnbaum et al. indicate that, when COX-2 is both acetylated and S-nitrosylated, the enzyme is inactive, thus providing potential adverse interactions among statins, thiazolidinediones, and high-dose aspirin. It remains of interest whether these results, demonstrated both in vitro and in murine models in vivo, translate to humans. It is likely that this mechanism will also impact the biosynthesis of the aspirin triggers forms of the resolvins and protectins (vide infra).
Resolvins Are Novel Endogenous Mediators: 18R E Series and 17R D Series Resolvins
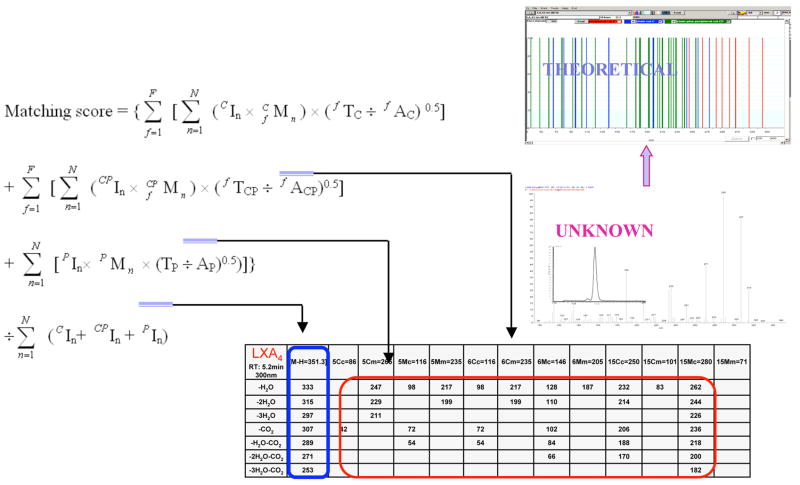
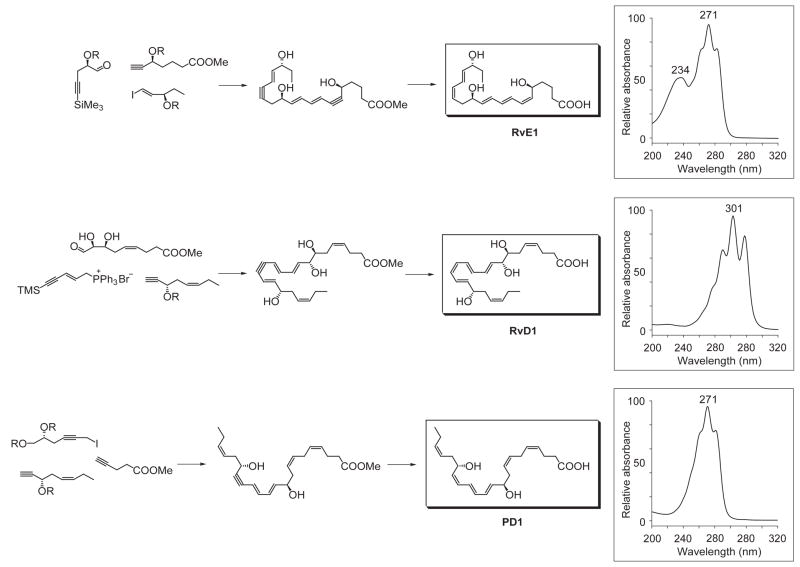
In view of the role of LX in resolution and reported beneficial actions of omega-3 in humans, it was of interest to determine whether specialized lipid mediators are involved in the resolution of self-limited inflammation. The mouse air pouch was selected for systematic analysis because acute inflammation and exudate formation are spontaneously resolved in this dorsal skin cavity, which permitted kinetic analysis of chemical mediators and leukocyte traffic. The novel lipid mediators produced from EPA were first isolated from resolving exudates that proved to contain 18R-hydroeicosapentaenoic acid (18R-HEPE) as well as several other related bioactive compounds (7). The first bioactive product isolated from exudates, coined resolvin E1, reduced inflammation (Fig. 4) and blocked human PMN transendothelial migration (13). Structural elucidation was carried out together with both GC-MS and MS-MS-based lipidomic analysis of bioactive fractions obtained following extraction and RP-HPLC. The basic structure of this potent bioactive product in the resolving exudates proved to be 5,12,18R-trihydroxyeicosapentaenoic acid (7) (Fig. 4). As outlined in Figure 5, databases were constructed containing both known and theoretical fragments produced by MS-MS of putative lipid mediators. These databases were systematically researched in a stepwise fashion using UV chromophores and MS-MS spectra, then LC retention times to identify the basic structures of new compounds in the inflammatory exudates. These procedures required constructing algorithms (Fig. 5) used together with library software for mass spectral analyses (79). Following assessment of potential bioactions, the complete stereochemistry of the bioactive and related isomers and compounds was confirmed using total organic synthesis as outlined in Fig. 8 (8, 80, 81).
Figure 5.
Lipid mediator lipidomics: searches of databases and algorithms for identifying lipid mediators. Databases contain theoretical fragmentation post-mass spectrometry analysis. Known lipid mediators are matched according to fragments generated and their tandem MS-MS spectra stepwise to UV absorbance λ maxima and then to relative retention times. Databases were constructed using known lipid mediators, eicosanoids, prostaglandins, leukotrienes and lipoxins, as well as theoretical fragmentations expected for lipid mediators derived from other potential precursors.
Figure 8.
General scheme for total organic synthesis of resolvins and protectins. Insets depict the characteristic UV spectrum associated with each of the key bioactive resolvins and protectins. The stereochemistry of endogenous RvE1, RvD1, and PD1 has been established, and the biological actions and physical properties of the endogenous compounds confirmed via total organic synthesis. In addition to the compounds shown, double bond isomers and chiral epimers have been synthesized to address the impact of stereochemistry and potency of the natural products. See text for details.
Recombinant COX-2 treated with aspirin generates 18R-HEPE as well as 15R-HEPE from EPA, which are blocked by selective COX-2 inhibitor (7). Of interest, at clinically used doses both acetaminophen and indomethacin permitted oxygenation of EPA to both 18R-HEPE and 15R-HEPE with isolated recombinant COX-2, albeit the levels of these hydroxy products were significantly reduced yet nonetheless actively generated by the COX-2. These results indicate that the oxygenation of omega-3 PUFA to generate novel bioactive mediators can also involve certain of the widely used anti-inflammatory drugs but not selective COX-2 inhibitors (Figure 3) (7).
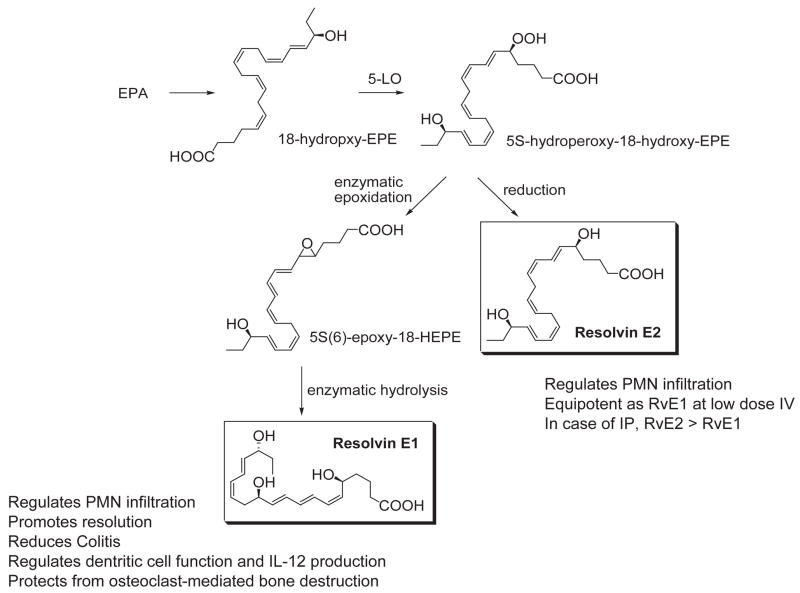
Next, the most likely human pathways were reconstructed in vitro that biosynthesize these bioactive mediators within the resolving exudates, which involve cell-cell interactions in vivo within the exudates (Fig. 4). Isolated human cells, vascular endothelial cells treated with aspirin convert EPA to 18R-HEPE that is released and then rapidly converted by activated human PMN to a 5(6) epoxide-containing intermediate that is converted to the bioactive 5,12,18R-trihydroxy-eicosapentaenoic acid, denoted Resolvin E1 (RvE1). RvE1 possesses a distinct structure consisting of a conjugated triene plus conjugated diene chromophore present within the same molecule (Figure 4). Both biogenic (7) and total organic synthesis was achieved and its complete stereochemical assignment was recently established (80). RvE1 proved to be 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid (Figs. 4–8). The synthetic RvE1 displayed potent stereoselective actions in vivo and with isolated cells confirming the original structural assignment.
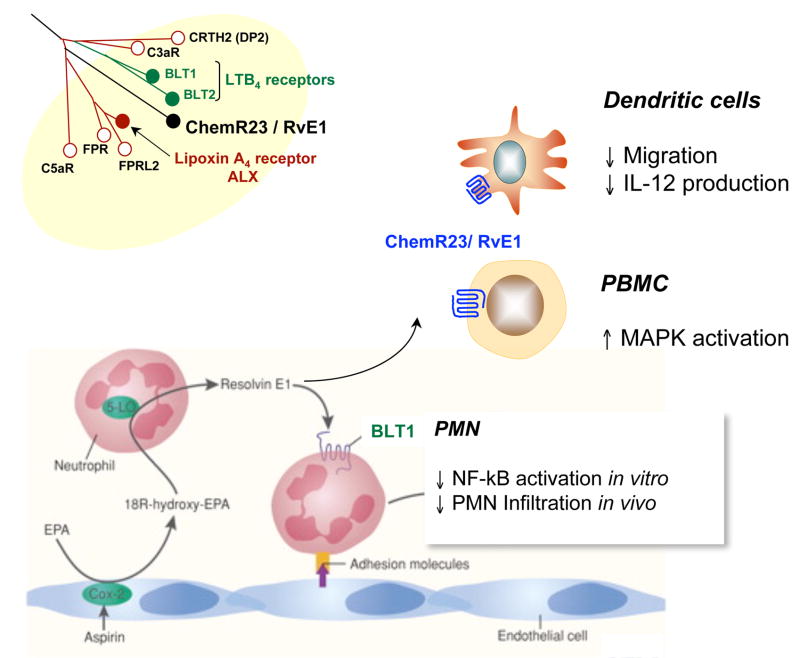
Next, to determine the receptors involved in RvE1 stereoselective actions, a screening library of G-protein-coupled receptors was devised employing counter regulation of TNFα, a task that one might expect a resolvin to carry out in vivo (80). The orphan receptor ChemR23 specifically bound to [3H]-labeled RvE1, and attenuate TNF activated nuclear factor (NF)-κB signaling (80). The labeled RvE1 was synthesized using the acetylenic compound shown in Figure 8 (upper panel) and isolated using RP-HPLC. The main second messenger for RvE1 agonist actions via these GPCRs appears to be activation of intracellular phosphorylation pathways. This contrasts pro-inflammatory mediators that use mobilization of intracellular calcium or cyclic AMP (80, 82). Using a structure-function approach, a second high affinity GPCR was identified that signals in response to RvE1. RvE1 also interacts with LTB4 receptor BLT1 and attenuates LTB4-induced pro-inflammatory signals by acting as partial agonist/antagonist on LTB4 receptor present on human PMN (83). ChemR23 is expressed on DC and monocytes (Fig. 9).
