Abstract
Objective
Cytomegalovirus (CMV) is an important pathogen in healthy neonates and individuals with human immunodeficiency virus (HIV-1). The objective of this study was to determine whether the detection of CMV DNA (CMV DNAemia) in maternal plasma was associated with mortality in HIV-1 infected women or their infants.
Methods
A longitudinal study was designed to examine the relationship between maternal CMV DNAemia and maternal-infant mortality during two years postpartum. Sixty-four HIV-1 infected women and their infants were studied. CMV DNA loads were quantified in plasma from the mothers near the time of delivery. Baseline maternal CD4 counts, CD4%, HIV-1 RNA, and CMV DNAemia were evaluated as covariates of subsequent maternal or infant mortality in univariate and multivariate Cox regression.
Results
CMV DNA was detected in 11/64 (17%) of the HIV-1 infected women. HIV-1 and CMV viral load were strongly correlated in CMV DNAemic women (ρ=0.84, p=0.001). Detection of CMV DNAemia was associated with decreased maternal survival at 24 months postpartum (log-rank p=0.006). Additionally, HIV-1 infected infants born to CMV DNAemic women had a 4-fold increased risk of mortality during 24 months of follow-up. Maternal CMV DNAemia remained a significant risk factor for mortality in HIV-1 infected infants after adjusting for maternal CD4 cells/mm3 (adjusted HR=4.3, CI=1.4–13), CD4% (HR=3.2, CI=1.0–10), HIV-1 viral load (HR=4.1, CI=1.4–12) or maternal death (HR=3.7, CI=1.0–13).
Conclusions
Maternal plasma CMV DNAemia identified a subgroup of Kenyan women and infants at high risk for death in the two years following delivery.
Keywords: cytomegalovirus, vertical transmission, viral load, infant mortality
Introduction
Cytomegalovirus (CMV) is the most frequent viral cause of congenital disease globally, affecting 0.2–3% of live births in higher-income nations, with higher rates reported in sub-Saharan Africa [1–3]. Congenital CMV infection is associated with severe developmental disease such as microcephaly, sensorineural hearing loss and mental retardation [4, 5]. Sequelae are present in ~10–20% of congenitally infected neonates who are born to women undergoing a primary infection, but maternal immunity confers significant protection from infection and disease in infants born to women with chronic CMV infection [6–8]. Postnatally acquired CMV is typically asymptomatic, but may be accompanied by transient mononucleosis and flu-like symptoms [9, 10]. CMV is also major opportunistic pathogen in patients with human immunodeficiency virus (HIV-1) infection. In the absence of highly active antiretroviral therapy (HAART), patients with CD4 counts less than 50–100 cells/mm3 are at risk of CMV retinitis, gastrointestinal and neurological disease [11–14]. Vertical CMV transmission is more frequent in the setting of maternal HIV-1 infection and CMV infection has been associated with increased disease progression and mortality in HIV-1 infected infants [15–17].
Infants may acquire CMV close to the time they acquire HIV-1 infection, as a result of maternal primary CMV infection or recurrent infection [7, 8, 18]. Advancing maternal HIV-1 disease can also have important consequences for vertical HIV-1 transmission and infant survival. Maternal CD4 measurements, HIV-1 RNA viral load, and death have been shown to correlate with subsequent infant disease progression and mortality, though the precise mechanisms governing these relationships are not known [19–23]. Timing of vertical transmission, transfer of maternal antibody, exposure to co-infecting pathogens, and the ability to provide childcare may all be influenced by the mother’s stage of HIV-1 disease. The role of maternal CMV replication and subsequent maternal-infant HIV-1 disease progression is unknown. In this report we examine the impact of maternal HIV-1 replication, immunosuppression and CMV replication on virus transmission and maternal-infant mortality.
Methods
Participants and study design
A longitudinal cohort study was designed to evaluate the relationship between maternal CMV DNA replication near the time of delivery and subsequent maternal and infant HIV-1 disease progression. The study protocol was approved by the Kenyatta National Hospital Ethics Review Committee and the Institutional Review Board of the University of Washington. A subset of 64 women and infants were selected from a previously described perinatal HIV-1 cohort [21, 24, 25]. HIV-1 seropositive pregnant women were recruited before their 28th week of gestation; the women received short-course zidovudine for prevention of HIV-1 transmission. Following delivery women and infants received no further antiretroviral therapy. Serial blood specimens were obtained in pregnancy, at delivery, and months 1, 3, 6, 9, 12, 15, 18, 21, and 24 postpartum. HIV-1 infected women and children were followed until death or exit from the study at two years postpartum. Infants who did not acquire HIV-1 during the study were followed for 1 year postpartum. The infants studied were part of a subset selected for intensive evaluation of immunological and viral factors as correlates of infant HIV-1 disease progression and survival. The selection of subjects from the original study cohort was therefore based upon specimen availability and follow-up of the infants. Inclusion criteria for HIV-1 infected infants and their mothers were: 1) well defined timing of HIV-1 acquisition 2) availability of an infant plasma specimen by 1 month of age, and 3) infant survival to at least 3 months of age. Twenty HIV-1 uninfected infants and their HIV-1 infected mothers were also selected, based upon these criteria. Thirteen HIV-1 uninfected pregnant women were selected from a cohort with similar demographics as negative controls [26].
