Abstract
Reactive oxygen species (ROS) and reactive carbonyl species (RCS) are the major cause of biological tissue damage during the exposure to ionizing radiation (IR). The existing strategies to protect normal tissues from detrimental effects of IR suffer from several shortcomings including high toxic side effects, unfavorable administration routs or low efficacy. These shortcomings emphasize a need for radioprotective treatments that combine effectiveness with safety and ease of use. In this paper, we demonstrate that pyridoxamine, a ROS and RCS scavenger with a very favorable safety profile, can inhibit IR-induced gastrointestinal endothelial apoptosis in cell culture and in animal model. Pyridoxamine was more effective at protecting from radiation-induced apoptosis compared to Amifostine, a synthetic thiol compound and the only FDA approved radioprotector. We suggest that PM has a potential as an effective and safe radioprotective agent.
Keywords: ionizing radiation, radioprotection, reactive oxygen species, reactive carbonyl species, gastrointestinal tract, radiation toxicity, apoptosis, pyridoxamine
Introduction
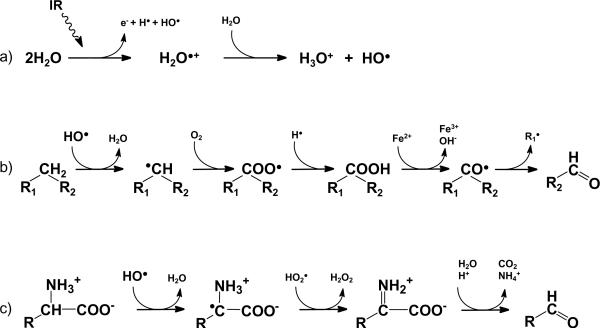
Exposure to ionizing radiation (IR) can produce severe health impairments due to injury and failure to susceptible organs. Detrimental effects of IR on biological tissues are, in major part, mediated via increased production of reactive oxygen species (ROS) and reactive carbonyl species (RCS). Hydroxyl radical produced during radiolysis of water can trigger oxidation of lipids, amino acids, and saccharides leading to formation of various secondary free radicals and reactive carbonyl compounds as shown in Fig. 1 [1-3]. These toxic products chemically modify DNA, proteins, and lipids causing cellular damage.
Figure 1.
Pathways of ionizing radiation-induced generation of ROS and RCS in biological tissues. The scheme is based on references [1-3] and shows: formation of hydroxyl radical via radiolysis of water (a); formation of RCS during hydroxyl radical-induced lipid peroxidation (b) and oxidation of amino acids (c).
Since the intestinal epithelium is one of the fastest proliferating tissues in the body, the gastrointestinal (GI) tract is injured in intended or accidental radiation exposure [4-7]. In the GI tract, epithelial cells of the small intestinal crypts are the most susceptible to radiation-induced apoptosis [8]. While only occasional apoptotic cells can be observed in the intestinal crypts of healthy mice and man, as low as 1 Gy of radiation induces dramatic increase in apoptosis in mouse small intestinal crypt within three to six hours after exposure, predominantly in the stem cell region [9].
Radioprotectors are essential in safeguarding the normal tissue during intended radiation exposure. Various radioprotective strategies have been explored including thiol compounds that can scavenge free radicals and modulate DNA repair process, or growth factors and cytokines that function through receptor mediated mechanisms and can modify cellular response to radiation [10-12]. At present, thiol compounds is the most effective class of radioprotectors; however, they have significant shortcomings including relatively high toxicity and unfavorable routes of administration, which negatively affect their application and efficacy [13]. Therefore, there is a need for safer and even more effective radioprotective treatments.
Pyridoxamine (PM) is one of the natural forms of vitamin B6 and an intermediate in transamination reactions catalyzed by vitamin B6-dependent enzymes. PM can also inhibit pathogenic oxidative reactions (reviewed in [14]), and is a prospective pharmacological agent for treatment of chronic conditions involving oxidative and carbonyl stress such as diabetic complications [15-17]. Clinical trials have also demonstrated that PM is safe at the effective pharmacological doses [18, 19]. Investigation of its mechanism of action showed that PM can scavenge RCS and ROS derived from oxidation of sugars and lipids [20-27].
Since ROS and RCS are important mediators of IR-induced damage in biological systems (Fig. 1), we reasoned that PM might be protective against detrimental effects of radiation. We demonstrated here that PM treatment inhibited IR-induced GI endothelial apoptosis in cell culture and in animal model. PM was more effective at protecting from radiation-induced apoptosis compared to Amifostine (Ethyol), a synthetic thiol compound and the only FDA approved radioprotector. PM, therefore, has potential as an effective and safe radioprotective agent.
Materials and Methods
Chemicals
Bovine serum albumin, pyridoxamine hydrochloride, xanthine, and glycolaldehyde were purchased from Sigma-Aldrich. Xanthine oxidase was from Roche; and Amifostine was from Medimmune Oncology Inc., Gaithersburg, MD.