Figure 9.
Resolvin E1 acts via ChemR23 and BLT1 peripheral blood leukocytes: Actions mediated via two distinct GPCRs. Agonist at ChemR23 and partial agonist/antagonist at BLT1.
A second bioactive member of the E series resolvins was recently identified that is produced during RvE1 biosynthesis and shares anti-inflammatory properties with RvE1 (84). The basic structure of the novel dihydroxyeicosapentaenoid was determined and shown to be 5S,18(R/S)-dihydroxy-eicosapentaenoic acid, denoted resolvin E2 (RvE2), the reduction product of 5S-hydroperoxy,18-hydroxy-eicosapentaenoic acid (Figure 4). Human 5-lipoxygenase has a pivotal role since it catalyzes the initial 5-hydroperoxide generation from 18-hydroxy-eicosapentaenoic acid as well as the epoxide required for RvE1 formation. Human neutrophils can biosynthesize RvE2 in greater amounts than RvE1. Resolvin E2 is equipotent to RvE1 when administered intravenously and additive at low doses when given intraperitoneally, suggesting actions mediated via different receptors than RvE1 (84).
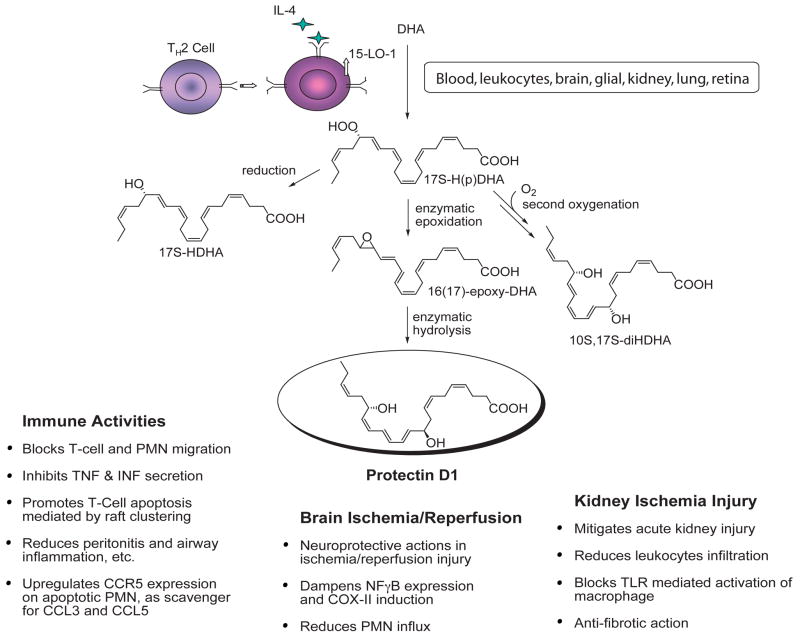
Resolvins and Protectins Biosynthesized from DHA: The D Series Resolvins
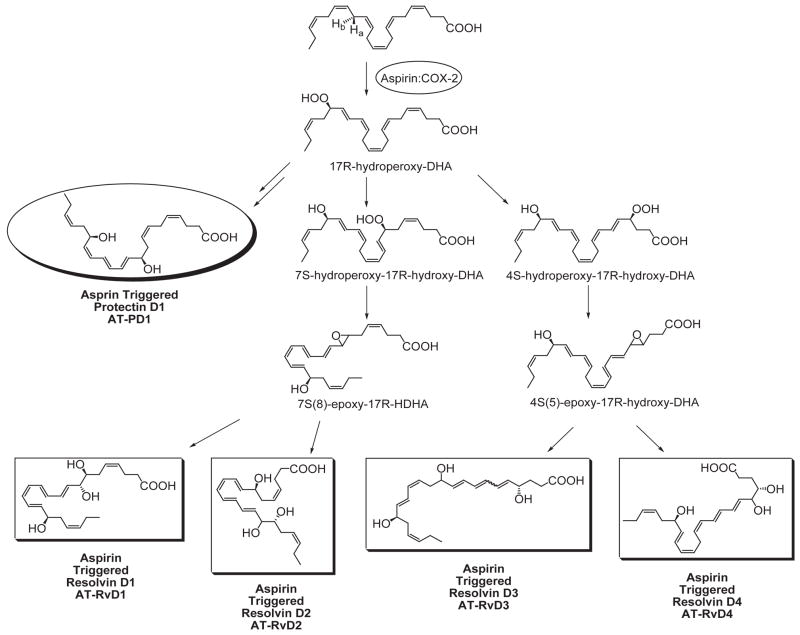
Mice given aspirin plus DHA contained novel 17R-hydroxy-DHA (17R-HDHA) (Fig. 6) and two separate novel families of bioactive compounds in their resolving exudates (Figures 6 and 7). Again, the biosynthetic pathways were reconstructed in vitro to establish potential cellular origins for these novel compounds. Hypoxic human microvascular endothelial cells treated with aspirin release 17R-HDHA. DHA is a substrate for isolated human recombinant cyclooxygenase-2 (COX-2) producing 13-hydroxy-DHA (8). With aspirin treatment, COX-2 switches to 17R-oxygenation with molecular oxygen to give an epimeric or aspirin-triggered form in exudates and also in blood and brain with both families of resolvins and protectins (8, 9). The aspirin-triggered forms carry a 17R alcohol group configuration instead of the carbon 17S as when biosynthesized via lipoxygenase mechanisms (Figure 6).
Figure 7.
Biosynthesis of protectin D1/neuroprotectin D1 and related compounds.
Even in the absence of aspirin, endogenous DHA is converted to a 17S alcohol-containing series of resolvins (RvD1 through RvD4; shown in Figure 6), as well as docosa-conjugated triene-containing structures via lipoxygenase-initiated mechanisms (9, 11). The complete stereochemistry of protectin D1, which carries a base 10,17-dihydroxydocosatriene structure (8, 9) was established (Figs. 7 and 8) and confirmed the original assignment (8, 10). Total organic synthesis of related isomers and matching studies with biologically derived materials showed that endogenous protectin D1, denoted neuroprotectin D1 (NPD1) when produced by neural tissues, was established in isolated human cells and murine cells in vivo as 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid (10). The geometry of the double bonds in PD1 and their positions during biosynthesis from the key intermediates in its biosynthesis in situ, namely via an epoxide intermediate formed at the 16(17) position, indicated that biosynthesis of PD1 requires enzymatic steps to generate the potent bioactive molecule (Figure 7). On a molar basis, PD1 proved to be log orders of magnitude more potent than its native precursor DHA (9, 10, 85).
Upon activation, DHA contained in tissues or taken into the inflammatory site produces 17S-hydroxy-containing protectins and resolvins. The complete stereochemistry via matching studies and total organic synthesis of RvD1 (7S,8R,17S-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid) and the aspirin triggered form shown in Figure 6, AT-RvD1 (7S,8R,17R-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid) were recently achieved (8, 81). Results from these studies not only confirmed their potent anti-inflammatory actions and original structural elucidation but also now open the possibility to study the formation and actions of these novel mediators in a variety of addition system relevant to inflammatory diseases.
Resolvins and Protectins in Complex Models of Disease
RvE1, in low doses (i.e., only nanogram levels), reduces neutrophil transendothelial migration, dermal inflammation (8), peritonitis, dendritic cell migration and interleukin (IL)-12 production (Table 4). Synthetic RvE1 blocks PMN infiltration, and protects from bone destruction in a rabbit model of periodontal disease (86) and protects against the development of colitis (82). Hence, in several animal models of inflammatory diseases, RvE1 is a potent counter-regulator that protect against leukocyte-mediated tissue injury. Resolvins of the D series also block tumor necrosis factor TNF-α-induced IL-1β transcripts in microglial cells and are potent regulators of PMN limiting infiltration into inflamed brain, skin, and peritonitis (8, 9, 11) (Table 5). Direct comparisons between the resolvin E versus both of the D series (17S and 17R epimer aspirin-triggered series) at equal doses demonstrated that the 17S series generated by lipoxygenase-initiated mechanisms and those of the 17R series RvDs triggered by aspirin treatment when administered intravenously at 100 ng [~3 μg/kg] in mice display essentially similar actions reducing PMN infiltration by ~50% in peritonitis.
Table 4.
Impact of E-Series And D-Series Resolvins in Disease Models or Organ Systems
| Disease Model | Actions | Mediator | References |
|---|---|---|---|
| Acute inflammation (murine peritonitis and dermal air pouch inflammation) | • Reduces PMN infiltration | Resolvin E1 | Serhan et al. (7, 8), Arita et al., (80) Bannenberg et al. (31) |
| • Upregulates CCR5 expression on late apoptotic human leukocytes | Resolvin E1 Protectin D1 ATL/LXA4 |
Ariel et al. (37) | |
| • Acts as “terminator” of chemokine signaling during resolution | Resolvin D1 | ||
| Colitis | • Decreases PMN recruitment and proinflammatory gene expression | Resolvin E1 | Arita et al. (82) |
| • Improves survival | |||
| • Reduces weight loss | |||
| • Genetically engineered fat-1 mice possess high levels of DHA and EPA, generate resolvins during colitis and demonstrate reduction in GI tissue damage | Resolvin E1 Resolvin D |
Hudert et al. (92) | |
| Periodontitis | • Reduces PMN infiltration, stops inflammation-induced tissue and bone loss | Resolvin E1 | Hasturk et al. (86) |
| Acute inflammation (murine peritonitis) | • Stops PMN infiltration | Resolvin E2 | Tjonahen et al. (84) |
| Acute inflammation (murine peritonitis) | • Stops PMN recruitment | Resolvin D1 | Serhan et al. (8) Sun et al, 2007 (81) |
| Microglial cells | • Reduces microglial cell cytokine expression in vitro | Hong et al. (9) | |
| Kidney | • Protects in renal ischemic injury by limiting PMN infiltration | Resolvin D1 and Protectin D1 | Duffield et al. (85) |
| • Regulates macrophages | |||
| Acute inflammation (murine peritonitis and dermal air pouch inflammation) | • Stops PMN recruitment | Resolvins D2, D3, D4 | Serhan et al., 2002 (8) |
| • Reduces peritonitis |
Table 5.