HIV-1 diagnosis and monitoring of immunologic parameters
HIV-1 RNA viral loads were measured in this cohort as previously described using the Gen-Probe transcription mediated assay [24, 27, 28]. Infant HIV-1 infection was diagnosed by nested PCR amplifying HIV-1 gag proviral DNA from dried blood spotted onto filter paper [29]. CD4 measurements were performed on whole blood using TriTest CD3FITC/CD4PE/CD45PerCP antibodies (BD Biosciences, San Jose, California, USA) and FACScan analysis with CELLQuest Software (BD Biosciences).
Infant HIV-1 infection in utero was defined as the detection of either HIV-1 DNA or RNA within 48 hours of birth, followed by a positive specimen (viral RNA or DNA) at the subsequent clinic visit. The peak HIV-1 viral load was defined as the highest measurement obtained during the first 6 months of infection, and the set-point viral load was defined as the first viral load measured at least 6 weeks after the peak.
CMV diagnosis and quantification
Nucleic acids were extracted from 50–200 μl of plasma using the Qiagen UltraSens virus extraction kit (Qiagen, Valencia, California, USA). CMV DNA loads were measured using a real time PCR to detect the glycoprotein B gene (gB) as previously described [30]. The lower limit of detection was 1 copy/reaction. Negative (no DNA detection) and indeterminate (<1 copy/reaction) PCR assays were not included in calculations of median or peak viral load, and were categorized as negative. CMV DNAemia was defined as the detection of CMV DNA in plasma. Timing of CMV acquisition was estimated as the mid-point between the last negative and first positive measurement. The peak CMV viral load was defined as the highest measurement within the first 6 months of infection.
Statistical analyses
Stata SE v9 (Stata Corp. College Station, Texas, USA) was used for analysis. Viral loads were base 10 log-transformed (log10) before comparisons and inclusion in regression models. T tests were used to compare mean log10 HIV-1 viral load and CD4 values between groups. Fisher exact tests were used to compare proportions between groups. Maternal factors were examined as covariates for maternal and infant survival in univariate and multivariate survival analyses. Cox regression was used to estimate time to death and time to CMV infection, and the log-rank test was used to compare time to death between groups of subjects. All reported p values are for two-tailed tests.
Results
Baseline parameters of HIV-1 infected women
CMV DNAemia was measured in 64 HIV-1 infected women and 13 HIV-1 negative controls. Women were screened near to the time of delivery (57 women screened at delivery, 5 at 32 weeks gestation and 2 at 1 month postpartum) and infants were screened longitudinally during the first two years of life. CMV DNA was detected in 11/64 (17%) of HIV-1 infected women and none of the HIV-1 negative women (0/13, p=0.2). The median CMV DNA load was low in CMV DNAemic women; median 1.8 log10 DNA copies/ml (range=1.6–2.2). Comparisons of the HIV-1 infected women at 32 weeks gestation revealed that neither baseline CD4 counts nor HIV-1 RNA levels differed significantly between CMV DNAemic and non-CMV DNAemic women (mean CD4 count 335 versus 420 cells/mm3 respectively, p=0.2; mean 5.2 vs 4.9 log10 RNA copies/ml, respectively, p=0.2; Table 1). There was a trend for lower CD4% in the CMV DNAemic women (mean 16 vs 21, p=0.08).
Table 1.
Comparison of women based on plasma CMV DNAemia.
| Maternal CMV DNA |
|||
|---|---|---|---|
| Detected | Not detected | P | |
| Number of women | 11 | 53 | |
| Median age at enrolment in years (IQR) | 25 (20–30) | 25 (22–28) | 0.8 |
| Median months of follow-up in study (IQR) | 21 (7–24) | 15 (12–24) | 0.4 |
| Breastfed their infants | 91% (10/11) | 89% (47/53) | 0.7 |
| Immunologic parameters 32 weeks gestationa | |||
| Mean Log10 HIV-1 viral load (± SE) | 5.2 ± 0.30 | 4.9 ± 0.12 | 0.2 |
| Mean CD4 cells/mm3 (± SE) | 335 ± 75 | 420 ± 27 | 0.2 |
| Mean CD4% (± SE) | 16 ± 3.2 | 21 ± 1.1 | 0.08 |
| Maternal mortality at 24 months post partum | 27% (3/11) | 3.8% (2/53) | 0.03 |
| Mean survival time (months post partum) [95%CI] | 21 [17–25] | 24 [23–24] | 0.006 |
SE, standard error of mean; IQR, interquartile range; 95%CI, 95% confidence interval.
At 32 weeks, CD4 measurements were available for 9 CMVDNA+ women and 50 CMVDNA- women, HIV-1 viral loads were available for 9 CMVDNA+ women and 52 CMVDNA- women.
