Abstract
Objective
Previous studies demonstrated that the dynorphin/κ opioid system was up-regulated upon repeated cocaine self-administration. In the present study, we tested the hypothesis that increased cocaine self-administration with extended access was associated with increased activity of the κ opioid system in rats.
Materials and methods
Rats self-administered 0.5 mg/kg per injection of cocaine on a fixed-ratio (FR) schedule in either 1-h (short access, ShA) or 6-h (long access, LgA) sessions. After cocaine intake in the LgA rats increased to a maximum, the effects of κ opioid receptor antagonists and a partial agonist were tested on cocaine intake in ShA and LgA rats.
Results
Cocaine self-administration increased under FR and progressive-ratio (PR) schedules in LgA rats. Nor-BNI (15–30 mg/kg), a κ receptor antagonist, decreased cocaine intake in LgA rats under a PR schedule (ShA, +1.7%; LgA, −27.4% from baseline), whereas naltrexone (0.3–10 mg/kg) and SG-II-49 (0.025–0.1 mg/kg), a nonspecific opioid receptor antagonist and a partial agonist, respectively, decreased cocaine intake in both groups (PR data: SG-II-49, ShA -28.6%, LgA -19.8%; naltrexone, ShA −34.6%, LgA −11.8% compared with vehicle data).
Conclusions
The present study demonstrated that the antagonism of κ opioid receptors attenuated only the increased cocaine intake in LgA rats under a PR schedule, whereas the antagonism of μ and κ receptors decreased cocaine intake in both ShA and LgA groups. The data suggest that increased motivation for cocaine in rats with extended access may be related to increased κ opioid activity and may contribute to compulsive use.
Keywords: Kappa opioid receptor, Cocaine, Self-administration, Addiction, Rat
Introduction
Drug addiction is a brain disease that results from neuroplasticity to brain motivation circuits during repeated drug abuse. Therefore, to understand drug addiction, it is important to identify neural adaptations that occur during repeated drug abuse. Drug addiction is characterized by compulsive drug intake and drug withdrawal symptoms, such as craving, depression, and dysphoria (American Psychiatric Association 2000). It has been hypothesized that the emergence of a negative emotional state during drug withdrawal not only provides a key marker for the development of dependence but may also be etiological for compulsive drug taking associated with addiction (Koob 2008; Koob and Le Moal 1997). Such negative emotional states can contribute to relapse. In fact, one of the most frequent determinants of relapse is reported to be a negative emotional state in alcoholism, heroin addiction, and binge eating disorder (Marlatt 1985). Additionally, abstinent cocaine-dependent patients showed enhanced negative emotions and reported that stress/cue-induced drug craving was accompanied by increased negative affect (Fox et al. 2008). Similar results are also found in opioid-dependent individuals (Hyman et al. 2007). Therefore, adaptations in neurotransmitter systems that are involved in negative emotional states may underlie the development of drug addiction.
Among neurotransmitter systems, the κ opioid system has been linked to depression or dysphoric-like behaviors in laboratory animals and in humans (Mague et al. 2003; Pfeiffer et al. 1986; Tomasiewicz et al. 2008). Stimulation of the κ opioid system with acute exogenous k agonist appears to produce an aversive effect when measured in a conditioned place preference task (McLaughlin et al. 2006; Mucha and Herz 1985; Pliakas et al. 2001), and the aversive effects of κ opioid agonists were hypothesized to be mediated centrally in rats (Bals-Kubik et al. 1989; Mucha and Herz 1985). Dynorphin, an endogenous ligand for κ opioid receptors, is also implicated in stress mechanisms (Land et al. 2008), which is an important factor in compulsive drug intake (Miczek et al. 2004) and relapse (Shaham et al. 2000). Focusing on psychostimulants, research shows that repeated administration of psychostimulants activates the dynorphin system in rats (Hanson et al. 1988; Turchan et al. 1998). Frankel et al. (2008) also found increased immunoreactivity for dynorphin in the caudate and ventral pallidum of chronic cocaine abusers, regions related to drug abuse. Similarly, increased amounts of dynorphin and κ opioid receptors in cocaine addicts were previously reported (Hurd and Herkenham 1993). Accordingly, we hypothesized that enhanced κ opioid activity during prolonged cocaine abuse contributes to intensifying negative emotional states during drug withdrawal, which leads to cocaine dependence in humans.
In the present study, therefore, we investigated a relationship between the κ opioid system and increased cocaine intake in rats with extended access to cocaine. Our hypothesis was that increased cocaine intake with extended access was associated with increased κ opioid activity in rats. Accordingly, the blockade of the κ opioid receptors would decrease increased cocaine intake in rats with extended access. The literature suggests that the inhibition of κ opioid receptors has no effect on non-escalated cocaine intake in rats and monkeys (Glick et al. 1995; Negus et al. 1997). Therefore, we hypothesized that the inhibition of κ opioid receptors would have no effect on cocaine intake in rats with short access in the present study.
Materials and methods
All animal use procedures were approved by The Scripps Research Institute Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines (NIH publication no. 85-23, revised 1996).
