Conspectus
Magnetic resonance imaging (MRI) has become increasingly popular in molecular imaging and clinical radiology because it is non-invasive and capable of producing three-dimensional representations of opaque organisms with high spatial and temporal resolution. While approximately 35% of all clinical MR scans utilize contrast media, a primary limitation of MR imaging is the low sensitivity to detect contrast agents requiring high concentrations of agent for enhanced signal intensity (0.1-0.6 mM).1
A number of strategies have been employed to amplify the observed in vivo signal of MR contrast agents. Approaches include attachment of Gd(III) chelates to polymers, proteins and particles, encapsulation into micelles and caged structures, and targeting to receptors. While each of these approaches has yielded significant increases in the relaxivity of MR contrast agents (and therefore sensitivity), all of these classes of complexes possess intrinsic background signal and function solely as anatomical reporters due to their constitutive activity.
In order to reduce the background signal and simultaneously create probes that are modulated by biochemical events, caged complexes were designed to coordinatively saturate the paramagnetic ion. Coupled with amplification strategies, these agents represent a means to selectively modulate the observed MR signal and function as in vivo biochemical reporters. For example, to create an in vivo MR assay of enzymatic activities and secondary messengers, agents have been designed and synthesized with removable protection groups that largely prevent access of water to a paramagnetic center. By limiting the access of bulk water (q-modulation) the unprocessed agent is designed to be an ineffective contrast agent, and hence serves as a reliable marker for regions of enzyme activity or secondary messengers.
In this Account we describe our results toward designing new classes of MR agents that are i. responsive to in vivo physiological or biochemical events ii. cell-permeable to increase local concentration, and iii. attached to large molecules or are synthesized with multiply labeled conjugates for signal amplification.
Magnetic Resonance Imaging
Imaging with nuclear magnetic resonance (NMR) spectroscopy was first introduced by the pioneering work of Lauterbur in 1973.2 MRI exploits the differences in the relaxation rates of nuclear spins due to an applied magnetic field. MR detects the electromagnetic radiation emitted from the transition of higher energy nuclei to a lower energy level. While nuclei with a spin quantum number of I = ½ such as 1H, 13C, 19F and 31P are used, the majority of current MR technology is based on 1H nuclei. The NMR signal is relatively low compared to other spectroscopic methods, however due to the large number of protons in a typical sample, detection is possible. Current MR images are acquired using magnetic field strengths from 1.5 to 7 Tesla in clinical applications, and as high as 19T for high-resolution molecular imaging.3
MRI takes advantage of the most abundant molecule in biological tissues, water. It measures the proton NMR signal of water in which the signal intensity is proportional to the relaxation rates of the nuclear spins. In the presence of a magnetic field the magnetic moments of water protons orient themselves along the magnetic field both in a parallel and antiparallel orientation. An applied radiofrequency pulse inverts the magnetization vector away from the axial field, and reorientation with the magnetic field occurs through both longitudinal (T1) and transverse relaxation pathways (T2).
Due to the heterogeneity of most MR samples, intrinsic contrast between organs can be observed due to the varying water concentrations and local environments.3,4 Signal resolution and sensitivity can be greatly enhanced with the use of paramagnetic contrast agents. For T1-weighted MR images, Gd(III)-based agents are most common due to the seven unpaired electrons of Gd(III), its high magnetic moment and long electron spin relaxation time (10−9s). However, the Gd(III) ion is toxic, potentially effecting Ca(II) signaling as Gd(III) has an ionic radius similar to Ca(II). Therefore, the Gd(III) ion is chelated to render it biologically inert (−log Kd ∼ 17-25).
