Introduction
Stem cell therapy appears to be a promising modality for myocardial repair of both hearts post myocardial infarction and those with other forms of structural cardiac disease (eg. congestive heart failure). In fact recent experimental and clinical work has suggested that stem cell therapy contributes to cardiac regeneration. Unfortunately at this time we contend that stem cell therapy is proarrhythmic. Accordingly, in this review we will approach this subject by restating this potential in the framework of traditional mechanisms of arrhythmias (automaticity and reentry). Lastly we will address recent clinical work with stem cells commenting on the proarrhythmic outcomes.
A. The Players
Before assessing the proarrhythmic potential of stem cells, we think it important to first address the nature of the players. After all these “players” are the cells selected for use in trials.
Embryonic stem cells (ESC)
Obviously since this cell population has the capacity to develop into differentiated cardiac cells, they have been phenotyped in terms of ionic current makeup, intracellular Ca2+ handling, and connexin expression.1 ESCs have been shown to have at least fast sodium current, L type Ca2+ current, If, and IK12,3 and immature excitation-contraction (EC) coupling.4 Thus, implanting them into damaged myocardium you would be implanting areas of additional excitable cells that presumably would form gap junctions not only with fellow ESCs, but also with surviving myocytes of the damaged substrate. Evidence for resident cardiac stem cells 5,6 has surfaced and these excitable cells could show promise for use in stem cell therapy. For example, c-Kit+ cardiac derived cells isolated from a normal rat heart when delivered via the aortic root, seem to invade the infarcted myocardium and regenerate muscle to improve left ventricular (LV) function.7 Unfortunately, there was no mention of rhythm instability (or stability) of the injected hearts in this study. Some have suggested that Kit+ expressing cells are actually not heart cells but bone marrow cells out of place. Importantly human ESCs (hESCs) can be cultured as three dimensional differentiating cell aggregates called embryoid bodies (Figure 1).8
Figure 1.
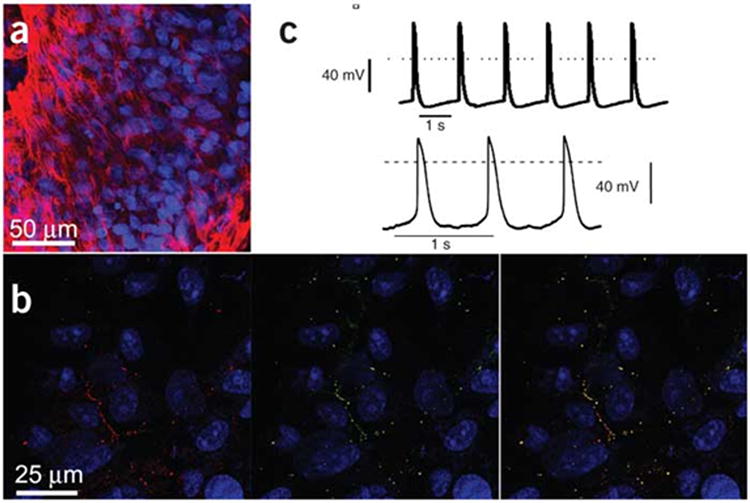
Morphology and function of hESCs. A, Immunostaining with anti-Troponin I (red). Nuclei are in blue. Cardiac cells are within the embroyoid body. B, Staining with Cx43 (red, left side) and Cx45 (green, right side). Gap junction containing both seen in right panel (yellow dots). C, Spontaneous electrical activity in embryoid bodies. Repinted from Kehet et al8 with permission of the publisher. Copyright ©2004, Nature Publishing Group.
Skeletal Myoblasts (SkMs)
Perhaps one of the first classes of cell types used for replacement therapy was the autologous skeletal myoblast derived cell. While these cells are able to contract and show some excitability, the phenotype of EC coupling in these cells differs completely from that of normal cardiac cells.9 Importantly undifferentiated myoblasts can express connexins and form gap junctions. However, after time in the new substrate, the myoblast tends to lose this capacity.10 Thus myoblasts may survive where replacement tissue is needed but could then lack both mechanical synchronization and electrical integration forming islands of tissue.
Bone marrow cells (BMC) and Mesenchymal stem cell (MSCs)
Various types of bone marrow cells can differentiate into important stem cells. One population is the mesenchymal stem cell (MSC). They can be differentiated into neuronal type cells and have been implanted into the ischemic brain.11 Studies have described a complement of ionic currents in both the undifferentiated and differentiated MSC12;13 resulting in cells with resting potentials in the −30 to −40mV range. Other studies have described critical cardiac proteins such as troponin I and connexin 43 (Cx43).14 Whether MSCs fully differentiate into cardiac cell types remains controversial.15,16
B. Theoretical Considerations
1. Factors that would lead to enhanced Automaticity
If stem cells are implanted into myocardium for replacement or to become the pacemaker of the heart, they should functionally couple with remaining myocytes of the substrate to allow for a more homogeneous myocardium. As we know from numerous years of study of the pacemaker cells of the normal sinus node, if implanted cells have intrinsic abnormal electrophysiology and/or show spontaneous electrical activity, they then can become the source of electrical excitation. Cell electrophysiology studies of ESCs have shown slow upstroke action potentials and triggered activity (Figure 1).8,17-19 This inherent pacemaking activity is thought to be due to high input resistance and high sodium current density.3 In culture studies of both neonatal rat and hESCs, as the implanted cells, intrinsic pacemaker activity of the implanted cells cannot overcome normal rhythm since they were only located in a small area (eg. 200μm by 20μm).8 In contrast, larger denser areas of cell implantation can cause pacemaker potentials derived from the hESCs, a strategy often used for biopacemaker treatment of bradyarrhythmias.20-22 Interestingly, in a rather exhaustive study using mouse hearts post myocardial infarction (MI),23 there is no mention of enhanced spontaneous automatic rhythms after in vivo engraftment of ESCs, fibroblasts, or SkMs alone. Perhaps these events may have happened but were not reported. Only inducible rhythms were reported.