Impact of Protectin D1/Neuroprotectin D1 in Disease Models or Organ Systems
| Organ System/Disease Model | Actions | References | |
|---|---|---|---|
| Protectin D1 | Acute inflammation (peritonitis) | • Reduces PMN infiltration | Hong et al. (9) |
| • Upregulates CCR5 expression on late apoptotic human leukocytes; “terminator” of chemokine signaling during resolution | Ariel et al. (37) | ||
| • Regulates T-cell migration | Ariel et al. (132) | ||
| Liver | • Correlates supplements with biosynthesis of PD1 and organ protection in vivo | González-Périz et al. (33) | |
| • PD1 and 17S-HDHA attenuate peroxide-induced DNA damage and oxidative stress in hepatocytes and protect from necroinflammatory liver injury in mice | |||
| Lung | • PD1 formation is reduced in murine models of asthma | Levy et al. (101) | |
| • PD1 protects from lung damage in vivo | |||
| • PD1 is generated in human asthma, protects from airway inflammation and hyperresponsiveness | |||
| Kidney | • PD1 formed in murine kidney | Duffield et al. (85) | |
| • Protects from ischemia-reperfusion-induced kidney damage and loss of function | |||
| PD1 | Acute inflammation (peritonitis) | • Reduces PMN infiltration | Bannenberg et al. (31) |
| • Shortens resolution interval (Ri)a | |||
| • Downregulates pro-inflammatory cytokines and chemokines | |||
| • Stimulates anti-inflammatory cytokines and chemokines | |||
| NPD1 | Retina | • Protects from injury | Bazan (133) |
| NPD1 | Alzheimer’s disease | • Diminished production in human Alzheimer’s disease | Lukiw et al. (12) |
| • Promotes neural cell survival in vivo | |||
| NPD1 | Stroke | • Limits ischemic damage | Marcheselli et al. (11) |
| • Reduces PMN entry into the brain |
See Table 3A.
Indomethacin, the still widely used anti-inflammatory, was tested for direct comparison at the same doses and gave only ~25% reduction in leukocyte infiltration (8, 9). The RvDs (17S-series) and aspirin-triggered RvDs (17R-series) are, hence, potent regulators of PMN infiltration in vivo, and the S to R switch with aspirin treatment in the biosynthesis of the 17 position alcohol in the omega side chain does not diminish their activity. This implies that the aspirin-triggered 17R epimers of protectins and resolvins may each serve as the body’s own anti-inflammatory mediators in response to aspirin. Their biosynthesis with aspirin gives this drug the ability to jump-start resolution, cutting off prostaglandins and triggering resolvins.
The protectins possess the conjugated triene structure, a key feature of this family. PD1 is referred to as neuro-protectin D when generated in the nervous system (Fig. 7), and possesses potent actions in vitro and in vivo (Table 5). For example, synthetic PD1 at 10 nM attenuates human neutrophil transmigration by ~50% in vitro, whereas its Δ15-trans-isomer is essentially inactive. PD1 is also a potent regulator of PMN in vivo by reducing PMN infiltration (~40% at 1 ng/mouse) in murine peritonitis. PD1 also reduced PMN infiltration when administered after the initiation of inflammation in vivo as well as acts in an additive fashion with RvE1 to stop PMN infiltration. PD1 is thus a potent, stereoselective anti-inflammatory molecule in vivo (8–10). Moreover, these results and those obtained with Bazan and colleagues in neural tissues demonstrate that PD1 displays potent immunoregulatory (Table 5) (8–10) and neuroprotective actions (11, 12, 87) as well as promotes wound healing capacity (88) and is antifibrotic in kidney (85).
Mouse kidneys produce RvDs and PD1 in response to bilateral ischemia/reperfusion injury from endogenous sources of substrate. This may play a role in protection against and resolution of acute kidney injury along with protection from eventual organ fibrosis (85). Of interest, fish, a source of omega-3 PUFA, also biosynthesize both resolvins and protectins (89). Hence, the resolvins and protectins possess anti-inflammatory actions, are log orders more potent than their precursors EPA and DHA, and are conserved structures in evolution. Their roles in fish pathology or physiology, however, remain to be elucidated but may be related to some of the beneficial actions implicated for various fish oils used in clinical studies.
Acute inflammation (peritonitis, dermal inflammation)
Zymosan-induced peritonitis is a self-resolving exudate and was used to identify anti-inflammatory and pro-resolving circuits. Administration of ATL analog, RvE1, or NPD1 in this system activated and/or accelerated resolution. The murine air pouch, a widely used model for assessing dermal inflammation, is a cavity lined by fibroblast-like and macrophage-like cells. Intrapouch administration of TNF-alpha initiates leukocyte infiltration by stimulating release of chemokines and chemoattractants. In this system, RvE1 is produced in subnanogram amounts. In a TNF-alpha-induced air pouch dermal inflammation model, administration of 100 ng/mouse of synthetic RvE1 stopped leukocyte infiltration into inflammatory loci by 50–70%. For comparison, topical application of dexamethasone (10 μg/mouse) gave ~60% reduction, and aspirin (1.0 ng/mouse) gave 70% inhibition of leukocyte recruitment. Thus, RvE1 at nanomolar levels is as potent as higher doses of glucocorticoids or aspirin in stopping leukocyte trafficking into an inflammatory site.
In zymosan-induced murine peritonitis, RvE1 (100 ng/mouse) gives 50~60% inhibition of leukocyte infiltration compared to only 25% inhibition of infiltration with indomethacin (100 mg/mouse). Systemic administration of RvE1 dramatically attenuates leukocyte recruitment (80). RvE2 shares anti-inflammatory actions with RvE1. Of interest, intravenous administration of 1 ng of RvE2 resulted in 11.3% ± 4.8% reduction of PMN infiltration. PMN infiltration was reduced by 17.7% ± 9.9% and 33.7% ± 5.0% at 10 and 100 ng, respectively (Fig. 4). RvE2 was not significantly different than RvE1 at any of the doses since they both significantly reduced PMN infiltration in zymosan-induced peritonitis. When given together, RvE1 and RvE2 had an additive anti-inflammatory impact. When administered intraperitoneally at a 10 ng dose per mouse, RvE2 reduced PMN infiltration by 34.5% ± 4.5%. Unlike RvE2, RvE1 did not stop PMN recruitment when administered intraperitoneally (84).
D-series resolvins are DHA-derived local mediators and exhibit potent anti-inflammatory actions (Tables 4 and 5). In murine model of peritonitis, both RvD1 and AT-RvD1 showed a dose-dependent decrease in PMN infiltration into the inflammatory site. Maximal inhibition was ~35% and occurred at a 10–100 ng dose. RvD1 and AT-RvD1 are equally efficacious in decreasing total leukocyte infiltration, yet AT-RvD1 is statistically more potent at the 10 ng dose than RvD1. After 4h of incubation post IV-administration of compound, the leukocytic infiltration was composed of ~70% PMNs and ~30% monocytes. Since AT-RVD1 and RvD1 have similar actions on PMNs, the two may have different actions on monocytes (81).
In the setting of acute inflammation, intraperitoneal administration of 1 ng PD1/mouse reduces infiltration of PMN 2 h after zymosan challenge. Once PD1 was administered, > 90% of PMN infiltration was blocked. Interestingly, when administered together, RvE1 and PD1 have additive effects. Differential counts on light microscopy revealed that PD1 and its chemical analog 15,16-dehydro-PD1 reduced PMN infiltration and increased nonphlogistic recruitment of monocytes and lymphocytes, all in the context of reducing inflammation and promoting resolution.
The 17S series resolvins and PD1 are potent inhibitors of TNF-alpha-induced leukocyte infiltration in the air pouch model of dermal inflammation via both topical and systemic application. The 17S series resolvins reduced PMN recruitment by 82.2 ± 5.6% when applied topically and by 49.6% ± 8.2% when given intravenously. Systemic treatment with the 17S series resolvins also reduced zymosan A-induced PMN recruitment to the peritoneum by 45.1 to 0.8%, which was equipotent to the 17R series resolving value. PD1 and synthetic PD1 were also potent systemic inhibitors reducing PMN trafficking into the exudates by ~40%. This was of similar potency with indomethacin, at essentially equal doses (9).
In a model of murine zymosan peritonitis, mice were injected i.p. with ATLa, RvE1, or PD1 (300 ng/mouse). These compounds all reduce PMN traffic into cells. PD1 stands apart, displaying different kinetics (Table 3B). RvE1 and PD1 give maximal reduction at 12 h, while the actions of ATLa appear earlier, at ~4 h. FACS analysis demonstrated that these compounds, at 12h, did not change the percentage of macrophage populations. As for the resolution indices given in Table 3, ATLa lowered Ψmax without changing Ri or Tmax. In contrast, RvE1 and PD1 initiated Ri at earlier time intervals. Furthermore, PD1 reduced the duration of Ri. As for their chemokine/cytokine regulation, ATLa reduced pro-inflammatory cytokines/chemokines [e.g., IL-6, TNF-beta, and others; see ref. (31) for complete list and proteomic analyses], most strikingly at 4 h, while RvE1 and PD1 had maximal inhibitory actions at ~12 h. It is important to note that in vivo generation of LXA4 was at 2–4 h whereas PD1 levels peak at 12 h, coinciding with the time points of their bioaction. Interestingly, ATLa evoked TGF-beta release in the context of peritonitis only (31).
Gastrointestinal disease and colitis
Both lipoxins and resolvins control inflammation in a wide range of experimental inflammatory disorders. Lipoxins are produced in gut mucosa and serve to limit persistent inflammation (55). Patients with ulcerative colitis have low to absent synthesis of LXA4 and lower mucosal 15-lipoxygenase-2 enzyme levels (55). Interestingly, 3-oxa-LXA4 analogs have potent oral efficacy (90) in promoting resolution of 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis, a Crohn’s disease model (58). Mice sensitized to TNBS by skin painting developed colitis characterized by severe wasting and bloody diarrhea. Treating mice with RvE1 (1 μg/mouse ~ 0.05 mg/kg) improved mortality: RvE1-treated mice had 25% mortality and TNBS alone had 62.5% mortality. RvE1-treated mice experienced less weight loss and level of shortening of the colonic mucosa. Mice treated with vehicle alone showed ulcerations and significant transmural infiltration with PMNs, monocytes, and lymphocytes. RvE1-treated mice had less severe histological features of colitis. For comparison, ATLa, which was proven to be protective against dextran sodium sulfate-induced colitis (91), was also similarly protective in TNBS colitis.