Relaxation Theory
The theory of relaxation in the presence of paramagnetic ions has been described by Solomon, Bloembergen, and Morgan.5,6 Use of Gd(III) complexes in MR imaging produces an increase in the T1 relaxation rates and thus an increase in signal intensity. The observed relaxation rate of protons is the result of the sum of both diamagnetic (d) and paramagnetic (p) contributions.
| Equation 1 |
The diamagnetic term arises from the relaxation rate of water proton nuclei in the absence of a paramagnetic ion. The paramagnetic term can be expressed as the relaxation rate enhancement induced by the paramagnetic species which is linearly proportional to its concentration (Equation 2). Therefore, the efficacy of Gd(III) complexes in decreasing T1 of local water protons is measured by its relaxivity value, r1 (mM−1s−1), the slope of the line of 1/T1 versus [Gd].3,4
| Equation 2 |
While both the inner-sphere and outer-sphere water protons contribute equally to the relaxation process, for the development of MR contrast agents the inner-sphere term can be modified and therefore represents the major contribution to the overall proton relaxation rate. The inner-sphere relaxation mechanism arises from the chemical exchange of the water molecules bound to the first coordination sphere of the metal center to the bulk water solvent and can be expressed by Equation 3. PM is the mole fraction of the gadolinium ion, q is the number of bound water molecules in the first coordination sphere, T1M is the relaxation time of the bound inner-sphere water protons, and τm is the mean residence lifetime of the bound water molecule.
| Equation 3 |
The correlation time that defines dipole-dipole relaxation is τc and is determined by Equation 4 where T1e is the electron spin relaxation time and τR is the rotational correlation time.
| Equation 4 |
Relaxivity is maximized when τc equals the inverse of the Larmor frequency.3 Optimization of the parameters shows that τm should be decreased but not such that it begins to limit T1M.
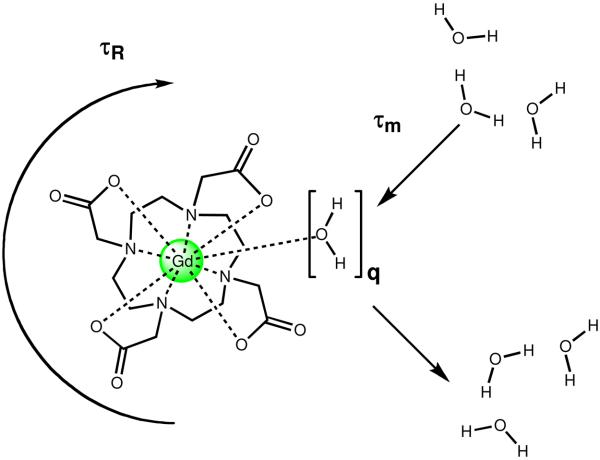
The above equations demonstrate that proton relaxivity is influenced by a number of parameters and is dependent on magnetic field strength and temperature. Most contrast agents used in clinical applications have relaxivity values approximately 4-10 mM−1s−1, far below the theoretical maximum of ∼100 mM−1s−1.3 Due to the low relaxivity values of commercial contrast agents, their effective range requires concentrations greater than 0.1 mM.7 In order to increase the relaxivity of Gd(III)-based contrast agents, researchers focus primarily on optimization of three parameters: q, τm, and τc (Figure 1). It is the goal of our research to optimize all three of these parameters and in addition to develop MR probes that are highly sensitive, cell permeable and are activated by biochemical processes.
Figure 1.
Three important factors that determine the relaxivity of Gd(III)-based MR contrast agents: hydration number (q), the mean residence lifetime of bound water molecules (τm), and the rotational correlation time (τR).
Next Generation MR Probes
Despite efforts to improve the proton relaxation rates of Gd(III)-chelates through manipulation of the relaxivity parameters discussed above, the majority of agents are limited to the blood pool and extracellular space and are constitutively detectable (i.e., possess a background signal). This limits the scope of these agents to highlighting anatomical features rather than biochemical events. We pioneered the development of MR contrast agents where the relaxivity is modulated by changes in the coordination of the chelate.8 This approach has provided the means to non-invasively image biochemical events such as enzyme activity8-12 and to detect important ions including Ca(II) and Zn(II).13-16 This work has inspired the development of a large number of responsive, or bioactivated contrast agents, that are sensitive to pH,17,18 proteins,19 temperature,20 oxygen21,22 and has been recently summarized in an excellent review.23
Current MR contrast agents are typically restricted to the extracellular environment. With the advance of MR imaging techniques in providing cellular resolution, a great deal of research has focused on the development of cell permeable contrast agents. The employment of activated MR contrast agents is greatly dependent on the ability of these agents to cross cell membranes, as the majority of physiological processes of interest take place in the intracellular environment.
