Abstract
Background:
Human T-lymphotropic virus (HTLV) type I is the causative agent of HTLV-associated myelopathy (HAM)/tropical spastic paraparesis, and a number of HAM cases with HTLV-II infection have also been reported. However, despite some reports, it is unclear whether HTLV-I or -II infection is associated with other neurologic manifestations.
Methods:
An analysis of medical histories and screening neurologic examinations from a prospective cohort of 153 HTLV-I, 388 HTLV-II, and 810 HTLV-seronegative individuals followed up for means of 11.5, 12.0, and 12.2 years was performed. Participants diagnosed with HAM were excluded. We calculated odds ratios (ORs) and 95% confidence intervals (CIs), adjusting for age, sex, race or ethnicity, income, educational attainment, body mass index, alcohol and cigarette consumption, injection drug use, diabetes, and hepatitis C virus status, using generalized estimating equations for repeated measures.
Results:
HTLV-I and -II participants were more likely than seronegative participants to have leg weakness (ORs 1.67 [95% CI 1.28–2.18] and 1.44 [1.16–1.78]), impaired tandem gait (ORs 1.25 [95% CI 1.07–1.47] and 1.45 [1.27–1.64]), Babinski sign (ORs 1.54 [95% CI 1.13–2.08] and 1.51 [1.18–1.93]), impaired vibration sense (ORs 1.16 [95% CI 1.01–1.33] and 1.27 [1.14–1.42]), and urinary incontinence (ORs 1.45 [95% CI 1.23–1.72] and 1.70 [1.50–1.93]). For both HTLV-I and -II participants, higher odds of sensory neuropathy by monofilament examination were no longer significant after adjustment for confounding.
Conclusions:
These results provide strong evidence that human T-lymphotropic virus (HTLV)-I and -II are associated with a spectrum of predominantly motor abnormalities in patients without overt HTLV-associated myelopathy. Further investigation of the clinical course and etiology of these abnormalities is warranted.
GLOSSARY
- ATL
= adult T-cell leukemia/lymphoma;
- CI
= confidence interval;
- HAM
= human T-lymphotropic virus–associated myelopathy;
- HOST
= HTLV Outcomes Study;
- HTLV
= human T-lymphotropic virus;
- OR
= odds ratio;
- ORa
= adjusted odds ratio.
Human T-lymphotropic virus (HTLV) types I and II are human retroviruses first described in the early 1980s.1,2 HTLV-I is the causative agent of HTLV-associated myelopathy (HAM; also known as tropical spastic paraparesis), a progressive neurologic disorder characterized by leg weakness, diffuse hyperreflexia, clonus, loss of vibration sense, and detrusor insufficiency leading to bladder dysfunction. Of the millions of individuals infected with HTLV-I worldwide,3 it is estimated that approximately 4% will develop HAM during their lifetimes.4 Although the role of HTLV-II in HAM is somewhat controversial, there is increasing evidence that supports an association, and a recent critical review has recognized the entity.5
Several studies suggest that HTLV may be associated with a wider spectrum of neurologic manifestations that do not meet diagnostic criteria for HAM. These symptoms and conditions may later progress to HAM or constitute isolated neurologic syndromes associated with HTLV infection. Sensory neuropathy,6–8 gait abnormalities,9,10 bladder dysfunction,6,9–12 erectile dysfunction,13,14 ALS,15 mild cognitive deficits,16 and rarely, motor neuropathies6,8,13,17–19 have all been reported among HTLV-I–infected individuals without HAM. Although less research has focused on HTLV-II, sensory neuropathy has been observed with HTLV-II alone20 and with HIV coinfection.20–23 A spinocerebellar syndrome has also been documented in a few case reports of HTLV-I– and -II–infected patients.24
A better understanding of the neurologic abnormalities associated with HTLV infection is important for the clinical care of infected patients. The etiology and pathogenesis of these abnormalities are poorly defined, and it is unclear whether they are a precursor to the development of HAM or part of a broader spectrum of HTLV-associated neurologic morbidity. Previous findings on HTLV-associated neurologic abnormalities other than HAM are derived from case series and cross-sectional studies and have been mostly limited to the study of HTLV-I. As part of the HTLV Outcomes Study (HOST), we investigated the association of HTLV-I– and -II–infected individuals with neurologic abnormalities in a large cohort of HTLV-I– and -II–infected individuals followed prospectively with standardized neurologic screening examinations for more than 15 years.
METHODS
Study design and participants.
This was a prospective, multicenter cohort study of individuals with HTLV-I or -II infection detected at the time of attempted blood donation at 5 major US blood centers (Baltimore/Washington, Detroit, Oklahoma City, San Francisco, and Los Angeles) and HTLV-seronegative donors enrolled at the same centers. Details of the cohort enrollment and follow-up procedures have been previously published.25 Briefly, 155 HTLV-I, 387 HTLV-II, and 799 HTLV-seronegative persons were enrolled into the cohort in 1990 through 1992 and were followed up every 2 years. Five HTLV-I, 4 HTLV-II, and 11 HTLV-seronegative persons were additionally enrolled during the sixth visit in 2002 through 2003. HTLV-seronegative controls were matched 2:1 to HTLV-I– and -II–infected persons within each stratum based on age, sex, race or ethnicity, blood center, and type of blood donation (community, autologous, or directed). For this analysis of neurologic abnormalities, the inclusion criterion was completion of the interview at any visit. Subjects were excluded if they had a diagnosis of HAM or adult T-cell leukemia/lymphoma (ATL).