Maternal disease progression and mortality
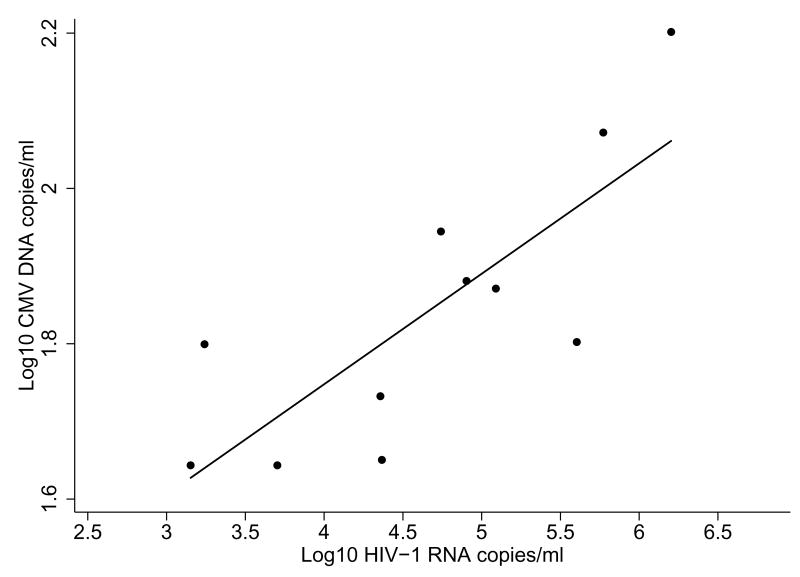
At 24 months, the group of CMV DNAemic women had a higher frequency of deaths than the non-CMV DNAemic women (3/11 vs 2/53, p=0.03), and shorter survival time (21 months vs 24 months, p=0.006). HIV-1 RNA and CMV DNA loads were highly correlated in the 11 HIV-1 infected women who were CMV DNAemic (ρ=0.84, p=0.001; Figure 1). Since CD4 measurements were not performed at delivery (the time at which most CMV viral loads were measured), we were unable to examine concurrent correlation between CD4 measurements and CMV loads.
Figure 1. CMV and HIV-1 loads in co-infected women.
Scatter-plot and linear fit line show data for HIV-1 infected, CMV DNAemic women (ρ=0.84, p=0.001).
Cox regression was used to examine the relationship between CD4 measurements, HIV-1 RNA viral load, CMV DNAemia, and survival in the HIV-1 infected women. To establish a constant sample size, only women with data for all covariates were included in the univariate and multivariate Cox regression (59 women with HIV-1 viral load, CD4 count, CD4% and CMV DNA measured). Univariate predictors of maternal death included CD4 cells/mm3 at 32 weeks gestation (HR=0.99, 95%CI=0.98–1.00, p=0.004), CD4% at 32 weeks gestation (HR=0.78, 95%CI=0.68–0.91, p=0.001), HIV-1 RNA viral load at 32 weeks gestation (HR=7.2, 95%CI=1.5–34, p=0.01) and maternal CMV DNAemia dichotomised as detected/not detected (HR=9.7, 95%CI=1.6–59, p=0.01). After adjusting for maternal CD4%, maternal CMV DNAemia was no longer a significant risk factor for death (adjusted HR=1.9, 95%CI=0.17–21, p=0.6). Results were similar if alternatively adjusting for maternal CD4 cells/mm3, or HIV-1 RNA viral load (data not shown).
Virus transmission
We next examined the association between maternal CMV DNAemia and mother-infant CMV transmission. The frequency of CMV detection at birth was similar between HIV-1 infected infants born to mothers with and without CMV DNAemia (Table 2, 20% vs 10%, respectively, p=0.5), however there was a trend for women who were CMV DNAemic to transmit CMV to their (HIV-1 infected) infants earlier than women who were not CMV DNAemic (mean 1.2 months for CMV DNAemic women, 3.1 months for non-DNAemic women, p=0.1). The cumulative prevalence of infant CMV detection at 12 months was similar between HIV-1 infected infants born to CMV DNAemic and non-DNAemic women (100% vs 92%, p=0.6). There was no difference between prevalence of infant CMV, in utero transmission of CMV, or timing of CMV transmission in the HIV-1 uninfected infants born to women with and without CMV DNAemia.
Table 2.
Virus acquisition and disease progression of infants born to women with and without plasma CMV DNAemia.