Biologically Activated or Responsive Contrast Agents
The strategy for the design of responsive contrast agents involves the modulation of one or more parameters (q, or τm) affecting relaxivity to produce distinct relaxation states before and after activation. These agents conditionally modulate the access of water through changes in the structure and coordination environment of the Gd(III)-chelates due to their biological environment. Relaxation theory shows that doubling the number of bound water molecules would double the relaxivity by providing an increase in the observed signal in an MR image. An ideal responsive agent will have two states where (q = 0) before activation, and a higher relaxation state (q = 1 or 2) post activation.
Enzyme-Responsive Contrast Agents
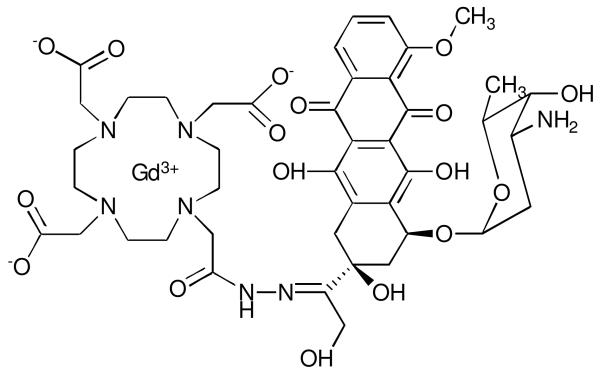
The first responsive MR agent, EGad, was designed to be biologically activated in the presence of an enzyme.10 This complex, and subsequent generations, are based on sterically inhibiting water access to the Gd(III) ion (coordinatively saturated or q = 0 complex) by appending a galactopyranose moiety to a Gd-DO3A chelate.9-12 This series of agents was designed to behave as enzyme reporters with the goal of imaging gene expression in vivo.24 In the presence of the enzyme β-galactosidase, the galactopyranose sugar is cleaved from the Gd(III) chelate creating a water-accessible conformation of the agent. The observed value of q changes from 0 to 1 or 2, triggering an increase in relaxivity.
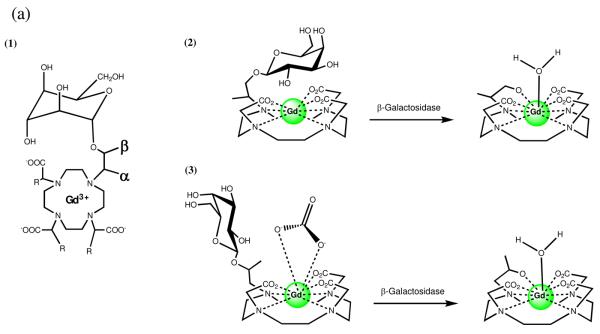
To investigate the enzymatic activation of this class of complexes, α-EGadMe and β-EGadMe (a methyl group on the appending arm is α or β to the macrocycle, respectively) were synthesized (Figure 2a, 2b).10-12 Based on characterization of the isomers by 2D NMR, luminescence lifetime, and τm measurements we proposed two coordination environments for the Gd(III) ion, which suggests two mechanisms for enzymatic activation based on the structures of the complexes. In the case of α-EGadMe, a q = 0 complex is observed due to the steric blocking of water to the Gd(III) center from the galactopyranose moiety. After enzymatic cleavage of the galactopyranose by β-galactosidase, an open coordination environment is observed in which water can access Gd(III) resulting in an increase in relaxivity (Figure 2a). In the case of β-EGadMe, a q = 0 complex is observed due to the binding of endogenous carbonate to the Gd(III) center. Cleavage of the sugar leaves an available hydroxyl group on the appending arm that displaces the carbonate and binds Gd(III) producing a q = 1 to 2 complex with an increase in relaxivity. Since carbonate is a bidentate ligand and the hydroxyl is monodentate, a coordination space is open creating a q = 1 complex (Figure 2b). Each of these stereoisomers involves different mechanisms based on the structure of the initial complex and provide changes in relaxivity from 40 to 50% upon enzyme activation.