Laboratory testing for HTLV-I or -II seropositivity has been previously described. HTLV serologic status was determined by enzyme immunoassay, followed by confirmatory Western blot. A central laboratory performed HTLV-I vs -II typing with a type-specific serologic assay, PCR, or both. Serologic typing correlated with results from a type-specific PCR assay. All participants at baseline and at visit 5 were tested and found seronegative for HIV. Neither vitamin B12 nor syphilis serology was measured systematically, but subjects with overt neurologic findings suggestive of HAM underwent testing for these potential causes of neurologic disease, and most were negative.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the University of California San Francisco Committee on Human Research and by institutional review boards at other participating institutions, and all subjects gave written informed consent.
Neurologic history and examination.
Each visit included a standardized questionnaire focused on symptoms of neurologic, urinary tract, and hematologic disease; a standardized screening examination; and phlebotomy for complete blood count and repository specimens. The neurologic portion of the screening examination was performed by trained study nurses and tested heel, toe, and tandem gait; biceps reflex, patellar reflex, and extensor plantar response (Babinski sign); and vibration sensation, as previously described4 (see appendix e-1 on the Neurology® Web site at www.neurology.org). Leg muscle weakness was also screened for by instructing the subject to rise from a chair of standard height without using the hands. Nurses received training in the examination procedures at the beginning of each set of visits by a board-certified academic neurologist (J.E.) and were supervised by the study physician at each center.
Beginning at visit 5, a 5.07 (10-g) Semmes–Weinstein monofilament was used to test for sensory neuropathy at 3 sites on the distal lower extremity using procedures adapted from those recommended by a consensus conference on sensory neuropathy.26,27 For the monofilament examination outcome variable, subjects were required to have decreased sensation at a minimum of 2 of the 6 lower extremity sites examined to be considered abnormal, to minimize false-positive readings. Body mass index was calculated from self-reported height and weight, and diabetes was determined by self-report. The examinations were not performed blinded to HTLV status because the subjects were aware of their infection status and counseling was providing during these examinations.
Statistical methods.
Data through visit 7 conducted in 2004 through 2007 were available for analysis. Visit 4 in 1998 was excluded from the analysis because no examinations were performed during that visit. The Kaplan–Meier method was used to generate survival curves for the onset and recurrence of neurologic abnormalities, and the log-rank test was used to test for associations with HTLV status. Gait examination findings (impaired heel, toe, and tandem gait), reflex findings (biceps reflex, patellar reflex, and Babinski sign), and urinary tract symptoms (prevoid and postvoid urgency and incontinence) were each combined to derive survival curves. Leg weakness and impaired vibration sense were also assessed using survival curves. Subjects were censored at the visit number during which the abnormality was detected or at the last follow-up visit for subjects without an abnormality. Time-to-event analyses were conducted using STATA 10 software (StataCorp LP, College Station, TX).
To account for repeated examination of the same subjects, odds ratios (ORs) and 95% confidence intervals (CIs) for HTLV-I and -II associations with neurologic signs and symptoms compared with seronegative subjects were calculated using generalized estimating equations for repeated measures. We first calculated unadjusted ORs using reduced models that included only HTLV status, visit number, and blood center as independent variables. We then generated adjusted ORs for HTLV-I and -II associations with neurologic outcomes using multivariate models that included HTLV status, visit number, blood center, age at baseline, sex, race, educational attainment, cigarette and alcohol consumption, injection drug use, body mass index, diabetes, and hepatitis C virus status as independent variables. We also assessed HTLV-I and -II associations with work-loss days using adjusted multivariate models. Repeated-measures analyses were conducted using SAS 9.1 software (SAS Institute, Cary, NC).
We compared the proportions of HTLV-infected and HTLV-seronegative participants with impaired sensation by monofilament examination using χ2 and Fisher exact tests. We also compared their mean number of work-loss days for each visit using the Student t test. The χ2 test, Fisher exact test, and Student t test were performed as 2-sided tests using STATA 10 software.
RESULTS
Study population and follow-up.
After excluding 9 participants with HAM and 1 patient with ATL, we included 153 HTLV-I, 388 HTLV-II, and 810 HTLV-seronegative participants in this analysis. The baseline characteristics of the study population are provided in table 1. The HTLV groups and seronegative participants were comparable with respect to age, sex, race or ethnicity, blood center, type of blood donation (allogeneic vs autologous), and body mass index except for slightly higher proportions of African-Americans among HTLV-I participants. HTLV-seronegative participants had the highest socioeconomic status, as indicated by educational attainment and income. Pack-years of cigarette smoking and amount of alcohol intake were higher in HTLV-II participants, and they also more frequently reported a lifetime history of injection drug use or current injection drug use. However, most injection drug use was remote, with current injection drug use reported by only 2% of HTLV-II participants and none of the HTLV-I or HTLV-seronegative participants. Hepatitis C seropositivity was substantially higher among HTLV-II participants.
