Abstract
Objective:
To investigate whether internuclear ophthalmoparesis (INO) due to demyelination of the medial longitudinal fasciculus (MLF) provides a model for studying the poorly understood symptom of fatigue in multiple sclerosis (MS). We asked whether repetitive horizontal saccades increased eye movement disconjugacy in patients with MS with INO, but not in healthy subjects.
Methods:
We compared conjugacy of horizontal saccades in 9 patients with INO (4 bilateral, total 13) and 8 controls during minute 1 and minute 10 of a fatigue test; we measured the ratio of abducting/adducting peak velocity (versional disconjugacy index [VDI]).
Results:
VDI values were greater in patients than controls. During the fatigue test, controls showed no changes of VDI, but patients did (p < 0.005) for 10/13 INOs, with increased ratios in 5 cases and a decrease in the other 5.
Conclusion:
Fatigue-induced worsening of conjugacy was observed in milder internuclear ophthalmoparesis (INO), and may reflect deteriorated fidelity of saccadic pulse transmission along demyelinated medial longitudinal fasciculus. Improved conjugacy was observed in the more severe INOs, and may be due to adaptive mechanisms, such as recruitment of vergence to aid gaze shifts. INO may provide an accessible, reductionist model to study how decreased neural transmission influences fatigue in multiple sclerosis, how the brain adapts to it, and whether drugs may prove therapeutic.
GLOSSARY
- AR
= amplitude ratio;
- INO
= internuclear ophthalmoparesis;
- IVD
= interocular velocity difference;
- MLF
= medial longitudinal fasciculus;
- MS
= multiple sclerosis;
- PI
= prediction interval;
- VDI
= versional disconjugacy index.
Fatigue is a disabling symptom of MS but its pathogenesis is not understood.1,2 Internuclear ophthalmoparesis (INO) is common in multiple sclerosis (MS), being evident when patients make rapid gaze shifts (saccades) between 2 horizontally separated targets.3,4 Normal subjects show conjugate horizontal saccades but, in INO, the saccade made by the adducting eye is slowed more than the abducting eye (“adduction lag”). The pathogenesis of INO is well understood (figure 1).4–6 Burst neurons lying in the paramedian pontine reticular formation generate a pulse of innervation that projects to 2 populations of neurons in the abducens nucleus: abducens motor neurons and abducens internuclear neurons. The pulse of innervation passes on axons of abducens motoneurons to the lateral rectus muscle, and the eye accelerates to high speeds.7,8 Abducens internuclear neurons convey the pulse of innervation, via the medial longitudinal fasciculus (MLF), to medial rectus motoneurons in the contralateral oculomotor nucleus that cause the medial rectus muscle to contract rapidly. In normal subjects, the eyes turn rapidly together, as a conjugate saccade. The initial portion of horizontal saccades, which corresponds to the saccadic pulse, is machine-like and normal limits can be defined for the speed of abducting and the adducting eyes.4,7 INO in MS is due to demyelination of the MLF, which causes slowing of the adducting eye, which can no longer conduct high-frequency signals.4,9 The goal of this study was to determine whether the circuit for conjugate horizontal saccades in figure 1 provides an accessible model for studying the phenomenon of fatigue in MS.
