Abstract
Objective:
To evaluate the influence of the single nucleotide polymorphism rs1080985 in the cytochrome P450 2D6 (CYP2D6) gene on the efficacy of donepezil in patients with mild to moderate Alzheimer disease (AD).
Methods:
This was a multicenter, prospective cohort study of 127 white patients with AD according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association Work Group criteria. Patients were treated with donepezil 5–10 mg/daily for 6 months. Cognitive and functional statuses were evaluated at baseline and at 6-month follow-up. Response to therapy was defined according to the National Institute for Health and Clinical Excellence criteria. Compliance and drug-related adverse events were also evaluated. The analyses identifying the CYP2D6 and APOE polymorphisms were performed in blinded fashion.
Results:
At 6-month follow-up, 69 of 115 patients (60%) were responders and 46 patients (40%) were nonresponders to donepezil treatment. A significantly higher frequency of patients with the G allele of rs1080985 was found in nonresponders than in responders (58.7% vs 34.8%, p = 0.013). Logistic regression analysis adjusted for age, sex, Mini-Mental State Examination score at baseline, and APOE demonstrated that patients with the G allele had a significantly higher risk of poor response to donepezil treatment (odds ratio 3.431, 95% confidence interval 1.490–7.901).
Conclusions:
The single nucleotide polymorphism rs1080985 in the CYP2D6 gene may influence the clinical efficacy of donepezil in patients with mild to moderate Alzheimer disease (AD). The analysis of CYP2D6 genotypes may be useful in identifying subgroups of patients with AD who have different clinical responses to donepezil.
GLOSSARY
- AChE
= acetylcholinesterase;
- AD
= Alzheimer disease;
- ADAS-Cog
= Alzheimer’s Disease Assessment Scale–Cognitive Section;
- ADL
= activities of daily living;
- bp
= base pair;
- CDR
= Clinical Dementia Rating Scale;
- CI
= confidence interval;
- CYP
= cytochrome P450;
- DM
= dextromethorphan;
- DX
= dextrorphan;
- HW
= Hardy–Weinberg;
- IADL
= Instrumental Activities of Daily Living;
- ICD-9
= International Classification of Diseases, 9th Revision;
- MCI
= mild cognitive impairment;
- MMSE
= Mini-Mental State Examination;
- NICE
= National Institute for Health and Clinical Excellence;
- NINCDS-ADRDA
= National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association Work Group;
- OR
= odds ratio;
- SNP
= single nucleotide polymorphism.
Donepezil is a specific piperidine-based inhibitor of acetylcholinesterase (AChE) currently used for the treatment of mild to moderate Alzheimer disease (AD).1,2 Recent studies reported a significant benefits of donepezil vs placebo on cognitive function, activities of daily living (ADL), and behavior.3,4 These improvements, however, are not always detectable in clinical practice.4,5
Most studies reported interindividual differences in drug response that may be due to variability in drug metabolism related to behavioral, clinical, and genetic factors, mainly hereditary polymorphisms of drug-metabolizing enzymes.6,7 Among the hepatic cytochrome P450 (CYP) enzymes, CYP2D6 and CYP3A4 have been shown to be the main CYP isoenzymes involved in the metabolism of donepezil.1 In particular, polymorphisms of the CYP2D6 seem to play a role in the pharmacokinetics of donepezil, which may influence the efficacy of treatment in patients with AD.8 A large number of allelic variants causing absent, decreased, or increased CYP2D6 enzyme activity have been described.9 Recent data demonstrated that the G allele of the single nucleotide polymorphism (SNP) rs1080985 (C-1584→G) in the CYP2D6 gene is associated with a higher enzymatic activity in vivo as a consequence of a higher gene expression associated with the G allele.10,11 It has been suggested that the presence of the G allele of rs1080985 is associated with a more rapid drug metabolism, and that the analysis of rs1080985 may be useful to rule out the CYP2D6 poor metabolizer phenotype in white individuals.10 The aim of this study was to evaluate the influence of this SNP on the clinical efficacy of donepezil in patients with mild to moderate AD.
METHODS
Patient recruitment.