Experimental work with SkMs have reported that grafted myoblasts differentiate into peculiar hyperexcitable cells24 with EC coupling independent of the host cardiac cells. Experimental work with inexcitable MSCs has not led to increased automaticity of implanted cultures, but did alter conduction.25 However, if MSCs are made to express HCN channels and show pacemaker function, then good escape rhythms exist in the injected hearts. 21,22 hESCs, when transplanted in AV blocked animals, also show the potential for pacemaker activity. Interestingly, these experiments required only hundreds of hESCs for an effect.20 Thus, depending on your outlook, implanted cells can show enhanced automaticity and be arrhythmic, or can show enhanced automaticity and be antiarrhythmic.
2. Factors that could lead to Reentry
a. Stem cells could lead to an increase in the area of conduction block in the damaged heart if and only if the stem cells DO NOT electrically couple to surviving myocytes. So is there evidence that stem cells electrically couple to nonmyocytes?
Most in vitro experimental work has been done using neonatal myocytes and implanted stem cells. These cells couple differently than the typical adult cell surviving in a host myocardium. In fact, hESC cells did show positive staining for Cx43 but no functional electrical coupling (note here; it was presumed based on “normal mechanical contractions”).8 Furthermore, when bone marrow derived cells were efficiently grafted into the ischemic region of the adult heart, they were located in clusters within the infarct scar or border zone, but showed no electronically evoked Ca2+ transients.16 Staining for gap junction proteins was absent in these studies.
The efficacy and arrhythmia occurrence of stem cell therapy depends on the cell number as well as the cells' delivery route. An intramyocardial route tends to cause cell clusters embedded in nonmyocardium10,24 leading to heterogeneity in conduction and perhaps conduction block. The intracoronary route could provide more homogeneous delivery, but hopefully cells will aggregate in sufficient quantity at the correct anatomical locale. In fact, in rat hearts post MI,26 intramyocardial BMC injections, while improving cardiac function, increased the risk of ventricular premature complexes (VPCs) for 28 days post injection. When the intracoronary route was used in these studies, VPC occurrence was markedly decreased. Importantly these animal studies were done in the ABSENCE of antiarrhythmic drugs, which is often not the case for patients in clinical trials (see below).
b. Stem cells could promote slowed conduction between substrate myocytes. What is the nature of propagation between “normal” myocardium and stem cells if they are coupled? Is there INa and gap conductance?
Some implanted cells have intrinsic INa function (eg. ESCs2,3) and when implanted could provide reasonable fast sodium dependent conduction between host myocardium and stem cell areas. On the other hand, if propagation is only Ca2+ dependent (so called slow response conduction27) or purely electronic, it may be that the implanted stem cells would provide areas of slowed conduction, setting the stage for reentry.
While experimental work has shown a temporal increase in conduction velocity (CV) over a combined culture of human MSCs (hMSCs) and neonatal host cells,28 the actual values of CV measured are quite slow, ranging from 4 to 17 cm/sec. Furthermore, there was still a 4 fold difference in CV between the graft and host sites even at the longest time post culture (14 days). Presumably cells under these conditions have reduced and differing resting potentials (MSCs −40mV vs Host −67mV), suggesting that this preparation is potentially arrhythmogenic.
Direct calculation of conduction paths and velocities of excitatory waves over integrated hMSCs with rat cell cultures again suggest that hMSCs, which show Cx43 positive staining, do indeed provide conduction between two channels of neonatal cells, therefore conduction block is relieved. However, propagation is extremely slow (0.9 cm/s), perhaps electronic29 and the hMSCs in the conducting channel show reduced resting potentials and action potential amplitude. As above, the implanted stem cells seem to provide areas of slowed conduction setting the stage for reentry.
When others have transplanted SkMs into adult canine myocardium with and without MI and then mapped conduction30, they also found clear conduction slowing particularly in the epicardium of the SkM transplanted wedge sections.
c. Is refractoriness or action potential duration (APD) dispersion promoted with stem cell replacement?
While there has been emphasis of the repair of conduction between the disparate areas of host myocardium by the implanted stem cells, there has been little appraisal of the changes in refractoriness or APD dispersion of substrate with the grafted cells on board. Experimental work has suggested APDs differ considerably between host and graft cells28,29 and other important work has shown that the increase in tissue heterogeneities of host/graft (MSCs) cell cultures do not align with altered APD restitution curves but with reduced conduction velocity, and easily inducible spiral wave reentry (Figure 2).25 In these MSC/neonatal cocultures, MSCs expressed Cx43 and were coupled to the host cells. However in cocultures containing >10% MSCs, transplanted cells became areas of inexcitable sinks and delayed activation and repolarization, which led to a proarrhythmogenic substrate.
Figure 2.
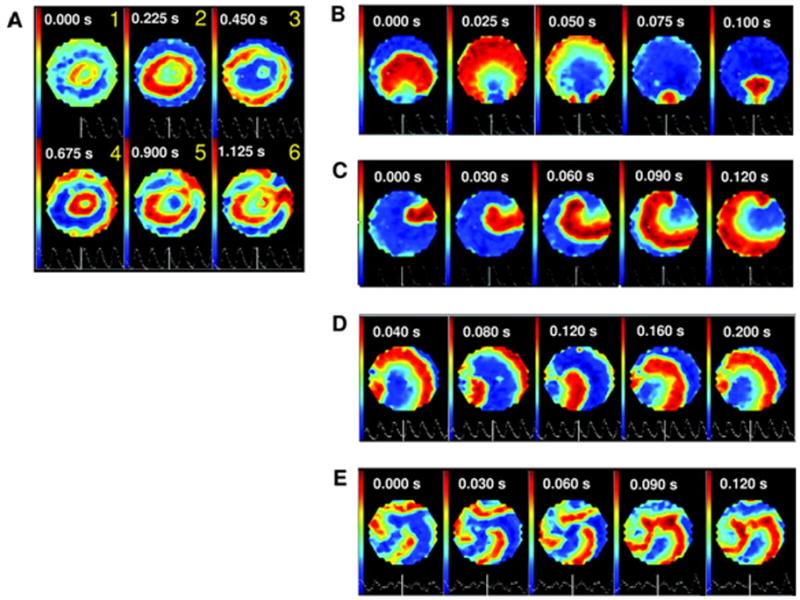
Induction of spiral wave reentry of various forms in cocultures of MSCs and neonatal rat cells (1:4). A, Reentry conditions exist for initiation. B through E, Different simple and complex forms of sustained reentry induced. Reprinted from Chang et al25 with permission of the publisher. Copyright © 2006, the American Heart Association.