| Maternal CMV DNA |
|||
|---|---|---|---|
| Detected | Not detected | P | |
| Number of infants | 11 | 53 | |
| HIV-1 infected | 7 | 37 | |
| HIV-1 uninfected | 4 | 16 | |
| CMV acquisition (HIV-1 infected infants) | |||
| CMV detection at birtha | 20% (1/5) | 10% (3/30) | 0.5 |
| CMV detection by 12 months | 100% (7/7) | 95% (34/37) | 0.6 |
| Mean month of CMV acquisition [95%CI] | 1.2 [0.57–1.7] | 3.1 [1.6–4.7] | 0.1 |
| CMV acquisition (HIV-1 uninfected infants) | |||
| CMV detection at birtha | 0% (0/4) | 8.3% (1/12) | 0.8 |
| CMV detection by 12 months | 100% (4/4) | 94% (15/16) | 0.8 |
| Mean month of CMV acquisition [95%CI] | 1.6 [1.0–2.3] | 2.9 [1.3–4.4] | 0.6 |
| Summary immunologic parameters (HIV-infected infants) | |||
| Mean Peak log10 HIV-1 viral load (± SE) | 6.7 ± 0.30 | 6.9 ± 0.11 | 0.7 |
| Mean Set-point log10 HIV-1 viral load (± SE) | 6.3 ± 0.34 | 6.1 ± 0.15 | 0.7 |
| Mean CD4% at 6 months post partum (± SE)b | 25 ± 3.9 | 25 ± 2.0 | 0.97 |
| Median peak log10 CMV DNA copies/ml | |||
| HIV-1 infected infants (IQR) | 3.0 (2.6–3.5) | 3.1 (2.6–3.5) | 0.7 |
| HIV-1 uninfected infants (IQR) | 3.2 (2.6–3.5) | 2.5 (2.0–3.0) | 0.3 |
| Mortality in HIV-1 infected infants | |||
| Infant mortality at 24 months | 86% (6/7) | 38% (14/37) | 0.03 |
| Mean survival time in months [95%CI] | 10 [5.6–15] | 19 [16–21] | 0.003 |
Restricted to infants tested for CMV DNA at birth.
At 6 months, CD4 measurements were available for 5 infants born to CMV DNA+ women and 20 infants born to CMV DNA− women.
Infant HIV-1 disease progression
HIV-1 infected infants born to HIV-1 infected women with CMV DNAemia were similar to non-DNAemic women in their levels of HIV-1 replication and immunosuppression, as indicated by CD4 measurements and HIV-1 peak and set-point viral load (Table 2). CD4 counts were available for a 25 of the children at 6 months, and there was no significant difference between CD4% in children born to CMV DNAemic women and non-DNAemic women (mean 25% vs 25%, p=0.97). Peak CMV load was also similar in HIV-1 infected infants born to women with CMV DNAemia and without CMV DNAemia (median 3.0 vs 3.1 log10 copies/ml, p=0.7).
Infant mortality
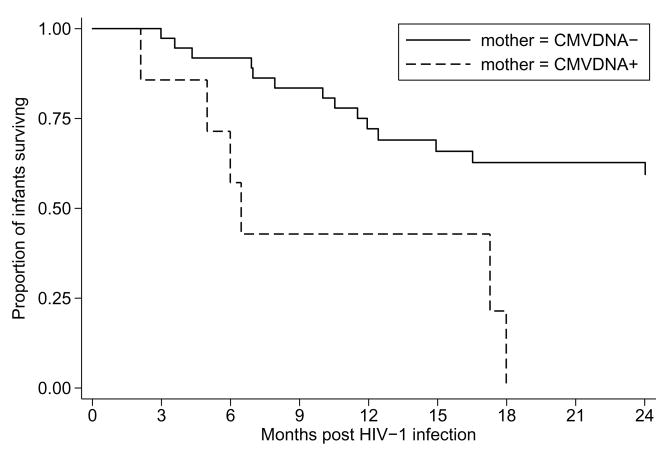
Though we did not detect differences in levels of HIV-1 replication, immunosuppression, or CMV replication between children grouped by maternal CMV DNAemia, the difference in infant mortality was striking. By 24 months post partum 6 of the 7 (86%) HIV-1 infected infants born to CMV DNAemic mothers had died, and 14 of the 37 (38%) HIV-1 infected infants born to the non-CMV DNAemic women had died (p=0.03). Infants born to CMV DNAemic women had a shorter mean survival time (10 months 95%CI=5.6–15) compared to infants born to non-CMV DNAemic women (19 months, 95%CI=16–21, p=0.003, Figure 2).
Figure 2. Maternal CMV DNAemia and 2-year infant survival.
Kaplan-Meier curves showing time to death (log-rank p=0.003) for HIV-1 infected infants grouped by maternal CMV DNAemia. Solid line, infants born to mothers who were not CMV DNAemic; dashed line, infants born to mothers who were CMV DNAemic.
We next used Cox regression to examine the relationship between the detection of maternal CMV DNA and mortality in the HIV-1 infected infants. To establish a constant sample size in the univariate and multivariate analyses, we restricted the analysis to subjects who had measurements available for all covariates (42 HIV-1 infected mother-infant pairs). In univariate Cox regression, maternal factors significantly predicting time to infant death were maternal CD4% at 32 weeks gestation (HR=0.92, p=0.03) and maternal CMV DNAemia (HR=4.4, p=0.006, Table 3). Maternal CD4 cells/mm3 at 32 weeks gestation and maternal HIV-1 viral load at 32 weeks gestation were not significant predictors of mortality in this subset of patients. A trend for association was found between maternal death and infant survival (HR=3.5, p=0.06). Multivariate Cox regression models were subsequently constructed to examine maternal CMV DNAemia as a cofactor for infant mortality while controlling for maternal disease progression and HIV-1 replication (Table 3). Since CD4 measurements, HIV-1 viral load, and death are collinear [21], these were not included together in the same model. The detection of maternal CMV DNA remained a predictor of infant mortality after adjusting for either baseline maternal CD4 count (HR=4.3, p=0.009), maternal CD4% at 32 weeks (HR=3.2, p=0.05), maternal HIV-1 RNA viral load (HR=4.1, p=0.01) or maternal death (HR=3.7, p=0.04).