Animals and apparatus
Fifty-seven male Wistar rats (Charles River, Hollister, CA; 16 rats for SG-II-49 and 15 mg/kg of nor-BNI, 12 rats for 30 mg/kg of nor-BNI, nine rats for the vehicle of nor-BNI, and 20 rats for naltrexone), each weighing between 300 and 400 g at the time of testing opioid receptor ligands in the study, served as subjects. Eight of 12 rats that were used to test 30 mg/kg of nor-BNI had previously been tested with CRF1 receptor antagonists (Wee and Koob, unpublished data), and there was at least 1 week of baseline cocaine self-administration between the two studies. Rats were housed in groups of two or three in plastic cages with a reversed 12:12 h light/dark cycle with lights on at 8:00p.m. Food and water were available ad libitum. During experimental sessions, each rat was placed in an operant conditioning chamber (28×26×20 cm; Med Associates Inc., St Albans, VT). The chamber had two retractable response levers mounted on a sidewall, and a stimulus light was mounted above each lever. Drug injection was delivered by a syringe pump (Razel™ Scientific Instruments, Georgia, VT) located on top of the cubicle. Experimental sessions were controlled and recorded by a PC computer with custom interface and software in the experimental room. Experimental sessions were conducted once a day during the dark (active) cycle. At the start of a session, two response levers were presented into the chamber, and responding on the right lever resulted in the delivery of 0.1 ml of a drug solution over 4 s. During an injection, a stimulus light above the active lever was illuminated and lasted throughout the time-out period (20 s) that followed each injection. Pressing the left lever was counted but had no other programmed consequences. The session ended by the withdrawal of the levers.
Self-administration procedure
Detailed surgical methods were previously described (Wee et al. 2007). Briefly, rats were implanted with silastic catheters (0.3 mm ID×0.64 mm OD; Dow Corning Co. Midland, MI) into the right external jugular vein. After recovery from the surgery, rats were trained to self-administer 0.5 mg/kg per injection of cocaine in daily 1-h sessions under a fixed-ratio (FR) 1 schedule for 10 days. Following these baseline sessions, rats were separated into two groups and balanced for cocaine self-administration in the last baseline session. The session length was kept to 1 h for one group (short access, ShA, n=8) and was increased to 6 h for the other group (long access, LgA, n=8; escalation period). Sessions in this escalation period lasted for 15 days before testing the effect of opioid receptor ligands on cocaine self-administration. After 15 escalation sessions, the effect of SG-II-49 on cocaine self-administration was first tested under an FR1 schedule and then under a progressive-ratio (PR) schedule. For the PR schedule, the response requirement began at one response/injection and increased according to the following equation: (Richardson and Roberts 1996). When a rat failed to achieve the response requirement within 1 h, the session ended. A session length under a PR schedule was always set at 6 h, and PR sessions lasted an average of 3 h across rats. Test sessions were separated by at least two escalation sessions (ShA rats, 1-h session; LgA rats, 6-h session), and the doses of SG-II-49 were tested in a counter-balanced manner across rats with three vehicle test sessions interspersed. If re-determined vehicle data differed from the previous data, a vehicle session was repeated to re-establish the control data, and the doses of SG-II-49 were retested. This was not necessary in the present study because all the rats maintained less than mean±2 injections or mean±15% under PR and FR schedules, respectively, throughout the test period. One ShA rat was excluded from the study because of unexpected death. Two LgA rats were excluded from the study of SG-II-49 under a PR schedule because of compromised health. After testing SG-II-49 on cocaine self-administration, the rats were injected with 15 mg/kg of nor-BNI, and the effect of nor-BNI on cocaine self-administration was measured in eight consecutive sessions. In detail, 30 min after rats were subcutaneously injected with nor-BNI, they were allowed to self-administer cocaine under an FR schedule. On the following day, the rats were allowed to self-administer cocaine under a PR schedule. Consequently, rats self-administered cocaine under FR and PR schedules on alternating days for a total of 8 days with no further injection.
With respect to 30 mg/kg of nor-BNI, another group of rats (n=6 per group) was tested for the effect of 30 mg/kg of nor-BNI. Rats were injected with 30 mg/kg of nor-BNI 30 min before they were allowed to self-administer cocaine under an FR schedule. On the following day, the rats were allowed to self-administer cocaine under a PR schedule. The effect of 30 mg/kg of nor-BNI on cocaine self-administration was evaluated under alternating FR and PR schedules for 10 days. Nor-BNI was tested at the end of the study because its antagonism of κ-opioid antinociceptive effects is reported to last over 21 days in mice, rat, and rhesus monkeys, suggesting an extremely long half-life of the drug in the body (Butelman et al. 1993; Horan et al. 1992; Jones and Holtzman 1992). As a control for the nor-BNI paradigm, nine naive rats (ShA n=3, LgA n=6) were injected with sterile water (nor-BNI vehicle) 30 min before an FR session and were allowed to self-administer cocaine under alternating FR and PR schedules for 10 days.
The effect of naltrexone on cocaine self-administration under a PR schedule was evaluated in a separate group of naive rats (n=10 per group). The general procedure is as described above. Briefly, rats were trained to self-administer cocaine under an FR1 schedule for an hour per day for 13 days. After 13 days, the rats were divided into two groups balanced by cocaine intake and allowed to self-administer cocaine in 1- or 6-h sessions for 15 sessions. After 15 sessions, cocaine self-administration under a PR schedule was examined in both groups, and the effect of naltrexone on the PR cocaine intake was evaluated. Test sessions were separated by at least two escalation sessions (ShA rats, 1-h session; LgA rats, 6-h session), and the doses of naltrexone were tested in a counter balanced manner. Two rats were trained to self-administer cocaine for 19 days because of a slow acquisition of cocaine self-administration, and each was added to the ShA and LgA groups.
Opioid receptor binding assay
Receptor binding studies were conducted on human opioid receptors transfected into Chinese hamster ovary cells. The mu (μ) cell line was maintained in Ham's F-12 medium supplemented with 10% fetal bovine serum (FBS) and 400μg/mL Geneticin (G418). The delta (δ) and κ cell lines were maintained in Dulbecco's minimal essential medium supplemented with 10% FBS, 400μg/mL G418, and 0.1% penicillin/streptomycin. All cell lines were grown to confluence, then harvested for membrane preparation.