From January 2005 to December 2006, a total of 1,252 elderly subjects (768 men, 484 women) aged ≥65 years who consecutively attended the Istituto di Ricovero e Cura a Carattere Scientifico Casa Sollievo della Sofferenza in San Giovanni Rotondo (Geriatric Unit), the Catholic University School of Medicine in Rome (Department of Neurology), or the University of Perugia in Perugia (Institute of Gerontology and Geriatrics) were screened for possible enrollment in the study.
Standard protocol approvals, registrations, and patient consents.
This was a prospective multicenter cohort study fulfilling the Declaration of Helsinki, the guidelines for Good Clinical Practice, and the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.12 The approval of the study for experiments using human subjects was obtained from the local ethics committees on human experimentation. Written informed consent for research was obtained from each patient or from relatives or a legal guardian in the case of critically disabled patients with dementia.
Inclusion/exclusion criteria.
Inclusion criteria were 1) white race, 2) age ≥65 years, 3) diagnosis of probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association Work Group (NINCDS-ADRDA) criteria13 with a mild to moderate degree of disease severity, and 4) written informed consent for research. Patients were excluded from the study if they were unwilling or unable to fulfill the requirements of the study, had clinically significant and unstable medical illnesses, or had undergone medical/surgical hospitalization within 1 month before the study. In particular, patients were excluded who 1) had a known or suspected history of dementia secondary to abuse of psychoactive substances; 2) had non-AD dementia (normal pressure hydrocephalus, subdural hematoma, Parkinson disease, frontotemporal dementia, primary progressive aphasia, vascular dementia, mixed dementia); 3) had symptoms of depression, obsessive–compulsive disorders, and anxiety; 4) had a diagnosis of mild cognitive impairment (MCI) (Mini-Mental State Examination [MMSE] score 24–27, Clinical Dementia Rating Scale [CDR] score 0.5); or 5) were taking drugs that are extensively metabolized by CYP2D6 (anticholinergics, anticonvulsants, antidepressants, β-blockers, antipsychotic drugs).14
Data collection.
Baseline demographic and clinical characteristics were collected by a structured interview, clinical evaluation, and review of records from patient’s general practitioners. All included patients were initially treated with donepezil 5 mg/daily for 1 month. Thereafter, patients who had followed the treatment with satisfactory or good compliance and without clinically relevant drug-related adverse events increased the dosage of donepezil to 10 mg/daily for the following 5 months. At the 6-month follow-up, the clinical assessment, including the evaluation of cognitive and functional status, compliance, and drug-related adverse events, was repeated.
Cognitive evaluation and diagnosis of AD.
Cognitive status was evaluated by means of the Alzheimer’s Disease Assessment Scale–Cognitive Section (ADAS-Cog),15 the MMSE,16 and the CDR.17 Diagnoses of probable AD was made according to the NINCDS-ADRDA criteria.13 Differential diagnosis among AD, vascular dementia, and mixed dementia was based on the Hachinski Ischemia Score and neuroimaging evidence.18,19 Diagnosis of MCI was made according to Petersen criteria for amnestic MCI.20,21 Functional status was evaluated using the ADL index and the Instrumental Activities of Daily Living (IADL) scales.22,23
Responder/nonresponder assessment criteria.
According to the National Institute for Health and Clinical Excellence (NICE) requirements,24 a responder was defined as a patient who showed improvement or no deterioration in cognition as evaluated by means of ADAS-Cog and MMSE, and improvement in functional status as evaluated by means of ADL or IADL.
Genetic analysis.
Genotype analysis of the SNP rs1080985 was made as already described with minor modifications.10 Briefly, by means of PCR-mediated site-directed mutagenesis, a 327–base pair (bp) fragment was amplified from genomic DNA with a forward primer containing 2 mismatch (lower cases) 5′>GAATTCAAGACCAGCCTGGACAACTTGGAAGggCC>3′ introducing an ApaI restriction site in presence of the C allele (reverse primer 5′>GTGGCTCCCCTCCATTGTGC>3′). This 327-bp product was digested into 292- and 35-bp fragments, whereas the fragment containing the G allele remained uncut. To cut the fragment containing the G allele, we generated a 283-bp fragment with a reverse primer 5′>CAATCCCAGCTAATTTTGTATTTTTTGTAGgGgCC>3′ containing 2 mismatch (lower cases) also introducing an ApaI restriction site in presence of the G allele (forward primer 5′>GCAGCTGCCATACAATCCACCTG>3′). This 283-bp product was digested into 248- and 35-bp fragments, whereas the fragment containing the C allele remained uncut. Analysis of the APOE polymorphisms was performed as previously described.25
The CYP allele nomenclature committee assigned the CYP2D6*41 label to the rs1080985 C allele and the CYP2D6*2A label to the rs1080985 G allele.9 Because the described genotyping procedure does not discriminate CYP2D6*2 variants from A to K, in this study we specifically refer to the C and the G allele of rs1080985.