Figure 2.
(a) Schematic representations of the two isomers of enzyme-responsive MR agents activated by β-galactosidase: (1) Flat projection with α and β positions labeled (2) α-EGadMe and (3) β-EGadMe. Each isomer provides an increase in relaxivity following cleavage of the sugar by different mechanisms. (b) MRI detection of β-galactosidase mRNA expression in living X. laevis embryos. MR images of two embryos injected with EgadMe at the two-cell stage. (A) Unenhanced MR image. The embryo on the right was injected with β-gal mRNA, resulting in the higher intensity regions. The signal strength is 45–65% greater in the embryo on the right containing β-gal (contrast-to-noise ratio ranges from 3.5 to 6). The cement gland has intrinsically short T1, thus is visible as a bright structure on both embryos. (B) Pseudocolor rendering of same image in (A) with water made transparent. The image correction makes it possible to recognize the eye, and brachial arches in the injected embryo: d, dorsal; v, ventral; r, rostral; e, eye; c, cement gland; s, somite; b, brachial arches. Scale bar = 1 mm.
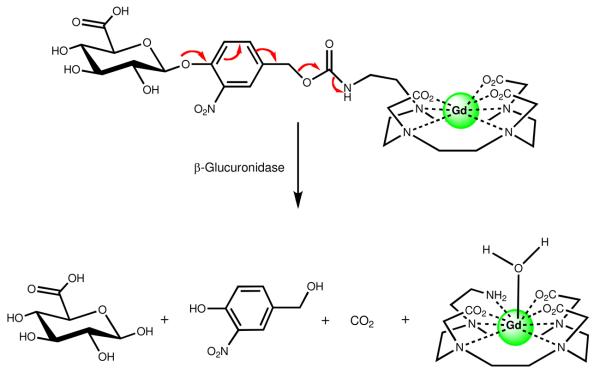
In order to increase the kinetics of enzyme activation we designed a MR probe responsive to β-glucuronidase.25 The prototype of this class is a Gd(III) agent designed to function within the β-glucuronidase prodrug family. This agent's effect on water proton T1 relaxation is modulated by hydrolysis of β-glucuronic acid and is designed to report on the activity of this oncologically upregulated enzyme. The enzyme substrate β-glucuronic acid is attached to a Gd-DO3A chelate through a self-immolative linker. Hydrolysis of β-glucuronic acid initiates a cascade reaction that releases the Gd(III) chelate, carbon dioxide, and the bridging arm (Figure 3).
Figure 3.
A responsive MR contrast agent activated by β-glucuronidase via a self-immolative linker. As with β-EGadMe (Figure 2), endogenous carbonate acts as the ligand to reduce water coordination prior to enzyme activation.
Ion-Responsive Contrast Agents
The first example of an ion-activated agent was designed to report on fluctuations in Ca(II) concentrations for imaging in vivo signal transduction events.4 This agent incorporated the well-known Ca(II)-binding motif, BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), bridging two Gd-DO3A chelates (Figure 4). In the absence of Ca(II), four aminoacetate arms from the Ca(II)-binding domain bind to Gd(III) creating a closed q = 0 complex with the low relaxivity of 3.3 mM−1s−1 at 500 MHz and 25 °C. Upon addition of Ca(II), a 75% increase in relaxivity is observed due to a conformational change in which the aminoacetate arms selectively bind Ca(II) allowing access of water to Gd(III). This agent is responsive to the presence of Ca(II) ions in the range of 0.1 to 10 μM, which is physiologically relevant concentrations for intracellular Ca(II) signaling in neurons. Recently, a calcium responsive agent was reported where the structure is a derivative of the agent described in Figure 4. Activation of the agent occurs at [Ca2+] = 10−3 M, which may be suitable for extracellular environment where the Ca(II) concentration is high.26,27 The relaxation enhancement of these agents is approximately 15%, likely insufficient for in vivo detection.
