Abstract
A total of 238 isolates of Providencia stuartii obtained from infected patients in six Dublin hospitals were grouped by using serological and bacteriocin typing methods and tested for sensitivity to a number of antimicrobial agents. Most isolates were resistant to several of these agents. Resistance to tetracycline, resistance to penicillin, resistance to polymyxin, and probably resistance to nitrofurantoin was intrinsic. Plasmid screening coupled with resistance transfer studies showed that both chromosome-encoded and plasmid-coded resistance mechanisms were clinically important. Ampicillin resistance was both chromosomally and plasmid encoded, whereas resistance to kanamycin and resistance to carbenicillin were exclusively plasmid encoded. Gentamicin resistance was more common than kanamycin resistance, and although gentamicin-resistant strains contained aminoglycoside acetyltransferase activity, no association could be demonstrated with plasmid deoxyribonucleic acid in the strains tested. Unlike minimal inhibitory concentrations for kanamycin, minimal inhibitory concentrations for gentamicin varied over a wide range. P. stuartii isolated obtained from several different countries were tested for comparison. As a group, these strains were less resistant, but they did exhibit similar resistance properties.
Full text
PDF

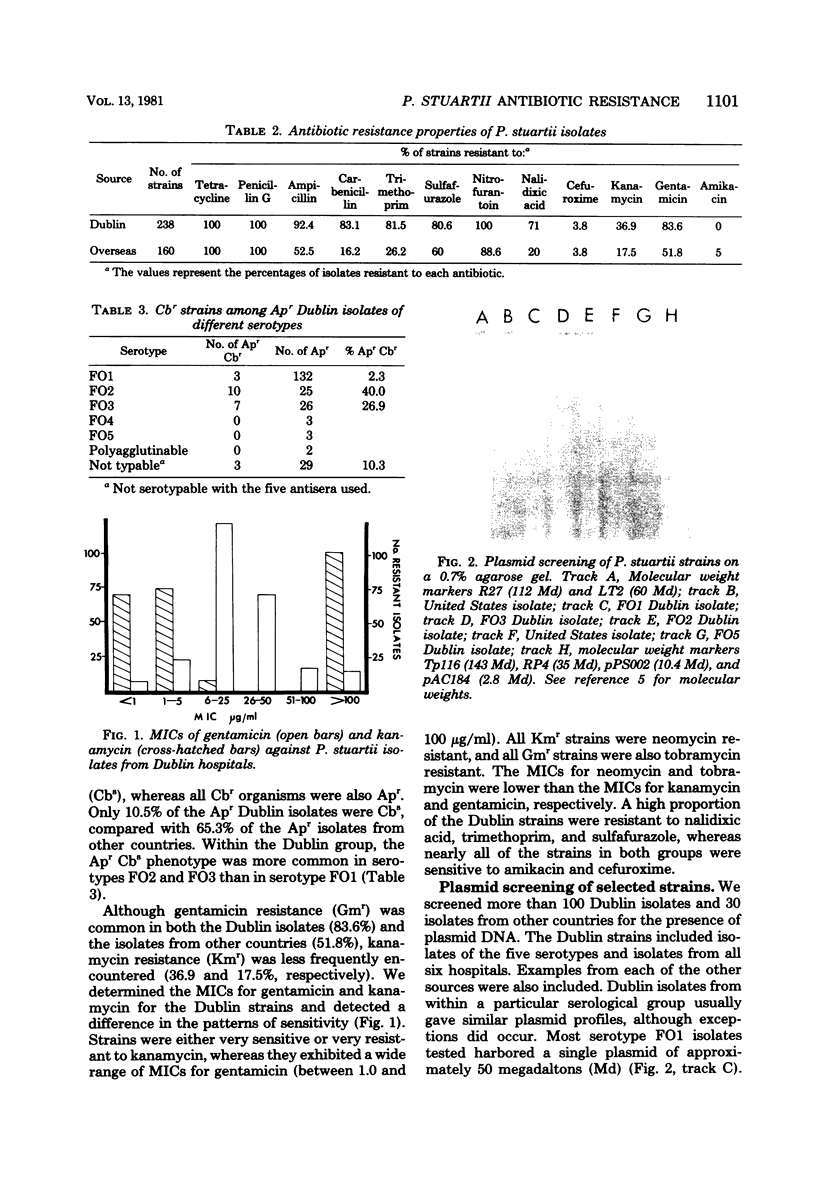



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W. R factors from Providence. J Gen Microbiol. 1974 Mar;81(1):171–181. doi: 10.1099/00221287-81-1-171. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, Rubens C. E., Farrar W. E., Jr Characteristics of gentamicin resistance in nosocomial infections. Am J Med Sci. 1980 Jan-Feb;279(1):25–30. doi: 10.1097/00000441-198001000-00003. [DOI] [PubMed] [Google Scholar]
- Keane C. T., English L. F., Wise R. Letter: Providencia stuartii infections. Lancet. 1975 Nov 22;2(7943):1045–1045. doi: 10.1016/s0140-6736(75)90341-4. [DOI] [PubMed] [Google Scholar]
- Kocka F. E., Srinivasan S., Mowjood M., Kantor H. S. Nosocomial multiply resistant Providencia stuartii: a long-term outbreak with multiple biotypes and serotypes at one hospital. J Clin Microbiol. 1980 Feb;11(2):167–169. doi: 10.1128/jcm.11.2.167-169.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale P., English L., Speekenbrink A., Keane C. Kanamycin resistance in Providencia stuartii. J Antimicrob Chemother. 1978 May;4(3):273–278. doi: 10.1093/jac/4.3.273. [DOI] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent M. E., Bone D. H., Datta N. A transposon, Tn732, encoding gentamicin/tobramycin resistance. Nature. 1979 Nov 22;282(5737):422–423. doi: 10.1038/282422a0. [DOI] [PubMed] [Google Scholar]
- Overturf G. D., Wilkins J., Ressler R. Emergence of resistance of Providencia stuartii to multiple antibiotics: speciation and biochemical characterization of Providencia. J Infect Dis. 1974 Mar;129(3):353–357. doi: 10.1093/infdis/129.3.353. [DOI] [PubMed] [Google Scholar]
- Penner J. L., Hinton N. A., Duncan I. B., Hennessy J. N., Whiteley G. R. O serotyping of Providencia stuartii isolates collected from twelve hospitals. J Clin Microbiol. 1979 Jan;9(1):11–14. doi: 10.1128/jcm.9.1.11-14.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price K. E., Pursiano T. A., DeFuria M. D. Activity of BB-K8 (amikacin) against clinical isolates resistant to one or more aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Feb;5(2):143–152. doi: 10.1128/aac.5.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C. E., McNeill W. F., Farrar W. E., Jr Transposable plasmid deoxyribonucleic acid sequence in Pseudomonas aeruginosa which mediates resistance to gentamicin and four other antimicrobial agents. J Bacteriol. 1979 Sep;139(3):877–882. doi: 10.1128/jb.139.3.877-882.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]