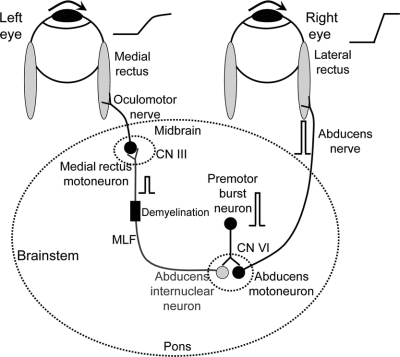
Figure 1 Summary of simple model for internuclear ophthalmoparesis
Premotor burst neurons, lying in the paramedian pontine reticular formation (PPRF), project a pulse of innervation (shown schematically as a frequency-firing histogram) to the abducens nucleus (CN VI). Abducens motoneurons project the pulse of innervation via the sixth nerve to the right lateral rectus, which contracts rapidly to generate an abducting saccade of the right eye. Abducens internuclear neurons project the pulse of innervation, via the medial longitudinal fasciculus (MLF, internuclear pathway) to medial rectus motoneurons that, in turn, innervate the left medial rectus via the third nerve, to generate a fast adducting saccade of the left eye. If the MLF is demyelinated, signals are low-pass filtered, thereby reducing the size of the pulse (as shown); consequently, the adducting saccade of the left eye will be slow. Thus, comparison of the initial acceleration of the abducting and adducting eyes (shown schematically to the right of each eyeball) provides direct information about transmission of high-frequency signals (pulses of innervation) in the MLF. Note that the step of innervation, which follows the pulse, is omitted for clarity.
METHODS
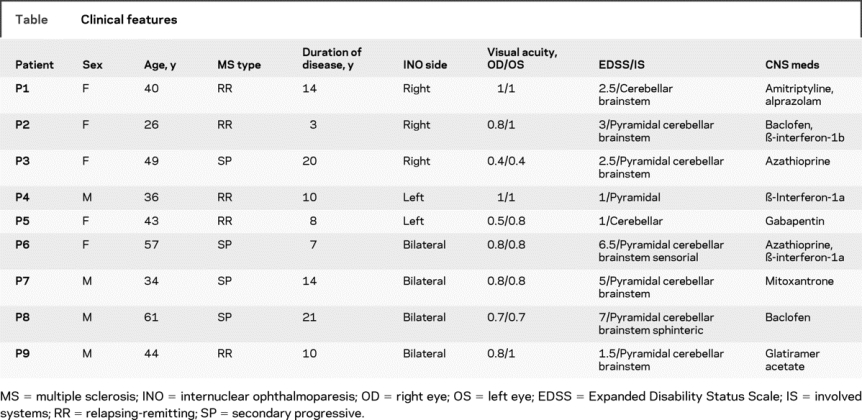
We studied a group of 9 patients (age range 26–61 years, mean 43.3; 5 female) with definite MS (5 relapsing-remitting, 4 secondary progressive, disease duration 3–21 years, mean 11.9), all of whom had clinically evident INO of varying severity (4 bilateral, total 13 INOs); their clinical features are summarized in the table. We also studied 8 healthy control subjects (age range 27–57 years, mean 36.7; 4 female).
Table Clinical features
Standard protocol approvals, registrations, and patient consents.
We received approval from our institutional review boards for our experiments using human subjects. All patients and control subjects gave written informed consent, in accordance with the Declaration of Helsinki and with the approval of our institutional review boards.
Patients and control subjects made horizontal saccades in response to 20-degree jumps, at 1.0 Hz across the midline, of a visual target (laser spot) viewed binocularly on a tangent screen at a distance of 1.2 m. This “fatigue test” lasted 10 minutes. Horizontal eye position was recorded using either infrared oculography or the magnetic search coil technique.4 Signals were filtered (0–150 Hz) prior to digitization at 500 Hz; eye velocity and acceleration were computed as previously described.10
The onset and the end of saccades were defined using a velocity threshold of 10 deg/s. We applied 3 measures of conjugacy of horizontal saccades from data segments collected during the first and last minute of the fatigue test: 1) amplitude ratio (AR) of the size of the abducting saccade to the size of the corresponding adducting saccade, measuring this ratio of change in eye position from the onset of the saccade until the time of the peak velocity, corresponding to the pulse of innervation; 2) the ratio of abducting/adducting peak velocity (versional disconjugacy index [VDI])9; and 3) the normalized, interocular velocity difference (IVD) measured at 20% of eye displacement on phase-plane plots. This last technique has been described previously,11 and is summarized briefly here. The displacement (change in position) and velocity of each eye were normalized by assigning a value of 1.0 to the maximum displacement, and to the peak velocity, of the eye making the larger movement. In this way, we were able to compare the velocity curve of each eye for the same normalized eye displacement during the entire saccade; the difference between these curves was velocity disconjugacy, which provided a value for IVD at 20% of eye displacement. We applied this technique to about 300 saccades at minute 1 and 300 saccades at minute 10 from the 8 age-matched normal subjects, and used linear regression to define 5%–95% prediction intervals (PI) of interocular velocity difference curves. Then, the average velocity disconjugacy was calculated from approximately 10 saccades at minute 1 and 10 saccades at minute 10 for each patient, and compared with PI of the control subjects.