C. Clinical experience
Cell-based therapy for cardiac regeneration has been evaluated in the clinic in three distinct clinical scenarios: (1) recent acute myocardial infarction, (2) chronic myocardial ischemia in no-option revascularization patients and (3) chronic infarct-related heart failure. Cell types that have been transplanted in these clinical settings include skeletal myoblasts (SkMs) and bone marrow-derived stem cells (BMCs) (mononuclear stem cells, hematopoietic stem cells, mesenchymal stem cells (MSCs), endothelial progenitor cells and circulating progenitor cells). Two cell delivery methods have been used: intracoronary and intramyocardial injections (transendocardial during cardiac catheterization and transepicardial during open-chest surgery). The amount of injected cells also varies among studies. Thus, a wide range of clinical situations, cell preparations, routes and doses employed make it difficult to totally interpret and compare the results from human trials. However, in this next section we will evaluate the consequences of stem cell transplantation and arrhythmia occurrence.
Skeletal Myoblasts
A number of features make myoblasts an attractive cell type for cardiac cell transplantation. They can be obtained in sufficient quantity directly from the patient and are resistant to ischemia, making them possible to survive in the low capillary environment of the infarcted myocardium.10 SkMs can differentiate into myotubes in vivo, but do not integrate with surviving cardiomyocytes. In addition, there is lack of evidence supporting their effectiveness in improving cardiac function.31
Several small trials investigating the safety and feasibility of myoblast transplantation in patients with ischemic cardiomyopathy have been published (Table 1).32-41 It is known that patients with left ventricular dysfunction and heart failure after a myocardial infarction have a favorable substrate for ventricular arrhythmias. However, these initial experiences suggested a proarrhythmic effect of SkM cell therapy. In the first phase I clinical trial with skeletal myoblasts, Menasche et al. reported sustained monomorphic ventricular tachycardia (VT) in 4 of 10 patients (one of them syncopal) early after the operation (11 to 22 days) that was not related to myocardial ischemia.32 The four patients had had an implanted cardioverter-defibrillator (ICD) implanted after the VT episode. At follow-up visits, two of these patients still experienced ventricular arrhythmias, despite antiarrhythmic drug therapy with beta-blockers and amiodarone. Indeed, due to the major concern of the potential arrhythmogenic effect of the new therapy, amiodarone was prophylactically instituted in the last three patients included in this study. Despite this, at a median follow-up 52 months after transplantation, there were 14 appropriate shocks for 3 arrhythmic storms in 3 patients.33 Shortly after these initial alarming data, Smits et al. reported episodes of sustained VT in one of five patients after transendocardial injection of SkMs for the treatment of ischemic heart failure.34 Subsequently, these same investigators have described an unpublished experience of two sudden cardiac deaths and three serious ventricular arrhythmias in eight additional patients.
Table 1. Arrhythmias after SkMs transplantation in non-randomized studies.
| Reference | Study design | Cell Route | n | F/u | Pre tx AAD/ICD | Rhythm monitoring | Pts with arrhythmias at baseline | Pts with arrhythmias post tx |
|---|---|---|---|---|---|---|---|---|
| Menaché et al32 Hagège et al.33 |
Previous MI Adjunct to CABG EF≤35% | Epicardial | 10 | 4 y | BB 3 amio |
Holter at baseline Holter/ICD interrogation every 3 m |
No mention | 1 NSVT, 4 SVT (50%) 5 ICD implants |
| Smits et al.34 | Previous MI EF 20-45% | Endocardial | 5 | 6 m | Optimal medical therapy | Holter at baseline, 1, 3 and 6 m | SVT, VF and syncope excluded | 1 NSVT runs (ICD implant), 1 SCD (40%) |
| Herreros et al.35 Gavira et al.36 |
Previous MI Adjunct to CABG EF >25% | Epicardial | 10 | 1 y | All amio | In-hospital monitoring Holter at baseline, 40 d and 3, 6 m | Malignant arrhythmias exclusion criteria | 1 NSVT (10%) |
| Pagani et al.37 | Heart transplant candidates. Adjunct to LVAD | Epicardial | 5 | 2 ICD | Holter at baseline | 1 AF, 4 NSVT (3pts, 60%) | 2 AF, 3 VT (4 pts, 80%) | |
| Dib et al.38 | Previous MI Adjunct to CABG EF <40% | Epicardial | 24 | 27m | 2 ICD | Holter at baseline and 1, 3, 6, 12, 24 w | 4 AF, 2 SVT, 14 NSVT | 1 AF, 1 SVT, 8 NSVT, 1 ICD activation, 5 ICD implant |
| Siminiak et al.39 | Previous MI Adjunct to CABG EF 25-40% | Epicardial | 10 | 1 y | 8 amio | Holter at 1, 2, 3, 4 w and 3, 12 m | No mention | 4 SVT |
| Siminiak et al.40 | Previous MI EF 25-40% | Percut trans-coronary-venous | 9 | 6 m | All BB 8 amio 2 ICD | 10-16 d of Holter monitoring Holter every w | No mention | 1 VT and ICD intervention No more SVT in f/up |
| Veltman et al.41 | Previous MI EF 20-45% | Endocardial | 14 treated 28 control | 4 y | All BB ICD: 9 treated, 8 control | ICD monitoring, Holter at end of f/up | No mention | ICD intervention 7 treated and 1 control 2 ARD treated and 1 control No dif in NSVT in Holter |
n: number of patients; EF: ejection fraction; LVAD: left ventricular assist device; ICD: implanted cardioverter defibrillator; ADD: antiarrhythmic drugs; BB: Betablockers; Amio: amiodarone; NSVT an SVT: nonsustained and sustained ventricular tachycardia; SCD: sudden cardiac death; AF: atrial fibrillation.; Holter: 24 hour ECG monitoring.