Table 1 Baseline characteristics of the study population by HTLV serologic status
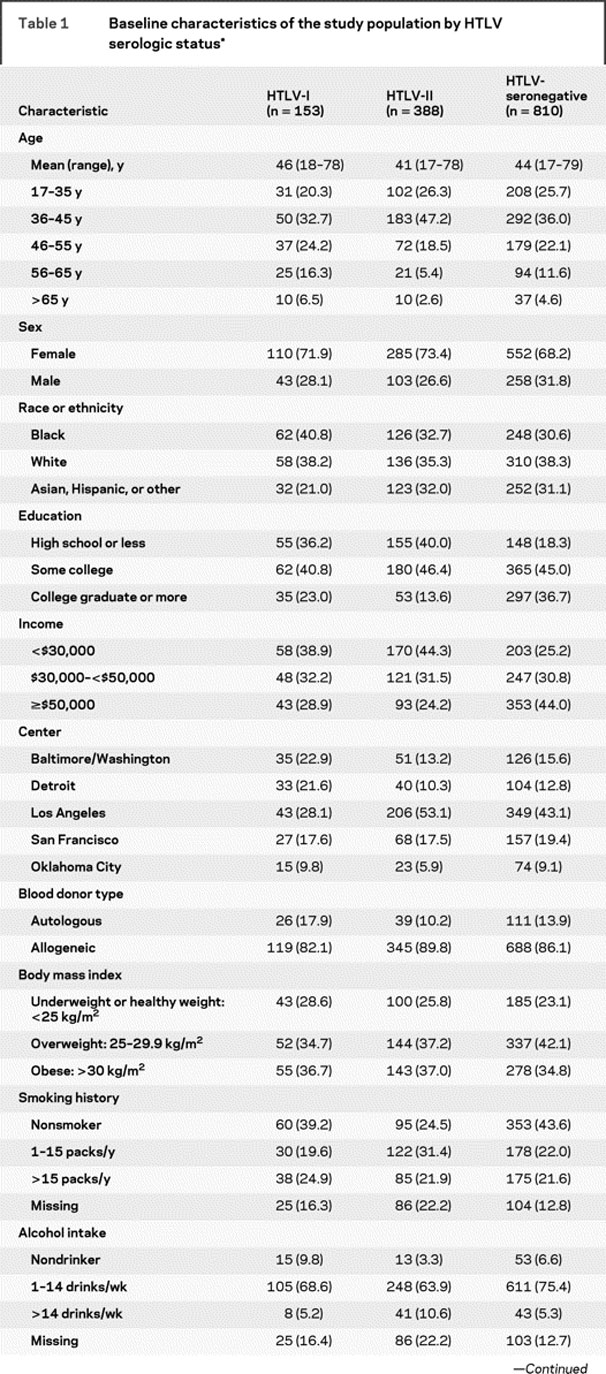
Table 1 Continued
The mean follow-up time was 12.0 years for all 1,351 participants, including late enrollees, and was 11.5 years for the HTLV-I group, 12.0 years for the HTLV-II group, and 12.2 years for the HTLV-seronegative group.
Clinical findings.
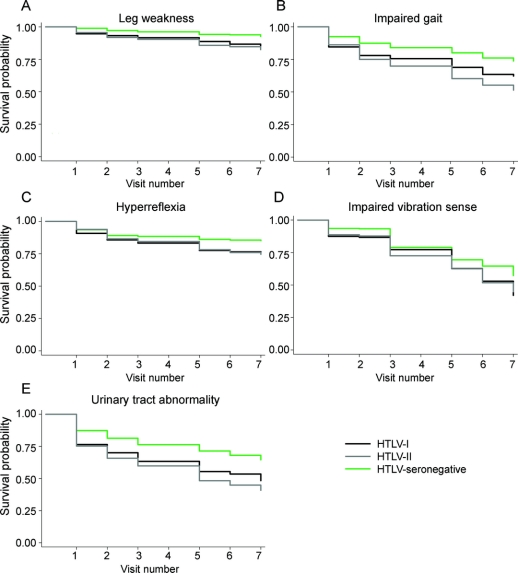
Disease-free survival curves for 5 types of neurologic abnormalities by HTLV status are presented in figure 1. Compared with HTLV-seronegative subjects, HTLV-I– and HTLV-II–infected subjects experienced lower survival to leg weakness, impaired gait, hyperreflexia, impaired vibration sense, and urinary tract abnormality (log-rank p < 0.01 for all abnormality types). There were no significant differences between HTLV groups in survival to any abnormality, nor did recurrence differ by HTLV status (data not shown).