RESULTS
Figure 2 shows representative records of horizontal saccades made during the first and 10th minute of the fatigue test from a control subject and 2 patients with INO (see Discussion for details). Figure 3 summarizes data from all patients and control subjects.
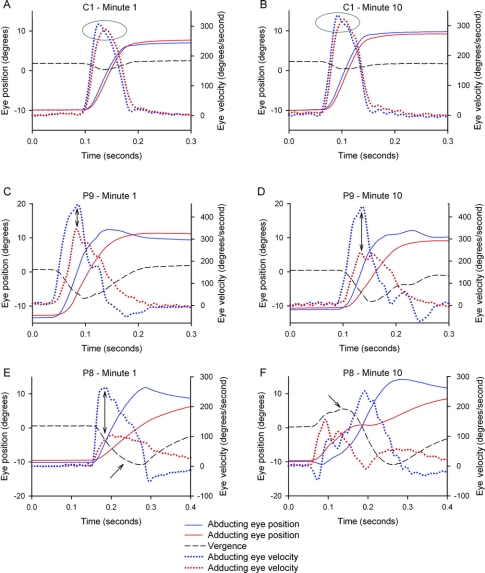
Figure 2 Representative records of horizontal saccades made during the first and 10th minute of the fatigue test for a control subject (C1) and two patients (P9 and P8)
Positive values indicate increased abduction or adduction and convergence. Note that position scales at left and velocity scales at right vary between different subjects but are constant for each subject. In A and B, the normal subject shows little difference between the peak velocities of the abducting vs adducting eyes (highlighted in ellipses) at 1 or 10 minutes; transient, minor divergence is evident. In C and D, P9 with mild INO (versional disconjugacy index [VDI] <1.9) shows greater peak velocity of the abducting vs adducting eye at 1 minute (adduction lag), and this increased further by 10 minutes (double-headed arrows); divergence is increased compared with the control subject. In E and F, P8 with more severe internuclear ophthalmoparesis (VDI >1.9) shows a large difference between the peak velocity of the abducting and adducting eye at 1 minute (double-headed arrow), which caused transient divergence (lower arrow), similar to Patient 9. However, by 10 minutes the eyes initially converge (upper arrow), so that an adduction lag is no longer evident.
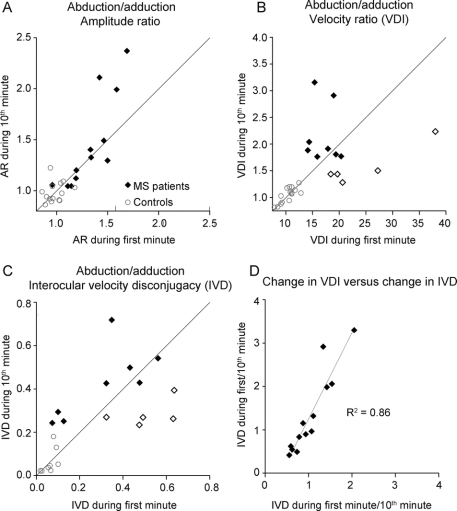
Figure 3 Comparison of abduction/adduction ratios during the first and 10th minute of testing
(A) Amplitude ratio (AR); (B) peak velocity (versional disconjugacy index [VDI]); (C) velocity disconjugacy on phase plane for eye displacement of 20% (interocular velocity difference [IVD]). D shows the tight correlation between ratios of measurements at first minute/10th minute for VDI and IVD. Closed diamonds in B and C represent patients whose disconjugacy worsened, and open diamonds represent patients whose disconjugacy improved, by 10 minutes; see text for details.