RvE1-treated mice had reduced leukocyte infiltration into the colon, as shown by lower levels of myeloperoxidase present. Serum levels of anti-TNBS IgG were decreased in the RvEl-treated mice, suggesting a decrease in antigen presentation and B-cell IgG production. Real time PCR showed that mice treated with RvE1 had a significant reduction in TNF-alpha, IL-12 p40, inducible NO synthase, and COX-2. No significant effect was seen on expression of INF-gamma, Il-4 and Il-10. Murine ChemR23 mRNA was shown to be slightly increased in colons obtained from TNBS-treated mice (82).
Transgenic fat-1 mice engineered to express the Caenorhabditis elegans fat-1 gene, which encodes an n-3 fatty acid desaturase, are capable of producing n-3 PUFA from n-6 PUFA. The fat-1 mice have a low ratio of n-6/n-3 fatty acids in their systems without the need for dietary interventions. Transgenic fat-1 mice generate higher tissue levels of DHA and EPA that are converted in vivo to resolvins and protectins. These fat-1 mice are protected from inflammation and show increased resistance to death from colitis (92.) Dextran sodium sulfate (DSS)-induced colitis is an IBD model and is characterized by infiltration of inflammatory cells into the lamina propria, lymphoid hyperplasia, epithelial ulceration and focal crypt damage. Induction of DSS colitis resulted in weight loss, bloody stools and changes in general status. Fat-1 mice showed significantly less weight loss and a delayed progression of diarrhea but no change in fecal bleeding. Wild type (WT) mice had more significant adhesions, strictures and massive thickening of the colon. Colon shortening in WT mice was 35% compared to 15% in fat-1 mice when compared to untreated controls. Hallmarks of colitis were alleviated in fat-1 mice except for minor punctuate erosions and few ulcerations. Using liquid chromatography-UV-tandem MS mediator informatics, RvE1, RvD3, and NPD1/PD1 were identified in colon of fat-1 transgenics at physiologically active levels. In addition to these, PGE3 and LTB5 were also found in fat-1 mice. Significant differences were not noted in the levels of PGE2 and LTB4. Fat1 mice also had a decrease in NF-κB protein activity monitored by activated p65 protein and reduced TNF-alpha mRNA levels. Inducible NO-synthase and IL-1 beta were reduced in fat-1 mice mRNA levels of intestinal trefoil factor 3 (TFF3) was increased in fat-1 mice, and mRNA levels of Toll-interacting protein (Tollip) were increased in fat-1 transgenes compared to their wild-type littermates (92).
Periodontitis
Periodontitis is a leukocyte-mediated inflammatory disease characterized by inflammation and bone loss, where the supporting bone of the dentition is resorbed by osteoclasts (93). There are many similarities in the pathogenesis of periodontitis and arthritis (see ref. 94). Overexpression of 15-LO in transgenic rabbits increased endogenous LXA4 formation, reduced the onset of periodontitis, and reduced atherosclerosis (95, 96). The differential actions of RvE1 and 16-parafluorophenoxy-LXA4 (ATLa) were recently studied on neutrophils derived from patients with localized aggressive periodontitis (LAP) and healthy individuals with no apparent periodontal disease. Localized aggressive periodontitis (LAP) is characterized by excessive neutrophil-mediated tissue and bone destruction. The impact of ATLa and RVE1 on superoxide generation by neutrophils was evaluated in response to TNF-alpha or fMLP. In both healthy subjects and LAP patient neutrophils, there was ~ 80% inhibition of superoxide generation when cells were treated with RvE1 (10−6–10−13M). LAP neutrophils, but not healthy neutrophils, were less responsive to ATLa (10−6–10−13M). Therefore, RvE1 is a potent counter-regulator of superoxide anion generation by neutrophils. A rabbit model of periodontitis was established with the application of Porphyromonas gingivalis together with ligatures to second premolars of rabbits. Topical treatment of RvE1, as little as ~4 μg per tooth, 3x per week, at the site of the ligature reduced >95% of alveolar bone destruction. Histological analysis revealed few if any neutrophils or tissue damage in the RvE1-treated rabbits. These sections were also stained for TRAP, an osteoclast marker, and showed less TRAP positive cells in the RvE1-treated rabbits and controls, compared to rabbits infected with P. gingivalis (86).
Stroke, neural and ocular tissues
Synaptic terminals and neuronal plasma membranes are enriched in DHA. NPD1 is generated in retinal pigment epithelium (RPE) from DHA and protects RPE cells from oxidative stress-induced apoptosis and pro-inflammatory gene expression. Photoreceptor cell integrity depends on the RPE, and photoreceptor cell degeneration is a feature of retinal degenerative diseases such as retinitis pigmentosa and age-related macular degeneration. RPE cells undergoing oxidative stress generate NPD1 and counteract oxidative stress-induced apoptotic DNA damage in RPE by upregulating anti-apoptotic proteins Bcl-2 and Bcl-x (L), and decreasing pro-apoptotic Bax and Bad. Additionally, NPD1 counteracts leukocyte infiltration and pro-inflammatory gene expression in brain ischemia-reperfusion (97). These features render NPD1 a potential therapeutic target for stoke and other neurodegenerative disease.
In Alzheimer’s disease, hippocampal cornu ammonis region 1 has decreased levels of DHA and NPD1. This trend is not seen in the thalamus or occipital lobes of the same brain. AD-hippocampus has decreased expression of key enzymes in NPD1 biosynthesis such as phospholipase A2 and 15-LO. NPD1 represses Abeta42-triggered activation of pro-inflammatory genes and upregulates anti-apoptotic genes Bcl2, Bcl-xL, and Bfl (A1). Soluble amyloid precursor protein-alpha stimulates NPD1 biosynthesis from DHA. NPD1 promotes brain cell survival via the induction of antiapoptotic and neuroprotective gene-expression program that suppress Abeta42-induced neurotoxicity (12).
Corneal damage: epithelial wound healing
LXA4 and NPD1 have roles in wound healing distinct from their PMN directed actions. Mouse cornea was shown to generate LXA4 and NPD1. Topical application of LXA4 or NPD1 (1 μg) increased the rate of re-epithelialization by ~75% and reduced the sequelae of thermal injury. Murine corneal epithelial removal induced neutrophil recruitment into the corneal stroma and increased levels of proinflammatory chemokine KC (the murine equivalent of IL-8 in humans). Local treatment with LXA4 or NPD1 increased PMNs in the cornea yet decreased KC formation by ~ 60%. 12/15-LOX-deficient mice had defective corneal re-epithelialization and PMN recruitment. These mice showed ~ 40% reduction in endogenous LXA4 levels (88).
Renal ischemia reperfusion injury
Acute kidney injury is an inflammatory process where even repair and regeneration following the acute event leads to interstitial fibrosis, scarring and chronic kidney failure (98). This may be partly due to the persistent leukocyte infiltration. Mice kidneys produce RvDs and protectin in response to bilateral renal ischemia reperfusion injury (85). Ischemia followed by 24 h reperfusion triggers endogenous biosynthesis and release of the precursor DHA into circulation. Postischemic kidneys also generate PD1, 17S-HDHA, and to a lesser extent RvD1 and RvD3. In the absence of added DHA, there was no increase in plasma levels of PD1 and RvD1, in contrast to kidney tissue, which demonstrated an increase even in the absence of DHA pre-treatment, but there was still an increase in the levels of RvD2, RvD3 and RvD4. Post-ischemic kidneys showed increased levels of 17S-HDHA in both the vehicle group and the DHA-treated group. This suggests that the enzyme 15-LO is induced in the setting of I/R. Exogenous addition of DHA to mice undergoing I/R of kidney increased the plasma and kidney tissue levels of RvD6 and RvD2. There was also an increase in the levels of 17S-HDHA, RvD1 and RvD3. Of interest, the administration of DHA did not further increase RvD4 levels in plasma or kidney tissue.
Mice were then treated with D-series resolvins 10 min before bilateral renal ischemia and continued during a 48 h interval. At 24 h, RvDs were shown to protect the kidneys from injury as evidenced by a lower creatinine level compared to vehicle-treated mice, a finding confirmed with synthetic RvD1. PD1 pre-treatment was also protective in the setting of I/R injury. Mice treated with 35 μg of PD1 or RvDs per mouse using a micropump had enhanced tubule cell survival, reduced renal inflammation, and decreased capillary occlusion, as noted in PAS-stained sections of post-ischemic renal tissue.
Mice treated with DHA, 17S-HDHA, PD1, and RvD1 had lower MPO activity compared to mice treated with vehicle alone. This feature was noted at both 24 h and 48 h. Both PD1 and RvDs reduced tissue PMNs and monocytes. When administered after ischemic kidney injury, RvD-treated mice showed marked protection from acute renal failure. This was not the case with protectin. RvDs and PD1 limit the deposition of interstitial collagen and therefore protect against fibrosis. Therefore, RvDs and PD1 play an effective role in activating resolution circuits in the setting of acute kidney injury (85).
Hepatic injury
Recent results indicate that dietary incorporation of DHA-derived lipid mediators is protective in murine liver necroinflammatory injury (33). Cultured hepatocytes supplemented with DHA (10 μM) have decreased hydrogen peroxide-induced DNA damage using the “comet assay” and demonstrate less oxidative stress by monitoring malondialdehyde levels. Mice fed a DHA-enriched diet showed less carbon tetrachloride-induced necroinflammatory hepatic damage compared to mice given a low DHA diet. Mice fed DHA and EPA had reduced ballooning degeneration in hepatocytes, and DHA-fed mice not only had lower levels of PGE2 and hepatic COX-2 expression in the liver, but also increased hepatic formation of 17S-HDHA and protectin D1. When synthetic 17-HDHA was administered, there was a decrease in both TNF-alpha release and 5-LO expression in murine macrophages. Of interest, in a transactivation assay, each DHA-derived lipoxygenase product 17-HDHA (17R-HDHA) and, to a lesser extent, 14-HDHA and 7-HDHA, activated PPAR-gamma in a concentration dependent fashion (33). These results suggest that in addition to the autacoid actions of resolvins and protectins on cell surface receptors, there may also be actions within their cells of origin when resolvin/protectin pathways are activated.
Airway inflammation
Lipoxins are generated in asthma and are potent inhibitors of airway inflammation and hyperresponsiveness (52). Lipoxins and their stable analogs are known to block pulmonary inflammation by decreasing leukocyte (neutrophil, eosinophil, lymphocyte) recruitment and activation and stimulating non-phlogistic clearance of apoptotic leukocytes. They are also known to block edema formation and to reduce pro-inflammatory mediators such as IL-5, IL-13, eotaxin, prostanoids, and cysteinyl leukotriene (52). Severe asthmatics have defects in lipoxin biosynthesis (99). LXs also play a role in resolution of acid-initiated acute lung injury (100).