Table 3.
Univariate and multivariate Cox regression models exploring the relationship between maternal CMV and survival of HIV-1 infected infants.
| Univariatea | HR | [95%CI] | p |
|---|---|---|---|
| Maternal CD4 cells/mm3 | 0.999 | [0.997–1.0] | 0.5 |
| Maternal CD4% | 0.92 | [0.86–0.99] | 0.03 |
| Maternal HIV-1 RNA viral load | 1.6 | [0.73–3.3] | 0.3 |
| Maternal death | 3.5 | [0.96–13] | 0.06 |
| Maternal CMV DNAemia | 4.4 | [1.5–13] | 0.006 |
| Multivariateb | HR | [95%CI] | p |
| Model 1: | |||
| Maternal CD4 cells/mm3 | 1.0 | [1.0-1.0] | 0.9 |
| Mother CMV DNAemic | 4.3 | [1.4–13] | 0.009 |
| Model 2: | |||
| Maternal CD4% | 0.95 | [0.88–1.0] | 0.1 |
| Mother CMV DNAemic | 3.2 | [1.0–10] | 0.05 |
| Model 3: | |||
| Maternal HIV-1 RNA viral load | 1.4 | [0.64–3.0] | 0.4 |
| Mother CMV DNAemic | 4.1 | [1.4–12] | 0.01 |
| Model 4: | |||
| Maternal death | 1.5 | [0.33–7.1] | 0.4 |
| Mother CMV DNAemic | 3.7 | [1.0–13] | 0.04 |
HR, hazard ratio.
Maternal CD4 cells/mm3, CD4% and HIV-1 viral load at 32 weeks gestation, death by 24 months.
Adjusted HR, [95%CI] and p values are shown for four Cox regression models including maternal CMV DNAemia and either maternal CD4 cells/mm3, CD4%, HIV-1 RNA viral load or death.
Discussion
Though CMV is typically asymptomatic when acquired postnatally in immunocompetent infants, CMV can cause disease and more rapid HIV-1 progression in HIV-1 infected infants [17]. At present, the role that maternal CMV replication plays in CMV transmission and outcome in the HIV-1 infected neonate is unknown. Our results demonstrate the important role that systemic CMV replication in the mother may play in mortality of the HIV-1 infected child. We found that women with CMV DNA detected in the plasma were more likely to die within 2 years postpartum, tended to transmit CMV to their infants earlier than non-DNAemic women, and their infants were less likely to survive the first 2 years of life.
We did not find a correlation between maternal CMV DNAemia and congenital CMV transmission. With so few in utero CMV transmission events, we are underpowered to show a difference between groups. However, other groups have reported that in healthy (HIV-1 negative) pregnant women with primary or chronic CMV infection, the presence of CMV DNA in the blood alone does not correlate well with in utero transmission [3, 31]. The detection of CMV from cervical secretions and breastmilk have been shown to correlate better with early CMV transmission than CMV in the blood [3]. CMV DNA appears in the blood during acute infection of healthy immunocompetent adults and decreases steadily to undetectable levels during the 6 month period post-infection in most subjects [31–33] and the detection of CMV in the plasma of healthy individuals with chronic CMV infection is uncommon [3]. Therefore, the appearance of CMV DNA in the peripheral blood suggests either recent primary infection, or that the host’s immune system is sufficiently compromised to enable systemic dissemination of CMV. From the information collected in this study we are unable to determine if the DNAemic women had primary infection, reactivation, or re-infection with a new strain of CMV.
The detection of CMV DNA in the blood of immunosuppressed subjects has previously been shown to predict CMV disease and mortality in transplant recipients [34], HIV-1 infected and uninfected infants [35, 36], and HIV-1 infected adults [37–39]. The strong correlation between levels of HIV-1 RNA and CMV DNA suggest that both HIV-1 and CMV are able to rapidly take advantage of immunosuppression at this level to replicate. Additionally, CMV could potentially act as a direct co-factor to increase HIV-1 replication, as reviewed by Griffiths [40]. A correlation between HIV-1 and CMV levels have also been reported in adult studies of blood [41] and breastmilk [42]; and the presence of HIV-1 shedding also correlates with the detection of CMV in the cervix [43] and semen [44]. In our study, plasma CMV DNAemia was associated with maternal death during the 2-year post partum period. In the multivariate model, the effect of maternal CMV DNAemia lost significance when adjusting for CD4%. This result is consistent with CMV DNAemia being a marker for more advanced HIV-1 disease in the mothers, but not necessarily an independent contributing factor to mortality.
To the best of our knowledge, this is the first report demonstrating a link between maternal CMV replication and infant mortality. HIV-1 infected infants born to HIV-1 infected women with CMV DNAemia were at a 4-fold greater risk of mortality compared to those born to CMV DNA-negative women. In multivariate regression adjusting for maternal immunosuppression or HIV-1 viral load, maternal CMV DNAemia remained the strongest predictor of infant mortality, with a hazard ratio varying from to 3.2–4.0 depending on the model. The relationship between maternal CMV DNAemia and infant mortality did not seem to be related to infant HIV-1 viral load or HIV-1 set-point, which were both comparable between infants born to women with or without CMV DNAemia.
