Abstract
Preclinical and clinical studies demonstrate that neurotrophic factors play critical roles in the etiology and treatment of depression. While the mechanisms underlying the therapeutic efficacy of antidepressants remain unknown, increasing evidence supports a role for increased trophic support in the treatment of depression. Furthermore, antidepressants block or reverse stress-induced down regulation of neurotrophic factor expression in limbic and cortical nuclei involved in the underlying pathophysiology of depression. Thus, components of neurotrophic factor-mediated signaling cascades or the signal transduction pathways that regulate neurotrophic factor expression may provide additional targets for the development of novel, more efficacious antidepressant drugs.
Keywords: hippocampus, neurogenesis, depression, brain-derived neurotrophic factor, vascular endothelial growth factor, insulin-like growth factor 1
Introduction
Major depressive disorder (MDD) is a prevalent, highly debilitating mental illness that affects 17% of the US population at least once in their lifetime and results in tremendous secondary costs to society (>$83 billion annually) [1,2]. Antidepressant drugs constitute the largest segment of the CNS market (approximately 24%) with global sales exceeding $17 billion per year. While a multitude of chemically diverse pharmacotherapies are currently available to treat MDD, the therapeutic benefit of these drugs is only realized in a subset (50-70%) of patients, but only following weeks to months of chronic treatment, and is often accompanied by numerous side effects. Although the acute pharmacological actions of available antidepressant medications are ascribed to modulation of serotonin and/or norepinephrine transmission in the brain, the cellular and biochemical mechanisms underlying the long-term adaptations that are required for the therapeutic actions of antidepressant treatments remain unclear. A growing body of evidence describing a critical role for neurotrophic factors in mediating the neural adaptations required for the therapeutic benefit of antidepressant treatments supports a neurotrophic and neurogenic hypothesis of treatment response [3,4]. Thus, targeting neurotrophic factors and their related signaling cascades represents a novel avenue in the development of MDD therapies.
Antidepressants Increase Neurotrophic Factor Expression in Patients with MDD
Clinical studies of adult patients with recurrent depressive episodes reveal reductions in overall volume of limbic and cortical structures involved in the underlying pathophysiology of MDD [5] and that these changes are reversed or reduced by chronic antidepressant treatment [6]. These results suggest that antidepressants may have neuroprotective effects on the development and/or progression of MDD. The structural changes observed in the hippocampus, amygdala and prefrontal cortex of depressed patients may be due to many factors, including death or atrophy of existing neurons, decreases in the size and number of glia, and decreases in neurogenesis [7-9]. While increasing evidence indicates that volumetric changes of the hippocampus and other limbic nuclei are involved in the pathophysiology of depression, the mechanism(s) underlying atrophy of these brain regions remain to be determined.
Postmortem analyses of brain tissue from depressed suicide victims provide insight into the cellular changes that may underlie atrophy of the hippocampus in these patients. Expression of neurotrophic factors, including brain-derived neurotrophic factor (BDNF) is decreased in brain tissue from depressed suicide victims when compared to healthy controls [10-12] which suggests that loss of trophic support may underlie structural changes observed in brain imaging studies of patients with MDD. Furthermore, expression of BDNF is increased [10] or unchanged [12] in postmortem tissue from suicide victims receiving antidepressant medications at the time of death. Taken together, these findings suggest that antidepressant treatments may have neuroprotective effects that are mediated, in part, by increases in trophic support with a time course that is consistent with that for the therapeutic and behavioral response to antidepressants.
Neurotrophic Factors, Antidepressants and Animal Models of Depressive Behavior
The neurotrophic factor hypothesis of depression and antidepressant therapeutic response is supported by an increasing body of evidence demonstrating that depression and stress reduce the expression of neurotrophic factors in limbic structures, including the hippocampus [3,13,14]. Clinical and pre-clinical studies indicate that expression of BDNF is decreased in postmortem brain tissue from patients with MDD [15] and in laboratory animals subjected to different types of chronic stress paradigms [for review see 4], respectively. Consistent with these findings, antidepressant treatment blocks or reverses the effects of stress and MDD on BDNF levels [4]. Moreover, a number of studies have shown that increased expression of BDNF plays a critical role in the behavioral and cellular efficacy of antidepressants [16-18]. Notably, these studies demonstrate that infusion of BDNF into the hippocampus is sufficient to produce an antidepressant response in behavioral models of depression [16]. In addition, these studies demonstrate that the behavioral actions of antidepressant treatment are blocked in BDNF deletion mutant mice or transgenic mice expressing a dominant negative form of the BDNF receptor (TrkB), indicating that BDNF is required for an antidepressant response [17-20]. However, it is notable that a depressive phenotype is not observed in the BDNF deletion mutants or dominant negative TrkB transgenic mice [17,19,20].