Figure 4.
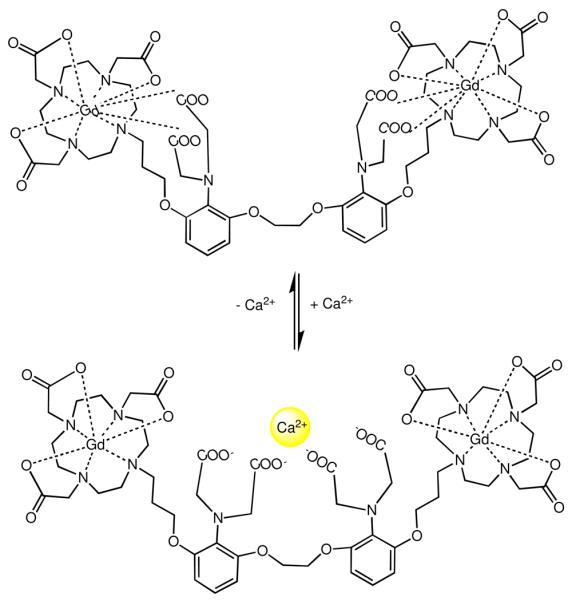
A Ca(II)-activated MR contrast agent Gd-DOPTA. The aminoacetate appended arms internally rearrange upon Ca(II) binding. As a result, a coordination site on the Gd(III) is made available to water (q = 0 to q = 1.5) with a subsequent increase in relaxivity. The agent is selective for Ca(II) only.
Zn(II) plays a critical role in cellular physiology and is involved in structural stability, catalytic activity, and signal transduction pathways.28-30 While much is known about the biochemistry of Zn(II) in metalloproteins, far less is understood about its specific mechanisms of cellular physiology and distribution, and pathology.31 Free Zn(II) concentrations in the extracellular brain tissue are about 150 nM while cellular zinc concentrations can be as high as 1 mM within synaptic nerves.32 Under abnormal stress such as a stroke or an epileptic seizure, 100-300 μM Zn(II) is released from the synaptic nerve into the somatic tissue resulting in a thousand-fold increase in free Zn(II) concentrations. Evidence suggests that these large and sudden increases in Zn(II) concentrations have been linked to the precipitation of amyloid plaques in Alzheimer's disease.29
Nagano and coworkers developed Gd-DTPA derivatives incorporating pyridyl Zn(II)-binding groups.33 However, these agents generate a decrease in signal intensity upon Zn(II)-binding. While a measurable change in proton relaxivity is observed, the decrease in relaxivity (and therefore absence of a signal) upon activation is not ideal.
Recently, a Zn(II)-responsive agent has been reported by Lippard and coworkers that employs a Mn(III)-porphyrin agent with both MR and fluorescent capabilities.34 In vitro studies demonstrate a decrease in relaxivity enhancement with the addition of Zn(II) while in vivo experiments show an increase in T1 relaxivity. Further investigation is required to understand the observed relaxation dynamics.
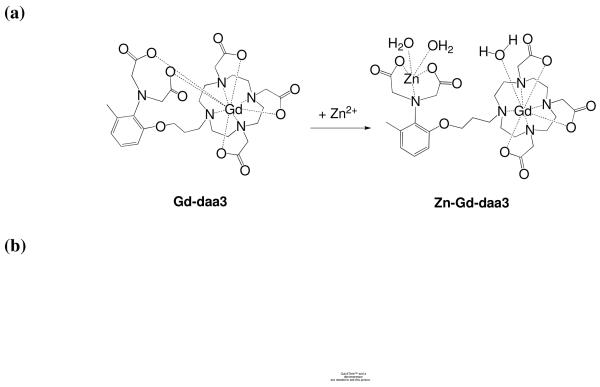
The first example of a MR contrast agent that exhibits an increase in relaxivity upon the addition of Zn(II) was recently reported (Figure 5).16 The design of Gd-daa3 was inspired by the Ca(II)-activated MR contrast agent, Gd-DOPTA. Gd-daa3 exhibits strong selectivity towards Zn(II) over other prevalent biological messengers such as Ca(II) and Mg(II) with a > 100% increase in relaxivity upon Zn(II) binding.