Binding assays were done using [3H]DAMGO, [3H]C1-DPDPE, and [3H]U69,593 at the μ, δ, and κ receptors, respectively. The assay was done in triplicate in a 96-well plate format. Nonspecific binding was determined with 1.0μM of the unlabeled counterpart of each radioligand. Cell membranes were incubated with the appropriate radioligand and test compound at 25°C for 60 min. The incubation was terminated by rapid filtration through glass fiber filter paper on a Tomtec cell harvester. The filters were dried overnight and bagged with 10 mL scintillation cocktail before counting for 2 min on a Wallac Betaplate 1205 liquid scintillation counter. Full characterization of compounds included analysis of the data for IC50 values and Hill coefficients by using the program Prism. Ki values were calculated using the Cheng Prusoff transformation:
where L was the radioligand concentration and Kd was the binding affinity of the radioligand, as determined previously by saturation analysis. The Ki value was expressed as the mean±standard deviation for three independent measurements.
[35S]GTPγS binding assay at opioid receptors
Triplicate assays were done in 96-well plates on ice with each incubation containing [35S]GTPγS (50 pM), cell membrane (10μg protein), guanosine diphosphate (GDP; 5μM), and SPA beads (0.5 mg) with assay buffer (pH7.5; 50 mM HEPES, 100 mM NaCl, 5 mM MgCl2, 10 mg/mL saponin) and the opioid ligands as before (Mohamed et al. 1986). Non-specific binding was determined in the presence of GTPγS (10μM). Single test agent dose–response curves (0.1 nM–10μM) of [35S]GTPγS-stimulated binding were done at each opioid receptor with each compound and compared to the standard opioid agonist compounds DAMGO, DPDPE, (–) and U50,488 for the μ, δ, and κ receptors, respectively. Membranes and GDP were incubated with the antagonists for 30 min before the [35S]GTPγS and SPA beads were added. Assay plates were shaken for 45 min at 25°C, and then centrifuged (1,500 rpm, 5 min, 25°C) before [35S]GTPγS-stimulated binding was assessed using the NXT Topcounter. The EC50 and Emax values were expressed as the mean±standard deviation of three independent measurements.
Data analysis
The data were expressed as the mean number of injections as well as the mean milligram per kilogram for each group of rats. The effect of access on cocaine self-administration per session as well as in the first hour of a session was examined over the initial 15 escalation sessions using a repeated measures two-way analysis of variance (ANOVA; access×daily session) with the Bonferroni post hoc test. After 15 cocaine self-administration sessions with extended access, an increase in cocaine self-administration under a PR schedule in LgA rats was examined in comparison with ShA rats using the Student's t test. The effect of SG-II-49 or naltrexone on cocaine self-administration was evaluated using a repeated measures two-way ANOVA (access×dose) with the Bonferroni post hoc test. With respect to nor-BNI, the mean values of cocaine self-administration from two FR and PR sessions that preceded nor-BNI treatment served as a control, and the effect of nor-BNI on cocaine self-administration was evaluated using a repeated measures two-way ANOVA (access×day) with the Bonferroni post hoc test. Software used for data analysis was Prism 4.0 (GraphPad, San Diego, CA).
Drugs
The National Institute on Drug Abuse (Rockville, MD) generously provided cocaine hydrochloride and nor-BNI bihydrochloride. Nor-BNI bihydrochloride and naltrexone hydrochloride were also purchased from Sigma-Aldrich (St. Louis, MO). SG-II-49 oxalate was synthesized at the Human BioMolecular Research Institute. Cocaine was dissolved in sterile saline for intravenous self-administration. Nor-BNI and SG-II-49 were dissolved in water and injected subcutaneously 30 min before a test session. Naltrexone was dissolved in saline and subcutaneously injected into rats 30 min before a session. All drug solutions were prepared for each rat based on its body weight and were updated twice a week. Doses of drugs were expressed in the salt form (Fig. 1).
Fig. 1.
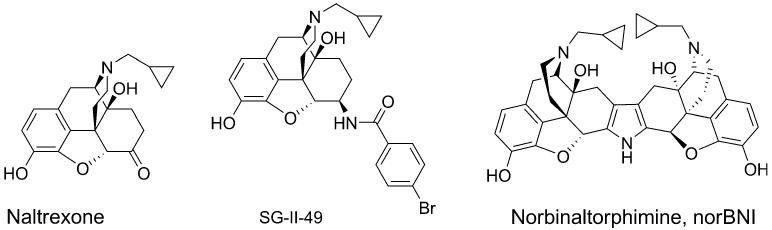
Structures of naltrexone, SG-II-49, and norBNI
Results
Effect of extended access to cocaine self-administration
Under all conditions, LgA rats produced a significant increase in cocaine self-administration, whereas ShA rats maintained a constant level of intake during the period of extended access for the LgA rats. For example, in the group of rats that were tested with SG-II-49 and 15 mg/kg of nor-BNI, cocaine self-administration in LgA rats significantly increased within a session as well as during the first hour of a session (Fig. 2a, session intake: session×access interaction, F14,196=6.84, p<0.001, session, F14,196=8.85, p<0.001, access, F1,196=195.0, p<0.001; Fig. 2b, first hour intake: session×access interaction, F14,196=2.26, p<0.01, session, F14,196=5.88, p<0.001, access, F1,196=4.79, p< 0.05). No significant change in cocaine self-administration was observed in ShA rats. After extended access to cocaine self-administration, LgA rats achieved a higher breakpoint for 0.5 mg/kg per injection of cocaine self-administration than ShA rats under a PR schedule in all groups [Student's t test, the SG-II-49/nor-BNI (15 mg/kg) group, p<0.05; the nor-BNI (30 mg/kg) group, p<0.01; the naltrexone group, p<0.01].
Fig. 2.