While these studies indicate that reduction of BDNF/TrkB signaling is not sufficient to produce a depressive phenotype, recent studies demonstrate that that decreased BDNF levels results in a state of increased vulnerability to depression [21]. When BDNF deletion mutant mice are exposed to mild stress, depressive behavior is observed even though the stress alone is not sufficient to have an effect [21]. This example of a gene x environment interaction has also been observed in humans, where a functional polymorphism of the BDNF gene is associated with increased risk for depression when patients have a prior history of stress or trauma [22,23]. These findings underscore the critical need for examining prior life history in genetic association studies and the importance of gene x environment interactions.
In addition to increasing expression of BDNF, chemical and somatic antidepressant administration increases expression of vascular endothelial growth factor (VEGF) mRNA and protein in the hippocampus [24-26]. Consistent with these results, intra-cranial infusions of recombinant VEGF peptide produce antidepressant behavioral responses in rodent models [24]. Furthermore, exposure to chronic stress down-regulates the expression of VEGF in the hippocampus [27] and microinfusions of a VEGF receptor inhibitor block the behavioral and neurogenic actions of antidepressant treatments [24].
Taken together, preclinical studies strongly suggest that decreased expression of BDNF and/or VEGF may contribute to altered structural plasticity observed in patients with MDD and that antidepressant treatments may block neuronal atrophy and cell loss via up-regulation of these neurotrophic factors (see Figure 1). In addition to BDNF and VEGF, other growth factors including insulin-like growth factor 1 (IGF-1) and fibroblast growth factor 2 (FGF-2) have been shown to play critical roles in the cellular and behavioral responses to stress and antidepressants [4]. This apparent redundancy in function may be due to overlapping but also different, complementary actions of these growth factors on overall cellular health, different stages of neurogenesis and the microenvironment in which neuroplasticity occurs. Future studies aimed at fully characterizing the interactions of these neurotrophic/growth factor systems will provide further insight into designing drug targets selective for the response to antidepressant treatment.
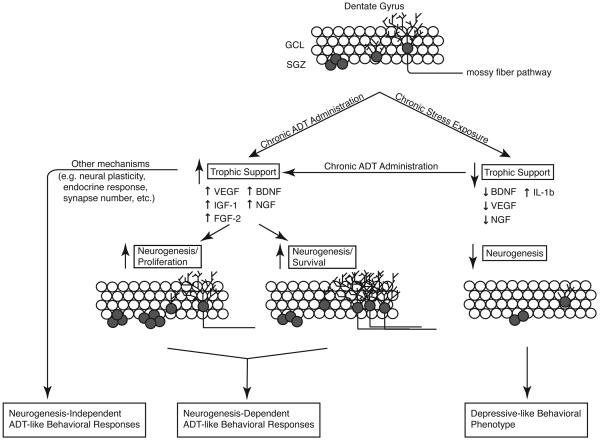
Figure 1. Neurotrophic/growth factor expression, neurogenesis and depressive-like behaviors are differentially regulated by antidepressants and exposure to stress.