Statistical analysis.
The estimated minimum number of both responders and nonresponders required for detecting a significant association among rs1080985 genotypes and nonresponders (i.e., an odds ratio [OR] >2), assuming a significance at the 5% level and a power of 75%, was n = 42. The Hardy–Weinberg (HW) equilibrium for both CYP2D6 and APOE loci was verified at baseline and after 6 months in both the responder and nonresponder subgroups. For dichotomous variables, differences between the groups were tested using the Fisher exact test. This analysis was made using the 2-Way Contingency Table Analysis available at the Interactive Statistical Calculation Pages (http://statpages.org/). For continuous variables, normal distribution was verified by the Shapiro–Wilk normality test and the 1-sample Kolmogorov–Smirnov test. For normally distributed variables, differences among the groups were tested by the Welch 2-sample t test or analysis of variance under general linear model. For nonnormally distributed variables, differences among the groups were tested by the Wilcoxon rank sum test with continuity correction or the Kruskal–Wallis rank sum test. Relative allelic frequencies were calculated by the gene-counting method.26 Binary logistic regression analyses were used to estimate crude and adjusted ORs and the 95% confidence interval (CI) for testing possible associations between response/nonresponse to treatment and 1) the CYP2D6 genotypes, evaluating age, sex, MMSE score at baseline, and APOE polymorphism as confounding factors, or 2) the APOE genotypes, evaluating age, sex, MMSE score at baseline, and CYP2D6 polymorphism as confounding factors. These analyses were made with the SPSS Version 10.1.3 software package (SPSS Inc., Chicago, IL). All the other analyses were made with the R Version 2.8.1 software package (The R Project for Statistical Computing; http://www.r-project.org/). Test results in which the p value was smaller than the type 1 error rate of 0.05 were declared significant.
RESULTS
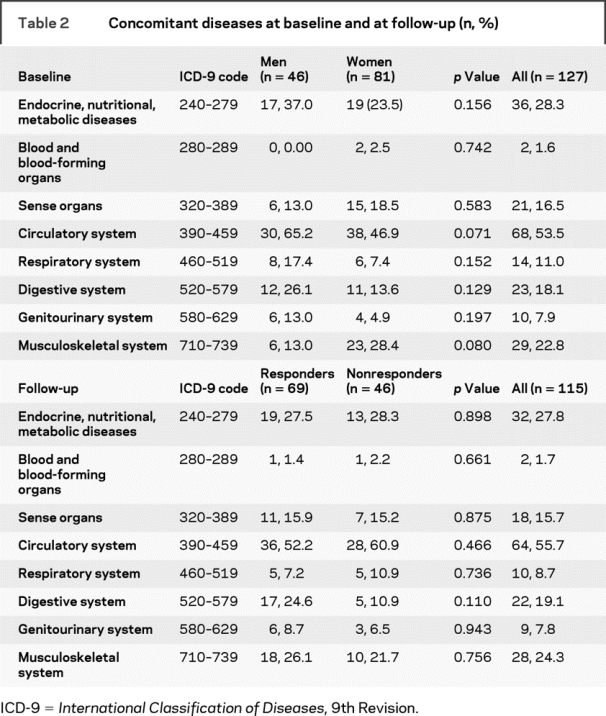
Among the 1,252 elderly subjects consecutively screened for the possible enrollment in the study, 683 patients did not fulfill the inclusion criteria. Among the remaining 569 patients, 442 patients were excluded because of 1) refusal to enter into the study or to sign the informed consent (n = 78); 2) presence of symptoms of depression, obsessive–compulsive disorders, and anxiety (n = 222); 3) diagnosis of MCI (MMSE score 24–27, CDR score 0.5) (n = 66); and 4) concomitant assumption of drugs extensively metabolized by CYP2D6 (n = 76). Thus, a total of 127 patients with AD (46 men and 81 women, mean age 74.09 ± 8.81 years, age range 65–94 years) were enrolled in the study. Demographic and clinical characteristics of patients at baseline according to sex are summarized in table 1. Women were significantly older than men (75.7 ± 8.5 vs 71.2 ± 8.6 years, p = 0.006), whereas no differences in mean values of ADL, IADL, ADAS-Cog, and MMSE between men and women were found. At baseline, no differences between men and women were also observed in the prevalence of concomitant therapies (table 1) and concomitant diseases (table 2).