Exhaled breath condensate from healthy human subjects contains both PD1 and 17S-hydroxy-docosahexaenoic acid. Condensates from patients in asthma exacerbation, when compared to healthy subjects, gave lower levels of PD1 (101). In a murine model of airway inflammation, PD1 was shown to be present in both control animals and those challenged with aerosolized allergens. Airway eosinophil and T lymphocyte recruitment was decreased in this model once PD1 was administered before the aeroallergen challenge. Similarly, levels of proinflammatory mediators like IL-13, cysteinyl leukotrienes, and PGD2 were also decreased. With PD1 on board, airway hyperresponsiveness to inhaled metacholine was reduced. Even with PD1 treatment after aeroallergen challenge, resolution of airway inflammation was still significantly accelerated. Compared to LX-analogs, PD1 is more potent in protecting from airway inflammation. Interestingly, IL-5 production, although reduced by LX stable analogs, was not reduced by PD1. This observation suggests a direct action of PD1 on eosinophils, lymphocytes, and other effector cells. Most importantly, PD1 led to a decrease in LX levels suggesting that the PD1 action in the airway is distinct from lipoxin actions (101).
A Potential for New Pro-Resolving Therapeutics
Additional studies are now called for in order to determine the link between the beneficial roles of omega-3-PUFAs relating to improved inflammatory states and the relation to protectins and resolvins. By identifying key players in resolution, promising new anti-inflammatory and pro-resolving drugs may be designed (4) without having the notorious side effects of COX-2 inhibitors (102) or anti-TNF therapies (103–105), and avoiding resolution toxicity (4, 34). In the era of COX-2 inhibitors (35, 106) deciphering the mechanisms of acute inflammation and its resolution is crucial. An ideal anti-inflammatory drug should be able to: 1) dampen the inflammatory response to reduce local tissue damage; 2) activate de novo resolution mechanisms; and 3) not compromise host defense to infectious disease.
Summation
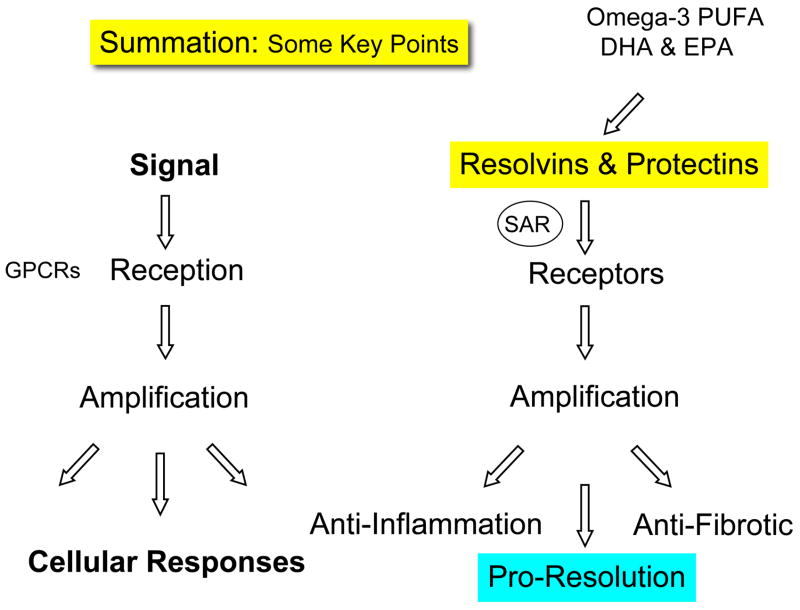
The recent evidence briefly summarized in this review indicates that a new era has emerged in the appreciation of acute inflammation and its progression to resolution, chronic inflammation, or fibrosis. The lipoxins, resolvins, and protectins each play an active role(s) in controlling and programming resolution of inflammation (7) by serving as agonists on their respective receptors stimulating endogenous anti-inflammatory and pro-resolving pathways (25). Lipoxins and ATLs have recently been shown to also attenuate nociception through a novel lipid mediator cascade regulating peripheral and spinal sensitization and hyperalgesia (63). Hence, the mapping of resolution to uncovering the key players and signaling pathways involving the resolvins and protectins (31) may yield clues for new treatment modalities aiming to reverse and/or prevent the chronic inflammatory state and possibly new therapeutic alternatives for a wide range of diseases (13).
The first steps of leukocytes in host defense in a delicate dance with vascular endothelial cells are now well appreciated, i.e., tethering, sticking, rolling adhesion molecules (107, 108). Whether these are movements orchestrated by chemoattractants generated via injury from within, as in ischemia-reperfusion injury, or in host defense against exogenous microbes, the engagement of chemoattractant G protein-coupled receptors, adhesion molecules and intracellular signal transduction events are all geared to rid the host of invading organisms and/or clear local tissues. During acute inflammation, lipid mediators such as prostaglandins and leukotrienes play pivotal roles in orchestrating the hemodynamic changes required as well as serve as potent chemoattractants to elicit neutrophils and call them into the tissues (Fig. 1), as sharks scent and respond to blood in the water. Until recently, clearance of the inflammatory tissue milieu of the debris from battle, i.e., host agonist invading microbes, was thought to be a passive event. As reviewed here, we’ve learned that biochemical programs are activated that generate potent lipid mediators such as lipoxins that serve both counter-regulatory and pro-resolving roles. For example, lipoxins stop leukocyte traffic, counter edema formation, stop pain signals, and stimulate the uptake of apoptotic neutrophils by macrophages. These are all events that culminate in the return of the tissue to homeostasis.
Catabasis is defined in medical dictionaries as the decline of the disease state. In this context, we initiated the elucidation of the biochemical, cellular, and molecular events in vivo (31) that enable the tissue to return to homeostasis; molecular catabasis, so to speak, at the nano and cellular levels. Aspirin appears to possess a unique property as an anti-inflammatory in that it not only blocks prostaglandins and thromboxane, which play a well known role in regulating the cardinal signs of inflammation, but also triggers the formation of potent endogenous lipid mediators such as the aspirin-triggered lipoxins (109). These are epimeric molecules at the carbon-15 position of LX and act at the lipoxin receptors and mimic their actions in vivo. They are also longer-acting because the carbon-15 position (C15) R epimers are less suitable substrates for dehydrogenation; in this context they resist the inactivating enzymes in the cascade by about 50%, resulting in a longer half-life. ATL stable analogs and LX have proven potent anti-inflammatory and pro-resolving actions in vivo (picogram-nanogram levels) as well as in vivo. In addition, they have more recently proven to treat complex diseases using industrial standard animal models (58, 110–112). Also, lipoxins and their stable analogs have antifibrotic actions demonstrated in recent studies. These findings lend assurance to the earlier notions that lipoxins could serve as biotemplates for the development of novel therapeutics that serve as agonists rather than the traditional inhibitor approach of anti-inflammatories. In this regard, screening for non-lipoxin agonists of the LXA4 receptor ALX/FPRL-1 gave orally active anti-inflammatory agents in murine models, providing additional evidence that receptor agonists can give new therapeutics for anti-inflammatory diseases.
From relatively recent results, the resolution of acute inflammation (7, 113) has emerged as a terrain rich in new pathways and therapeutic targets. Systematic analysis of resolution using a mass spectrometry-based lipid mediator-lipidomics approach and structural elucidation (using microchemical degradative analyses) demonstrate that active pathways are turned on during the resolution phase to generate novel pro-resolving and anti-inflammatory signals like the resolvins and protectins. Resolvins and protectins are biosynthesized from the essential omega-3 fatty acids (7, 8). The complete stereochemistry and total organic synthesis of RvE1, RvD1, and protectin D1/neuroprotectin D1, as well as their aspirin-triggered forms (Figs. 3 and 8), have recently been achieved. These new synthetic resolvins and protectins not only confirm their potent actions in vitro and in vivo, but also demonstrate the strict structure-activity relationships required for evoking their potent biological responses.
Receptors for RvE1 were demonstrated using a functional approach together with radioligand binding with synthetic RvE1. Resolvins and protectins are generated in vivo in murine systems as well as present in humans (80, 101). They were also identified in fish tissues (89), which raises questions about their role in fish and position in the food chain. In humans, DHA is enriched in neural tissues, and in this regard DHA has been considered to be a protective molecule in biomembranes (12). The finding that protectins and resolvins generated from precursor DHA led to a series of studies using resolvin D1 and protectin D1 with demonstrated anti-inflammatory actions already established, which go on to establish that resolvin D1 and protectin D1 have potent actions in neural tissues including retina as well as cornea (12, 88) as well as in liver (33). It is not surprising then that, in tissues where omega-3 fatty acids were suspected to play immunoregulatory and protective roles in earlier studies, the protectins and resolvins can carry out these responses in vivo (8, 114, 115).
Since the isolation of the first resolvin and its characterization in biological systems (7), it has become clear that resolvins and protectins have actions in a wide range of human tissues including neural, oral, airway, gastrointestinal, ocular, cardiovascular, and renal (Tables 4 and 5), where inflammation and/or reperfusion injury can occur. As early as 1929, in the original studies of Burr and Burr (14), essential fatty acids were defined by rigorous elimination of fats from the diets of rodents. Burr and Burr defined disease in rodents on depletion of fats from the laboratory diet, in particular polyunsaturated fatty acids, due to the absence of essential fatty acids. These hallmark studies of the last century have, for the most part, led to detailed analysis of lipids such as arachidonic acid and their transformation to local mediators such as prostaglandins and leukotrienes, which play pivotal roles in inflammation as well as in many other physiologic and pathophysiologic processes (24, 42). The other major fatty acids, omega-3 polyunsaturated fatty acids, were suspected to reduce inflammation and have been suggested to have a wide range of potential beneficial actions in human diseases including cardiovascular disease as well as cancers (18, 19, 116) and neural development (117). However, a number of mechanisms have been proposed over the years to describe the beneficial actions of polyunsaturated fatty acids, particular DHA and EPA, in human-derived cell lines and in epidemiologic studies.
These include the following.
First, arachidonic acid generates pro-inflammatory lipid mediators such as prostaglandins and leukotrienes as well as procoagulation signals such as thromboxane: omega-3 fatty acids (DHA and EPA) are thought to replace cellular stores of arachidonic acid that would then block biosynthesis of potent eicosanoids, reducing the tissue levels of pro-inflammatory mediators.