In other studies, arrhythmias after myoblast transplantation have been reported. Siminiak et al. gave prophylactic amiodarone to prevent ventricular arrhythmias to the last 8 patients included in their study of epicardial SkMs transplantation during CABG, after the first 2 had ventricular tachycardias in the early postoperative period.39 Two other studies also included prophylactic amiodarone as a standard pretransplantation therapy.35,40 In fact, in a different study by Siminiak et al., the only patient not receiving amiodarone developed episodes of ventricular tachycardia and experienced two interventions from its ICD at day 8 post procedure.40 This strongly suggests that the proarrhythmic effect of SkM transplantation might be prevented by amiodarone, even though it is not known how amiodarone may exert this effect.
To date, the only placebo-controlled randomized study evaluating the efficacy of SkMs has been the MAGIC II trial (Myoblast Autologous Grafting in Ischemic Cardiomyopathy). Here the efficacy of this cell-based therapy in patients with a history of myocardial infarction, left ventricular dysfunction and indication for coronary surgery was evaluated.31 Investigators tested two different doses of transepicardial injected SkMs versus placebo during CABG. An ICD and antiarrhythmic therapy were used in all patients. Even though the patients included in the trial are among the highest risk for ventricular arrhythmias, it seems that the investigators had significant concerns about the safety of the new procedure since they not only implanted an ICD, but they also recommended antiarrhythmic drugs. This trial failed to detect an incremental improvement in regional or global left ventricular function over that provided by CABG alone. However, at the 6 month follow-up, the number of ventricular arrhythmias was 2 times greater in patients of the treated groups. Notably these investigators called attention to the proarrhythmic risk of myoblast transplantation (Table 2). It is important to mention that the MAGIC II trial was the first large study providing exhaustive rhythm monitoring to the entire population.
Table 2. Ventricular arrhythmias at 6 months follow-up in the MAGIC II study.
| Placebo (n=34) |
Low dose (n=33) |
High dose (n=30) |
HR (95% CI) | P* | |
|---|---|---|---|---|---|
| Total ventricular arrhythmias, n (%) | 2 (6) | 4 (12) | 5 (17) | 2.7 (0.6; 12.6) | 0.18 |
| Sustained VF or polymorphic VT | 0 | 1 | 0 | - | - |
| Sustained monomorphic VT | 1 | 2 | 4 | - | - |
| Sustained monomorphic VT and sustained VF or polymorphic VT | 1 | 1 | 1 | - | - |
Adapted with permission from31
HR indicates hazard ratio of pooled treatment groups to placebo; VF: ventricular fibrillation; VT: ventricular tachycardia.
Log-rank test comparing pooled treatment groups vs placebo group.
In sum, small trials using SkMs have shown the treatment to be proarrhythmic.
Bone Marrow-Derived Stem Cells
The effects of adult bone marrow-derived progenitor cells have been investigated in patients with recent acute myocardial infarction after successful primary percutaneous coronary intervention. Other studies have also been performed in chronic MI and heart failure patients. Intracoronary, transendocardial and transepicardial administration routes have been used. Initially, the several small and non-randomized clinical trials which evaluated both safety and feasibility of BMCs in these situations, reported no obvious evidence of arrhythmic risk associated with the procedure or during follow-up care.42-49 Perin et al. reported one sudden cardiac death in a patient 14 weeks after transendocardial autologous BMC transplantation for chronic severe heart failure.50 When direct intramyocardial percutaneous delivery of autologous bone marrow cells in 10 patients with refractory myocardial angina was used, one patient experienced acute heart failure 7 days after the procedure due to acute atrial fibrillation.48 In another study, 4 of 12 patients developed transient atrial fibrillation after transepicardial injection of BMCs during coronary artery bypass grafting.49 Interestingly, no other arrhythmic episodes were described in these phase I safety studies. It is important to mention that arrhythmia monitoring was not continuous in any of these studies except during the periprocedural time. Only occasional 24-hour Holter ECGs and clinical evaluations were carried out and the follow-up period was no more than a few months.
So far, few of the randomized clinical BMC therapy studies suggests either no or a small benefit in patients with ischemic heart disease.51,52 From the published data of these randomized placebo-controlled trials, there does not seem to be an enhanced risk of clinical arrhythmias related to this type of cell transplantation, but again, the method of evaluating the arrhythmic risk is generally not exhaustive (Table 3).53-67 Only Wollert et al. tested arrhythmia inducibility with programmed ventricular stimulation 6 months after intracoronary BMC in 30 cell treated and 30 control patients.66 Most groups never report any specific rhythm monitoring during follow-up after transplantation thus arrhythmia occurrence is unknown.56, 59-63 On the other hand, most of the patients included in these few clinical trials were taking β blocker agents, as they are indicated for ischemic heart disease. Treatment with β blockers might mask a potential proarrhythmic effect of the transplanted cells in humans.
Table 3. Arrhythmias in randomized design clinical trials of BMC.