Amplitude measurements.
Normal subjects showed AR values close to 1.0 during both the first (0.97 ± 0.10) and 10th (1.00 ± 0.11) minute, and a paired t test showed no change (p = 0.475) (figure 3A). Patients showed larger AR values (p = 0.02) than controls at 1 minute (1.26 ± 0.27), with a further increase at 10 minutes (1.41 ± 0.43), although this increase was not significant (Wilcoxon rank sum test) due to large variance.
Velocity measurements.
VDI values in normal subjects were close to 1.0 (signifying near-perfect conjugacy or synchronicity of abducting and adducting movements during horizontal saccades) both during the first (1.04 ± 0.14) and 10th minute (1.05 ± 0.14), and a paired t test showed no change (p = 0.475) (figure 3B). Patients showed larger VDI values (1.96 ± 0.63) during the first minute than controls (p < 0.001 on Wilcoxon rank sum test). During the 10th minute of the fatigue test, changes in VDI occurred in 10/13 INOs, with increase in 5 cases (p < 0.001 in 4, p = 0.003 in 1) who had milder INO (VDI <1.9) at presentation, and a decrease in the other 5 (p < 0.001) who had more severe INO (VDI >1.9); these 2 groups are shown within as closed and open diamonds, respectively, in figure 3B. These 2 different behaviors resulted in no change of VDI (1.94 ± 0.54) at 10 minutes for the group as a whole. Representative records of worsening of INO during the fatigue test are shown in figure 2, C and D, and of apparent improvement are shown in figure 2, E and F.
Phase-plane measurements of velocity disconjugacy.
Control subjects showed similar IVD values during both the first (0.06 ± 0.03) and 10th (0.64 ± 0.59) minute, and a paired t test showed no significant change (figure 3C). Patients with MS showed changes of IVD values in 10/13 INOs. However, no significant overall change of IVD values at 1 minute (0.35 ± 0.22) vs at 10 minutes (038 ± 0.14) was observed, because of an increase in 5 cases (closed diamonds in figure 3C) and a decrease in the other 5 (open diamonds in figure 3C). The 5 INOs with VDI increase after 10 minutes also showed increase in IVD, which was significant in 4/5 cases (p < 0.001), and the other 5 INOs with VDI decrease also showed similar IVD decrease after 10 minutes, which was significant in 2/5 cases. Representative examples of phase planes in figure 4 show either worsening (A) or improvement (B) of disconjugacy at 20% of eye displacement (IVD). Similar consistent changes in VDI for the same patients are also shown.
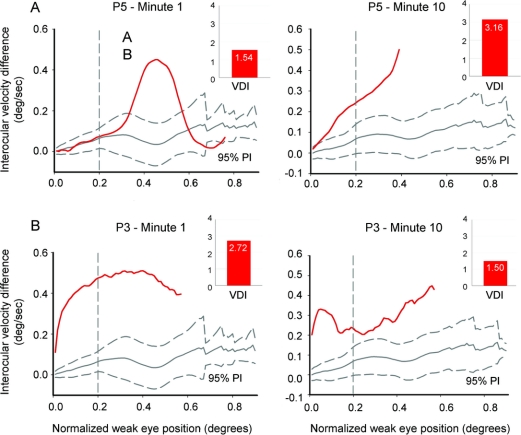
Figure 4 Phase-plane plots of velocity disconjugacy of saccades during the first minute (left plots) and during the 10th minute (right plots) of the fatigue test for representative patients P5 (A) and P3 (B)
The red curves are mean plots of velocity disconjugacy from several saccades; 95% prediction intervals (PI) for saccades from control subjects are shown in gray. The value of interocular velocity difference (IVD) corresponds to the normalized velocity difference between the eyes when normalized eye displacement reaches 0.2 (vertical dashed line). (A) Representative records from patient P5, who showed deterioration of INO (velocity disconjugacy at displacement of 0.2) at 10 minutes. A consistent change in VDI is also shown (red bars at right upper corner). (B) Example of a patient P3, whose INO improved during the fatigue test; note how the initial component is similar, but by displacement of 0.2 IVD value is reduced. Consistent change in VDI is also shown.