Table 1 Demographic and clinical characteristics of patients at baseline and at follow-up
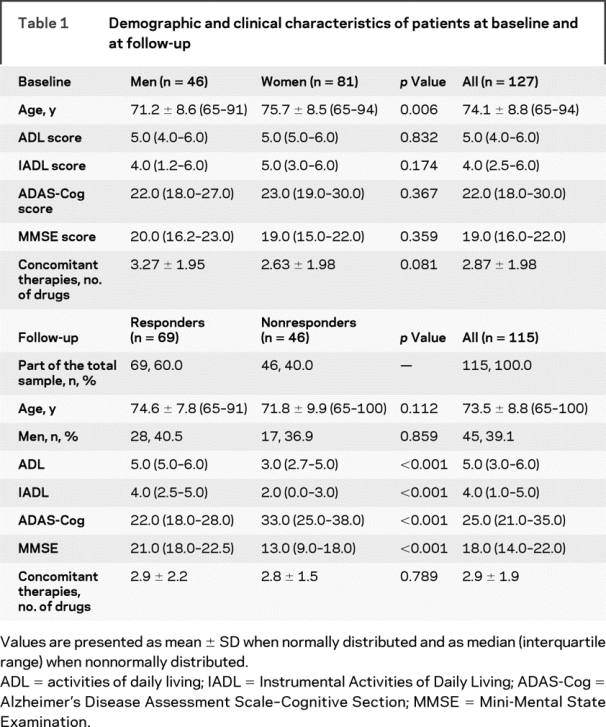
Table 2 Concomitant diseases at baseline and at follow-up (n, %)
The analysis of the SNP rs1080985 showed that 58.3% of patients (n = 74) were C/C wild-type, 33.9% were C/G heterozygotes (n = 43), and 7.9% were G/G homozygotes (n = 10). No differences were found between these observed frequencies and the expected HW frequencies for this locus (p = 0.229). The analysis of the APOE polymorphisms showed that 5.5% of patients were ɛ2/ɛ3 heterozygotes (n = 7), 1.6% were ɛ2/ɛ4 heterozygotes (n = 2), 48.0% were ɛ3/ɛ3 homozygotes (n = 61), 38.6% were ɛ3/ɛ4 heterozygotes (n = 49), and 6.3% were ɛ4/ɛ4 homozygotes (n = 8). No ɛ2/ɛ2 homozygotes were found. No differences were found between these observed frequencies and the expected HW frequencies for this locus (p = 0.999).
During the follow-up, 12 patients (1 man and 11 women, mean age 79.25 ± 7.4 years, age range 66–94 years) dropped out because of drug-related adverse events, i.e., nausea and vomiting (n = 3), bradycardia (n = 1), abdominal pain (n = 2), dizziness (n = 2), diarrhea (n = 3), and postural hypotension (n = 1). The analysis of the SNP rs1080985 in these 12 patients lost to follow-up showed that 83.3% of patients were C/C wild type (n = 10) and 16.7% were C/G heterozygotes (n = 2). No G/G homozygotes were observed in this group. The analysis of the APOE polymorphisms in the 12 patients lost to follow-up showed that 8.3% of patients were ɛ2/ɛ3 heterozygotes (n = 1), 41.7% were ɛ3/ɛ3 homozygotes (n = 5), 41.7% were ɛ3/ɛ4 heterozygotes (n = 5), and 8.3% were ɛ4/ɛ4 homozygotes (n = 1). No ɛ2/ɛ4 heterozygotes were observed in this group.