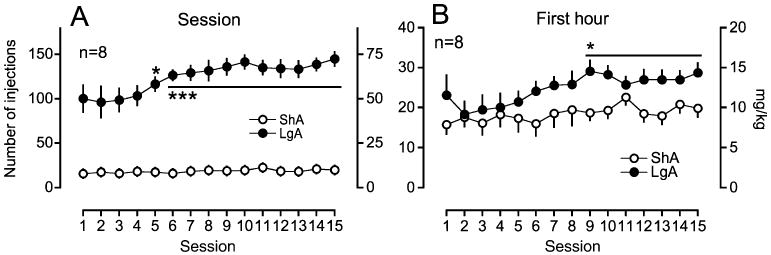
Cocaine self-administration under a fixed-ratio schedule of reinforcement. Data are expressed as the number of injections on the left axis and milligram per kilogram on the right axis. Error bars are SEM values. Open symbols are the data in rats with 1-h access to cocaine (ShA). Filled symbols are the data in rats with 6-h access (LgA). a Data from an entire session for each group, b data from the first hour of a 6-h session in LgA rats and from a 1-h session in ShA rats. *p<0.05, ***p<0.001 compared with session 1
Effect of nor-BNI, a selective κ opioid receptor antagonist, on cocaine self-administration
Because nor-BNI is long acting, the effect of each dose of the drug on cocaine self-administration was tested on alternating FR and PR sessions over 8 days after the injection of the drug in rats. The mean values of cocaine self-administration from two FR and PR sessions that preceded nor-BNI treatment served as baseline. The two baseline session data varied less than mean±15% or mean±2 injections (FR and PR data, respectively) in both groups of 15 and 30 mg/kg of nor-BNI [PR data (B1, B2): 15 mg/kg of nor-BNI, ShA (10.6±1.9, 10.6±1.9, n=5), LgA (15.5±2.9, 15.5±2.7, n=4); 30 mg/kg of nor-BNI, ShA (11.8±1.0, 10.2±0.6, n=6), LgA (15.2±1.5, 15.7±1.7, n=6)]. Fifteen milligrams per kilogram of nor-BNI did not alter cocaine self-administration under an FR schedule (Fig. 3-1). However, the dose of nor-BNI decreased cocaine self-administration in LgA rats on day 6 under a PR schedule (Fig. 3-1a, main effect of day, F4,28= 4.05, p<0.05). When rats were treated with 30 mg/kg of nor-BNI, cocaine self-administration in ShA rats significantly decreased on day 1 under an FR schedule, whereas cocaine self-administration in LgA rats did not alter under an FR schedule (Fig. 3-2b, first hour intake: access×day interaction F5,50=3.19, p<0.05, day F5,50=1.40, p>0.05, access F1,50= 27.3, p<0.001). The effect of 30 mg/kg of nor-BNI on cocaine self-administration in ShA rats was especially evident during the first 10 min of a session (between 30 and 40 min after the nor-BNI injection, Supplemental Fig. 1). In contrast, cocaine self-administration under a PR schedule was significantly decreased in LgA rats, but not in ShA rats, by 30 mg/kg of nor-BNI on days 2, 4, 6, and 10 in LgA rats (Fig. 3-2a, access×day interaction, F5,50=2.57, p<0.05). Cocaine self-administration after vehicle treatment was not altered for ten consecutive sessions under alternating FR and PR schedules, although all ShA and LgA rats uniformly showed a tendency of a decrease in cocaine intake only under a PR schedule on day 6 (Table 1: FR first hour intake, access×day interaction F5,35=1.80, p>0.05, day F5,35=1.09, p>0.05, access F1,35=11.9, p<0.05; PR data, access×day interaction F5,35=1.51, p>0.05, day F5,35=2.91, p<0.05, access F1,35=4.62, p>0.05).
Fig. 3.
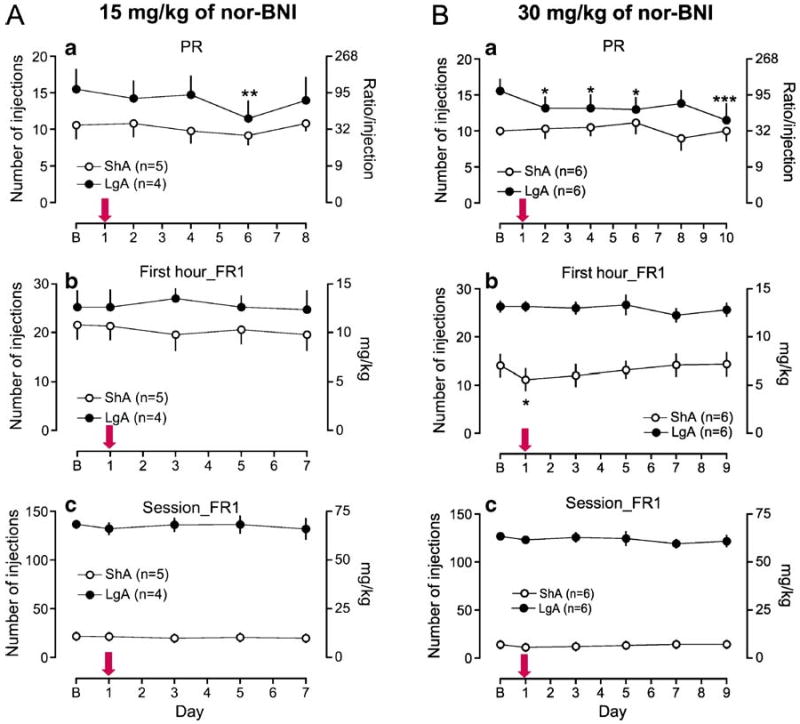
Effect of nor-BNI on cocaine self-administration. Data are expressed as the number of injections on the left axis and ratio/injection (a) or milligram per kilogram (b, c) on the right axis. Error bars are SEM values. The abscissa represents the nor-BNI treatment day, and the bold arrow indicates the day when rats were injected with nor-BNI 30 min before a session. FR and PR sessions were offered on odd and even days, respectively. a Data under a progressive-ratio schedule; b data from the first hour of a 6-h session in LgA rats and from a 1-h session in ShA rats under a fixed-ratio schedule; c data from an entire session. B baseline. The mean values of cocaine self-administration from two FR and PR sessions that preceded nor-BNI treatment served as baseline. *p<0.05, ***p<0.001 compared with baseline
Table 1.