| Reference | Design | Pts and Treatment | Cell route | F/u | Rhythm Monitoring | Arrhythmias |
|---|---|---|---|---|---|---|
| Chen et al.53 | Rand, placebo-controlled. 18 d after PCI for AMI | 34 MSC 35 placebo |
IC | 6 m | Holter at 3 m | No arrhythmias |
| Kang et al.54,55 | Rand, controlled. 3-270 d after AMI | 10 G-CSF + PBSC 10 G-CSF 7 control |
IC | 2 y | Clinical assessment and treadmill test at 1, 2, 4 and 6 m | No substantial arrhythmias |
| Erbs et al.56 | Rand, placebo-controlled. 10 d after opening of a chronic total occlusion | 12 G-CSF + PBPC 11 G-CSF + placebo |
IC | 3 m | Clinical assessment once a week. No specific monitoring | No mention |
| Schächinger et al.57,58 | Rand, placebo-controlled. 3-6 d after reperfusion for AMI | 101 BMC 103 placebo |
IC | 1 y | Holter at 4 m and 1 y | Treatment group: 5 VT, 1 SCD Control group: 4 VT, 1 syncope, 1 SCD |
| Janssens et al.59 | Rand, placebo-controlled. 24 h after PCI for AMI | 33 BMC 34 placebo |
IC | 4 m | In-hospital Holter monitoring | Treatment group: 5 SVA Control group: 6 SVA, 3 NSVT No late potentials |
| Assmus et al.60 | Rand, controlled, crossover. 3 m after AMI | 24 PBSC 28 BMC 23 control |
IC | 3 m | Holter after procedure No mention after discharge | In-hospital VT: 1 PBSC, 0 BMC, 1 control No VT after discharge |
| Meluzin et al.61;62 | Ran, controlled. 3-8 d after PCI for AMI | 20 low-dose BMC 20 high-dose BMC 20 control |
IC | 1 y | No mention | No mention |
| Kang et al.63 | Rand, controlled. After PCI for AMI (7 d) or OMI (517 d) | - AMI: 25 G-CSF + PBSC, 26 control - OMI: 16 G-CSF + PBSC, 16 control |
IC | 6 m | No mention | No mention |
| Lunde et al.64,65 | Rand, controlled. 4-8 d after PCI for AMI | 47 BMC 50 control |
IC | 1 y | Signal-averaged ECG at baseline and 3 m Holter at baseline and 6 m |
BMC: 2 SVA, 3 syncope Control: 1 SVA No differences in NSVT, PVC and signal-averaged ECG in f/up |
| Wollert et al.66 Meyer et al.67 |
Rand, controlled. 5 d after PCI for AMI | 30 BMC 30 control |
IC | 18 m | Holter before discharge, at 6 w, 3 and 6 m ProgVS at 6 m Clinical evaluation at 18 m |
No dif in PVC or NSVT ProgVS: 1 NSVT treatment 1 NSVT and 1 VF control |
AMI: acute myocardial infarction; OMI: old myocardial infarction; PCI: percutaneous coronary intervention; IC:intracoronary; ProgVS: programmed ventricular stimulation; G-CSF: granulocyte-colony stimulating factor; PBSC: peripheral blood stem cell; BMC: bone marrow stem cell; MSC: mesenchymal stem cells; PVC: premature ventricular complex; NSVT: Non-sustained ventricular tachycardia; VF: ventricular fibrillation; SCD: sudden cardiac death; SVA: supraventricular arrhythmias.
Two other non-randomized studies have specifically evaluated the electrophysiological and arrhythmogenic effects of transplantation of autologous bone marrow-derived progenitor cells.68;69 The study by Beeres et al. was carried out in 20 patients with drug-refractory angina and myocardial ischemia. Immediately before intramyocardial BMC injection, 3-dimensional electroanatomical LV mapping was performed to evaluate the local bipolar electrogram characteristics of the myocardial region with ischemia in which BMCs were to be injected. Three months later, mapping was repeated in the same area and electrograms showed no prolongation, no decrease in amplitude or increase in fragmentation suggesting conduction was not affected.68 Twenty-four hour Holter monitoring was performed at baseline and 3 and 6 months later. The total number of ventricular premature beats remained unchanged. However, this was a non-randomized study, without a control group and with no programmed ventricular stimulation protocol to evaluate the inducibility of ventricular tachycardia. Also, the authors state that the measurement of electrogram duration by the electrophysiological mapping is influenced by the direction of the wavefront in relation to the bipole, which could limit the interpretation of the results. In the second study, Katritsis et al. followed patients with a history of myocardial infarction and ICD for ventricular arrhythmias in whom intracoronary transplantation of MSCs and endothelial progenitor cells was performed.69 Before stem cell transplantation, clinical non-sustained ventricular tachycardia and inducible monomorphic ventricular tachycardia or ventricular flutter were demonstrated in all 5 patients. At 16-36 months follow-up, the interrogation of the ICD failed to detect sustained or non-sustained ventricular arrhythmias in any patient and a repeated electrophysiological study induced sustained ventricular arrhythmias in only two patients. This was a small and non-randomized study and should be regarded as a preliminary experience and not as proof of an antiarrhythmic potential of this type of stem cell.
The exact mechanism of “electrical” action following BMC transplantation is unknown. With intracoronary administration, fewer than 5% of cells are retained in the infarcted myocardium. If the cells do not remain in the areas of interest, neither important long lasting effects nor arrhythmic potential might be expected. In fact, a lack of sustained long-term beneficial effects of BMC has recently been reported.55,65,67
Further clinical experience and more exhaustive studies are necessary before reaching valid conclusions regarding the electrical safety of BMCs or MSCs.
Other Stem Cells/Future Clinical Directions
SkMs and BMCs might have some beneficial indirect effects on the myocardium (paracrine mechanisms, potential to induce angiogenesis),37 but they do not differentiate into cardiomyocytes. Thus, they may not have clinical significant long-term favorable effects over the heart pump function.31,55,65,67 Other sources of potential regenerative cells like ESCs and endogenous cardiac stem cells have not yet been tested in humans. Embryonic stem cells are the prototypical stem cells. However, there are several difficulties in using hESC. First, these cells are allogeneic, and immunosuppressive therapy might be needed. Second, they have the potential to form teratomas when injected in vivo, an issue that will be probably solved with technical advances to lead their differentiation only into cardiomyocytes.70 Finally, the use of hESC is still surrounded by ethical problems.
C. Conclusions
In conclusion, given both the experimental and clinical data available so far we content that stem cell therapy is arrhythmogenic. Experimental studies have provided some electrical basis for such in that stem cells can show intrinsic pacemaker function and provide for areas of slowed conduction. These latter changes in the substrate could set the stage for arrhythmias. Clinical studies so far are not exhaustive in their rhythm monitoring and usually have some type of antiarrhythmic therapy accompanying the treatment. This is a wise idea since stem cell therapy can be proarrhythmic.
Acknowledgments
Sources of Funding: Supported by grant HL58860 from the National Heart Lung and Blood Institute Bethesda, Maryland; Dr. Macia is supported by Medtronic fellowship.