Generation and determination of hydroxyl radical
Hydroxyl radical was generated using xanthine:xanthine oxidase:Fe3+ system [1]. Quantification of hydroxyl radical was performed using salicylate hydroxylation method [28]. Briefly, xanthine oxidase (16 mU) was added to 150 mM potassium phosphate buffer, pH 7 supplemented with 0.1 mM FeCl3, 0.1 mM EDTA, 0.2 mM hypoxanthine, and 0.2 mM sodium salicylate. After a 90-min incubation at room temperature reaction was stopped by addition of concentrated HCl and NaCl extracted by cold diethyl ether. Organic phase was evaporated and the residue dissolved in 250 μL of water. After the addition of 125 μL TCA, 250 μL 10% sodium tungstate, 250 μL 0.5% sodium nitrite, and 0.5 M potassium hydroxide, the formation of hydroxylated product, 2,3-dihyroxybenzoate, was determined by measuring absorbance at 510 nm. Calibration curve was generated using 2,3-dihydroxybenzoate standard solution.
Carbonyl protein modification and determination of protein-bound carboxymethyllysine
Glycolaldehyde (6.7 mM) and BSA (7.5 mg/ml, 6.7 mM amino groups) were incubated alone or with different concentrations of pyridoxamine for 10 days. The incubations were carried out at 37°C in 150 mM sodium phosphate buffer, pH 7.5 containing 0.02% sodium azide. CML-modified BSA was measured by ELISA as previously described [20].
Cell cultures and treatment
Rat small intestine epithelium cells IEC-6 were obtained from ATCC (CRL-1592) and maintained in DMEM with 1.5 g/L sodium bicarbonate 10% FBS and 1% penicillin/streptomycin (Life Technologies, Gaithersburg, MD). Cells were grown in a humidified 5% CO2 incubator at 37°C. For irradiation of cells, Therapax DXT 300 X-ray machine (Pantak) delivering 2.04 Gy/min at 80 kVP was used. For radioprotective studies, cells were treated with 1 mM PM 1 h prior to IR or 1 mM Amifostine for 30 min prior to IR.
Clonogenic survival
Colony-forming assay was performed as previously described [29]. Briefly, calculated numbers of cells were plated to enable normalization for plating efficiencies. Cells were allowed to attach for 5 h, treated with PBS, PM or Amifostine and then irradiated with 0, 2, 4, 6 or 8 Gy. After 7-10-day incubation plates were fixed with 70% EtOH and stained with 1% methylene blue. Colonies consisting of >50 cells were counted under microscope. The survival fractions were calculated as (number of colonies / number of cells plated) / (number of colonies for corresponding control / number of cells plated).
Apoptosis assays for cultured cells
Apoptosis was determined by 4',6-diamidino-2-phenylindole (DAPI) staining. Cells were grown on slides. After treatment, cells were washed with PBS, fixed in 4% paraformaldehyde at room temperature for 10 min, and stained with 5 μg/ml DAPI at room temperature for 10 min. The nuclear morphology was observed using Olympus BX60 fluorescent microscope equipped with Retiga 2000R digital camera. Apoptosis was quantified by scoring the percentage of cells with apoptotic nuclear morphology at the single cell level. Condensed or fragmented nuclei were scored as apoptotic; average percentage of apoptotic cells (+/- SEM) was calculated in 5-7 randomly selected high power field (HPF).
Alternatively, cell death was determined by Annexin V-APC and propidium iodide staining using Apoptosis Detection Kit (BD PharMingen, San Diego, CA). Briefly, aliquots of 105 treated cells were incubated with Annexin V-APC and propidium iodide for 15 minutes at room temperature. Cells were then analyzed by flow cytometry, using a two-color FACS analysis (BD LSR II). For each treatment, the average fold-increase of apoptotic cells over control (+/- SEM) was calculated.
Western blot analysis
Western blot analysis was performed using antibodies for the detection of phospho-GSK-3βSer-9 and Tyr-216, GSK-3β, phospho-AktSer-473, Akt, Bax (B-9), Bcl-2 (N-19) (Santa Cruz Biotechnologies, SantaCruz, CA) and actin (Sigma, St. Louis, MO) as described previously [29].
Mice and treatment
All animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Mice were housed up to five per cage on a 12-h light/dark cycle (lights on at 0600 h). Food (Purina Rodent Chow) and water were provided ad libitum. C57/BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). For experiments with Amifostine, 10-12 week-old mice were treated intraperitoneally (IP) with PBS or 400.0 mg/kg Amifostine in PBS 30 min before radiation. For experiments with PM, mice were given 150 mg/kg/day in drinking water for 24 h prior to radiation. Whole-body mouse irradiations were carried out using a Therapax DXT 300 X-ray machine (Pantak) delivering 2.04 Gy/min at 80 kVP. Mice were irradiated in a holder designed to immobilize unanaesthetized mice such that the abdomens were presented to the beam.
Histochemistry
Mice were sacrificed at indicated times after irradiation by cervical dislocation under isoflurane anesthesia. The jejunum was fixed intact in 10% formalin fixative for 24 h prior to storage in 70% ethanol. The ileal region of the small intestine was cut into 5 segments, embedded vertically and sectioned to provide 5 transverse sections of the ileum. Five μm sections were then taken and placed on Superfrost Gold Plus slides (Erie Scientific, Portsmouth, NH). Tissue sections were stained with the DeadEnd™ Colorimetric TUNEL System (Promega, Madison, WI) and counterstained with hematoxylin and eosin in the standard fashion. For the TUNEL experiments, at least three animals were used in each experimental group. TUNEL positive cells (TPC) were counted under a light microscope (400x). At least three high power fields per animal were scored. The average number of TUNEL positive cells per HPF (+/- SEM) was calculated.