Figure 1 Survival to leg weakness (A), impaired gait (B), hyperreflexia in the lower limbs (C), impaired vibration sense (D), and urinary tract abnormality (E) by visit number and HTLV status
HTLV-I– and HTLV-II–infected subjects experienced lower survival to leg weakness, impaired gait, hyperreflexia, impaired vibration sense, and urinary tract abnormality compared with HTLV-seronegative subjects (log-rank p < 0.01 for all abnormality types). Neurologic examinations were not performed in visit 4.
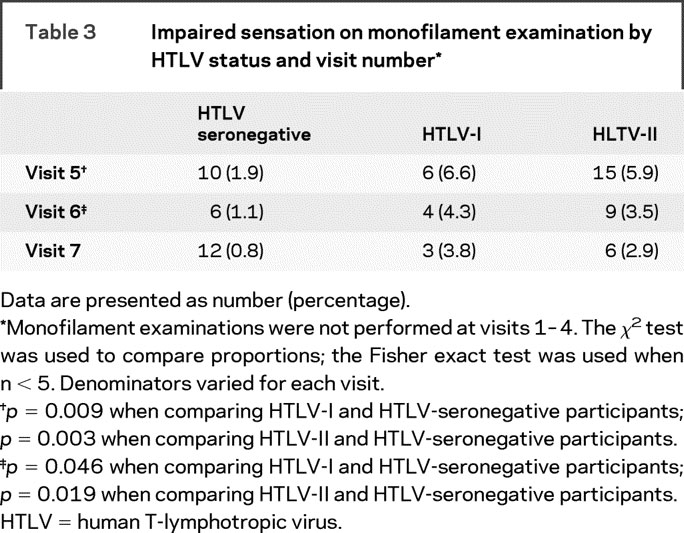
In unadjusted comparisons with seronegative participants, HTLV-I and -II infections were both associated with leg weakness, impaired heel walking, impaired toe walking, impaired tandem gait, Babinski sign, and impaired vibration sense (table 2). All of these associations persisted after adjustment for confounding, except for that between HTLV-I infection and impaired toe walking. Although the proportions of participants with impaired sensation by monofilament examination differed by HTLV status for visits 5 and 6 using the χ2 test (table 3), adjusted ORs from the repeated-measures analysis were not significant (table 2). Self-reported urinary tract symptoms indicated higher rates of prevoid and postvoid urgency and incontinence in HTLV-I and -II participants in both the unadjusted and adjusted analyses (table 2).
Table 2 Crude and adjusted ORs and 95% CIs of neurologic signs and symptoms in HTLV-I– and HTLV-II–infected participants compared with HTLV-seronegative participants, visits 1–7

Table 3 Impaired sensation on monofilament examination by HTLV status and visit number
The overall mean number of work-loss days was 5.5 for HTLV-I, 14.5 for HTLV-II, and 6.1 for HTLV-seronegative subjects. At each of the 6 visits with complete data (visit 4 was not included), the mean number of work-loss days was greater for HTLV-II participants when compared with that for HTLV-I and HTLV-seronegative participants (p < 0.0001 at all visits; figure 2). Using multivariate repeated-measures analysis to control for confounding, HTLV-II participants were shown to report 14.8 (95% CI 4.9–44.6, p < 0.0001) more work-loss days on average as compared with HTLV-seronegative participants. There was no significant difference in mean number of work-loss days between HTLV-I and HTLV-seronegative participants (OR 0.88, 95% CI 0.11–7.0, p = 0.9015).
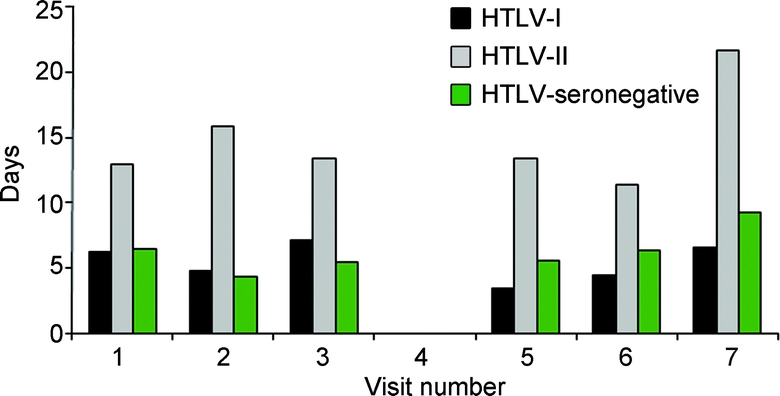
Figure 2 Self-reported mean number of work-loss days by HTLV status and visit number
The mean number of work-loss days was greater at all visits for human T-lymphotropic virus (HTLV)-II subjects when compared with that for HTLV-I and HTLV-seronegative subjects (p < 0.0001). Neurologic examinations were not performed at visit 4.