Exposure to chronic stress decreases expression of trophic factors including BDNF, VEGF and NGF in the dentate gyrus, increases expression of the inflammatory cytokine IL-1β, and decreases neurogenesis in the dentate gyrus. In contrast, chronic antidepressant administration increases expression of trophic factors including BDFN, VEGF, NGF, IGF-1 and FGF-2 in the dentate gyrus and increases neurogenesis. Furthermore, chronic antidepressant administration reverses or blocks stress-induced downregulation of trophic factor expression and neurogenesis. Neurotrophic/growth factors may have differential effects on stages of adult hippocampal neurogenesis. Increased expression of VEGF, IGF-1 and FGF-2 in the dentate gyrus result in increased rates of proliferation of neural progenitor cells in the subganular zone. Moreover, increased expression of BDNF and NGF in the dentate gyrus result in increased survival and maturation of newborn neurons. Chronic antidepressant administration produces neurogenesis-dependent and neurogenesis-independent behavioral responses that coincide with increased expression of trophic factors. ADT, antidepressant; BDNF, brain-derived neurotrophic factor; FGF-2, fibroblast growth factor 2; GCL, granule cell layer; IGF-1; insulin-like growth factor 1; IL-1β, interleukin-1β; NGF, nerve growth factor; SGZ, subgranular zone; VEGF, vascular endothelial growth factor;
Neurotrophic Factors Have Differential Roles in Adult Hippocampal Neurogenesis
Stress-induced plasticity in the hippocampus includes decreased rates of neurogenesis in the dentate gyrus and remodeling of CA3 pyramidal cell processes. Hippocampal neurogenesis is required for the behavioral response to antidepressant treatments in some, but not all, behavioral paradigms [28-30]. While the exact role of BDNF in the proliferation of adult progenitor cells remains unknown [18,31], the survival rate of immature neurons in the dentate gyrus is significantly decreased in heterozygote BDNF knockout mice and transgenic mice expressing a dominant-negative TrkB receptor [18]. Consistent with these results, the increased survival of newborn granule cells in the dentate gyrus in response to antidepressant treatment is blocked in mice with impaired TrkB signaling, indicating a requirement for this neurotrophic factor for the cellular effects of antidepressants [18]. However, a recent study has demonstrated that deletion of TrkB in neural progenitor cells blocks the ability of antidepressant treatment to increase the proliferation of newborn neurons [32], indicating that BDNF-TrkB could influence both proliferation and survival of newborn neurons. In addition to increasing survival of neuronal progenitor cells, chronic antidepressant administration increases the maturation of newborn neurons and enhances long-term plasticity in the dentate gyrus [33].
BDNF is not the only neurotrophic factor that is regulated by antidepressants and that has neurogenic actions. VEGF/Flk-1 signaling increases the proliferation rate of neuronal progenitor cells [34], stimulates adult hippocampal neurogenesis [35], and promotes neurite outgrowth [36]. VEGF may promote neuronal cell proliferation directly through mitogenic effects on neural progenitor cells or through an indirect mechanism that stimulates endothelial cell proliferation, which, in turn, induces neural progenitor cell division. Different pharmacological classes of antidepressants increase the expression of VEGF in the hippocampus [26,37]. Taken together these findings suggest that BDNF and VEGF may have differential roles, but also some overlapping effects on the proliferation and survival of newborn granule cells in the hippocampus. Future studies aimed at delineating the pathways underlying the regulation of BDNF and VEGF, and the precise neurogenic actions of these growth factors may facilitate the development of novel antidepressant medications that target specific stages of adult neurogenesis. Toward this goal, it is also important and useful to characterize the mechanisms underlying the actions of stress. A recent study from our laboratory has found that the anti-neurogenic and anhedonic effects of chronic unpredictable stress are blocked by inhibition of the inflammatory cytokine, interleukin-1b (IL-1b), identifying another potential target for the development of novel antidepressant agents.
Targeting Neurotrophic Factors to Modulate Neurogenesis-Dependent Cellular and Behavioral Effects of ADTs
The neurotrophic and neurogenic hypothesis of antidepressant response is supported by a large body of evidence demonstrating that chronic antidepressant administration blocks or reverses stress-induced down regulation of neurotrophic factor expression and adult hippocampal neurogenesis, effects that could contribute to the altered morphology observed in the hippocampus of patients with MDD [4]. More direct evidence that neurogenesis is required for the behavioral response to some, but not all, antidepressants comes from recent preclinical studies in which x-ray irradiation was used to block basal rates of cell proliferation in the hippocampus of adult mice [29,33,38]. One important caveat of these studies is that irradiation of the hippocampus and cortex may have affected expression of neurotrophic/growth factors including BDNF and this loss of neurotrophic support may have contributed to the inability of antidepressants to produce behavioral responses in irradiated mice.
New methods for measuring neurogenesis in vivo will allow researchers to evaluate the putative antidepressant effects of novel pharmacotherapies that have neurogenic properties in human patients with MDD. One recent study using high-resolution magnetic resonance imaging (MRI) techniques in rodents demonstrated that changes in hippocampal cerebral blood volume provide an in vivo correlate of neurogenesis [39]. Moreover, inhibition of adult hippocampal neurogenesis using x-ray irradiation blocks exercise-induced changes in hippocampal cerebral blood volume which suggests that neurogenesis may be required for the antidepressant and cognitive-enhancing effects of exercise [39]. Brain imaging techniques that measure neurogenesis-dependent changes in hippocampal structure and function following antidepressant administration may serve as useful tools for screening novel, efficacious pharmacotherapies in patients with MDD.