Statistical analyses
The mean and standard error of the mean (SEM) of each treatment group were calculated for all experiments. The number of samples is indicated in the description of each experiment. Statistical analysis was performed using Kruskal-Wallis One Way Analysis of Variance (ANOVA). All pairwise comparison procedures including calculation of P value were done using Student-Newman-Keuls method. A P value of <0.05 was considered significant.
Results
Pyridoxamine inhibits both accumulation of hydroxyl radical and carbonyl-induced protein oxidation
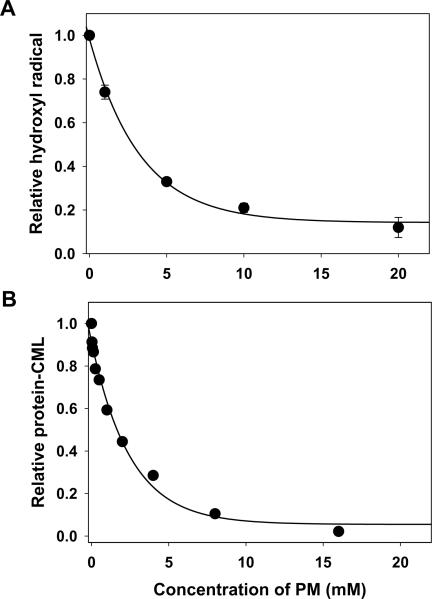
Significant amount of hydroxyl radical was generated in xanthine/xanthine oxidase system (Fig. 2A). PM inhibited detectable hydroxyl radical in concentration dependent manner (Fig. 2A), consistent with earlier report of PM inhibitory effect towards hydroxyl radical in Fenton reaction [14]. In a similar concentration dependent fashion, PM inhibited oxidative carboxylation of albumin lysine residues induced by reactive carbonyl glycolaldehyde (Fig. 2B). This data reflects PM ability to readily form covalent adducts with different reactive carbonyl species, thus protecting protein side chains from carbonyl-derived modifications [20, 21, 23, 27].
Figure 2.
Scavenging of hydroxyl radical and inhibition of protein carbonyl damage by pyridoxamine. (A) Hydroxyl radical generated by xanthine/xanthine oxidase system was determined in the absence of PM or in the presence of the indicated concentrations of PM as described under Materials and Methods. (B) Effect of PM on glycolaldehyde-induced formation of CML-BSA was determined using ELISA as described under Materials and Methods.
Pyridoxamine increases clonogenic survival of irradiated IEC-6 cells
To study radioprotective effects of PM or Amifostine in cell culture, we examined viability of irradiated IEC-6 small intestine epithelium cells using clonogenic survival assay. Pretreatment of IEC-6 cells with 0.1-2 mM PM for 1 h before irradiation significantly increased cell survival as compared to cells treated with radiation alone at all examined doses (up to 8 Gy) with a maximum protection achieved at 1 mM PM (Fig. 3A). This PM effect was comparable with radioprotection by Amifostine at doses from 4 to 8 Gy, however, at a dose of 2 Gy, PM demonstrated significantly better protection of cell survival compared to that by Amifostine (Fig. 3B).
Figure 3.
Pyridoxamine increases clonogenic survival of irradiated IEC-6 cells. (A) Concentration-dependent effect of PM on survival of irradiated IEC-6 cells. (B) Comparative effect of 1 mM PM and 1 mM Amifostine on survival of irradiated IEC-6 cells. IEC-6 cells were treated with PBS (◯), 0.1 mM PM (■), 0.5 mM PM (▲), 1 mM PM (●) 2 mM PM (▼) 1 h prior to IR or 1 mM Amifostine (◆) 30 min prior to IR. The treated cells were irradiated with 0, 2, 4, 6 and 8 Gy and plated for clonogenic survival assay. Shown are the surviving fractions and SEM from three experiments; *, P<0.05, denotes differences from the PBS group (excluding Amifostine pretreatment at 2 Gy).
Pyridoxamine attenuates apoptosis in irradiated IEC-6 cells
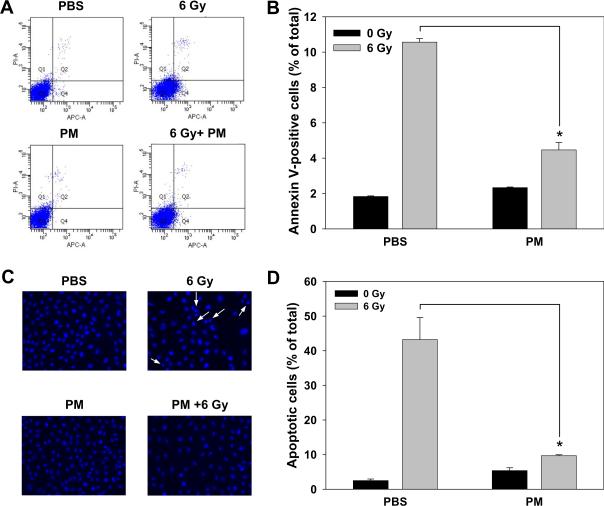
To elucidate the mechanism of PM-induced increase in survival of irradiated IEC-6 cells, we analyzed treated cells by using Annexin V-APC and propidium iodide staining in flow cytometry assay. IEC-6 cells showed 3-fold decrease in radiation-induced apoptosis when pretreated with PM as compared to cells pretreated with vehicle PBS (Fig. 4A and B, 3.2% vs. 9.0%, respectively). We further evaluated the nuclear morphology of dying cells using DAPI staining (Fig. 4C). Irradiated IEC-6 cells pretreated with PM demonstrated a protective effect with reduced number of apoptotic cells to 9.7% as compared to 43.2 % in PBS-pretreated irradiated cells (Fig. 4D). These data suggest that radioprotective effect of PM in small intestine epithelial cells is due to attenuation of radiation-induced apoptosis.