Thus, a total of 115 patients with AD (45 men and 70 women, mean age 73.56 ± 8.79 years, age range 65–91 years) were evaluable at follow-up. Of 115 patients, 69 patients (60.0%) were classified as responders and 46 patients (40.0%) were classified as nonresponders to donepezil treatment. Demographic and clinical characteristics of patients at follow-up according to their response to treatment with donepezil are summarized in table 1. As expected, significant differences in ADL (p < 0.001), IADL (p < 0.001), ADAS-Cog (p < 0.001), and MMSE (p < 0.001) scores were observed between responders and nonresponders. Conversely, no significant differences were observed between the 2 groups in mean age, sex distribution, concomitant therapies (table 1), and concomitant diseases (table 2).
Genotype frequencies at the CYP2D6 and APOE loci are summarized in table 3. No differences were found between the observed genotype frequencies and the expected HW frequencies for these loci. When compared, a significant difference was found in the overall distribution of the rs1080985 genotype between the 2 groups (p = 0.006). The frequency of C/G heterozygotes was higher in nonresponders than in responders (41.3% vs 31.9%). This difference, however, did not reach significance (p = 0.098). Similarly, the frequency of the G/G homozygotes was higher, but significant, in nonresponders than in responders (17.4% vs 2.9%, p = 0.004). Thus, whereas the C/G heterozygotes did not show a significant association with donepezil response (OR = 2.045, 95% CI 0.912–4.592), G/G homozygotes demonstrated a significant risk for a poor response to donepezil treatment (OR = 9.474, 95% CI 2.039–42.833). Logistic analysis, adjusted for age, sex, MMSE score at baseline, and APOE polymorphism, revealed that both C/G heterozygotes (p = 0.035, OR = 2.588, 95% CI 1.072–6.249) and G/G homozygotes (p = 0.003, OR = 15.768, 95% CI 2.482–100.158) had a significant risk for a poor response to donepezil treatment.
Table 3 Observed genotype frequencies at the CYP2D6 and APOE loci
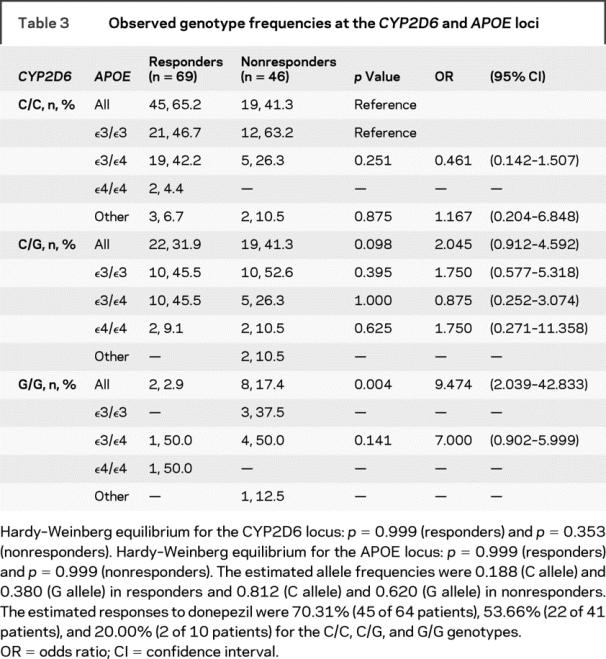
Notably, no significant differences were found in the distribution of the APOE genotypes divided according to the CYP2D6 genotypes. In particular, no differences were found between responders and nonresponders in the distribution of the ɛ3/ɛ4 genotypes in the C/C (p = 0.251), C/G (p = 1.000), or G/G (p = 0.141) subgroups. Similarly, no differences were found between responders and nonresponders in the distribution of the ɛ4/ɛ4 genotypes in the C/G group (p = 0.625). No ɛ4/ɛ4 homozygotes were found in the C/C and G/G nonresponders (table 3). Logistic analysis, adjusted for age, sex, MMSE score at baseline, and CYP2D6 polymorphism, did not show significant differences between responders and nonresponders in the distribution of ɛ3/ɛ4 (0.111) or ɛ4/ɛ4 (p = 0.305) genotypes.