Footnotes
Disclosures: None
References
- 1.Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-Based Therapy for Myocardial Ischemia and Infarction: Pathophysiological Mechanisms. Annual Review of Pathology: Mechanisms of Disease. 2007;2:307–339. doi: 10.1146/annurev.pathol.2.010506.092038. [DOI] [PubMed] [Google Scholar]
- 2.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental Changes in Cardiomyocytes Differentiated from Human Embryonic Stem Cells: A Molecular and Electrophysiological Approach. Stem Cells. 2007;25:1136–1144. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 3.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I, Gepstein L. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. The Journal of Physiology Online. 2004;559:479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O. Functional Properties of Human Embryonic Stem Cell-Derived Cardiomyocytes: Intracellular Ca2+ Handling and the Role of Sarcoplasmic Reticulum in the Contraction. Stem Cells. 2006;24:236–245. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Smith RR, Barile L, Messina E, Marbβn E. Stem cells in the heart: What's the buzz all about?--Part 1: Preclinical considerations. Heart Rhythm. 2008;5:749–757. doi: 10.1016/j.hrthm.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. PNAS. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotech. 2004;22:1282–1289. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 9.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical Coupling between Skeletal and Cardiac Muscle: Implications for Infarct Repair. J Cell Biol. 2000;149:731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinecke H, Poppa V, Murry CE. Skeletal Muscle Stem Cells Do Not Transdifferentiate Into Cardiomyocytes After Cardiac Grafting. Journal of Molecular and Cellular Cardiology. 2002;34:241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 11.Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human Bone Marrow Stem Cells Exhibit Neural Phenotypes and Ameliorate Neurological Deficits after Grafting into the Ischemic Brain of Rats. Experimental Neurology. 2002;174:11–20. doi: 10.1006/exnr.2001.7853. [DOI] [PubMed] [Google Scholar]
- 12.Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E, Ravens U. Electrophysiological properties of human mesenchymal stem cells. The Journal of Physiology Online. 2004;554:659–672. doi: 10.1113/jphysiol.2003.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li GR, Deng XL, Sun H, Chung SSM, Tse HF, Lau CP. Ion Channels in Mesenchymal Stem Cells from Rat Bone Marrow. Stem Cells. 2006;24:1519–1528. doi: 10.1634/stemcells.2005-0307. [DOI] [PubMed] [Google Scholar]
- 14.Amado LC, Saliaris AP, Schuleri KH, John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. PNAS. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menasche P. Challenging the Cardiac Differentiation of Bone Marrow Cells: A Clinical Perspective. Mol Ther. 2008;16:1000–1001. doi: 10.1038/mt.2008.75. [DOI] [PubMed] [Google Scholar]
- 16.Scherschel JA, Soonpaa MH, Srour EF, Field LJ, Rubart M. Adult Bone Marrow-derived Cells Do Not Acquire Functional Attributes of Cardiomyocytes When Transplanted into Peri-infarct Myocardium. Mol Ther. 2008;16:1129–1137. doi: 10.1038/mt.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YM, Hartzell C, Narlow M, Dudley SC., Jr Stem Cell-Derived Cardiomyocytes Demonstrate Arrhythmic Potential. Circulation. 2002;106:1294–1299. doi: 10.1161/01.cir.0000027585.05868.67. [DOI] [PubMed] [Google Scholar]
- 18.Singla DK, Lyons GE, Kamp TJ. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1308–H1314. doi: 10.1152/ajpheart.01277.2006. [DOI] [PubMed] [Google Scholar]
- 19.Igelmund P, Fleischmann BK, Fischer IR, Soest J, Gryshchenko O, B+¦hm-Pinger MM, Sauer H, Liu Q, Hescheler J. Action potential propagation failures in long-term recordings from embryonic stem cell-derived cardiomyocytes in tissue culture. Pfl++gers Archiv European Journal of Physiology. 1999;437:669–679. doi: 10.1007/s004240050831. [DOI] [PubMed] [Google Scholar]
- 20.Xue T, Cho HC, Akar FG, Tsang SY, Jones SP, Marban E, Tomaselli GF, Li RA. Functional Integration of Electrically Active Cardiac Derivatives From Genetically Engineered Human Embryonic Stem Cells With Quiescent Recipient Ventricular Cardiomyocytes: Insights Into the Development of Cell-Based Pacemakers. Circulation. 2005;111:11–20. doi: 10.1161/01.CIR.0000151313.18547.A2. [DOI] [PubMed] [Google Scholar]
- 21.Plotnikov A, Sosunov EA, Qu J, Shlapakova I, Anyukhousky EP, Liu L, Janse MJ, Brink P, Cohen IS, Robinson RB, Danilo P, Jr, Rosen MR. Biological Pacemaker implanted in canine left bundle branch provides ventricular escape rhythms that have physiologically acceptable rates. Circ. 2004;109:506–512. doi: 10.1161/01.CIR.0000114527.10764.CC. [DOI] [PubMed] [Google Scholar]
- 22.Qu J, Plotnikov A, Danilo P, Jr, Shlapakova I, Cohen IS, Robinson RB, Rosen MR. Expression and function of a biological pacemaker in canine heart. Circ. 2004;107:1106–1109. doi: 10.1161/01.cir.0000059939.97249.2c. [DOI] [PubMed] [Google Scholar]
- 23.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 24.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. PNAS. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marban E, Abraham MR. Proarrhythmic Potential of Mesenchymal Stem Cell Transplantation Revealed in an In Vitro Coculture Model. Circulation. 2006;113:1832–1841. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 26.Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, Barton PJR, Terracciano CMN, Yacoub MH, Suzuki K. Direct Intramyocardial But Not Intracoronary Injection of Bone Marrow Cells Induces Ventricular Arrhythmias in a Rat Chronic Ischemic Heart Failure Model. Circulation. 2007;115:2254–2261. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 27.Cranefield PF. The Conduction of the Cardiac Impulse. The slow response and Cardiac arrhythmias. Futura; Mt.Kisco: 1975. [Google Scholar]