Figure 4.
Pyridoxamine attenuates radiation-induced apoptosis in IEC-6 cells. IEC-6 cells were treated with PBS or 1 mM PM 1 h prior to irradiation with 6 Gy. (A, B) Cells were collected 24 h after irradiation, stained with Annexin V-APC and propidium iodide and analyzed by flow cytometry. Shown are representative diagrams of distribution of stained cells (A) and a bar graph of apoptotic cells expressed as a percent of total cells for each treatment with SEM from three experiments (B). (C, D) Cells were stained with DAPI 24 h after irradiation and apoptotic cells indicated by arrows were counted in multiple randomly selected fields. Shown are representative micrographs (C) and a bar graph of apoptotic cells expressed as a percent of total cells for each treatment with SEM from three experiments; *, P<0.05 (D).
Pyridoxamine does not induce anti-apoptotic activation of Bcl-2/Bax and Akt/GSK-3β in IEC-6 cells
We explored a possibility that PM treatment alone may induce cellular anti-apoptotic signaling, thus, making cells more resistant to radiation-induced apoptosis. To this end, we measured the expression of Bax/Bcl-2 and activation of Akt and its down-stream target GSK-3β in rat small intestine epithelium cells IEC-6 after 6 h of treatment with 1 mM PM. Western blot analysis of cell lysates demonstrated that PM treatment did not affect expression and/or activation of these proteins (data not shown). These results are consistent with the notion that PM protection is due to scavenging of radiation-induced cytotoxic ROS and RCS rather than induction of anti-apoptotic cellular defenses.
Pyridoxamine protects mouse small intestine epithelium in vivo from radiation-induced apoptosis
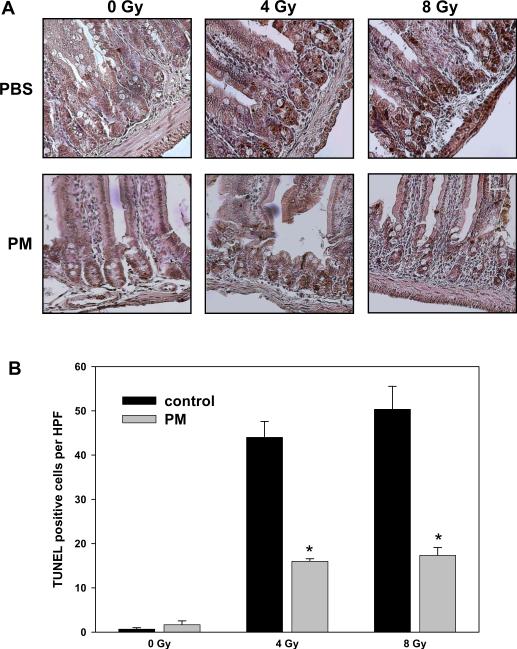
To determine whether PM could be used to protect from radiation-induced apoptosis in the mouse intestine, 10-week old mice were given 150 mg/kg/day in drinking water for 24 h prior to radiation. This dose is within the range of safe and effective PM doses of 100-400 mg/kg/day that have been used previously in diabetic mouse models [15, 30, 31]. Then mice were treated with a single dose of 4 or 8 Gy of whole body irradiation. Twelve hours later, the proximal jejunum was analyzed using a TUNEL staining. In small intestinal epithelium of irradiated mice, we observed dramatic (~ 45-fold) increase of TUNEL-positive cells (TPC) at the stem cell region at both radiation doses (Fig. 5, 44 and 55 TPC at 4 and 8 Gy vs. 1 TPC in sham-irradiated control). PM pretreatment significantly inhibited radiation-induced apoptosis at both radiation doses (Fig. 5, 16 and 17 TPC; 4 and 8 Gy, respectively).
Figure 5.
Pyridoxamine protects mouse small intestine epithelium from radiation-induced apoptosis. Ten-week old mice were given PM (150 mg/kg/day in drinking water) for 24 h prior to whole body irradiation with either 4 or 8 Gy. Twelve hours later, proximal jejunum was fixed, sectioned and stained using TUNEL kit. Shown are representative micrographs of TUNEL staining (A, cells with dark brown nuclei) and a bar graph of the average numbers of TUNEL-positive cells per HPF in each treatment group with SEM from three experiments; *, P < 0.05 (B).