One explanation for the association we have observed between maternal CMV DNAemia and infant mortality is earlier transmission of CMV from the CMV DNAemic women. We have some evidence to support this hypothesis; we were underpowered to precisely examine the timing of infant CMV acquisition, but we observed a trend for earlier CMV transmission from women who were DNAemic. Several mechanisms may explain the proposed relationship. First, cellular activation initiated by acute CMV infection may influence HIV-1 replication and dissemination by increasing the pool of CCR5-expressing targets for HIV-1 infection [45], thereby accelerating CD4 depletion. As both CMV and HIV-1 are transmitted via breastmilk [46], CD4 depletion in the neonatal gut could conceivably be accelerated by the synchronous introduction of both viruses to this site of infection [47]. Additionally, if the neonatal gut is compromised by HIV-1 infection, CMV replication may occur more readily, and potentially lead to the development of CMV gastrointestinal disease.
Our study has several strengths and some important limitations. Strengths include the prospective assessment of maternal factors and the collection of longitudinal data to assess the relative influence of maternal immunosuppression, HIV-1 viral load, mortality, and CMV DNAemia on infant mortality. An important limitation in the study was the use of plasma specimens for the measurement of CMV DNAemia. The use of whole blood or cell specimens would have increased our sensitivity to detect virus, but these specimens were not available. We are thus likely to have both underestimated levels of CMV replication in the blood, and to have underestimated the true frequency of individuals with active CMV replication. Secondly, we were not able to screen specimens from delivery for all of the infants; because of this we may have underestimated the true frequency of in utero CMV acquisition. Thirdly, because the original cohort was not enrolled to study CMV, we do not have data regarding infant CMV-related morbidities. Finally, our exclusion of mother-infant pairs with less than 3 months of follow-up prohibits extrapolation of our results to infants who died in the first 3 months of life. Our selection criteria were designed to ensure adequate sampling and follow up for HIV-1 diagnosis, CMV diagnosis, and survival analysis, and these criteria may have led to selection bias. We found that maternal CMV strongly predicted mortality among infants who survived beyond 3 months. While it is likely that the same phenomenon occurs earlier in life, our selection criteria do not allow extrapolation to very early infant mortality.
Our study has important clinical implications. CMV DNAemia during pregnancy identified a subgroup of women and infants with a high risk of death in the two years following delivery. The value of CMV screening, prevention and therapy of pregnant HIV-1 infected women needs further study in regions where HIV-1/CMV co-infection is highly prevalent. Additionally, increased access to antiretrovirals will likely have indirect effects on the epidemiology of CMV in these regions, and the role of CMV in neonatal HIV-1 disease will need to be re-evaluated among women and infants receiving antiretroviral therapy.
Acknowledgments
Funding source
Supported by the US National Institutes of Child Health and Disease (NICHD) through grant #RO1 HD-23412 and MRC grant to the Human Immunology Unit of the Weatherall Institute of Molecular Medicine. JS, BL-P, EO and CF were scholars in the AIDS International Training and Research Program, NIH Research Grant D43 TW000007, funded by the Fogarty International Center and the Office of Research on Women’s Health. DM-N is an Elizabeth Glaser Pediatric AIDS Foundation International Leadership awardee. GJ-S and SR-J have each received the Pediatric AIDS Foundation Elizabeth Glaser Scientist Award. VCE is funded by a grant from the MRC Centre for Clinical Virology. The funding sources were not involved in the analyses or interpretation of data.
The authors would like to thank the CTL Study clinical, laboratory and data teams; Julie Overbaugh who provided facilities, reagents and staff for the HIV-1 viral load and infant diagnostic assays, and Sandy Emery, who performed the HIV-1 Gen-Probe assays and assisted with laboratory work pertaining to the CMV studies; Dana DeVange Panteleef for the diagnostic filter paper PCRs; and Ken Tapia for assistance with the data analysis.