Second, omega-3 polyunsaturated fatty acids would replace arachidonic acid in the eicosanoid cascade, generating, instead of potent prostaglandins and leukotrienes, similar compounds derived from EPA and DHA that are devoid of potent biologic action such as leukotriene B5 and/or thromboxane A3, which are unable to evoke responses akin to the arachidonic acid native compound.
These are the two main hypotheses that have gained credence to date (118) and, together with the low levels of omega-3 found in Western diet (119), warrant an increase in omega-3 intake.
Our results demonstrate that each omega-3, EPA and DHA, is transformed within the resolution phase of acute inflammatory responses to resolvins and protectins, novel families of mediators that are local-acting with unique chemical structures (Fig. 11). Specific resolvins have tight structure-activity relationships and, as in the case of RvE1, interact with specific receptors that amplify the intracellular signals, leading to anti-inflammation (80, 83), pro-resolution, and anti-fibrotic phenotypes in complex disease models (Tables 4 and 5).
Figure 11.
Summation: Key points in summation.
Fish produce resolvins and protectins, suggesting not only that these are primordial structures that are highly conserved along evolutionary lines (89), but also that these polyunsaturated fatty acid pathways likely evolved in parallel and that omega-3 fatty acid uptake, biosynthesis, and generation to resolvins and protectins are events that occur in tandem/parallel to the biosynthesis of eicosanoids and appear to be temporally and functionally dissociated in experimental models where t0 can be fixed (31). They are not simply crisscrossed pathways blocking conversion or overwhelming the arachidonic acid in cellular stores. Establishing these pathways and their precise structure-activity relationships for the chemical mediators they generate indicates that resolution is an active rather than a passive process. They also provide additional evidence that resolution of acute inflammation and the use of resolvin and protectin stable analogs may represent novel therapeutic approaches (120) to expedite the natural return of inflamed tissue to homeostasis.
Establishing the structure-activity relationship for the resolvins and protectins, specifically resolvin E1, resolvin D1, and protectin D1, to date now opens new avenues of opportunity to investigate mechanisms of resolution. We can appreciate the means by which inflammatory exudates can trigger the return or resolve of inflamed tissue, the delicate balance of which dictates the outcome of successful resolution (i.e., complete resolution). It follows that deficiencies in substrate enzymes and/or receptors in these resolution pathways and/or mutations could lead to chronic inflammatory disease states that are the well-recognized tissue basis of many inflammatory diseases.
Summary Points List Highlighting The Central Issues Addressed In This Review
Resolvins and Protectins are novel enzymatically-generated families of local chemical mediators
PMN change their phenotype and generate anti-inflammatory and pro-resolving lipid mediators in resolving exudates
Resolution is an active process
Rv and PD dampen inflammation and PMN injury “from within”
Rv and PD1 are potent in picogram to nanogram range in vivo; stereoselective; GPCR agonists
Promote resolution: “stop PMN infiltration” and activate pro-resolving circuits
Regulate chemokine-cytokine axis and are anti-fibrotic
Resolvins and protectins are the likely active endogenous mediators of essential omega-3 PUFA
Acknowledgments
We thank M. Halm Small for manuscript preparation. This study was supported, in part, by National Institutes of Health grant nos. GM38765, DK074448 and P50-DE016191 (C.N. Serhan).
List of Key Terms used in text and their definitions
- aspirin-triggered
bioactive compounds initiated via modified COX-2 when the enzyme remains active
- eicosanoids
from Greek twenty, family of arachidonic-acid-derived mediators with carbon-based structure
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- leukotrienes
bioactive leukocyte-derived conjugated triene-containing structures biosynthesized from arachidonic acid that are potent proinflammatory mediators
- lipoxins
arachidonate-derived eicosanoids possessing conjugated tetraenes, trihydroxy bioactive structures
- lipoxygenase (LO)
inserts molecular oxygen and abstract hydrogen in a stereoselective reaction with 1, 4-cis-pentadiene units present in polyunsaturated fatty acids. The major human lipoxygenases, 5-LO, 12-LO and 15-LO, are defined by the carbon position with arachidonic acid substrate, where molecular oxygen in enzymatically inserted to form hydroperoxy-containing intermediates, i.e., 15S-HpETE or 17S-HpDHA
- non-phlogistic
non-inflammatory, non-fever-producing
- prostanoids
branch of the eicosanoid family having the general prostaglandin cyclic structures. They are bioactive products of cyclooxygenases, isoprostanoids can be produced via non-enzymatic oxidative mechanisms
- Protectin (PD)
the family of DHA-derived mediators possessing a conjugated triene structure as a distinguishing feature
- Resolvin (Rv)
resolution phase interaction products carrying bioactivity
List of most important acronyms used in text
- AT-RvD1
aspirin-triggered-resolvin D1 (7S, 8, 17R-trihydroxy-docosa-4Z, 9E, 11E, 13Z, 15E, 19Z-hexaenoic acid)
- AT-RvD2
aspirin-triggered-resolvin D2 (7S, 16, 17R-trihydroxy-docosa-4Z, 8E, 10Z, 12E, 14E, 19Z-hexaenoic acid)
- AT-RvD3
aspirin-triggered-resolvin D3 (4S, 11, 17R-trihydroxy-docosa-5, 7E, 9E, 13Z, 15E, 19Z-hexaenoic acid)
- AT-RvD4
aspirin-triggered-resolvin D4. (4S, 5, 17R-trihydroxy-docosa-6E, 8E, 10Z, 13Z, 15E, 19Z-hexaenoic acid)
- LC-UV-MS/MS
liquid chromatography-ultraviolet spectrometry-tandem mass spectrometry
- LM
lipid mediator
- LTB4
leukotriene B4 (5S, 12R-dihydroxy-eicosa-6Z, 8E, 10E, 14Z-tetraenoic acid)
- LXA4
lipoxin A4 (5S, 6R, 15S-trihydroxy-eicosa-7E, 9E, 11Z, 13E-tetraenoic acid)
- LXB4
lipoxin B4 (5S, 14R, 15S-trihydroxy-eicosa-6E, 8Z, 10E, 12E-tetraenoic acid)
- PD1/NPD1
protectin D1/neuroprotectin D1 (10R, 17S-dihydroxy-docosa-4Z, 7Z, 11E, 13E, 15Z, 19Z-hexaenoic acid)
- PGE2
9-oxo-11a, 15S-dihydroxy-prosta-5Z, 13E-dien-1-oic acid
- PUFA
polyunsaturated fatty acid
- RvE1
Resolvin E1 (5S, 12R, 18R-trihydroxy-eicosa-6Z, 8E, 10E, 14Z, 16E-pentaenoic acid)
- RvD1
Resolvin D1 (7S, 8R, 17R-trihydroxy-4Z, 9E, 11E, 13Z, 15E, 19Z-docosahexaenoic acid)
LITERATURE CITED
- 1.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. Philadelphia: W.B. Saunders Co; 1999. p. 1425. [Google Scholar]
- 2.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature Immunol. 2001;2:612–19. doi: 10.1038/89759. This is the original report demonstrating temporal separation between individual classes of eicosanoids, i.e., dissociation between the formation and actions of prostaglandins, leukotrienes, and lipoxins in resolution. [DOI] [PubMed] [Google Scholar]
- 3.Calder PC. Long-chain polyunsaturated fatty acids and inflammation. Scand J Food Nutr. 2006;50(S2):54–61. [Google Scholar]
- 4.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LAJ, Perretti M, Rossi AG, Wallace JL. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, editor. Prostaglandins Leukot Essent Fatty Acids. 3–4. Vol. 73. 2005. Special Issue on Lipoxins and Aspirin-Triggered Lipoxins; pp. 139–321. This special issue features nineteen reviews by experts covering the anti-inflammatory actions of lipoxins, aspirin-triggered lipoxins, and therapeutic potential of stable metabolic analogs. This is a detailed resource for original research and critical review of results on lipoxins and their mechanism of action. [DOI] [PubMed] [Google Scholar]
- 6.Morris T, Rajakariar R, Stables M, Gilroy DW. Not all eicosanoids are bad. Trends Pharmacol Sci. 2006;27:609–11. doi: 10.1016/j.tips.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–204. doi: 10.1084/jem.192.8.1197. This is the original report on the generation of arrays of bioactive compounds via aspirin-triggered pathways and omega-3 fatty acids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. The isolation and identification of bioactive compounds generated during the resolution phase of acute inflammatory response; identification and characterization of the resolvins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. This is the first detailed report of the docosatrienes, novel DHA-derived compounds containing conjugated triene structures, and the resolvins, formed de novo from DHA in human blood, glial cells, and also murine brain. These endogenous 17S-series resolvins have actions equivalent to the aspirin-triggered 17R-series resolvins reported in Refs. 8 and 11. [DOI] [PubMed] [Google Scholar]
- 10.Serhan CN, Gotlinger K, Hong S, Lu Y, Siegelman J, Baer T, Yang R, Colgan SP, Petasis NA. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–59. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 11.Marcheselli VL, Hong S, Lukiw WJ, Hua Tian X, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. This is the original report demonstrating formation of 10,17-docosatriene and resolvin D in stroke and their actions in regulating neutrophil infiltration in brain and protecting from tissue damage incurred during stroke. [DOI] [PubMed] [Google Scholar]
- 12.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–83. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007:25. doi: 10.1146/annurev.immunol.25.022106.141647. In press. [DOI] [PubMed] [Google Scholar]
- 14.Burr GO, Burr MM. A new deficiency disease produced by the rigid exclusion of fat from the diet. J Biol Chem. 1929;82:345–67. doi: 10.1111/j.1753-4887.1973.tb06008.x. The original report demonstrating the importance of fatty acids and lipids in the diet of rodents, and most importantly defines for the first time the multi-organ disease impact of fatty acid/lipid dietary exclusion. [DOI] [PubMed] [Google Scholar]
- 15.James MJ, Proudman SM, Cleland LG. Dietary n-3 fats as adjunctive therapy in a prototypic inflammatory disease: issues and obstacles for use in rheumatoid arthritis. Prostaglandins Leukot Essent Fatty Acids. 2003;68:399–405. doi: 10.1016/s0952-3278(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 16.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(suppl):1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 17.Hamazaki K, Itomura M, Sawazaki S, Hamazaki T. Fish oil reduces tooth loss mainly through its anti-inflammatory effects? Med Hypotheses. 2006;67:868–70. doi: 10.1016/j.mehy.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 18.GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–55. [PubMed] [Google Scholar]
- 19.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 20.Harris WS, Reid KJ, Sands SA, Spertus JA. Blood omega-3 and trans fatty acids in middle-aged acute coronary syndrome patients. Am J Cardiol. 2007;99:154–58. doi: 10.1016/j.amjcard.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Michelson AM, McCord JM, Fridovich I. Superoxide and Superoxide Dismutases. London: Academic Press; 1977. [Google Scholar]
- 22.Halliwell B. Superoxide and hydroxylation reactions. In: Michelson AM, McCord JM, Fridovich I, editors. Superoxide and Superoxide Dismutases. London: Academic Press; 1977. pp. 335–49. [Google Scholar]