Figure 5.
(a) Proposed mechanism of a Zn(II)-responsive MR contrast agent. The coordinating acetate arms internally rearrange to bind Zn(II) which opens a coordination site on the Gd(III) center and therefore increases relaxivity. (b) Left: Gd-daa3 Selectivity. T1-weighted MR images of a 1 mM solution of Gd-daa3 in HEPES buffer. (A) HEPES buffer; (B) Gd-daa3; (C) Gd-daa3 with 1 mM ZnCl2; (D) Gd-daa3 with 1 mM MgCl2; (E) Gd-daa3 with 1 mM CaCl2. (b) Right Gd-daa3 Sensitivity. T1-weighted MR images of a 1 mM solution of Gd-daa3 in HEPES buffer with varying zinc concentrations. (A) 0μM Zn; (B) 50 μM Zn; (C) 100 μM Zn; (D) 500 μM Zn; (E) 1 mM Zn.
The relaxivity of Gd-daa3 in the absence of Zn(II) measured in HEPES buffer (pH = 7.4) was 2.3 mM−1s−1, consistent with a q = 0 complex. In the presence of Zn(II), an increase in relaxivity was observed; a value of 5.1 mM−1s−1 was measured upon addition of one equivalent of Zn(II). Relaxivity studies conducted in human blood serum prove the capability of translation to in vivo experiments. Importantly, the zinc-dissociation constant (Kd = 2.4 × 10−4 M) calculated from fluorescent competitive binding assays reveals that Gd-daa3 is responsive towards physiologically relevant Zn(II) concentrations released in pathological brain tissue. Phantom MR images of Gd-daa3 with varying concentrations of Zn(II) confirm these results (Figure 5b).
The observed doubling of the relaxivity in the presence of Zn(II) can be explained by the change in the coordination environment surrounding the Gd(III) allowing the access of water. The proposed mechanism of Gd-daa3 presented in Figure 5 suggests that a pair of diaminoacetate arms bind to the Gd(III) center to create a coordinatively saturated complex in the absence of Zn(II). Upon addition of Zn(II), the diaminoacetates preferentially bind Zn(II) allowing one water molecule to bind to the Gd(III) center.
To develop Zn(II)-activated agents with a range of Zn(II) binding constants and to provide direct evidence of the above mechanism, we investigated a series of complexes with varying coordinating groups.15 The systematic variation of Gd(III)-coordinating aminoacetates and non-coordinating pyridyl groups allowed the investigation of the coordination geometry of Gd-daa3. Two structural requirements for the development of Zn(II)-activated contrast agents were determined. First, at least one aminoacetate must be present to coordinatively saturate the complex before the addition of Zn(II). Second, there must be at least two coordinating groups present for the binding of Zn(II). These criteria will allow for the development of a series of contrast agents with a range of Zn(II)-binding constants through variation of one of the aminoacetate arms.
Theranostic-Responsive Agents
We have prepared the first prodrug-procontrast MR probe by conjugating doxorubicin to a Gd(III) chelate using an acid-labile linker (Figure 6).35 Doxorubicin (adriamycin) is a widely used anticancer drug of the anthracycline class and was discovered more than three decades ago. Several reports have appeared that exploit the conjugation of doxorubicin to macromolecules (monoclonal antibodies, humans serum albumin and polymers) via this type of linker. This complex is designed to release doxorubicin when exposed to low pH while simultaneously undergoing a change in relaxivity. This first generation agent is one representative of a class of probes known as theranostics, a form of diagnostic therapy to personalize treatment. The challenge for this class of bifunctional agents is to amplify the signal from the contrast agent to be detectable in a range where significantly lower concentrations of the drug are required for efficacy.
Figure 6.
An example of a prodrug-procontrast complex by conjugating Doxorubicin to a Gd(III) chelate using an acid labile linker. The complex undergoes a change in relaxivity while simultaneously producing an active anticancer drug.