Both IVD and VDI showed consistent changes in patients with MS between the first and 10th minute (figure 3D), confirming the result that patients with MS showed 2 distinct forms of behavior in response to the fatigue test. In the Discussion, we provide possible explanations for general worsening of conjugacy (increased VDI and IVD values) in INOs with initial VDI <1.9, and general improvement of conjugacy (decreased VDI and IVD values) with initial VDI >1.9.
DISCUSSION
We investigated whether the syndrome of INO might provide an accessible and sensitive model for studying the phenomenon of primary fatigue in MS. We found that 10/13 INO in 9 patients with MS showed substantial changes in conjugacy with a fatigue test, during which subjects made 20-degree horizontal saccades at 1.0 Hz for 10 minutes. Two types of change in the ratio of abducting/adducting movements were encountered. In 5 cases, INO became significantly worse, as judged by changes in the peak velocity ratio (VDI) and velocity disconjugacy evaluated on phase planes (IVD). In another 5 cases, INO appeared to improve. How can these apparently disparate results be explained?
Our hypothesis was that abduction/adduction velocity ratio would increase with repetitive saccades due to progressive failure of transmission of the saccadic pulse by the demyelinated MLF. Such transmission would be expected to be tenuous based on the demonstration that increase in body temperature makes INO worse,6,12 and 5 cases of INO indeed showed significant deterioration of INO during the fatigue test. But how can we account for the paradoxical effect of improvement of INO during the fatigue test? We propose that such improvement was an adaptive response of the visual system to repetitive transient diplopia exacerbated by the fatigue test. Thus, it has been shown that patients with unilateral INO whose normal eye is patched, imposing habitual viewing with the weak eye, develop adaptive changes in innervation over the course of a few days.13 In these experiments, adduction improved on the side of INO, whereas the covered normal eye developed overshoots, reflecting an increase in the conjugate saccadic pulse in response to visual demands. We considered the possibility that improvement might have occurred in our patients if the eye with impaired adduction also had better vision (preferred viewing); however, our patients did not show large differences in the visual acuity of each eye (table), and the changes in disconjugacy developed over 10 minutes rather than days. Inspection of phase planes (figure 4) offers another possibility: vergence eye movements may have been recruited to substitute for impaired conjugate movements. Convergence movements are variably spared in INO, but may be elicited even in patients who have exotropia.4 Although convergence movements are unlikely to have much effect on the initial, high-acceleration portion of the saccade, after about 20% of eye displacement they may be able to correct the developing exotropia imposed by slowed saccadic adduction. In a prior study of patients with slow horizontal saccades due to brainstem stroke, we have provided evidence that vergence movements may play a substantial role in gaze shifts.11 Furthermore, improvement of conjugacy during the fatigue test was observed in those patients with a more severe degree of INO (initial VDI >1.9), who may already have well developed substitution of vergence eye movements for saccades. These different forms of behavior during the fatigue test were evident in some time plots, such as the representative data shown in figure 2. Thus, the normal subjects (figure 2, A and B) showed little difference between the peak velocity of the abducting vs adducting eyes (highlighted in ellipses) at 1 or 10 minutes. Patient 9 (VDI <1.9; figure 2, C and D) showed greater difference between the peak velocity of the abducting vs adducting eye at 1 minute (adduction lag), and this increased further by 10 minutes (double-headed arrows). Patient 8 (VDI >1.9; figure 3, E and F) showed a large difference between the peak velocity of the abducting and adducting eye at 1 minute (double-headed arrow), which caused transient divergence (lower arrow), similar to patient 9. However, by 10 minutes, his behavior had changed and the eyes initially converged (upper arrow), so that adduction lag was no longer evident. Small, high-frequency oscillations, evident in the velocity record, are reported to occur when saccades and vergence movements are combined.10 Adaptive changes in vergence behavior occurred for both rightward and leftward saccades in P8, and were also encountered in P3. A second mechanism that appeared to contribute to improved conjugacy was a modest decrease in the velocity of the abducting eye at 10 minutes; this was most evident in P6 and P9 (for an example of an amplitude/peak velocity plot, see figure e-1 on the Neurology® Web site at www.neurology.org). Such changes seem more likely to be due to a central adaptive response than fatigue.14 Further studies are required to investigate the mechanisms that improve conjugacy in INO during a fatigue test.