Neurotrophic Factors Activate Complementary Intracellular Signaling Cascades
BDNF, VEGF, IGF-1 and FGF-2 bind to and activate tyrosine kinase receptors that are coupled to similar, interconnected signal transduction pathways. These pathways influence numerous cellular functions, including the expression of genes that are integrally involved in regulating neuroplasticity and cellular health. Recent postmortem analyses of brain tissue from depressed suicide victims indicate that decreased mitogen-activated protein (MAP) kinase signaling may contribute to the pathophysiology of MDD [40,41]. Consistent with these results, preclinical studies demonstrate that chronic antidepressant administration, which increases expression of neurotrophic factors, activates the MAP kinase signaling cascade [42-44] and pretreatment with the MEK inhibitor PD184161, which blocks signaling through the MAPK pathway, blocks the behavioral response to ADTs [21]. Acute and chronic antidepressant administration also increases neurotrophin-mediated activation of phospholipase C (PLC) gamma 1 and phosphorylation of the transcription factor cAMP response element-binding protein (CREB) [45]. Furthermore, CREB expression and transcriptional activity are increased by chronic, but not acute, antidepressant administration [46,47]. Collectively, these and other studies indicate that chronic antidepressant administration increases neurotrophic/growth factor-mediated signaling cascades including the MAPK, PLC and phosphatidylinositol 3′-kinase (PI3K) networks which converge to activate CREB-mediated gene transcription [48].
Despite producing antidepressant behavioral effects, exogenously delivered neurotrophic/growth factors have limited therapeutic applications in humans due to poor pharmacokinetics and adverse side effect profiles [49]. However, the intracellular signaling cascades that mediate neurtrophic/growth factor receptor signaling provide numerous drug targets. For instance, MAPKs are inactivated by dual specificity phosphatases (DUSPs), enzymes that dephosphorylate threonine and tyrosine sites on ERK and other MAPKs [50]. Expression of DUSP isoforms is increased in the hippocampus and frontal cortex following acute or chronic antidepressant administration suggesting a negative feedback mechanism that functions to offset antidepressant-enhanced BDNF-ERK-CREB signaling [21]. Other targets for antidepressant drug design that regulate MAPK signaling are phosphatases such as striatal enriched phosphastase (STEP), protein phosphatase 1 (PP1) and 2A (PP2A) that deactivate ERK by dephosphorylating tyrosine or serine/theronine residues. While designing selective and efficacious small molecule activators of the kinases and other enzymes that comprise neurotrophic/growth factor receptor signaling cascades has proven difficult, a more successful approach has been to develop small molecule inhibitors of enzymes that negatively regulate these networks [51]. Thus, drugs that inhibit DUSP, STEP, PP1 or PP2A activity may produce antidepressant responses on their own or enhance the efficacy of antidepressants that upregulate MAPK signaling.
Conclusion
Antidepressant drugs and stress exert opposing modulatory influences on structural plasticity in the hippocampus and these effects are mediated, in part, by altered expression of neurotrophic factors including BDNF and VEGF. A large body of evidence suggests that the cellular response of antidepressants includes increased expression of neurotrophic/growth factors, increased proliferation, survival, and maturation of neuronal progenitor cells, and enhanced synaptic plasticity in the dentate gyrus. Increased structural and synaptic plasticity in the adult hippocampus in response to ADT treatment may result in cognitive flexibility and, subsequently, an increased ability to adapt/cope with stressful situations.