Control data for nor-BNI under alternating FR and PR schedules
| ShA (n=3) | LgA (n=6) | ||||
|---|---|---|---|---|---|
| Day | FR | PR | FR (first hour) | FR (session) | PR |
| B | 11.5±2.8 | 8.8±2.8 | 27.1±2.1 | 135.3±8.4 | 12.6±0.9 |
| 1 | 10.3±3.4 | n.d. | 25.2±2.4 | 132.2±9.0 | n.d. |
| 2 | n.d. | 9.7±3.7 | n.d. | n.d. | 14.0±1.0 |
| 3 | 13.0±3.6 | n.d. | 25.7±2.2 | 129.5±9.5 | n.d. |
| 4 | n.d. | 7.3±3.3 | n.d. | n.d. | 15.0±0.6 |
| 5 | 11.3±2.8 | n.d. | 24.2±2.2 | 130.5±6.7 | n.d. |
| 6 | n.d. | 6.3±0.7 | n.d. | n.d. | 11.0±1.1 |
| 7 | 11.3±3.8 | n.d. | 25.2±2.4 | 128.5±6.6 | n.d. |
| 8 | n.d. | 9.3±3.5 | n.d. | n.d. | 13.5±0.4 |
| 9 | 13.7±4.6 | n.d. | 24.0±2.3 | 123.3±6.6 | n.d. |
| 10 | n.d. | 10.0±4.0 | n.d. | n.d. | 12.8±0.9 |
Data are the number of injections±SEM. Rats were injected with vehicle of nor-BNI on day 1 and were allowed to self-administer cocaine under a FR schedule 30 min after the vehicle injection. On the following day, the rats were allowed to self-administer cocaine under a PR schedule. Therefore, the rats self-administered cocaine under FR and PR schedules on alternating days for a total of 10 days with no further injection. The mean values of cocaine self-administration from two FR and PR sessions that preceded the vehicle treatment served as baseline. The two baseline data varied less than mean±15% or mean±2 injections (FR and PR data, respectively) in both groups of rats
B baseline session, n.d. not determined
Effect of naltrexone, a non-selective opioid receptor antagonist, on cocaine self-administration
Naltrexone decreased cocaine self-administration under a PR schedule in a dose-dependent manner in both groups (Fig. 4; dose×access interaction, F4, 72=1.06, p>0.05, dose, F4, 72=7.10, p<0.001, access, F1, 72=9.62, p<0.01). However, naltrexone decreased cocaine self-administration in ShA rats at lower doses of naltrexone than in LgA rats (1 vs 10 mg/kg, respectively).
Fig. 4.
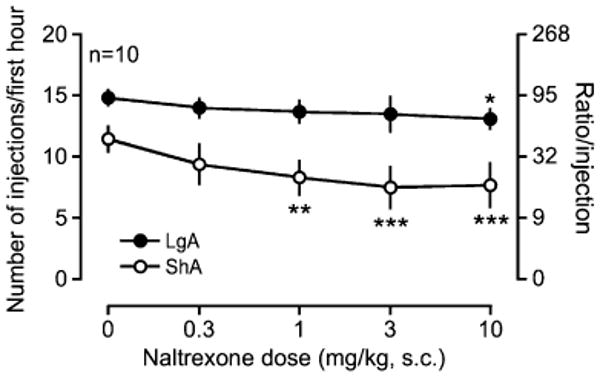
Effect of naltrexone on cocaine self-administration under a progressive-ratio schedule. Data are expressed as the number of injections on the left axis and ratio/injection on the right axis. Error bars are SEM values. The abscissa represents the dose of naltrexone. Doses of naltrexone were subcutaneously injected into rats 30 min before each test session. *p<0.05, **p<0.01, ***p<0.001 compared with vehicle
Effect of SG-II-49, a non-selective opioid receptor partial agonist, on cocaine self-administration
Pretreatment with SG-II-49 significantly decreased cocaine intake in both ShA (69.7%) and LgA rats (72.0%) under an FR schedule (Fig. 5b, first hour intake: dose×access interaction, F4, 52=2.49, p<0.05, dose, F4, 52=7.5, p<0.001, access, F1, 52=3.0, p>0.05; Fig. 5c, session intake: dose×access interaction, F4, 52=3.69, p<0.05, dose, F4, 52=7.47, p<0.001, access, F1, 52=177.0, p<0.001). SG-II-49 (0.1 mg/kg) decreased cocaine intake in both ShA and LgA rats throughout a session under an FR schedule but with a presentation of the regular pattern of responding (regular inter-injection interval) in rats, implying that the drug did not disturb general motor behaviors (Supplemental Fig. 2). Similarly, the compound dose-dependently decreased cocaine self-administration under a PR schedule in both ShA (71.0%) and LgA (73.5%) groups (Fig. 5a, dose, F3,33=6.98, p<0.001) with no significant interaction between dose and access.
Fig. 5.