- 28.Pijnappels DA, Schalij MJ, van Tuyn J, Ypey DL, de Vries AAF, van der Wall EE, van der Laarse A, Atsma DE. Progressive increase in conduction velocity across human mesenchymal stem cells is mediated by enhanced electrical coupling. Cardiovascular Research. 2006;72:282–291. doi: 10.1016/j.cardiores.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 29.Beeres SLMA, Atsma DE, van der Laarse A, Pijnappels DA, van Tuyn J, Fibbe WE, de Vries AAF, Ypey DL, van der Wall EE, Schalij MJ. Human Adult Bone Marrow Mesenchymal Stem Cells Repair Experimental Conduction Block in Rat Cardiomyocyte Cultures. Journal of the American College of Cardiology. 2005;46:1943–1952. doi: 10.1016/j.jacc.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 30.Fouts K, Fernandes B, Mal N, Liu J, Laurita KR. Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm. 2006;3:452–461. doi: 10.1016/j.hrthm.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagege AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial: First Randomized Placebo-Controlled Study of Myoblast Transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 32.Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. Journal of the American College of Cardiology. 2003;41:1078–1083. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 33.Hagege AA, Marolleau JP, Vilquin JT, Alheritiere A, Peyrard S, Duboc D, Abergel E, Messas E, Mousseaux E, Schwartz K, Desnos M, Menasche P. Skeletal Myoblast Transplantation in Ischemic Heart Failure: Long-Term Follow-Up of the First Phase I Cohort of Patients. Circulation. 2006;114:I-108. doi: 10.1161/CIRCULATIONAHA.105.000521. [DOI] [PubMed] [Google Scholar]
- 34.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EEM, Lee CH, Maat APWM, Serruys PW. Catheter-Based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: Clinical experience with Six-Month Follow-Up. Journal of the American College of Cardiology. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Herreros J, Prosper F, Perez A, Gavira JJ, Garcia-Velloso MJ, Barba J, Sanchez PL, Canizo C, Rabago G, Marti-Climent JM, Hernandez M, Lopez-Holgado N, Gonzalez-Santos JM, Martin-Luengo C, Alegria E. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. European Heart Journal. 2003;24:2012–2020. doi: 10.1016/j.ehj.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Gavira JJ, Herreros Js, Perez A, Garcia-Velloso MJ, Barba J, Martin-Herrero F, Ca±izo C, Martin-Arnau A, MartØ-Climent JM, Hernβndez M, L≤pez-Holgado N, Gonzβlez-Santos JM, MartØn-Luengo C, Alegria E, Prosper F. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. The Journal of Thoracic and Cardiovascular Surgery. 2006;131:799–804. doi: 10.1016/j.jtcvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge ASB, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans: Histological analysis of cell survival and differentiation. Journal of the American College of Cardiology. 2003;41:879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 38.Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, Binkley P, Buchele D, Anand I, Swingen C, Di Carli MF, Thomas JD, Jaber WA, Opie SR, Campbell A, McCarthy P, Yeager M, Dilsizian V, Griffith BP, Korn R, Kreuger SK, Ghazoul M, MacLellan WR, Fonarow G, Eisen HJ, Dinsmore J, Diethrich E. Safety and Feasibility of Autologous Myoblast Transplantation in Patients With Ischemic Cardiomyopathy: Four-Year Follow-Up. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 39.Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzezniczak J, Rozwadowska N, Kurpisz M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: Phase I clinical study with 12 months of follow-up. American Heart Journal. 2004;148:531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 40.Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kalmucki P, Kurpisz M. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. European Heart Journal. 2005;26:1188–1195. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- 41.Veltman CE, Soliman OII, Geleijnse ML, Vletter WB, Smits PC, ten Cate FJ, Jordaens LJ, Balk AHHM, Serruys PW, Boersma E, van Domburg RT, van der Giessen WJ. Four-year follow-up of treatment with intramyocardial skeletal myoblasts injection in patients with ischaemic cardiomyopathy. European Heart Journal. 2008;29:1386–1396. doi: 10.1093/eurheartj/ehn171. [DOI] [PubMed] [Google Scholar]
- 42.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of Infarcted Myocardium by Autologous Intracoronary Mononuclear Bone Marrow Cell Transplantation in Humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 43.Perin EC, Dohmann HFR, Borojevic R, Silva SA, Sousa ALS, Silva GV, Mesquita CT, Belem L, Vaughn WK, Rangel FOD, Assad JAR, Carvalho AC, Branco RVC, Rossi MID, Dohmann HJF, Willerson JT. Improved Exercise Capacity and Ischemia 6 and 12 Months After Transendocardial Injection of Autologous Bone Marrow Mononuclear Cells for Ischemic Cardiomyopathy. Circulation. 2004;110:II-213. doi: 10.1161/01.CIR.0000138398.77550.62. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs S, Kornowski R, Weisz G, Satler LF, Smits PC, Okubagzi P, Baffour R, Aggarwal A, Weissman NJ, Cerqueira M, Waksman R, Serrruys P, Battler A, Moses JW, Leon MB, Epstein SE. Safety and Feasibility of Transendocardial Autologous Bone Marrow Cell Transplantation in Patients With Advanced Heart Disease. The American Journal of Cardiology. 2006;97:823–829. doi: 10.1016/j.amjcard.2005.09.132. [DOI] [PubMed] [Google Scholar]
- 45.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, de la Fuente L, Gomez-Bueno M, Cantalapiedra A, Fernandez J, Gutierrez O, Sanchez PL, Hernandez C, Sanz R, Garcia-Sancho J, Sanchez A. Experimental and Clinical Regenerative Capability of Human Bone Marrow Cells After Myocardial Infarction. Circ Res. 2004;95:742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 46.Schechinger V, Assmus B, Britten MB, Honold Jr, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the TOPCARE-AMI Trial. Journal of the American College of Cardiology. 2004;44:1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, K÷gler G, Wernet P, Mnller HW, K÷stering M. Regeneration of Human Infarcted Heart Muscle by Intracoronary Autologous Bone Marrow Cell Transplantation in Chronic Coronary Artery Disease: The IACT Study. Journal of the American College of Cardiology. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 48.Briguori C, Reimers B, Sarais C, Napodano M, Pascotto P, Azzarello G, Bregni M, Porcellini A, Vinante O, Zanco P, Peschle C, Condorelli G, Colombo A. Direct intramyocardial percutaneous delivery of autologous bone marrow in patients with refractory myocardial angina. American Heart Journal. 2006;151:674–680. doi: 10.1016/j.ahj.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Stamm C, Kleine HD, Westphal B, Petzsch M, Kittner C, Nienaber CA, Freund M, Steinhoff G. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac Cardiovasc Surg. 2004;52:152–158. doi: 10.1055/s-2004-817981. [DOI] [PubMed] [Google Scholar]