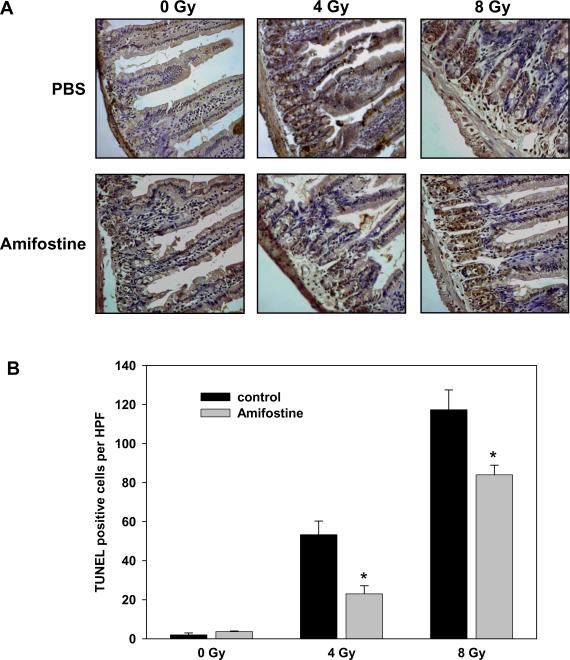
Amifostine is less effective compared to PM in protecting from radiation-induced small intestine epithelium apoptosis
In a separate set of experiments, mice were injected IP with PBS or Amifostine (400 mg/kg) 30 min prior to whole body irradiation with either 4 or 8 Gy. Twelve hours later, proximal jejunum was fixed, sectioned and stained using TUNEL kit. At a sublethal 8 Gy radiation dose, we consistently observed a higher degree of apoptosis in irradiated controls from Amifostine experiment compared to corresponding controls from PM experiment (Figs. 6 and 5, respectively). This effect appears to be due to the use of a more stressful administration method in Amifostine experiment, an IP injection. It is consistent with the phenomenon of combined injury which is not observed at low radiation doses but become evident at higher doses (> 5 Gy) [32]. However, this detrimental consequence of the administration method has affected equally all the animals in the Amifostine experiment and, thus, has been accounted for by our experimental design. Amifostine protected mouse small intestine epithelium from IR-induced apoptosis (Fig. 6). At the radiation dose of 4 Gy, the degree of Amifostine protection was similar to that of PM (Figs. 5B and 6B). However, at a dose of 8 Gy, Amifostine was less effective than PM showing a significantly lower degree of protection (Figs. 5B and 6B, 3.2-fold protection vs. 1.4-fold protection at 8 Gy for PM and Amifostine, respectively).
Figure 6.
Protective effect of Amifostine in mouse small intestine epithelium from radiation-induced apoptosis is less pronounced as compared to pyridoxamine. Ten-week old mice were injected IP with PBS or Amifostine (400 mg/kg) 30 min prior to whole body irradiation with either 4 or 8 Gy. Twelve hours later, proximal jejunum was fixed, sectioned and stained using TUNEL kit. Shown are representative micrographs of TUNEL staining (A, cells with dark brown nuclei) and a bar graph of the average numbers of TUNEL-positive cells per HPF in each treatment group with SEM from three experiments; *, P < 0.05 (B).
Discussion
Radioprotective treatments that have been proposed over the past several decades include thiol compounds that can scavenge free radicals; growth factors and cytokines that function through receptor mediated mechanisms to modify cellular response to radiation; and natural antioxidants and extracts [10-12, 33]. Thiol compounds are the most effective and the longest studied radioprotectors. Synthetic thiol Amifostine (Ethyol) is the only FDA approved radioprotective treatment available today [34]. However, it has significant shortcomings including relatively high toxicity, unfavorable routes of administration, and narrow protection time window [13, 33]. Because of its adverse side effects in humans, effectiveness of Amifostine in clinical trials is significantly diminished compared to that in animal studies [35]. Another class of radioprotectors, cytokines and immunomodulators, can only be effective if significant fraction of target cell population survives after the radiation exposure, and, thus, should be used with low radiation doses and/or in combination with radical scavengers and antioxidants [36]. These radioprotectors have common adverse side effects related to their proinflammatory activity and immunogenicity [33]. Natural antioxidants, such as vitamin E, melatonin, flavonoids and others, have fewer toxic side effects but also a lower degree of protection compared to thiol agents [33, 35]. One approach to address these shortcomings has been the use of combinations of natural and synthetic compounds to increase effectiveness while lowering toxic side effects [33, 35]. However, the availability of effective and safe therapeutic strategies to ameliorate radiation-induced damage is still lacking [33, 35].
Using cell culture and animal model experimental systems, we found that PM is a potent inhibitor of radiation-induced apoptosis in small intestine epithelial cells, which is a major component in radiation damage in the GI tract (Figs. 3-5). Importantly, PM inhibited only IR-induced apoptosis in mouse intestinal crypts and did not affect the spontaneous apoptosis in the upper villi (Fig. 5), which occurs during normal epithelial cell renewal in the GI tract [4, 8, 9].