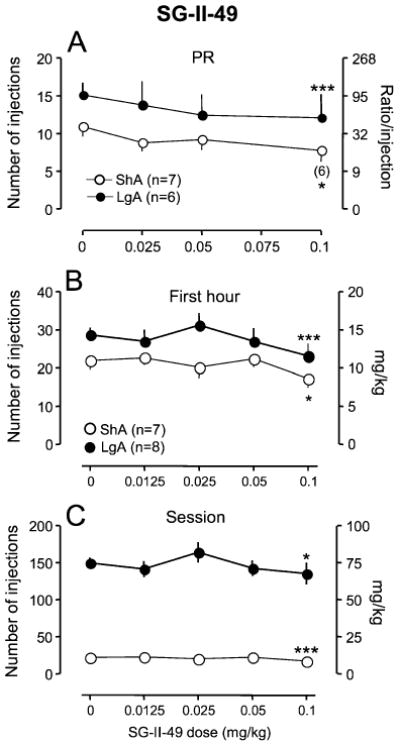
Effect of SG-II-49 on cocaine self-administration. Data are expressed as the number of injections on the left axis and ratio/injection (a) or milligram per kilogram (b, c) on the right axis. Error bars are SEM values. The abscissa represents the dose of SG-II-49. Doses of SG-II-49 were subcutaneously injected into rats 30 min before each test session. a Data under a progressive-ratio schedule; b data from the first hour of a 6-h session in LgA rats and from a 1-h session in ShA rats under a fixed-ratio schedule; c data from an entire session. Number in parenthesis in the top panel indicates the number of animals tested. *p<0.05, ***p<0.001 compared with vehicle
Pharmacodynamic profiles of SG-II-49 at opioid receptors
In the opioid receptor binding assay, SG-II-49 showed high affinity for μ, δ, and κ receptors with approximately a threefold difference in affinity among receptors (Table 2). In contrast, nor-BNI had approximately a 168-fold greater affinity for κ opioid receptors than for μ opioid receptors (Takemori et al. 1988). Published data by the authors Ghirmai et al. (2008) and Takemori et al. (1988) indicate that naltrexone had approximately a 2.7-fold or 22.7-fold greater affinity for μ receptors, respectively, in in vitro opioid receptor binding assays (Table 2).
Table 2.
Opioid receptors binding
In the GTPγS functional binding assay, SG-II-49 stimulated all three opioid receptors with 38% to 46% efficacy, whereas nor-BNI had no agonistic activity at the receptors (Table 3). Naltrexone had approximately 20% and 5% efficacy in stimulating δ and κ receptors, respectively.
Table 3.
Stimulation of [35S] GTPγS binding at opioid receptors
| Drug | μ | δ | κ | |||
|---|---|---|---|---|---|---|
| EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | EC50 (nM) | Emax (%) | |
| SG-II-49 | 4.5±0.5 | 38±4.3 | 0.2±0.1 | 46±3.7 | 0.1±0.1 | 42±3.9 |
| Nor-BNI | >10,000 | 0 | >10,000 | 0 | >10,000 | 0 |
| Naltrexonea | >10,000 | 0 | 230.0±0 | 20.4±0 | 2.1±0 | 5.3±0 |
Discussion
One hypothetical contribution to the compulsive drug searching observed in drug addiction is that the development of negative emotional states during drug withdrawal helps drive drug-seeking and drug-taking behaviors leading to excessive drug intake. The literature suggests that the κ opioid system is related to dysphoria, depression, and an aversive effect in humans and laboratory animals. Additionally, increased dynorphin and κ opioid receptors were found in chronic cocaine abusers. Therefore, in the present study, we hypothesized that enhanced κ opioid activity with repeated cocaine administration may contribute to excessive drug intake associated with extended access and drug dependence. Previous studies showed that rats with extended access exhibited increased cocaine intake, which was correlated with an increased intracranial self-stimulation threshold, suggestive of a dysphoric-like state (Ahmed et al. 2002; Ahmed and Koob 1998). To test the hypothesis, we evaluated the effects of κ opioid receptor antagonists on increased cocaine self-administration in rats with extended access.
When rats were treated with 15 mg/kg of nor-BNI, a long-acting selective κ opioid receptor antagonist, cocaine intake did not change in either group except that it decreased on the sixth day after the treatment in LgA rats under a PR schedule. However, at 30 mg/kg of nor-BNI, LgA rats showed significantly decreased cocaine intake over days under a PR schedule, whereas ShA rats showed significantly decreased cocaine intake only right after the injection of 30 mg/kg of nor-BNI under an FR schedule. It was reported that, when the dose of nor-BNI increased to a high level, the drug was able to inhibit the antinociceptive effects of μ and δ receptor agonists for an hour right after the intracerebroventricular injection, while the action of nor-BNI at κ receptors gradually increased to a maximum over 24 h and lasted 21 days in mice (Horan et al. 1992). Over 21 days of the action, duration of nor-BNI was also noted in rats and monkeys (Butelman et al. 1993; Jones and Holtzman 1992). Moreover, it was shown that up to 20 mg/kg of nor-BNI (s.c.) selectively antagonized the κ antinociceptive effect of U50,488 in mice without influencing the antinociceptive effects of μ and δ receptor agonists (Takemori et al. 1988). These findings suggest that 15 mg/kg of nor-BNI is selective for κ opioid receptors in rats, whereas 30 mg/kg may be high enough to non-selectively block μ and δ receptors at least within the initial hours after treatment. Consequently, the present data indicate that the increased motivation for cocaine in LgA rats under a PR schedule is attenuated by the action of nor-BNI at κ opioid receptors, whereas cocaine intake in ShA rats may have been decreased by the action of nor-BNI at μ or δ opioid receptors.