- 23.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 24.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–6. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–97. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN. Mediator lipidomics. Prostaglandins Other Lipid Mediat. 2005;77:4–14. doi: 10.1016/j.prostaglandins.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by IL-13 and IFN-γ and inhibits TNF-α-induced IL-8 release. J Exp Med. 1998;187:1285–94. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnans C, Mainprice B, Chanez P, Bousquet J, Urbach V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J Biol Chem. 2003;278:10879–84. doi: 10.1074/jbc.M210294200. [DOI] [PubMed] [Google Scholar]
- 29.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt B, Szczeklik W, Drazen JM, Serhan CN. Lipoxins and aspirin-triggered lipoxins in airway responses. Adv Exp Med Biol. 2003;525:19–23. doi: 10.1007/978-1-4419-9194-2_5. [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN. On the relationship between leukotriene and lipoxin production by human neutrophils: evidence for differential metabolism of 15-HETE and 5-HETE. Biochimica et Biophysica Acta. 1989;1004:158–68. doi: 10.1016/0005-2760(89)90264-6. [DOI] [PubMed] [Google Scholar]
- 31.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55. doi: 10.4049/jimmunol.174.7.4345. This is of special interest because it is the first report to systematically address in quantitative terms the cellular and molecular events involved in resolution. [DOI] [PubMed] [Google Scholar]
- 32.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–16. doi: 10.1038/nrd1383. Authoritative review that addresses whether the process of resolution holds the promise of new targets and potential drug treatment. [DOI] [PubMed] [Google Scholar]
- 33.González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, Rodés J, Clària J. Docosahexanenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–39. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 34.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cycloxygenase may have anti-inflammatory properties. Nature Med. 1999;5:698–701. doi: 10.1038/9550. This paper demonstrates for the first time that cyclooxygenase-2 can play a key role in resolution and endogenous anti-inflammation. It is a must-read in view of the impact of COX-2 inhibitors found in clinical trials in recent years. [DOI] [PubMed] [Google Scholar]
- 35.Rajakariar R, Yaqoob MM, Gilroy DW. COX-2 in inflammation and resolution. Mol Interv. 2006;6:199–207. doi: 10.1124/mi.6.4.6. [DOI] [PubMed] [Google Scholar]
- 36.Gilroy DW, Perretti M. Aspirin and steroids: new mechanistic findings and avenues for drug discovery. Curr Op Pharmacol. 2005;5:405–11. doi: 10.1016/j.coph.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Ariel A, Fredman G, Sun Y-P, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution via modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–84. doi: 10.1074/jbc.M605146200. This report demonstrates the signal transduction and suppression of pro-inflammatory eicosanoids while turning on anti-inflammatory lipid mediator pathways, 15-LO and lipoxin A4 production regulating NO synthesis and macrophages. [DOI] [PubMed] [Google Scholar]
- 39.Flower RJ. Cyclooxygenase inhibitors: an overview. In: Bailey JM, editor. Prostaglandins, Leukotrienes and Lipoxins: Biochemistry, Mechanism of Action, and Clinical Applications. New York: Plenum; 1985. pp. 583–91. A superb review and historical perspective on nonsteroidal anti-inflammatory drugs; an authoritative and important contribution. [Google Scholar]
- 40.Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147:S182–S92. doi: 10.1038/sj.bjp.0706506. A wonderful historical perspective on the race to establish the structures of potent bioactive mediators and the multidisciplinary approach to physiologic chemistry of the prostaglandins. An authoritative review with enormous resources. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vane JR. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Stockholm: Almqvist & Wiksell; 1982. Adventures and excursions in bioassay: the stepping stones to prostacyclin; pp. 181–206. A “must-read” account for students of inflammation of the Nobel lecture in 1982 encompassing the tour de force of physiology and pharmacology in the hands of an expert. [Google Scholar]
- 42.Samuelsson B. Les Prix Nobel: Nobel Prizes, Presentations, Biographies and Lectures. Stockholm: Almqvist & Wiksell; 1982. From studies of biochemical mechanisms to novel biological mediators: prostaglandin endoperoxides, thromboxanes and leukotrienes; pp. 153–74. A “must-read” account of the 1982 Nobel lecture on the structural elucidation of leukotrienes and identification of thromboxane. Emphasis is on the importance of transient biosynthetic intermediates in these pathways and the biosynthesis of prostaglandins from arachidonic acid. [DOI] [PubMed] [Google Scholar]
- 43.Weissmann G. Pathogenesis of rheumatoid arthritis. J Clin Rheumatol. 2004;10(3 Suppl):S26–S31. doi: 10.1097/01.rhu.0000130687.75646.44. [DOI] [PubMed] [Google Scholar]
- 44.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–42. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titos E, Chiang N, Serhan CN, Romano M, Gaya J, Pueyo G, Clària J. Hepatocytes are a rich source of novel aspirin-triggered 15-epi-lipoxin A4 (ATL) Am J Physiol. 1999;277:C870–C77. doi: 10.1152/ajpcell.1999.277.5.C870. [DOI] [PubMed] [Google Scholar]
- 46.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devchand PR, Arita M, Hong S, Bannenberg G, Moussignac R-L, Gronert K, Serhan CN. Human ALX receptor regulates neutrophil recruitment in transgenic mice: Roles in inflammation and host-defense. FASEB J. 2003;17:652–59. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- 48.Hashimoto A, Murakami Y, Kitasato H, Hayashi I, Endo H. Glucocorticoids co-interact with lipoxin A4 via lipoxin A4 receptor (ALX) up-regulation. Biomed Pharmacother. 2007;61:81–85. doi: 10.1016/j.biopha.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Chiang N, Serhan CN, Dahlén S-E, Drazen JM, Hay DWP, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–87. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 50.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: A role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–68. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 51.Brezinski DA, Nesto RW, Serhan CN. Angioplasty triggers intracoronary leukotrienes and lipoxin A4. Impact of aspirin therapy. Circulation. 1992;86:56–63. doi: 10.1161/01.cir.86.1.56. Initial report documenting in humans the intraluminal generation of leukotrienes and lipoxin A4 on PCTA-induced plaque rupture. [DOI] [PubMed] [Google Scholar]
- 52.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM, Serhan CN. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nature Med. 2002;8:1018–23. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 53.Kieran NE, Maderna P, Godson C. Lipoxins: Potential anti-inflammatory, proresolution, and antifibrotic mediators in renal disease. Kidney Int. 2004;65:1145–54. doi: 10.1111/j.1523-1755.2004.00487.x. [DOI] [PubMed] [Google Scholar]
- 54.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nature Immunol. 2004;5:388–92. doi: 10.1038/ni1056. The analysis of nonresolving airway inflammation in cystic fibrosis as a treatment paradigm for anti-inflammatory lipid mediators. [DOI] [PubMed] [Google Scholar]
- 55.Mangino MJ, Brounts L, Harms B, Heise C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Oth Lipid Mediat. 2006;79:84–92. doi: 10.1016/j.prostaglandins.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Serhan CN, Maddox JF, Petasis NA, Akritopoulou-Zanze I, Papayianni A, Brady HR, Colgan SP, Madara JL. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–15. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 57.Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein linked lipoxin A4 receptor. J Biol Chem. 1997;272:6972–78. doi: 10.1074/jbc.272.11.6972. [DOI] [PubMed] [Google Scholar]
- 58.Guilford WJ, Parkinson JF. Second-generation beta-oxidation resistant 3-oxa-lipoxin A4 analogs. Prostaglandins Leukot Essent Fatty Acids. 2005;73:245–50. doi: 10.1016/j.plefa.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006;5:91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- 60.Bandeira-Melo C, Serra MF, Diaz BL, Cordeiro RSB, Silva PMR, Lenzi HL, Bakhle YS, Serhan CN, Martins MA. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol. 2000;164:1029–36. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- 61.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–26. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hachicha M, Pouliot M, Petasis NA, Serhan CN. Lipoxin (LX)A4 and aspirin-triggered 15-epi-LXA4 inhibit tumor necrosis factor 1α-initiated neutrophil responses and trafficking: regulators of a cytokine-chemokine axis. J Exp Med. 1999;189:1923–29. doi: 10.1084/jem.189.12.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin stop inflammatory pain processing. J Exp Med. 2007 doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serhan CN, Fierro IM, Chiang N, Pouliot M. Nociceptin stimulates neutrophil chemotaxis and recruitment: Inhibition by aspirin-triggered-15-epi-lipoxin A4. J Immunol. 2001;166:3650–54. doi: 10.4049/jimmunol.166.6.3650. [DOI] [PubMed] [Google Scholar]
- 65.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–67. doi: 10.4049/jimmunol.164.4.1663. The first report establishing that lipoxins are potent stimuli for nonphlogistic phagocytosis by macrophages. [DOI] [PubMed] [Google Scholar]
- 66.Mitchell S, Thomas G, Harvey K, Cottell D, Reville K, Berlasconi G, Petasis NA, Erwig L, Rees AJ, Savill J, Brady HR, Godson C. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–507. doi: 10.1097/01.asn.0000032417.73640.72. Demonstration that lipoxins and lipoxin stable analogs stimulate resolution in vivo via the demonstration of accelerated uptake of apoptotic PMN; a technical tour de force and must-read. [DOI] [PubMed] [Google Scholar]
- 67.Rossi AG, Sawatzky DA, editors. The Resolution of Inflammation. Basel: Birkhäuser Verlag AG; 2007. in press. [Google Scholar]
- 68.Ward C, Dransfield I, Chilvers ER, Haslett C, Rossi AG. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends Pharmacol Sci. 1999;20:503–09. doi: 10.1016/s0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- 69.Brezinski ME, Gimbrone MA, Jr, Nicolaou KC, Serhan CN. Lipoxins stimulate prostacyclin generation by human endothelial cells. FEBS Letters. 1989;245:167–72. doi: 10.1016/0014-5793(89)80214-5. [DOI] [PubMed] [Google Scholar]
- 70.Paul-Clark MJ, van Cao T, Moradi-Bidhendi N, Cooper D, Gilroy DW. 15-epi-lipoxin A4-mediated induction of nitric oxide explains how aspirin inhibits acute inflammation. J Exp Med. 2004;200:69–78. doi: 10.1084/jem.20040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: Aspirin-triggered-15R-lipoxin A4 and lipoxin A4. J Pharmacol Exp Ther. 2002;300:385–92. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 72.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Villela CG, Fierro IM. Novel lipid mediator aspirin-triggered lipoxin A4 induces heme oxygenase-1 in endothelial cells. Am J Physiol Cell Physiol. 2005;289:C557–C63. doi: 10.1152/ajpcell.00045.2005. [DOI] [PubMed] [Google Scholar]