DISCUSSION
Current data on the clinical response to donepezil in patients with AD are not homogeneous, mainly because of the inclusion of patients with different degrees of disease severity, duration of treatments, and criteria to identify responders or nonresponders. In this study, we enrolled only highly selected patients with mild to moderate AD. Patients were treated for 6 months, and responders to treatment were defined conservatively according to the NICE criteria as patients who showed improvement or no deterioration in cognition, and improvement in functional status.24
Our study showed an association of rs1080985 G allele in the CYP2D6 gene with a poor response to donepezil treatment. In an a priori sample-size estimation, we set the power of the study ≥75%, and the post hoc calculated power was 85.0%, with an effect-size h of 0.57. Thus, the findings of the study may be considered quite solid. At 6 months, we observed a 60% response to donepezil. This rate is in agreement with meta-analyses of randomized clinical trials of donepezil 5–10 mg/daily reporting 30% to 68% of response to treatment after 6 months.3,4,24
Most clinical studies tried to identify risk factors for nonresponse to donepezil, including age,27 sex,28 baseline severity of cognitive or functional status,29 drug-related adverse events,30 and the ɛ4 allele of the APOE polymorphism.31–33 Even if CYP2D6 pharmacogenetics have been claimed to play a significant role in explaining variability in response to donepezil,34 only 1 study reported data about the impact of CYP2D6 polymorphisms on the clinical outcome of donepezil in patients with AD.8 The authors included 42 patients with probable AD who were treated for 3 months, mostly (31 patients, 74%) with 5 mg daily. Moreover, response to therapy was evaluated according to changes in the MMSE and Clinician Interview-Based Impression of Change Plus Caregiver Input scores.8
In our study, we selected patients with AD without confounding factors that might influence the CYP2D6 metabolism of donepezil, such as the concomitant use of other CYP2D6-metabolized drugs. Moreover, these patients showed the typical AD-related ΑPOE genotype distribution.19,21
Recent studies suggested that the ɛ4 allele of the APOE polymorphism seems to improve the responsiveness to donepezil treatment in patients with AD.32,33 In agreement with other studies,31 our study did not find a significant role of the ɛ4 allele in improving the clinical response to donepezil. Indeed, multivariate analyses demonstrated that the significant role of CYP2D6 polymorphism in influencing the clinical response to donepezil was independent of the age, sex, and MMSE score at baseline as well as the APOE polymorphism of patients. In particular, in the stratification of APOE genotypes according to CYP2D6 genotypes, no significant differences were observed for the ɛ4 allele. Furthermore, multivariate analysis did not show a significant role of APOE polymorphism in improving clinical responsiveness to donepezil, even after adjustment for sex, age, MMSE score at baseline, and CYP2D6 polymorphism. All these findings suggest that the APOE gene is unrelated to the AChE inhibitor metabolism and do not support the hypothesis of a direct interaction between APOE and CYP2D6 polymorphisms.33
The genotype frequencies of the SNP rs1080985 in the study cohort were comparable to the CYP2D6 genotype distribution reported in white individuals.10 Moreover, the observed genotype frequencies at the CYP2D6 and APOE loci did not differ from the expected HW frequencies, also after categorizing patients according to response or nonresponse to donepezil treatment. These conditions minimize the risk of a genetic bias in patient enrollment.
Recently, a correlation between the CYP2D6 enzyme activity, expressed as the urinary metabolic ratio of dextromethorphan (DM)/dextrorphan (DX), and rs1080985 was reported.10 The presence of the G allele was found only in extensive metabolizers (patients showing a DM/DX metabolic ratio <0.3) and not in poor metabolizers (patients with a DM/DX metabolic ratio >0.3).10 This finding was in agreement with the reported higher microsomal CYP2D6 protein expression in liver biopsies from individuals having the G rather than the C allele.11 In agreement with these data, our findings suggest that in patients with AD, the G allele is significantly associated with a poor response to donepezil treatment. Indeed, whereas G/G homozygotes demonstrated a significant risk of a poor response to donepezil, confirmed by a significance in both crude and adjusted analyses, C/G heterozygotes had a minor risk, as suggested by significance in the adjusted analysis only. These findings, together with the estimated response rates to donepezil, which were 53.6% and 20.0% for C/G and G/G genotypes, suggest a possible dose effect for the G allele that may influence the CYP2D6 metabolic phenotype. Data from this study cannot explore this hypothesis.