As previously reported, a cumulative 4% of HTLV-I–infected participants and 1% of HTLV-II–infected participants have developed HAM over the course of the HOST study, which is now in its 16th year.4 Although they were excluded from the current analysis, we examined the records of all incident cases of HTLV-I HAM (n = 2) and HTLV-II HAM (n = 2) for neurologic abnormalities before their diagnosis of overt HAM. All 4 participants experienced at least some signs of leg weakness, impaired gait, impaired vibration sense, or urinary tract abnormality at visits before diagnosis.
DISCUSSION
In this large prospective cohort study, HTLV-I– and -II–infected participants had increased odds of motor and sensory neurologic signs and bladder symptoms compared with HTLV-seronegative participants. In general, both HTLV-I– and -II–infected participants were more likely than HTLV-seronegative participants to report bladder dysfunction and manifest examination abnormalities, including leg weakness, impaired gait, hyperreflexia, and impaired vibration sense. Neither group had a higher frequency of sensory peripheral neuropathy compared with HTLV-seronegative participants after adjusting for potential confounding variables. These results support some, but not all, previous studies that demonstrated the association of HTLV with a spectrum of neurologic abnormalities other than HAM.
These data also provide the strongest evidence to date that HTLV-II, as well as HTLV-I, is associated with an array of predominantly motor and bladder neurologic findings. Although a limited number of studies have shown that HTLV-II–infected individuals are more likely than uninfected individuals to develop neurologic disability,20 peripheral neuropathy,21,22 and progressive myelopathy,23 the role of HIV and/or drug-related factors could not be excluded. In this study, subjects were seronegative for HIV and only a small percentage reported injection drug use, which we adjusted for in our analysis. Although the role of HTLV-II in the development of neurologic disorders has been less clear than that of HTLV-I in the literature, our findings show similar odds of neurologic abnormalities across both groups of infected subjects. In addition, HTLV-II subjects had a significantly higher number of work-loss days compared with HTLV-seronegative subjects, even after adjusting for socioeconomic factors, which provides support for increased morbidity, though not necessarily neurologic. HTLV-II subjects may have had more work-loss days as a result of their higher frequency of neurologic abnormalities or because of their higher incidence of respiratory tract infections and arthritis.10 We have previously reported 4 cases of HAM among HTLV-II patients in the HOST cohort,4 and there are a number of other HTLV-II HAM case reports in the literature.28–34 These results, in addition to the spectrum of milder abnormalities found in our study, support the neuropathologic effects of this virus.
Our negative results on HTLV-I infection and peripheral sensory neuropathy contradict those of previous studies.6–8,13 However, all but one of the studies consisted of uncontrolled case reports, so results could not be appropriately compared with HTLV-seronegative persons. Because peripheral neuropathy has multiple causes,35 the use of a control group is critical. Confounding by other causes of peripheral neuropathy may account for some reports in the literature that lacked the kind of multivariate analysis we performed.
In contrast to our negative findings on impaired fine touch, we did find a higher incidence of impaired vibration sense among HTLV-I and -II subjects compared with seronegative subjects. Potential age-related differences in vibration sensation between HTLV groups and seronegative subjects were excluded by multivariate analysis. Decreased vibration sense is among the main neurologic manifestations of HAM as defined by the World Health Organization,36 but it has rarely been studied in HTLV-infected individuals without HAM. It is noteworthy that we found abnormality in vibration sense but not fine touch. The anatomy of spinal cord structures involved in advanced HAM is well known and includes the corticospinal tracts, anterior horn cells, and posterior columns.37 The clinical findings among our subjects suggest that spinal cord involvement associated with HTLV-I or -II infection may affect single or multiple spinal cord structures across a clinical spectrum from asymptomatic to mild or severe involvement. Our data also suggest that peripheral sensory nerves are not a focus of HTLV neuropathology. The reasons for these sensory differences could be explored more fully in animal models.
Our current finding of a highly significant increased likelihood of prevoid and postvoid urgency and incontinence in both HTLV-I– and -II–infected subjects is consistent with other reports of HTLV-related neurogenic bladder in the literature, including our own.10–12,38 Because we did not perform evaluations for urinary tract infections (UTI), it is conceivable that unrecognized UTI could account for our findings. However, a recent cross-sectional study of 157 HTLV-I–infected individuals found that only 19% of 64 subjects with bladder symptoms had positive urine cultures, indicating that the majority of urinary symptoms were due to neurogenic bladder.12 Furthermore, this study performed urodynamic studies on a subgroup of 21 symptomatic individuals with negative urine cultures and found evidence of neurogenic bladder in 81% of these individuals. These results argue that HTLV-I and -II subjects should have careful, periodic neurologic evaluation to document and symptomatically treat bladder as well as gait manifestations. Future prospective studies should include urodynamic evaluations and urine cultures.
Strengths of this study include its large sample size, prospective cohort design, standardized examinations, and long-term follow-up of both HTLV-infected and seronegative individuals. In addition, we conducted multivariate repeated-measures analyses to control for confounders that could obscure the relationship of HTLV with its neuropathologic features, although residual confounding may still have influenced the magnitude of the associations we observed. A potential weakness is the lack of blinding of participants and research nurses, which may have biased the association of HTLV infection and neurologic abnormalities upward. However, because our cohort consisted of voluntary blood donors, who are known to be healthier than the general population, it is likely that the absolute rates of neurologic abnormalities reported in our study are underestimates. Although we used rising from a chair without using the hands to screen for leg weakness, it is possible that balance, muscle, or joint abnormalities unrelated to motor function may explain difficulty in performing this maneuver.