Since our patients’ INO showed variable effects during the fatigue test, can the simple model in figure 1 be used to explain fatigue’s pathogenesis in MS and, furthermore, to investigate efficacy of drugs available for treatment? Our findings point to a worsening signal conduction in the setting of nerve demyelination to account for increased ocular disconjugacy (fatigue) in MS, with possible central adaptive mechanisms acting to correct failed transmission along the MLF. It should be noted that the focus of this study was on the saccadic pulse, but it seems likely that the step (eye position signal) that follows the pulse is also affected by fatigue in MS. In future studies, we plan to extend our methods of analysis to account for changes in both saccadic pulse and step, using an approach such as the first-pass amplitude measurement pioneered by Frohman et al.,15 which compares the abducting eye and adducting eye position at the time point when the abducting eye approximates the visual target. It also seems possible that an index of fatigue in INO can be developed that combines multiple measures of saccade disconjugacy. We propose that similar, and more complicated, effects are likely to be operating in more complex motor systems, such as those involved in gait. For example, a recent study reported that gait in patients with MS improved, rather than fatigued, with task-repetitive gait training.16
Although our results are based on a relatively small sample, we found substantial changes in conjugacy in over 75% of cases. Our results suggest the need for further studies of this simple fatigue test, which was well tolerated by all patients, both before and after administration of drugs to combat fatigue. Trials of drug treatment of fatigue in MS, with agents like modafinil and 4-aminopyridine, have not shown consistent benefits compared to placebo,17 when evaluated by a standard fatigue score.18 We suggest that our saccade fatigue test provides the advantages of dependence upon a known circuit (MLF) affected by MS, and easy access to reliable measurements of the effects of drugs that can improve nerve transmission.
ACKNOWLEDGMENT
The authors thank Dr. Elliott Frohman for comments and advice and Dr. Ke Liao for programming. Dr. Matta and Dr. Serra thank Professor Giulio Rosati for support of this research.
DISCLOSURE
Dr. Leigh serves as a member of the NIH/NEI Central Visual Processing study section; served as Editor of Progress in Brain Research (volume 171, 2008); receives royalties from publishing Neurology of Eye Movements (Oxford University Press, 2006); and receives research support from the NIH (NEI R01-EY06717, Principal Investigator), the Department of Veterans Affairs, the Office of Research and Development, Medical Research Service (Investigation and Treatment of Vestibulo-Visual Disorders, Principal Investigator), and the Evenor Armington Fund. Dr. Pugliatti, Dr. Aiello, Dr. Serra, and Dr. Matta report no disclosures.
Supplementary Material
Address correspondence and reprint requests to Dr. Alessandro Serra, Department of Neurology, 11100 Euclid Avenue, Cleveland, OH 44106-5040 alessandro.serra@Uhhospitals.org
Supplemental data at www.neurology.org
Supported by NIH R01EY06717, Department of Veterans Affairs, the Evenor Armington Fund, and Regione Autonoma della Sardegna (Assessorato dell’Igiene e Sanità e dell’Assistenza Sociale), Fondazione Italiana Sclerosi Multipla (Cod. 2002/R/43 e Cod. 97/R/69).