The lack of effect of nor-BNI on non-extended access cocaine self-administration has been previously reported in rats and monkeys (Glick et al. 1995; Negus et al. 1997). Similarly, although nor-BNI (3 mg/kg) inhibited the acquisition of a very low dose of cocaine self-administration in rats, the drug had no effect on a higher dose of cocaine (approximately 0.15 mg/kg per injection), which is still lower than the one (0.5 mg/kg per injection) in the present study (Kuzmin et al. 1998). In contrast, decreased cocaine self-administration by the inhibition of the selective μ opioid receptor antagonist, β-funaltrexamine, was noted under a PR schedule, although not under an FR schedule, in rats (Ward et al. 2003). Similarly, selective μ receptor antagonists were shown to decrease the rewarding effect of cocaine in a conditioned place preference paradigm in rats (Rademacher and Steinpreis 2002; Schroeder et al. 2007). Therefore, we postulate that cocaine self-administration in ShA rats involves, at least in part, increased μ opioid receptor activity. In contrast, the involvement of μ opioid activity in cocaine self-administration decreases in LgA rats, resulting in the lack of effect of nor-BNI during the initial hours after treatment, while extended access to cocaine self-administration induces an increase in κ opioid activity in rats, which is associated with increased motivation to self-administer cocaine.
To further support our hypothesis, we tested naltrexone, a mixed μ and κ opioid receptor antagonist. Previous data indicate that naltrexone has a similar or approximately a 22-fold greater affinity to μ receptors than to κ receptors (Ghirmai et al. 2008; Takemori et al. 1988). Similarly, in vivo study with naltrexone demonstrated that the drug has an approximate tenfold greater affinity at antagonizing the analgesic effect of a μ agonist than that of κ receptors in rhesus monkeys employing a pA2 analysis (Ko et al. 1998). Consistent with the nor-BNI data, naltrexone decreased cocaine self-administration under a PR schedule in both ShA and LgA groups, suggesting that the blockade of μ and κ receptors decreased cocaine self-administration in both groups. Moreover, cocaine self-administration in ShA rats was more sensitive to the naltrexone treatment than that of LgA rats, which might be related to the preferential binding of naltrexone at μ receptors over κ receptors. The decreased cocaine self-administration by naltrexone has been previously observed in rats with limited access to cocaine (Corrigall and Coen 1991; Ramsey et al. 1999), although there are conflicting reports (Ettenberg et al. 1982; Hemby et al. 1996).
Additionally, we tested SG-II-49 on cocaine self-administration in rats with extended access. SG-II-49 was developed as a pharmacotherapeutic candidate for alcoholism based on previously described opioids (Ghirmai et al. 2008), and the opioid receptor binding data show that the drug is a non-selective partial agonist at three opioid receptors. Several studies have previously focused on the identification of therapeutic agents with partial agonistic property at μ receptors to avoid withdrawal symptoms after the cessation of the treatment. Buprenorphine, a potent μ receptor partial agonist with κ receptor antagonistic activity (Dum and Herz 1981; Romero et al. 1999), inhibits cocaine self-administration in monkeys (Mello et al. 1989; Mello et al. 1990). Similar results were reported in rats (Carroll and Lac 1992; Comer et al. 1996). Buprenorphine also decreases the rewarding effect of cocaine in rats when measured in conditioned place preference (Kosten et al. 1991; Suzuki et al. 1992). Thus, our hypothesis was that a partial blockade of both μ and κ opioid receptors would decrease cocaine self-administration both in ShA and LgA rats. Indeed, SG-II-49 decreased cocaine self-administration in both groups. More importantly, SG-II-49 dose-dependently decreased cocaine self-administration under a PR schedule to a similar extent in ShA and LgA rats, suggesting that the drug decreased the motivation to self-administer cocaine in both groups.
It is important to note that several studies also suggest that κ opioid receptor stimulation decreases the reinforcing or rewarding effect of cocaine. For example, a rightward/downward shift of the cocaine dose–response function by U69,593, a κ opioid receptor agonist, was shown in rats (Schenk et al. 1999). Moreover, κ opioid receptor activation decreased cocaine self-administration and blocked cocaine-induced conditioned place preference (Crawford et al. 1995; Schenk et al. 1999; Shippenberg et al. 2001). Similarly, pretreatment with κ opioid receptor agonists decreased cocaine self-administration in rats and rhesus monkeys, whereas a κ opioid receptor antagonist had no effect on cocaine self-administration (Glick et al. 1995; Negus et al. 1997). In contrast, the stimulation of κ opioid receptors reinstates responding for cocaine self-administration in laboratory animals. In monkeys, κ opioid receptor agonists induced reinstatement of responding for cocaine, which was blocked by naltrexone and a corticotropin-releasing factor 1 receptor antagonist (Valdez et al. 2007). Similarly, κ opioid receptor antagonists inhibited stress-induced, but not cocaine-induced, reinstatement of responding for cocaine in rodents (Beardsley et al. 2005; Redila and Chavkin 2008). Therefore, the data imply that the activation of κ opioid receptors may attenuate the rewarding effect of cocaine possibly via a punishment-like action (aversive effect) and reinstates responding for cocaine in rats with non-extended access by producing a stress-like effect (Mucha and Herz 1985; Todtenkopf et al. 2004; Tomasiewicz et al. 2008). Consistent with this argument, McLaughlin et al. (2006) showed that U50,488, a κ opioid receptor agonist, produced place aversion in control rats and decreased cocaine-induced place preference when tested 15 min after its injection, whereas it enhanced cocaine-induced conditioned place preference when tested 30 min after its injection.