- 50.Perin EC, Dohmann HFR, Borojevic R, Silva SA, Sousa ALS, Mesquita CT, Rossi MID, Carvalho AC, Dutra HS, Dohmann HJF, Silva GV, Belem L, Vivacqua R, Rangel FOD, Esporcatte R, Geng YJ, Vaughn WK, Assad JAR, Mesquita ET, Willerson JT. Transendocardial, Autologous Bone Marrow Cell Transplantation for Severe, Chronic Ischemic Heart Failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 51.bdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult Bone Marrow-Derived Cells for Cardiac Repair: A Systematic Review and Meta-analysis. Archives of Internal Medicine. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 52.Lipinski MJ, Biondi-Zoccai GGL, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of Intracoronary Cell Therapy on Left Ventricular Function in the Setting of Acute Myocardial Infarction: A Collaborative Systematic Review and Meta-Analysis of Controlled Clinical Trials. Journal of the American College of Cardiology. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 53.Chen Sl, Fang Ww, Ye F, Liu YH, Qian J, Shan Sj, Zhang Jj, Chunhua RZ, Liao Lm, Lin S, Sun Jp. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. The American Journal of Cardiology. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Lee DS, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. The Lancet. 2004;363:751–756. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 55.Kang HJ, Kim HS, Koo BK, Kim YJ, Lee D, Sohn DW, Oh BH, Park YB. Intracoronary infusion of the mobilized peripheral blood stem cell by G-CSF is better than mobilization alone by G-CSF for improvement of cardiac function and remodeling: 2-Year follow-up results of the Myocardial Regeneration and Angiogenesis in Myocardial Infarction with G-CSF and Intra-Coronary Stem Cell Infusion (MAGIC Cell) 1 trial. American Heart Journal. 2007;153:237. doi: 10.1016/j.ahj.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Erbs S, Linke A, Adams V, Lenk K, Thiele H, Diederich KW, Emmrich F, Kluge R, Kendziorra K, Sabri O, Schuler G, Hambrecht R. Transplantation of Blood-Derived Progenitor Cells After Recanalization of Chronic Coronary Artery Occlusion: First Randomized and Placebo-Controlled Study. Circ Res. 2005;97:756–762. doi: 10.1161/01.RES.0000185811.71306.8b. [DOI] [PubMed] [Google Scholar]
- 57.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM, for the REPAIR-AMI Investigators Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. European Heart Journal. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 58.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM, the REPA Intracoronary Bone Marrow-Derived Progenitor Cells in Acute Myocardial Infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 59.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. The Lancet. 367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 60.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary Transplantation of Progenitor Cells after Myocardial Infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 61.Meluzin J, Mayer J, Groch L, Janousek S, Hornβcek I, Hlinomaz O, Kala P, Panovsk2 R, Prβsek J, KamØnek M, StanØcek J, Klabusay M, KorØstek Z, Navrβtil M, Dusek L, Vinklβrkovβ J. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: The effect of the dose of transplanted cells on myocardial function. American Heart Journal. 2006;152:975. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Meluzin J, Janousek S, Mayer J, Groch L, Hornβcek I, Hlinomaz O, Kala P, Panovsk2 R, Prβsek J, KamØnek M, StanØcek J, Klabusay M, KorØstek Z, Navrβtil M, Dusek L, Vinklβrkovβ J. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. International Journal of Cardiology. 2008;128(2):185–92. doi: 10.1016/j.ijcard.2007.04.098. 18. [DOI] [PubMed] [Google Scholar]
- 63.Kang HJ, Lee HY, Na SH, Chang SA, Park KW, Kim HK, Kim SY, Chang HJ, Lee W, Kang WJ, Koo BK, Kim YJ, Lee DS, Sohn DW, Han KS, Oh BH, Park YB, Kim HS. Differential Effect of Intracoronary Infusion of Mobilized Peripheral Blood Stem Cells by Granulocyte Colony-Stimulating Factor on Left Ventricular Function and Remodeling in Patients With Acute Myocardial Infarction Versus Old Myocardial Infarction: The MAGIC Cell-3-DES Randomized, Controlled Trial. Circulation. 2006;114:I-145. doi: 10.1161/CIRCULATIONAHA.105.001107. [DOI] [PubMed] [Google Scholar]
- 64.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary Injection of Mononuclear Bone Marrow Cells in Acute Myocardial Infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 65.Lunde K, Solheim S, Forfang Kr, Arnesen H, Brinch L, Bj°rnerheim R, Ragnarsson A, Egeland T, Endresen K, Ilebekk A, Mangschau A, Aakhus S. Anterior Myocardial Infarction With Acute Percutaneous Coronary Intervention and Intracoronary Injection of Autologous Mononuclear Bone Marrow Cells: Safety, Clinical Outcome, and Serial Changes in Left Ventricular Function During 12-Months' Follow-Up. Journal of the American College of Cardiology. 2008;51:674–676. doi: 10.1016/j.jacc.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 66.Wollert KC, Meyer GP, Lotz J, Ringes Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. The Lancet. 364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 67.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary Bone Marrow Cell Transfer After Myocardial Infarction: Eighteen Months' Follow-Up Data From the Randomized, Controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) Trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 68.Beeres SLMA, Zeppenfeld K, Bax JJ, Dibbets-Schneider P, Stokkel MPM, Fibbe WE, van der Wall EE, Atsma DE, Schalij MJ. Electrophysiological and arrhythmogenic effects of intramyocardial bone marrow cell injection in patients with chronic ischemic heart disease. Heart Rhythm. 2007;4:257–265. doi: 10.1016/j.hrthm.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 69.Katritsis DG, Sotiropoulou P, Giazitzoglou E, Karvouni E, Papamichail M. Electrophysiological effects of intracoronary transplantation of autologous mesenchymal and endothelial progenitor cells. Europace. 2007;9:167–171. doi: 10.1093/europace/eul184. [DOI] [PubMed] [Google Scholar]
- 70.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotech. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]