Until longer follow-up of our cohort is achieved, it is difficult to speculate whether the neurologic abnormalities identified in subjects without HAM will remain stable or progress to a full diagnosis of HAM. Although our results suggest that some neurologic manifestations of HTLV-I and -II infection are isolated and do not reach the clinical threshold for the diagnosis of myelopathy, they remain consistent with the syndromic symptoms and signs of HAM. Our anecdotal data on abnormal neurologic examinations in 4 cohort participants who developed incident cases of HTLV-I or -II HAM further support the hypothesis that some participants with abnormal neurologic examinations will later progress to HAM.
Because only a small percentage of infected individuals develop HAM, HAM may simply be the “tip of the iceberg” of a broader spectrum of stable neurologic manifestations associated with HTLV infection.39 This view is supported by recent clinical evidence of increased HTLV-I proviral loads, similar to those of HAM patients, in patients with neurologic abnormalities other than HAM compared with asymptomatic carriers.40 These findings suggest that viral regulatory genes, genetic determination of the host’s immunologic response, or both may be responsible for both HAM and the more subtle spectrum of neurologic abnormalities that we report. Virologic and immunologic studies of symptomatic non-HAM cases may help to clarify the etiology of HTLV neurologic outcomes.
AUTHOR CONTRIBUTIONS
Ms. Hope Biswas performed the statistical analysis and drafted the manuscript. Dr. John Engstrom provided neurologic expertise regarding the study measurements and analysis, and also critically reviewed the manuscript. Ms. Zhanna Kaidarova prepared the data for analysis and critically reviewed the manuscript. Drs. George Garratty, Joan Gibble, Bruce Newman, James Smith, and Alyssa Ziman conducted the study at its multiple centers and also critically reviewed the manuscript. Drs. Joy Fridey and Ronald Sacher both provided outcome adjudication and critically reviewed the manuscript. Dr. Edward Murphy designed the study and directed its implementation, including the study’s analytic strategy, and assisted in drafting the manuscript.
ACKNOWLEDGMENT
The authors thank the study participants at all 5 centers for their ongoing participation in this long-term study.
DISCLOSURE
Ms. Biswas, Ms. Kaidarova, Dr. Garratty, Dr. Ziman, and Dr. Sacher report no disclosures. Dr. Engstrom receives research support from National Heart, Lung, and Blood Institute grant 2R01-HL-062235 (Coinvestigator). Dr. Gibble receives partial salary support from National Heart, Lung, and Blood Institute grant 2R01- HL-62235. Dr. Newman serves on the editorial board of the journal Transfusion. Dr. Smith serves as an Associate Editor of the Journal of Clinical Apheresis and receives research support from Osiris and National Heart, Lung, and Blood Institute grant 2R01-HL-062235 (Subcontractor Project Director). Dr. Fridey served on the medical advisory board of Blood Systems, Inc.; received royalties from publishing articles in Up-to-Date articles; and served as a consultant to a national blood collection organization for blood donor injury cases. Dr. Murphy receives research support from National Heart, Lung, and Blood Institute grant 2R01-HL-62235 (Principal Investigator) and from career award K24-HL-75036.
Supplementary Material
APPENDIX
The HTLV Outcomes Study (HOST) is the responsibility of the following persons:
Study Headquarters: University of California, San Francisco, CA: E.L. Murphy (Principal Investigator), J.W. Engstrom, D. DeVita, S. Yuen.
Blood Centers: American Red Cross Blood Services Greater Chesapeake and Potomac Region, Baltimore, MD: J.W. Gibble; American Red Cross Blood Services Southeastern Michigan Region, Detroit, MI: B.H. Newman; American Red Cross Blood Services Southern California Region, Pomona, CA: G. Garratty, A. Ziman, S.T. Hutching; Blood Centers of the Pacific, San Francisco, CA: M.P. Busch; Sylvan N. Goldman Center, Oklahoma Blood Institute, Oklahoma City, OK: J.W. Smith.
Central Laboratory: Blood Centers of the Pacific, San Francisco, CA: M.P. Busch, L. Pitina, L.H. Tobler.
Diagnostic Review Panel: E.L. Murphy, R.A. Sacher, J.L. Fridey.
Address correspondence and reprint requests to Dr. Edward L. Murphy, UCSF Departments of Laboratory Medicine and Epidemiology/Biostatistics and Blood Systems Research Institute, 270 Masonic Ave., San Francisco, CA 94118 murphy@ucsf.edu
Supplemental data at www.neurology.org
*See the appendix for HOST Investigators.
Supported by National Heart, Lung, and Blood Institute grant 2R01-HL-62235 and by career award K24-HL-75036 to Dr. Murphy.