In conclusion, the present study shows that the inhibition of κ opioid receptors selectively attenuated increased cocaine self-administration under a PR schedule in rats with extended access, whereas the inhibition of μ opioid receptors decreased cocaine self-administration in ShA and LgA rats. Therefore, the data suggest that increased motivation to self-administer cocaine in rats with extended access may be associated with enhanced activity of the κ opioid system.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical assistance of Jovy Quiocho who was an undergraduate student of the University of California, San Diego. This is publication number 20047 from The Scripps Research Institute. This study was supported by the National Institute on Drug Abuse grant DA004398 (G.F.K.) and the National Institute on Alcohol and Alcoholism grant AA016029 (J.R.C.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00213-009-1563-y) contains supplementary material, which is available to authorized users.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatry Press; Washington DC: 2000. [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, Woods JH. Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther. 1993;267:1269–1276. [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Effects of buprenorphine on self-administration of cocaine and a nondrug reinforcer in rats. Psychopharmacology (Berl) 1992;106:439–446. doi: 10.1007/BF02244812. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforcer on i.v. cocaine self-administration in rats maintained under FR schedules. Psychopharmacology (Berl) 1996;125:355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology (Berl) 1991;104:167–170. doi: 10.1007/BF02244173. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa agonist U-50, 488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Dum JE, Herz A. In vivo receptor binding of the opiate partial agonist, buprenorphine, correlated with its agonistic and antagonistic actions. Br J Pharmacol. 1981;74:627–633. doi: 10.1111/j.1476-5381.1981.tb10473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirmai S, Azar MR, Polgar WE, Berzetei-Gurske I, Cashman JR. Synthesis and biological evaluation of alpha- and beta-6-amido derivatives of 17-cyclopropylmethyl-3, 14beta-dihydroxy-4, 5alpha-epoxymorphinan: potential alcohol-cessation agents. J Med Chem. 2008;51:1913–1924. doi: 10.1021/jm701060e. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Characterization of methamphetamine effects on the striatal–nigral dynorphin system. Eur J Pharmacol. 1988;155:11–18. doi: 10.1016/0014-2999(88)90397-4. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. J Pharmacol Exp Ther. 1996;277:1247–1258. [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neo-striatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DN, Holtzman SG. Long term kappa-opioid receptor blockade following nor-binaltorphimine. Eur J Pharmacol. 1992;215:345–348. doi: 10.1016/0014-2999(92)90055-9. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, Woods JH. Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther. 1998;285:518–526. [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Marby DW, Nestler EJ. Cocaine conditioned place preference is attenuated by chronic buprenorphine treatment. Life Sci. 1991;49:PL201–PL206. doi: 10.1016/0024-3205(91)90490-3. [DOI] [PubMed] [Google Scholar]
- Kuzmin AV, Gerrits MA, Van Ree JM. Kappa-opioid receptor blockade with nor-binaltorphimine modulates cocaine self-administration in drug-naive rats. Eur J Pharmacol. 1998;358:197–202. doi: 10.1016/s0014-2999(98)00637-2. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Relapse prevention: theoretical rationale and overview of the model. In: Marlatt GA, Gordon JR, editors. Relapse prevention. Guilford Press; New York: 1985. pp. 37–38. [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50, 488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology. 2006;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine suppresses cocaine self-administration by rhesus monkeys. Science. 1989;245:859–862. doi: 10.1126/science.2772637. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1990;254:926–939. [PubMed] [Google Scholar]
- Miczek KA, Covington HE, 3rd, Nikulina EM, Jr, Hammer RP. Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev. 2004;27:787–802. doi: 10.1016/j.neubiorev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Mohamed MS, Larson DL, Takemori AE, Portoghese PS. Activity of N-methyl-alpha- and -beta-funaltrexamine at opioid receptors. J Med Chem. 1986;29:1551–1553. doi: 10.1021/jm00158a043. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Steinpreis RE. Effects of the selective mu(1)-opioid receptor antagonist, naloxonazine, on cocaine-induced conditioned place preference and locomotor behavior in rats. Neurosci Lett. 2002;332:159–162. doi: 10.1016/s0304-3940(02)00950-3. [DOI] [PubMed] [Google Scholar]
- Ramsey NF, Gerrits MA, Van Ree JM. Naltrexone affects cocaine self-administration in naive rats through the ventral tegmental area rather than dopaminergic target regions. Eur Neuropsychopharmacol. 1999;9:93–99. doi: 10.1016/s0924-977x(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl) 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Romero DV, Partilla JS, Zheng QX, Heyliger SO, Ni Q, Rice KC, Lai J, Rothman RB. Opioid peptide receptor studies. 12. Buprenorphine is a potent and selective mu/kappa antagonist in the [35S]-GTP-gamma-S functional binding assay. Synapse. 1999;34:83–94. doi: 10.1002/(SICI)1098-2396(199911)34:2<83::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology (Berl) 2007;195:265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Zapata A, Heidbreder CA. Modulation of the behavioral and neurochemical effects of psychostimulants by kappa-opioid receptor systems. Ann N Y Acad Sci. 2001;937:50–73. doi: 10.1111/j.1749-6632.2001.tb03558.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Takemori AE, Ho BY, Naeseth JS, Portoghese PS. Nor-binaltorphimine, a highly selective kappa-opioid antagonist in analgesic and receptor binding assays. J Pharmacol Exp Ther. 1988;246:255–258. [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69, 593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Lason W, Przewlocki R. Effects of repeated psychostimulant administration on the prodynorphin system activity and kappa opioid receptor density in the rat brain. Neuroscience. 1998;85:1051–1059. doi: 10.1016/s0306-4522(97)00639-8. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. Kappa agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Martin TJ, Roberts DC. Beta-funaltrexamine affects cocaine self-administration in rats responding on a progressive ratio schedule of reinforcement. Pharmacol Biochem Behav. 2003;75:301–307. doi: 10.1016/s0091-3057(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Wee S, Specio SE, Koob GF. Effects of dose and session duration on cocaine self-administration in rats. J Pharmacol Exp Ther. 2007;320:1134–1143. doi: 10.1124/jpet.106.113340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