Acknowledgements and author participation: The study was conceived and designed by Jennifer Slyker, Barbara Lohman-Payne, Grace C. John-Stewart, Sarah L. Rowland-Jones, and Vincent C. Emery. Vincent C. Emery, Jennifer Slyker, Barbrara Lohman Payne, Carey Farquhar and Sarah Rowland-Jones were responsible for the methodology and data collection. The study cohort from which these data are derived was funded, recruited and followed by Grace John-Stewart, Dorothy Mbori-Ngacha, Pelgona Otieno, Carey Farqhuar and Elizabeth Obimbo. Jennifer Slyker, Barbra Richardson, and Grace John-Stewart analysed the data. The bulk of the manuscript was written by Jennifer Slyker, and all co-authors were involved in manuscript revisions.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Conference presentation
Some of the data contained in this manuscript were presented orally at the Dominique Dormont International Conference: Maternal chronic viral infections transmitted to the infants, December 2007, Paris, France
References
- 1.Stagno S, Reynolds DW, Huang ES, Thames SD, Smith RJ, Alford CA. Congenital cytomegalovirus infection. N Engl J Med. 1977;296:1254–1258. doi: 10.1056/NEJM197706022962203. [DOI] [PubMed] [Google Scholar]
- 2.Bello C, Whittle H. Cytomegalovirus infection in Gambian mothers and their babies. J Clin Pathol. 1991;44:366–369. doi: 10.1136/jcp.44.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaye S, Miles D, Antoine P, Burny W, Ojuola B, Kaye P, et al. Virological and immunological correlates of mother-to-child transmission of cytomegalovirus in The Gambia. J Infect Dis. 2008;197:1307–1314. doi: 10.1086/586715. [DOI] [PubMed] [Google Scholar]
- 4.Pass RF, Stagno S, Myers GJ, Alford CA. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980;66:758–762. [PubMed] [Google Scholar]
- 5.Boppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. Pediatr Infect Dis J. 1992;11:93–99. doi: 10.1097/00006454-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Stagno S, Whitley RJ. Herpesvirus infections of pregnancy. Part I: Cytomegalovirus and Epstein-Barr virus infections. N Engl J Med. 1985;313:1270–1274. doi: 10.1056/NEJM198511143132006. [DOI] [PubMed] [Google Scholar]
- 7.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–667. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 8.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. Jama. 2003;289:1008–1011. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 9.Klemola E, Kaariainen L. Cytomegalovirus as a possible cause of a disease resembling infectious mononucleosis. Br Med J. 1965;2:1099–1102. doi: 10.1136/bmj.2.5470.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan MC, Rousseau W, Stewart JA, Noble GR, Chin TD. Spontaneous cytomegalovirus mononucleosis. Clinical and laboratory observations in nine cases. Ann Intern Med. 1973;79:153–160. doi: 10.7326/0003-4819-79-2-153. [DOI] [PubMed] [Google Scholar]
- 11.Drew WL. Nonpulmonary manifestations of cytomegalovirus infection in immunocompromised patients. Clin Microbiol Rev. 1992;5:204–210. doi: 10.1128/cmr.5.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerard L, Leport C, Flandre P, Houhou N, Salmon-Ceron D, Pepin JM, et al. Cytomegalovirus (CMV) viremia and the CD4+ lymphocyte count as predictors of CMV disease in patients infected with human immunodeficiency virus. Clin Infect Dis. 1997;24:836–840. doi: 10.1093/clinids/24.5.836. [DOI] [PubMed] [Google Scholar]
- 13.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. J Infect Dis. 1992;166:1223–1227. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 14.Salmon-Ceron D, Mazeron MC, Chaput S, Boukli N, Senechal B, Houhou N, et al. Plasma cytomegalovirus DNA, pp65 antigenaemia and a low CD4 cell count remain risk factors for cytomegalovirus disease in patients receiving highly active antiretroviral therapy. Aids. 2000;14:1041–1049. doi: 10.1097/00002030-200005260-00017. [DOI] [PubMed] [Google Scholar]
- 15.Chandwani S, Kaul A, Bebenroth D, Kim M, John DD, Fidelia A, et al. Cytomegalovirus infection in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J. 1996;15:310–314. doi: 10.1097/00006454-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1996;15:1102–1106. doi: 10.1097/00006454-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344:1366–1371. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis JP, Tatsioni A, Abrams EJ, Bulterys M, Coombs RW, Goedert JJ, et al. Maternal viral load and rate of disease progression among vertically HIV-1-infected children: an international meta-analysis. Aids. 2004;18:99–108. doi: 10.1097/00002030-200401020-00012. [DOI] [PubMed] [Google Scholar]
- 20.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 21.Obimbo EM, Mbori-Ngacha DA, Ochieng JO, Richardson BA, Otieno PA, Bosire R, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected african children. Pediatr Infect Dis J. 2004;23:536–543. doi: 10.1097/01.inf.0000129692.42964.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich KC, Fowler MG, Mofenson LM, Abboud R, Pitt J, Diaz C, et al. Maternal and Infant Factors Predicting Disease Progression in HIV Type 1-Infected Infants. Pediatrics. 2000;105:8–20. doi: 10.1542/peds.105.1.e8. [DOI] [PubMed] [Google Scholar]
- 23.Abrams EJ, Wiener J, Carter R, Kuhn L, Palumbo P, Nesheim S, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. Aids. 2003;17:867–877. doi: 10.1097/00002030-200304110-00012. [DOI] [PubMed] [Google Scholar]