A limitation of this study is the potential lack of generalizability of our findings, because our patients with AD were selected according to strict inclusion criteria. Moreover, the large CI associated with the G/G genotype in both crude (2.039–42.833) and adjusted analysis (2.482–100.158) could reflect imprecise OR values. However, with the high OR values associated with the G/G genotype (9.474 and 15.768 for the crude and adjusted analyses), it is difficult to draw negative conclusions.
In clinical practice, because the presence of a G allele may rule out patients with a poor metabolizer phenotype, analysis of the SNP rs1080985 may be a useful approach for predicting clinical response to donepezil in patients with AD. Further studies are needed to evaluate whether patients with AD who have a rapid metabolism may benefit from a higher dose of donepezil to achieve equivalent levels of cholinesterase inhibition.
ACKNOWLEDGMENT
The authors thank Prof. Munir Pirmohamed for comments and suggestions in reviewing the manuscript.
DISCLOSURE
Dr. Alberto Pilotto, Dr. Franceschi, Dr. D’Onofrio, Dr. Bizzarro, Dr. Mangialasche, Dr. Cascavilla, Dr. Paris, Dr. Matera, Dr. Andrea Pilotto, Dr. Daniele, Dr. Dallapiccola, and Dr. Seripa report no disclosures. Dr. Mecocci serves on the scientific advisory board for Lundbeck and serves as a Senior Editor of the Journal of Alzheimer’s Disease. Dr. Masullo serves as an Associate Editor of the Journal of Alzheimer’s Disease and has received research support from the Italian Ministry of Health (4FAN) and from the Italian Ministry of University and Research (Fondi di Ateneo).
Address correspondence and reprint requests to Dr. Alberto Pilotto, Geriatric Unit and Gerontology-Geriatrics Research Laboratory, Department of Medical Sciences, IRCCS Casa Sollievo della Sofferenza, Viale Cappuccini 1, 71013 San Giovanni Rotondo (FG), Italy alberto.pilotto@operapadrepio.it
Supported by “Ministero della Salute,” IRCCS Research Program, Ricerca Corrente 2006–2008, Linea n. 2 “Malattie di rilevanza sociale.”
Disclosure: Author disclosures are provided at the end of the article.
Received March 4, 2009. Accepted in final form June 9, 2009.
REFERENCES
- 1.Cummings JL. Alzheimer’s disease. N Engl J Med 2004;351:56–67. [DOI] [PubMed] [Google Scholar]
- 2.Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet 2002;41:719–739. [DOI] [PubMed] [Google Scholar]
- 3.Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst Rev 2006;CD001190. [DOI] [PubMed] [Google Scholar]
- 4.Hansen RA, Gartlehner G, Lohr KN, Kaufer DI. Functional outcomes of drug treatment in Alzheimer’s disease: a systematic review and meta-analysis. Drugs Aging 2007;24:155–167. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Snow V, Cross JT Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2008;148:370–378. [DOI] [PubMed] [Google Scholar]
- 6.Suh DC, Thomas SK, Valiyeva E, et al. Drug persistency of two cholinesterase inhibitors: rivastigmine versus donepezil in elderly patients with Alzheimer’s disease. Drugs Aging 2005;22:695–707. [DOI] [PubMed] [Google Scholar]
- 7.Cascorbi I. Pharmacogenetics of cytochrome P4502D6: genetic background and clinical implication. Eur J Clin Invest 2003;33(suppl 2):17–22. [DOI] [PubMed] [Google Scholar]
- 8.Varsaldi F, Miglio G, Scordo MG, et al. Impact of the CYP2D6 polymorphism on steady-state plasma concentrations and clinical outcome of donepezil in Alzheimer’s disease patients. Eur J Clin Pharmacol 2006;62:721–726. [DOI] [PubMed] [Google Scholar]
- 9.The Home Page of The Human Cytochrome P450 (CYP) Allele Nomenclature Committee. Available at: http://www.cypalleles.ki.se/. Accessed July 16, 2009.