The mechanism of protection is most likely based on the ability of PM to effectively inhibit oxidative reactions and scavenge major mediators of ionizing radiation toxicity - hydroxyl radical and reactive carbonyl species (Fig. 7). Previous mechanistic studies have demonstrated that 4-aminomethyl and/or 3-hydroxyl substituents of PM pyridinium ring are required for this activity [20, 21, 25, 27, 37, 38]. It appears that PM treatment does not induce cellular anti-apoptotic signaling since it did not affect expression of Bax/Bcl-2 and activation of Akt and its down-stream target GSK-3β in rat small intestine epithelium cells. These proteins are the major players in regulation of cell survival and apoptosis, and we have previously demonstrated that lithium exerts its radioprotective effects by inducing pro-survival signaling regulated by these proteins [29]. These results suggest different mechanisms of protection by PM and lithium and predict potential synergistic effect of lithium/PM combination therapy.
Figure 7.
Hypothetical mechanism of radioprotection of gastrointestinal epithelium by pyridoxamine (PM). PM scavenges or inhibits formation of ionizing radiation-induced reactive oxygen species (ROS) and/or reactive carbonyl species (RCS) thus inhibiting apoptotic cell death resulted from radiation.
In our study, PM provided better protection of epithelial cell clonogenicity at a lower radiation dose and was significantly more effective at protecting mouse GI epithelium from IR-induced apoptosis at a higher dose compared to synthetic thiol radioprotector Amifostine (Figs. 3, 5, 6). Amifostine is known to exhibit various toxicities, has to be administered either intraperitoneally or subcutaneously, and has a very narrow therapeutic time frame window (~30 min prior to irradiation) [33]. In contrast, PM is well tolerated with no significant treatment-related adverse effects [18, 19]. With an oral route of administration, PM could be easily self-administered repeatedly to maintain therapeutic PM levels during prolonged IR exposures. This circumvents another significant shortcoming of the existing radioprotective treatments, i.e. their narrow protective time window. Thus, PM has potential as a new and effective radioprotector with lower toxicity and a more favorable administration route compared to existing thiol compounds.
Ionizing radiation is a well known health hazard that affects specific population groups, for example, professionals that are subjects to occupational radiation exposure. Usually, this exposure is low (~0.3 mSv per worker per year in the US [39]) and is only slightly higher than the exposure due to background radiation. However, in these professional occupations there is a risk of exposure to higher doses of IR. Such excessive exposure can occur due to the accidents at nuclear power plants [7, 40, 41], exposure to contaminated waste [42], consequences of nuclear “dirty bombing” [42, 43] or due to industrial accidents during mining, milling and processing of radioactive materials [44]. In these situations, the risk of exposure to high doses of radiation increases significantly not only for radiation workers but also for personnel participating in emergency response.
The GI tract injury is a major cause of IR-induced severe health impairments or death. At the doses above 1 Gy, humans are at significant risk of developing acute and chronic symptoms related to intestinal epithelial damage such as denudation of the GI mucosa generally referred to as intestinal mucositis [4-6, 45]. Considerable morbidity is associated with mucositis, throughout the entire GI tract including pain, ulceration, vomiting and diarrhea, as well as bile salt malabsorption [46] and/or carbohydrate malabsorption [47]. Ultimately, radiation damage to GI system results in a progressively reduced survival with mean survival time of 3.5 days for radiation dose of 5 Gy [48]. Our results indicate that PM may be a new safe and effective radioprotective strategy to ameliorate these GI tract injuries as well as other health impairments induced by occupational or medicinal exposure to IR.
Acknowledgements
This work has been supported by the grants DK66415 (PV), DK65138 (BH), and CA89674 (DH) from National Institutes of Health and by the Research Grants from the Pardee Foundation (EY) and Whitmer Family Foundation (DH).
Abbreviations
- BSA
bovine serum albumin
- GI
gastrointestinal
- GLA
glycolaldehyde
- IR
ionizing radiation
- PBS
phosphate buffer saline
- PM
pyridoxamine
- ROS
reactive oxygen species
- RCA
reactive carbonyl species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. oxford University Press; London: 1999. [Google Scholar]
- [2].Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- [3].Fan X. Ionizing radiation induces formation of malondialdehyde, formaldehyde, and acetaldehyde from carbohydrates and organic acid. J Agric Food Chem. 2003;51:5946–5949. doi: 10.1021/jf0344340. [DOI] [PubMed] [Google Scholar]