- 24.Lohman BL, Slyker JA, Richardson BA, Farquhar C, Mabuka JM, Crudder C, et al. Longitudinal assessment of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon responses during the first year of life in HIV-1-infected infants. J Virol. 2005;79:8121–8130. doi: 10.1128/JVI.79.13.8121-8130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farquhar C, VanCott TC, Mbori-Ngacha DA, Horani L, Bosire RK, Kreiss JK, et al. Salivary secretory leukocyte protease inhibitor is associated with reduced transmission of human immunodeficiency virus type 1 through breast milk. J Infect Dis. 2002;186:1173–1176. doi: 10.1086/343805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosire R, Guthrie BL, Lohman-Payne B, Mabuka J, Majiwa M, Wariua G, et al. Longitudinal comparison of chemokines in breastmilk early postpartum among HIV-1-infected and uninfected Kenyan women. Breastfeed Med. 2007;2:129–138. doi: 10.1089/bfm.2007.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emery S, Bodrug S, Richardson BA, Giachetti C, Bott MA, Panteleeff D, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. J Clin Microbiol. 2000;38:2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otieno PA, Brown ER, Mbori-Ngacha DA, Nduati RW, Farquhar C, Obimbo EM, et al. HIV-1 disease progression in breast-feeding and formula-feeding mothers: a prospective 2-year comparison of T cell subsets, HIV-1 RNA levels, and mortality. J Infect Dis. 2007;195:220–229. doi: 10.1086/510245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeVange Panteleeff D, John G, Nduati RW, Mbori-Ngacha DA, Richardson BA, Kreiss JK, Overbaugh J. Rapid method for screening dried blood samples on filter paper for HIV type 1 DNA. J Clin Microbiol. 1999;37:350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattes FM, Hainsworth EG, Hassan-Walker AF, Burroughs AK, Sweny P, Griffiths PD, Emery VC. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191:89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]
- 31.Revello MG, Zavattoni M, Sarasini A, Percivalle E, Simoncini L, Gerna G. Human cytomegalovirus in blood of immunocompetent persons during primary infection: prognostic implications for pregnancy. J Infect Dis. 1998;177:1170–1175. doi: 10.1086/515277. [DOI] [PubMed] [Google Scholar]
- 32.Steininger C, Kundi M, Kletzmayr J, Aberle SW, Popow-Kraupp T. Antibody maturation and viremia after primary cytomegalovirus infection, in immunocompetent patients and kidney-transplant patients. J Infect Dis. 2004;190:1908–1912. doi: 10.1086/424677. [DOI] [PubMed] [Google Scholar]
- 33.Zanghellini F, Boppana SB, Emery VC, Griffiths PD, Pass RF. Asymptomatic primary cytomegalovirus infection: virologic and immunologic features. J Infect Dis. 1999;180:702–707. doi: 10.1086/314939. [DOI] [PubMed] [Google Scholar]
- 34.Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 35.Nigro G, Krzysztofiak A, Gattinara GC, Mango T, Mazzocco M, Porcaro MA, et al. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. Aids. 1996;10:1127–1133. [PubMed] [Google Scholar]
- 36.Lanari M, Lazzarotto T, Venturi V, Papa I, Gabrielli L, Guerra B, et al. Neonatal cytomegalovirus blood load and risk of sequelae in symptomatic and asymptomatic congenitally infected newborns. Pediatrics. 2006;117:e76–83. doi: 10.1542/peds.2005-0629. [DOI] [PubMed] [Google Scholar]
- 37.Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J Clin Invest. 1998;101:497–502. doi: 10.1172/JCI1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowen EF, Sabin CA, Wilson P, Griffiths PD, Davey CC, Johnson MA, Emery VC. Cytomegalovirus (CMV) viraemia detected by polymerase chain reaction identifies a group of HIV-positive patients at high risk of CMV disease. Aids. 1997;11:889–893. doi: 10.1097/00002030-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Dodt KK, Jacobsen PH, Hofmann B, Meyer C, Kolmos HJ, Skinhoj P, et al. Development of cytomegalovirus (CMV) disease may be predicted in HIV-infected patients by CMV polymerase chain reaction and the antigenemia test. Aids. 1997;11:F21–28. doi: 10.1097/00002030-199703110-00001. [DOI] [PubMed] [Google Scholar]
- 40.Griffiths PD. CMV as a cofactor enhancing progression of AIDS. J Clin Virol. 2006;35:489–492. doi: 10.1016/j.jcv.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Spector SA, Hsia K, Crager M, Pilcher M, Cabral S, Stempien MJ. Cytomegalovirus (CMV) DNA load is an independent predictor of CMV disease and survival in advanced AIDS. J Virol. 1999;73:7027–7030. doi: 10.1128/jvi.73.8.7027-7030.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gantt S, Carlsson J, Shetty AK, Seidel KD, Qin X, Mutsvangwa J, et al. Cytomegalovirus and Epstein-Barr virus in breast milk are associated with HIV-1 shedding but not with mastitis. Aids. 2008;22:1453–1460. doi: 10.1097/QAD.0b013e32830184f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lurain NS, Robert ES, Xu J, Camarca M, Landay A, Kovacs AA, Reichelderfer PS. HIV type 1 and cytomegalovirus coinfection in the female genital tract. J Infect Dis. 2004;190:619–623. doi: 10.1086/422533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheth PM, Danesh A, Sheung A, Rebbapragada A, Shahabi K, Kovacs C, et al. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J Infect Dis. 2006;193:45–48. doi: 10.1086/498576. [DOI] [PubMed] [Google Scholar]
- 45.King CA, Baillie J, Sinclair JH. Human cytomegalovirus modulation of CCR5 expression on myeloid cells affects susceptibility to human immunodeficiency virus type 1 infection. J Gen Virol. 2006;87:2171–2180. doi: 10.1099/vir.0.81452-0. [DOI] [PubMed] [Google Scholar]
- 46.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357:513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 47.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]