Disclosure: Author disclosures are provided at the end of the article.
Received March 2, 2009. Accepted in final form June 15, 2009.
REFERENCES
- 1.Mills RJ, Young CA. A medical definition of fatigue in multiple sclerosis. QJM 2008;101:49–60. [DOI] [PubMed] [Google Scholar]
- 2.Kos D, Kerckhofs E, Nagels G, et al. Origin of fatigue in multiple sclerosis: review of the literature. Neurorehabil Neural Repair 2008;22:91–100. [DOI] [PubMed] [Google Scholar]
- 3.Frohman EM, Frohman TC, Zee DS, et al. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol 2005;4:111–121. [DOI] [PubMed] [Google Scholar]
- 4.Leigh RJ, Zee DS. The Neurology of Eye Movements (Book/DVD), Fourth Edition. New York: Oxford University Press; 2006. [Google Scholar]
- 5.Frohman TC, Galetta S, Fox R, et al. Pearls & Oy-sters: The medial longitudinal fasciculus in ocular motor physiology. Neurology 2008;70:e57–e67. [DOI] [PubMed] [Google Scholar]
- 6.Leigh RJ, Serra A. Taking the temperature of MS with INO. Neurology 2008;70(13 Pt 2):1063–1064. [DOI] [PubMed] [Google Scholar]
- 7.Leigh RJ, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain 2004;127:460–477. [DOI] [PubMed] [Google Scholar]
- 8.Ramat S, Leigh RJ, Zee DS, et al. What clinical disorders tell us about the neural control of saccadic eye movements. Brain 2006;130:10–35. [DOI] [PubMed] [Google Scholar]
- 9.Frohman EM, Frohman TC, O’Suilleabhain P, et al. Quantitative oculographic characterisation of internuclear ophthalmoparesis in multiple sclerosis: the versional dysconjugacy index Z score. J Neurol Neurosurg Psychiatry 2002;73:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramat S, Somers JT, Das VE, et al. Conjugate ocular oscillations during shifts of the direction and depth of visual fixation. Invest Ophthalmol Vis Sci 1999;40:1681–1686. [PubMed] [Google Scholar]
- 11.Serra A, Liao K, Matta M, et al. Diagnosing disconjugate eye movements: phase-plane analysis of horizontal saccades. Neurology 2008;71:1167–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SL, Frohman TC, Crandall CG, et al. Modeling Uhthoff’s phenomenon in MS patients with internuclear ophthalmoparesis. Neurology 2008;70:1098–1106. [DOI] [PubMed] [Google Scholar]
- 13.Zee DS, Hain TC, Carl JR. Abduction nystagmus in internuclear ophthalmoplegia. Ann Neurol 1987;21:383–388. [DOI] [PubMed] [Google Scholar]
- 14.Ethier V, Zee DS, Shadmehr R. Changes in control of saccades during gain adaptation. J Neurosci 2008;28:13929–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohman EM, O’Suilleabhain P, Dewey RB, Jr., et al. A new measure of dysconjugacy in INO: the first-pass amplitude. J Neurol Sci 2003;210:65–71. [DOI] [PubMed] [Google Scholar]
- 16.Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair 2008;22:661–671. [DOI] [PubMed] [Google Scholar]
- 17.Rossini PM, Pasqualetti P, Pozzilli C. Fatigue in progressive multiple sclerosis: result of a randomised, double-blind, placebo controlled, crossover trial of oral 4-aminopyridine. Mult Scler 2001;7:354–358. [DOI] [PubMed] [Google Scholar]
- 18.Krupp LB, Alvarez LA, LaRocca NG, et al. Fatigue in multiple sclerosis. Arch Neurol 1988;45:435–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.