Disclosure: Author disclosures are provided at the end of the article.
Presented in part at the International HTLV Conference, Hakone, Japan, May 2007, and the HTLV European Research Network Conference, Bruges, Belgium, June 2008.
Received January 26, 2009. Accepted in final form June 10, 2009.
REFERENCES
- 1.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 1980;77:7415–7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalyanaraman VS, Sarngadharan MG, Robert-Guroff M, Miyoshi I, Golde D, Gallo RC. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 1982;218:571–573. [DOI] [PubMed] [Google Scholar]
- 3.de The G, Bomford R. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses 1993;9:381–386. [DOI] [PubMed] [Google Scholar]
- 4.Orland JR, Engstrom J, Fridey J, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes Study. Neurology 2003;61:1588–1594. [DOI] [PubMed] [Google Scholar]
- 5.Araujo A, Hall WW. Human T-lymphotropic virus type II and neurological disease. Ann Neurol 2004;56:10–19. [DOI] [PubMed] [Google Scholar]
- 6.Leite AC, Mendonca GA, Serpa MJ, Nascimento OJ, Araujo AQ. Neurological manifestations in HTLV-I-infected blood donors. J Neurol Sci 2003;214:49–56. [DOI] [PubMed] [Google Scholar]
- 7.Shimazaki R, Ueyama H, Mori T, et al. Chronic sensory neuronopathy associated with human T-cell lymphotropic virus type I infection. J Neurol Sci 2002;194:55–58. [DOI] [PubMed] [Google Scholar]
- 8.Leite AC, Silva MT, Alamy AH, et al. Peripheral neuropathy in HTLV-I infected individuals without tropical spastic paraparesis/HTLV-I-associated myelopathy. J Neurol 2004;251:877–881. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DJ, Caskey MF, Abbehusen C, et al. Brain magnetic resonance imaging white matter lesions are frequent in HTLV-I carriers and do not discriminate from HAM/TSP. AIDS Res Hum Retroviruses 2007;23:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy EL, Wang B, Sacher RA, et al. Respiratory and urinary tract infections, arthritis, and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis 2004;10:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro NM, Rodrigues W Jr, Freitas DM, Muniz A, Oliveira P, Carvalho EM. Urinary symptoms associated with human T-cell lymphotropic virus type I infection: evidence of urinary manifestations in large group of HTLV-I carriers. Urology 2007;69:813–818. [DOI] [PubMed] [Google Scholar]
- 12.Rocha PN, Rehem AP, Santana JF, et al. The cause of urinary symptoms among human T lymphotropic virus type I (HLTV-I) infected patients: a cross sectional study. BMC Infect Dis 2007;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caskey MF, Morgan DJ, Porto AF, et al. Clinical manifestations associated with HTLV type I infection: a cross-sectional study. AIDS Res Hum Retroviruses 2007;23:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro N, Oliveira P, Freitas D, Rodrigues W, Muniz A, Carvalho E. Erectile dysfunction and HTLV-I infection: a silent problem. Int J Impot Res 2005;17:364–369. [DOI] [PubMed] [Google Scholar]
- 15.Silva MT, Leite AC, Alamy AH, Chimelli L, Andrada-Serpa MJ, Araujo AQ. ALS syndrome in HTLV-I infection. Neurology 2005;65:1332–1333. [DOI] [PubMed] [Google Scholar]
- 16.Silva MT, Mattos P, Alfano A, Araujo AQ. Neuropsychological assessment in HTLV-1 infection: a comparative study among TSP/HAM, asymptomatic carriers, and healthy controls. J Neurol Neurosurg Psychiatry 2003;74:1085–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arakawa K, Umezaki H, Noda S, Itoh H. Chronic polyradiculoneuropathy associated with human T-cell lymphotropic virus type I infection. J Neurol Neurosurg Psychiatry 1990;53:358–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawa H, Nagashima T, Nagashima K, et al. Clinicopathological and virological analyses of familial human T-lymphotropic virus type I–associated polyneuropathy. J Neurovirol 2005;11:199–207. [DOI] [PubMed] [Google Scholar]
- 19.Douen AG, Pringle CE, Guberman A. Human T-cell lymphotropic virus type 1 myositis, peripheral neuropathy, and cerebral white matter lesions in the absence of spastic paraparesis. Arch Neurol 1997;54:896–900. [DOI] [PubMed] [Google Scholar]
- 20.Dooneief G, Marlink R, Bell K, et al. Neurologic consequences of HTLV-II infection in injection-drug users. Neurology 1996;46:1556–1560. [DOI] [PubMed] [Google Scholar]