- [4].Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58:925–973. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- [5].Withers HR. Regeneration of intestinal mucosa after irradiation. Cancer. 1971;28:75–81. doi: 10.1002/1097-0142(197107)28:1<75::aid-cncr2820280115>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- [6].Bowen JM, Gibson RJ, Cummins AG, Keefe DM. Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support Care Cancer. 2006;14:713–731. doi: 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- [7].Goldman M. The Russian radiation legacy: its integrated impact and lessons. Environmental health perspectives. 1997;105(Suppl 6):1385–1391. doi: 10.1289/ehp.97105s61385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. Journal of gastroenterology and hepatology. 2000;15:109–120. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- [9].Potten CS. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977;269:518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- [10].Vijayalaxmi, Reiter RJ, Tan DX, Herman TS, Thomas CR., Jr. Melatonin as a radioprotective agent: a review. International journal of radiation oncology, biology, physics. 2004;59:639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [11].Petrovecki M, Prager A, Terry NH, Murray D. Relationships between DNA damage and the survival of murine bone marrow cells irradiated in situ. Radiat Res. 1994;138:443–450. [PubMed] [Google Scholar]
- [12].Weiss JF, Landauer MR. Radioprotection by antioxidants. Ann N Y Acad Sci. 2000;899:44–60. [PubMed] [Google Scholar]
- [13].Maisin JR. Bacq and Alexander Award lecture--chemical radioprotection: past, present, and future prospects. Int J Radiat Biol. 1998;73:443–450. doi: 10.1080/095530098142284. [DOI] [PubMed] [Google Scholar]
- [14].Voziyan PA, Hudson BG. Pyridoxamine as a multifunctional pharmaceutical: targeting pathogenic glycation and oxidative damage. Cell Mol Life Sci. 2005;62:1671–1681. doi: 10.1007/s00018-005-5082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng F, Zeng YJ, Plati AR, Elliot SJ, Berho M, Potier M, Striker LJ, Striker GE. Combined AGE inhibition and ACEi decreases the progression of established diabetic nephropathy in B6 db/db mice. Kidney Int. 2006;70:507–514. doi: 10.1038/sj.ki.5001578. [DOI] [PubMed] [Google Scholar]
- [16].Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, Baynes JW. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002;61:939–950. doi: 10.1046/j.1523-1755.2002.00207.x. [DOI] [PubMed] [Google Scholar]
- [17].Stitt A, Gardiner TA, Alderson NL, Canning P, Frizzell N, Duffy N, Boyle C, Januszewski AS, Chachich M, Baynes JW, Thorpe SR. The AGE inhibitor pyridoxamine inhibits development of retinopathy in experimental diabetes. Diabetes. 2002;51:2826–2832. doi: 10.2337/diabetes.51.9.2826. [DOI] [PubMed] [Google Scholar]
- [18].Williams ME, Bolton WK, Khalifah RG, Degenhardt TP, Schotzinger RJ, McGill JB. Effects of Pyridoxamine in Combined Phase 2 Studies of Patients with Type 1 and Type 2 Diabetes and Overt Nephropathy. Am J Nephrol. 2007;27:605–614. doi: 10.1159/000108104. [DOI] [PubMed] [Google Scholar]
- [19].Williams ME. New potential agents in treating diabetic kidney disease: the fourth act. Drugs. 2006;66:2287–2298. doi: 10.2165/00003495-200666180-00002. [DOI] [PubMed] [Google Scholar]
- [20].Voziyan PA, Metz TO, Baynes JW, Hudson BG. A post-Amadori inhibitor pyridoxamine also inhibits chemical modification of proteins by scavenging carbonyl intermediates of carbohydrate and lipid degradation. J Biol Chem. 2002;277:3397–3403. doi: 10.1074/jbc.M109935200. [DOI] [PubMed] [Google Scholar]
- [21].Nagaraj RH, Sarkar P, Mally A, Biemel KM, Lederer MO, Padayatti PS. Effect of pyridoxamine on chemical modification of proteins by carbonyls in diabetic rats: characterization of a major product from the reaction of pyridoxamine and methylglyoxal. Arch Biochem Biophys. 2002;402:110–119. doi: 10.1016/S0003-9861(02)00067-X. [DOI] [PubMed] [Google Scholar]
- [22].Amarnath V, Amarnath K, Davies S, Roberts LJ. 2nd Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol. 2004;17:410–415. doi: 10.1021/tx0300535. [DOI] [PubMed] [Google Scholar]
- [23].Chetyrkin SV, Zhang W, Hudson BG, Serianni AS, Voziyan PA. Pyridoxamine protects proteins from functional damage by 3-deoxyglucosone: mechanism of action of pyridoxamine. Biochemistry. 2008;47:997–1006. doi: 10.1021/bi701190s. [DOI] [PubMed] [Google Scholar]
- [24].Onorato JM, Jenkins AJ, Thorpe SR, Baynes JW. Pyridoxamine, an inhibitor of advanced glycation reactions, also inhibits advanced lipoxidation reactions. Mechanism of action of pyridoxamine. J Biol Chem. 2000;275:21177–21184. doi: 10.1074/jbc.M003263200. [DOI] [PubMed] [Google Scholar]
- [25].Chetyrkin SV, Mathis ME, Ham AJ, Hachey DL, Hudson BG, Voziyan PA. Propagation of protein glycation damage involves modification of tryptophan residues via reactive oxygen species: inhibition by pyridoxamine. Free Radic Biol Med. 2008;44:1276–1285. doi: 10.1016/j.freeradbiomed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- [26].Jain SK, Lim G. Pyridoxine and pyridoxamine inhibits superoxide radicals and prevents lipid peroxidation, protein glycosylation, and (Na+ + K+)-ATPase activity reduction in high glucose-treated human erythrocytes. Free Radic Biol Med. 2001;30:232–237. doi: 10.1016/s0891-5849(00)00462-7. [DOI] [PubMed] [Google Scholar]