Future directions in developing novel, more efficacious antidepressant drugs may involve direct or indirect targeting of neurotophic/growth factors. Potential strategies include developing pharmacotherapies that modulate neurotrophic/growth factor receptors or components of neurotrophic/growth factor-mediated signaling cascades. Future generations of antidepressant drugs may act to increase proliferation of neural progenitor cells, survival of immature neuronal granule cells, and/or the local microenvironment in which neurogenesis occurs. Mechanisms that underlie these effects may include activating multiple neurotrophic/growth factors or signal transduction pathways involved in proliferation, survival, and maintenance of proliferating cells and their trophic environment. Future studies are required to elucidate the etiology of MDD and the role of neurotrohic/growth factors in the neuronal circuitry implicated in MDD. It will be interesting to determine if novel compounds that target neurotrophic/growth factors will be more efficacious and exhibit less side effects than currently available antidepressant drugs, improve remission in a larger percentage of patients with MDD, and have a rapid onset of therapeutic action.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greenberg PE, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64(12):1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 3.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(56):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 5.Campbell S, MacQueen G. An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry. 2006;19(1):25–33. doi: 10.1097/01.yco.0000194371.47685.f2. [DOI] [PubMed] [Google Scholar]
- 6.Sheline YI, et al. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 7.Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A. 2001;98(22):12320–12322. doi: 10.1073/pnas.231475998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempermann G. Regulation of adult hippocampal neurogenesis - implications for novel theories of major depression. Bipolar Disord. 2002;4(1):17–33. doi: 10.1034/j.1399-5618.2002.40101.x. [DOI] [PubMed] [Google Scholar]
- 9.Manji HK, et al. The cellular neurobiology of depression. Nat Med. 2001;7(5):541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 10.Chen B, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50(4):260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 11.Dwivedi Y, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 12.Karege F, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136(12):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 13.D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4(3):183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 14.Castren E, et al. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Sen S, et al. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirayama Y, et al. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saarelainen T, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sairanen M, et al. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25(5):1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteggia LM, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman CH, et al. A Role for MAP Kinase Signaling in Behavioral Models of Depression and Antidepressant Treatment. Biol Psychiatry. 2007;61(5):661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman J, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59(8):673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Kim JM, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62(5):423–428. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol. 2008;8(1):14–19. doi: 10.1016/j.coph.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton SS, et al. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci. 2003;23(34):10841–10851. doi: 10.1523/JNEUROSCI.23-34-10841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heine VM, et al. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21(5):1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 28.Meshi D, et al. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9(6):729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- 29.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 30.Holick KA, et al. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, et al. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82(6):1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JW, et al. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada T, et al. Vascular endothelial growth factor directly inhibits primitive neural stem cell survival but promotes definitive neural stem cell survival. J Neurosci. 2006;26(25):6803–6812. doi: 10.1523/JNEUROSCI.0526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin K, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khaibullina AA, et al. Vascular endothelial growth factor promotes neurite maturation in primary CNS neuronal cultures. Brain Res Dev Brain Res. 2004;148(1):59–68. doi: 10.1016/j.devbrainres.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Nibuya M, et al. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Surget A, et al. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 39.Pereira AC, et al. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dwivedi Y, et al. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry. 2006;11(1):86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- 41.Dwivedi Y, et al. Aberrant Extracellular Signal-Regulated Kinase (ERK) 5 Signaling in Hippocampus of Suicide Subjects. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301372. [DOI] [PubMed] [Google Scholar]
- 42.Kodama M, et al. Electroconvulsive seizures increase the expression of MAP kinase phosphatases in limbic regions of rat brain. Neuropsychopharmacology. 2005;30(2):360–371. doi: 10.1038/sj.npp.1300588. [DOI] [PubMed] [Google Scholar]
- 43.Mercier G, et al. MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci. 2004;24(2):207–216. doi: 10.1385/JMN:24:2:207. [DOI] [PubMed] [Google Scholar]
- 44.Bhat RV, et al. Region-specific targets of p42/p44MAPK signaling in rat brain. J Neurochem. 1998;70(2):558–571. doi: 10.1046/j.1471-4159.1998.70020558.x. [DOI] [PubMed] [Google Scholar]
- 45.Rantamaki T, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 46.Tardito D, et al. Signaling pathways regulating gene expression, neuroplasticity, and neurotrophic mechanisms in the action of antidepressants: a critical overview. Pharmacol Rev. 2006;58(1):115–134. doi: 10.1124/pr.58.1.7. [DOI] [PubMed] [Google Scholar]
- 47.Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59(12):1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Pittenger C, Duman RS. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 49.Price RD, et al. Advances in small molecules promoting neurotrophic function. Pharmacol Ther. 2007;115(2):292–306. doi: 10.1016/j.pharmthera.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Farooq A, Zhou MM. Structure and regulation of MAPK phosphatases. Cell Signal. 2004;16(7):769–779. doi: 10.1016/j.cellsig.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Tanis KQ, et al. Targeting neurotrophic/growth factor expression and signaling for antidepressant drug development. CNS Neurol Disord Drug Targets. 2007;6(2):151–160. doi: 10.2174/187152707780363276. [DOI] [PubMed] [Google Scholar]