- 73.Jin S-W, Zhang L, Lian Q-Q, Liu D, Wu P, Yao S-L, Ye D-Y. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth Analg. 2007;104:369–77. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- 74.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Huang M-H, Perez-Polo JR, Uretsky BF. Aspirin augments 15-epi-lipoxin A4 production by lipopolysaccharide, but blocks the pioglitazone and atorvastatin induction of 15-epi-lipoxin A4 in the rat heart. Prostaglandins Other Lipid Mediat. 2007;83:89–98. doi: 10.1016/j.prostaglandins.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Birnbaum Y, Ye Y, Lin Y, Freeberg SY, Nishi SP, Martinez JD, Huang M-H, Uretsky BF, Perez-Polo JR. Augmentation of myocardial production of 15-epi-lipoxin-A4 by pioglitazone and atorvastatin in the rat. Circulation. 2006;114:929–35. doi: 10.1161/CIRCULATIONAHA.106.629907. [DOI] [PubMed] [Google Scholar]
- 76.Birnbaum Y, Ye Y, Rosanio S, Tavackoli S, Hu ZY, Schwarz ER, Uretsky BF. Prostaglandins mediate the cardioprotective effects of atorvastatin against ischemia-reperfusion injury. Cardiovasc Res. 2005;65:345–55. doi: 10.1016/j.cardiores.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 77.Ye Y, Lin Y, Atar S, Huang M-H, Perez-Polo JR, Uretsky BF, Birnbaum Y. Myocardial protection by pioglitazone, atorvastatin, and their combination: mechanisms and possible interactions. Am J Physiol Heart Circ Physiol. 2006;291:H1158–H69. doi: 10.1152/ajpheart.00096.2006. [DOI] [PubMed] [Google Scholar]
- 78.Levy BD. Myocardial 15-epi-lipoxin A4 generation provides a new mechanism for the immunomodulatory effects of statins and thiazolidinediones. Circulation. 2006;114:873–75. doi: 10.1161/CIRCULATIONAHA.106.647925. [DOI] [PubMed] [Google Scholar]
- 79.Lu Y, Hong S, Tjonahen E, Serhan CN. Mediator-lipidomics: databases and search algorithms for PUFA-derived mediators. J Lipid Res. 2005;46:790–802. doi: 10.1194/jlr.D400020-JLR200. [DOI] [PubMed] [Google Scholar]
- 80.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–22. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17 Repimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J Biol Chem. 2007 doi: 10.1074/jbc.M609212200. http://www.jbc.org/cgi/doi/10.1074/jbc.M609212200. [DOI] [PubMed]
- 82.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci USA. 2005;102:7671–76. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007 doi: 10.4049/jimmunol.178.6.3912. in press. [DOI] [PubMed] [Google Scholar]
- 84.Tjonahen E, Oh SF, Siegelman J, Elangovan S, Percarpio KB, Hong S, Arita M, Serhan CN. Resolvin E2: Identification and anti-inflammatory actions: pivotal role of human 5-lipoxygenase in resolvin E series biosynthesis. Chem Biol. 2006;13:1193–202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 85.Duffield JS, Hong S, Vaidya V, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–11. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 86.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–03. doi: 10.1096/fj.05-4724fje. The first demonstration that RvE1 can protect in a rabbit model of periodontal disease. [DOI] [PubMed] [Google Scholar]
- 87.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–96. doi: 10.1073/pnas.0402531101. First evidence showing the neuroprotective actions of the novel 10,17-docosatriene and introduction of the term neuroprotectin D1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Schwartzman ML. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–78. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 89.Hong S, Tjonahen E, Morgan EL, Yu L, Serhan CN, Rowley AF. Rainbow trout (Oncorhynchus mykiss) brain cells biosynthesize novel docosahexaenoic acid-derived resolvins and protectins -- mediator lipidomic analysis. Prostaglandins Other Lipid Mediat. 2005;78:107–16. doi: 10.1016/j.prostaglandins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 90.Bannenberg G, Moussignac R-L, Gronert K, Devchand P, Schmidt B, Guilford WJ, Bauman JG, Subramanyam B, Perez HD, Parkinson JF, Serhan CN. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, Williams IR, Neish AS, Madara JL. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–67. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- 92.Hudert CA, Weylandt KH, Wang J, Lu Y, Hong S, Dignass A, Serhan CN, Kang JX. Transgenic mice rich in endogenouse n-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276–81. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- 94.Serhan CN. Clues for new therapeutics in osteoporosis and periodontal disease: new roles for lipoxygenases? Expert Opin Ther Targets. 2004;8:643–52. doi: 10.1517/14728222.8.6.643. [DOI] [PubMed] [Google Scholar]
- 95.Serhan CN, Jain A, Marleau S, Clish C, Kantarci A, Behbehani B, Colgan SP, Stahl GL, Merched A, Petasis NA, Chan L, Van Dyke TE. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–65. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 96.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim H-S, Kühn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–08. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–66. doi: 10.1111/j.1750-3639.2005.tb00513.x. Concise review of the author’s collaborative work and demonstration that NPD1 is a potent neuroprotective lipid mediator. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F99. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 99.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E Severe Asthma Research Program National Heart Lung and Blood Institute. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–30. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–72. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1653–56. doi: 10.1001/jama.296.13.jrv60011. [DOI] [PubMed] [Google Scholar]
- 103.Bongartz T, Sutton AJ, Sweeting JJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 104.De Bandt M, Saint-Marcoux B Club Rhumatismes et Inflammation. Tumour necrosis factor alpha blockade and the risk of vasculitis. Ann Rheum Dis. 2006;65:1534–35. doi: 10.1136/ard.2006.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hochman D, Wolff B. Risk of serious infections and malignancies with anti-TNF antibody therapy in rheumatoid arthritis. JAMA. 2006;296:2203. doi: 10.1001/jama.296.18.2203-a. [DOI] [PubMed] [Google Scholar]
- 106.Gallin JI, Snyderman R. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 1047–61. [Google Scholar]
- 107.Collins T, editor. Leukocyte Recruitment, Endothelial Cell Adhesion Molecules, and Transcriptional Control: Insights for Drug Discovery. Boston: Kluwer Academic Publishers; 2001. p. 327. [Google Scholar]
- 108.Gallin JI, Snyderman R, Fearon DT, Haynes BF, Nathan C, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia: Lippincott Williams & Wilkins; 1999. p. 1360. [Google Scholar]
- 109.Clària J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–79. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schottelius AJ, Giesen C, Asadullah K, Fierro IM, Colgan SP, Bauman J, Guilford W, Perez HD, Parkinson JF. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–70. doi: 10.4049/jimmunol.169.12.7063. The first detailed analysis using industry-standard in vivo pharmacologic assays to address the anti-inflammatory actions and potential of lipoxins. [DOI] [PubMed] [Google Scholar]
- 111.Wu S-H, Wu X-H, Liao P-Y, Dong L. Signal transduction involved in protective effects of 15(R/S)-methyl-lipoxin A4 on mesangioproliferative nephritis in rats. submitted. [Google Scholar]
- 112.Fiorucci S, Wallace JL, Mencarelli A, Distrutti E, Rizzo G, Farneti S, Morelli A, Tseng J-L, Suramanyam B, Guilford WJ, Parkinson JF. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–41. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–46. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Connor KM, SanGiovanni JP, Connor KM, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem NN, Jr, Serhan CN, Smith LEH. Increasing dietary omega-3 LC-PUFA reduces pathological angiogenesis through regulation of local resolvins, neuroprotectins and TNF-alpha. Nat Med. submitted. [Google Scholar]
- 115.Kang JX, Wang J, Wu L, Kang ZB. Fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 116.Xia S, Lu Y, Wang J, He C, Hong S, Serhan CN, Kang JX. Melanoma growth is reduced in fat-1 transgenic mice: Impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci USA. 2006;103:12499–504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Salem N, Jr, Litman B, Kim H-Y, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–59. doi: 10.1007/s11745-001-0805-6. Important contribution on the mechanisms of DHA from research at NIH. This is a mini-review; a must-read. [DOI] [PubMed] [Google Scholar]
- 118.Lands WEM, editor. Proceedings of the AOCS Short Course on Polyunsaturated Fatty Acids and Eicosanoids. Champaign, IL: American Oil Chemists’ Society; 1987. [Google Scholar]
- 119.Simopoulos AP, Leaf A, Salem N., Jr Workshop on the essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. J Am Coll Nutr. 1999;18:487–89. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- 120.Arita M, Oh S, Chonan T, Hong S, Elangovan S, Sun Y-P, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J Biol Chem. 2006;281:22847–54. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 121.Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clinical Science. 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 123.Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–69. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 and LXA4 stable analogs are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Menezes-de-Lima O, Jr, Kassuya CA, Nascimento AF, Henriques MG, Calixto JB. Lipoxin A4 inhibits edema in mice: Implications for the anti-edematogenic mechanism induced by aspirin. Prostaglandins Oth Lipid Mediat. 2006;80:123–35. doi: 10.1016/j.prostaglandins.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 126.Wada K, Arita M, Nakajima A, Katayama K, Kudo C, Kamisaki Y, Serhan CN. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–92. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- 127.Jain A, Batista EL, Jr, Serhan C, Stahl GL, Van Dyke TE. Role for periodontitis in the progression of lipid deposition in an animal model. Infect Immun. 2003;71:6012–18. doi: 10.1128/IAI.71.10.6012-6018.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thomas E, Leroux JL, Blotman F, Chavis C. Conversion of endogenous arachidonic acid to 5,15-diHETE and lipoxins by polymorphonuclear cells from patients with rheumatoid arthritis. Inflamm Res. 1995;44:121–24. doi: 10.1007/BF01782022. [DOI] [PubMed] [Google Scholar]
- 129.Wallace JL, Fiorucci S. A magic bullet for mucosal protection … and aspirin is the trigger! . Trends Pharmacol Sci. 2003;24:323–26. doi: 10.1016/S0165-6147(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 130.Kieran NE, Doran PP, Connolly SB, Greenan M-C, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–92. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 131.Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso JM, Badr KF. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci USA. 1999;96:13375–80. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ariel A, Li P-L, Wang W, Tang W-X, Fredman G, Hong S, Gotlinger KH, Serhan CN. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–86. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 133.Bazan NG. Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Adv Exp Med Biol. 2006;572:531–40. doi: 10.1007/0-387-32442-9_74. [DOI] [PubMed] [Google Scholar]