- 21.Zehender G, De Maddalena C, Osio M, et al. High prevalence of human T cell lymphotropic virus type II infection in patients affected by human immunodeficiency virus type 1–associated predominantly sensory polyneuropathy. J Infect Dis 1995;172:1595–1598. [DOI] [PubMed] [Google Scholar]
- 22.Zehender G, Colasante C, Santambrogio S, et al. Increased risk of developing peripheral neuropathy in patients coinfected with HIV-1 and HTLV-2. J Acquir Immune Defic Syndr 2002;31:440–447. [DOI] [PubMed] [Google Scholar]
- 23.Berger JR, Raffanti S, Svenningsson A, McCarthy M, Snodgrass S, Resnick L. The role of HTLV in HIV-1 neurologic disease. Neurology 1991;41:197–202. [DOI] [PubMed] [Google Scholar]
- 24.Castillo LC, Gracia F, Roman GC, Levine P, Reeves WC, Kaplan J. Spinocerebellar syndrome in patients infected with human T-lymphotropic virus types I and II (HTLV-I/HTLV-II): report of 3 cases from Panama. Acta Neurol Scand 2000;101:405–412. [DOI] [PubMed] [Google Scholar]
- 25.Murphy EL, Glynn SA, Fridey J, et al. Increased prevalence of infectious diseases and other adverse outcomes in human T lymphotropic virus types I- and II-infected blood donors. Retrovirus Epidemiology Donor Study (REDS) Study Group. J Infect Dis 1997;176:1468–1475. [DOI] [PubMed] [Google Scholar]
- 26.Proceedings of a consensus development conference on standardized measures in diabetic neuropathy: quantitative sensory testing. Neurology 1992;42:1829–1831. [PubMed] [Google Scholar]
- 27.Mayfield JA, Sugarman JR. The use of the Semmes-Weinstein monofilament and other threshold tests for preventing foot ulceration and amputation in persons with diabetes. J Fam Pract 2000;49:S17–S29. [PubMed] [Google Scholar]
- 28.Black FL, Biggar RJ, Lal RB, Gabbai AA, Filho JP. Twenty-five years of HTLV type II follow-up with a possible case of tropical spastic paraparesis in the Kayapo, a Brazilian Indian tribe. AIDS Res Hum Retroviruses 1996;12:1623–1627. [DOI] [PubMed] [Google Scholar]
- 29.Lehky TJ, Flerlage N, Katz D, et al. Human T-cell lymphotropic virus type II-associated myelopathy: clinical and immunologic profiles. Ann Neurol 1996;40:714–723. [DOI] [PubMed] [Google Scholar]
- 30.Toro C, Blanco F, Garcia-Gasco P, et al. Human T lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis in an HIV-positive patient coinfected with human T lymphotropic virus type 2 following initiation of antiretroviral therapy. Clin Infect Dis 2007;45:e118–e120. [DOI] [PubMed] [Google Scholar]
- 31.Harrington WJ Jr, Sheremata W, Hjelle B, et al. Spastic ataxia associated with human T-cell lymphotropic virus type II infection. Ann Neurol 1993;33:411–414. [DOI] [PubMed] [Google Scholar]
- 32.Sheremata WA, Harrington WJ Jr, Bradshaw PA, et al. Association of “(tropical) ataxic neuropathy” with HTLV-II. Virus Res 1993;29:71–77. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson S, Lehky T, Nishimura M, Robinson S, McFarlin DE, Dhib-Jalbut S. Isolation of HTLV-II from a patient with chronic, progressive neurological disease clinically indistinguishable from HTLV-I-associated myelopathy/tropical spastic paraparesis. Ann Neurol 1993;33:392–396. [DOI] [PubMed] [Google Scholar]
- 34.Biglione MM, Pizarro M, Salomon HE, Berria MI. A possible case of myelopathy/tropical spastic paraparesis in an Argentinian woman with human T lymphocyte virus type II. Clin Infect Dis 2003;37:456–458. [DOI] [PubMed] [Google Scholar]
- 35.England JD, Asbury AK. Peripheral neuropathy. Lancet 2004;363:2151–2161. [DOI] [PubMed] [Google Scholar]
- 36.WHO. Virus diseases: human T lymphotropic virus type I, HTLV-I. Wkly Epidemiol Rec 1989;64:382–383. [Google Scholar]
- 37.Sasaki S, Komori T, Maruyama S, Takeishi M, Iwasaki Y. An autopsy case of human T lymphotropic virus type I-associated myelopathy (HAM) with a duration of 28 years. Acta Neuropathol (Berl) 1990;81:219–222. [DOI] [PubMed] [Google Scholar]
- 38.Silva MT, Coutinho F, Leite AC, Harab RC, Araujo A, Andrada-Serpa MJ. Isolated bladder dysfunction in human T lymphotropic virus type 1 infection. Clin Infect Dis 2009;48:e34–e36. [DOI] [PubMed] [Google Scholar]
- 39.Araujo AQ, Silva MT. The HTLV-1 neurological complex. Lancet Neurol 2006;5:1068–1076. [DOI] [PubMed] [Google Scholar]
- 40.Silva MT, Harab RC, Leite AC, Schor D, Araujo A, Andrada-Serpa MJ. Human T lymphotropic virus type 1 (HTLV-1) proviral load in asymptomatic carriers, HTLV-1-associated myelopathy/tropical spastic paraparesis, and other neurological abnormalities associated with HTLV-1 infection Clin Infect Dis 2007;44:689–692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.