- [27].Metz TO, Alderson NL, Chachich ME, Thorpe SR, Baynes JW. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J Biol Chem. 2003;278:42012–42019. doi: 10.1074/jbc.M304292200. [DOI] [PubMed] [Google Scholar]
- [28].Halliwell B, Gutteridge JMC. Hydroxyl radicals assayed by aromatic hydroxylation and deoxyribose degradation. In: Greenwald RA, editor. Handbook of methods for oxygen radical research. CRC Press, Inc.; Boca Raton: 1986. pp. 177–180. [Google Scholar]
- [29].Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO, Jr., Boone B, Shinohara ET, Hallahan DE. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer research. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- [30].Tanimoto M, Gohda T, Kaneko S, Hagiwara S, Murakoshi M, Aoki T, Yamada K, Ito T, Matsumoto M, Horikoshi S, Tomino Y. Effect of pyridoxamine (K-163), an inhibitor of advanced glycation end products, on type 2 diabetic nephropathy in KK-A(y)/Ta mice. Metabolism. 2007;56:160–167. doi: 10.1016/j.metabol.2006.08.026. [DOI] [PubMed] [Google Scholar]
- [31].Canning P, Glenn JV, Hsu DK, Liu FT, Gardiner TA, Stitt AW. Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes. Exp Diabetes Res. 2007;2007:51837. doi: 10.1155/2007/51837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ran X, Cheng T, Shi C, Xu H, Qu J, Yan G, Su Y, Wang W, Xu R. The effects of total-body irradiation on the survival and skin wound healing of rats with combined radiation-wound injury. J Trauma. 2004;57:1087–1093. doi: 10.1097/01.ta.0000141885.72033.c7. [DOI] [PubMed] [Google Scholar]
- [33].Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007;12:794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- [34].Cassatt DR, Fazenbaker CA, Bachy CM, Hanson MS. Preclinical modeling of improved amifostine (Ethyol) use in radiation therapy. Semin Radiat Oncol. 2002;12:97–102. doi: 10.1053/srao.2002.31382. [DOI] [PubMed] [Google Scholar]
- [35].Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- [36].Herodin F, Drouet M. Cytokine-based treatment of accidentally irradiated victims and new approaches. Exp Hematol. 2005;33:1071–1080. doi: 10.1016/j.exphem.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [37].Voziyan PA, Khalifah RG, Thibaudeau C, Yildiz A, Jacob J, Serianni AS, Hudson BG. Modification of proteins in vitro by physiological levels of glucose: pyridoxamine inhibits conversion of Amadori intermediate to advanced glycation end-products through binding of redox metal ions. J Biol Chem. 2003;278:46616–46624. doi: 10.1074/jbc.M307155200. [DOI] [PubMed] [Google Scholar]
- [38].Davies SS, Brantley EJ, Voziyan PA, Amarnath V, Zagol-Ikapitte I, Boutaud O, Hudson BG, Oates JA, Ii LJ. Pyridoxamine Analogues Scavenge Lipid-Derived gamma-Ketoaldehydes and Protect against H(2)O(2)-Mediated Cytotoxicity. Biochemistry. 2006;45:15756–15767. doi: 10.1021/bi061860g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thompson MA. Maintaining a proper perspective of risk associated with radiation exposure. Journal of nuclear medicine technology. 2001;29:137–142. quiz 148-150. [PubMed] [Google Scholar]
- [40].Saenger EL. Radiation accidents. Annals of emergency medicine. 1986;15:1061–1066. doi: 10.1016/s0196-0644(86)80130-5. [DOI] [PubMed] [Google Scholar]
- [41].Miller KL. The nuclear reactor accident at Three Mile Island. Radiographics. 1994;14:215–224. doi: 10.1148/radiographics.14.1.8128063. [DOI] [PubMed] [Google Scholar]
- [42].Chin FK. Scenario of a dirty bomb in an urban environment and acute management of radiation poisoning and injuries. Singapore Med J. 2007;48:950–957. [PubMed] [Google Scholar]
- [43].Rosoff H, von Winterfeldt D. A risk and economic analysis of dirty bomb attacks on the ports of Los Angeles and Long Beach. Risk Anal. 2007;27:533–546. doi: 10.1111/j.1539-6924.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- [44].Brugge D, deLemos JL, Bui C. The Sequoyah corporation fuels release and the Church Rock spill: unpublicized nuclear releases in American Indian communities. American journal of public health. 2007;97:1595–1600. doi: 10.2105/AJPH.2006.103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Andreyev J. Gastrointestinal symptoms after pelvic radiotherapy: a new understanding to improve management of symptomatic patients. The lancet oncology. 2007;8:1007–1017. doi: 10.1016/S1470-2045(07)70341-8. [DOI] [PubMed] [Google Scholar]
- [46].Arlow FL, Dekovich AA, Priest RJ, Beher WT. Bile acids in radiation-induced diarrhea. South Med J. 1987;80:1259–1261. doi: 10.1097/00007611-198710000-00015. [DOI] [PubMed] [Google Scholar]
- [47].Weiss RG, Stryker JA. 14C-lactose breath tests during pelvic radiotherapy: the effect of the amount of small bowel irradiated. Radiology. 1982;142:507–510. doi: 10.1148/radiology.142.2.7054844. [DOI] [PubMed] [Google Scholar]
- [48].Anno GH, Baum SJ, Withers HR, Young RW. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5-30 Gy. Health physics. 1989;56:821–838. doi: 10.1097/00004032-198906000-00001. [DOI] [PubMed] [Google Scholar]