- 10.Gaedigk A, Ryder DL, Bradford LD, Leeder JS. CYP2D6 poor metabolizer status can be ruled out by a single genotyping assay for the -1584G promoter polymorphism. Clin Chem 2003;49(pt1):1008–1011. [DOI] [PubMed] [Google Scholar]
- 11.Zanger UM, Fischer J, Raimundo S, et al. Comprehensive analysis of the genetic factors determining expression and function of hepatic CYP2D6. Pharmacogenetics 2001;11:573–585. [DOI] [PubMed] [Google Scholar]
- 12.STROBE Statement: Strengthening the Reporting of Observational Studies in Epidemiology. Available at: http://www.strobe-statement.org/. Accessed July 16, 2009.
- 13.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Service Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 14.The P450 Table. Available at: http://medicine.iupui.edu/flockhart/table.htm. Accessed July 16, 2009.
- 15.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 16.Folstein M, Folstein S, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 17.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 18.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol 1975;32:632–637. [DOI] [PubMed] [Google Scholar]
- 19.Orsitto G, Seripa D, Panza F, et al. Apolipoprotein E genotypes in hospitalized elderly patients with vascular dementia. Dement Geriatr Cogn Disord 2007;23:327–333. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001;58:1985–1992. [DOI] [PubMed] [Google Scholar]
- 21.Orsitto G, Seripa D, Panza F, et al. Apolipoprotein E genotypes in mild cognitive impairment subtypes. J Am Geriatr Soc 2006;54:1965–1966. [DOI] [PubMed] [Google Scholar]
- 22.Katz S, Akpom CA. A measure of primary sociobiological functions. Int J Health Serv 1976;6:493–508. [DOI] [PubMed] [Google Scholar]
- 23.Lawton MP, Brody EM. Assessment of older people: self maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 24.NHS, National Institute for Health and Clinical Excellence. Available at: http://www.nice.org.uk. Accessed July 16, 2009.
- 25.Seripa D, Signori E, Gravina C, et al. Simple and effective determination of apolipoprotein E genotypes by positive/negative polymerase chain reaction products. Diagn Mol Pathol 2006;15:180–185. [DOI] [PubMed] [Google Scholar]
- 26.Gerdes LU, Klausen IC, Sihm I, Færgeman O. Apolipoprotein E polymorphism in a Danish population compared to findings in 45 other study populations around the world. Genet Epidemiol 1992;9:155–167. [DOI] [PubMed] [Google Scholar]
- 27.Bullock R, Bergman H, Touchon J, et al. Effect of age on response to rivastigmine or donepezil in patients with Alzheimer’s disease. Curr Med Res Opin 2006;22:483–494. [DOI] [PubMed] [Google Scholar]
- 28.Haywood WM, Mukaetova-Ladinska EB. Sex influences on cholinesterase inhibitor treatment in elderly individuals with Alzheimer’s disease. Am J Geriatr Pharmacother 2006;4:273–286. [DOI] [PubMed] [Google Scholar]
- 29.Caltagirone C, Bianchetti A, Di Luca M, et al. Guidelines for the treatment of Alzheimer’s disease from the Italian Association of Psychogeriatrics. Drugs Aging 2005;22(suppl 1):1–26. [DOI] [PubMed] [Google Scholar]
- 30.Takeda A, Loveman E, Clegg A, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry 2006;21:17–28. [DOI] [PubMed] [Google Scholar]
- 31.Rigaud AS, Traykov L, Latour F, et al. Presence or absence of at least one ɛ4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer’s disease. Pharmacogenetics 2002;12:415–420. [DOI] [PubMed] [Google Scholar]
- 32.Bizzarro A, Marra C, Acciarri A, et al. Apolipoprotein E ɛ4 allele differentiates the clinical response to donepezil in Alzheimer’s disease. Dement Geriatr Cogn Disord 2005;20:254–261. [DOI] [PubMed] [Google Scholar]
- 33.Choi SH, Kim SY, Na HR, et al. Effect of ApoE genotype on response to donepezil in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2008;25:445–450. [DOI] [PubMed] [Google Scholar]
- 34.Cacabelos R, Llovo R, Fraile C, Fernández-Novoa L. Pharmacogenetic aspects of therapy with cholinesterase inhibitors: the role of CYP2D6 in Alzheimer’s disease pharmacogenetics. Curr Alzheimer Res 2007;4:479–500. [DOI] [PubMed] [Google Scholar]