Cell uptake, Receptor targeted and Signal Amplification of MR Contrast Agents
While significant progress is being made in the development of responsive or activated contrast agents,23 the full potential of these complexes has yet to be realized due to their inability to penetrate cell membranes. As mentioned earlier, the inherent low relaxivity of the majority of contrast agents requires large doses for in vivo detection (0.1 – 0.3 mmol/kg). Improvement in amplification strategies and targeting of MR contrast agents will increase sensitivity.
Cell-Permeable MR Contrast Agents with Increased Cellular Retention
Transport of contrast agents across cellular membranes has been achieved with a variety of cell-penetrating peptides and transduction domains. For example, the human immunodeficiency virus (HIV) trans-activating protein (Tat) has been conjugated to Gd(III) MR contrast agents to achieve high cellular uptake of the agents and internalization into the nucleus.36 The relaxivity of the Tat-DOTA conjugate before addition to cells was 4.1 mM−1s−1. After cell internalization, a relaxivity of 2.2 mM−1s−1 was measured due to the limited water diffusion inside cells. With this decrease in observed relaxivity it is evident that large amounts of contrast agent will be required for cell imaging.
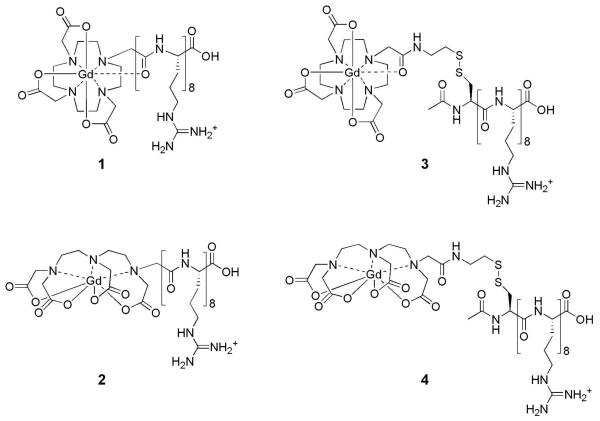
Typically, contrast agents are confined to vascular regions reducing their ability to provide information about cell physiology or molecular pathology. Arginine-rich peptides, such as the HIV-Tat peptide, have been well documented as membrane-permeable derivatives capable of intracellular protein and drug delivery.37 We have shown that a minimum of eight arginines within the peptide are required for sufficient uptake and developed a strategy to conjugate an arginine octamer to Gd(III)-DOTA and Gd(III)-DTPA agents (Figures 8).38-40 An arginine octamer was prepared by standard peptide synthesis techniques on solid-phase resins and coupled to a Gd(III) chelate.
Figure 8.
Structures of the arginine-modified (cell-permeable contrast agents) and disulfide bridged (cell-permeable contrast agents) respectively. (1) Gd(III)-DOTA-Arg8, (2) Gd(III)-DTPA-Arg8, (3) Gd(III)-DOTA-SS-Arg8, and (4) Gd(III)-DTPA-SS-Arg8.
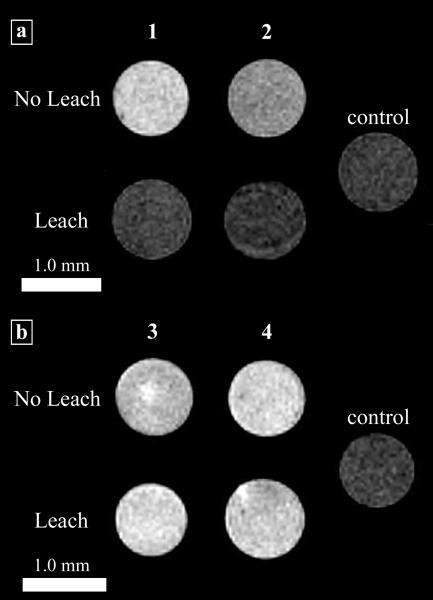
Once inside the cell, arginine-modified contrast agents rapidly efflux, decreasing the intracellular Gd(III) concentration and corresponding MR image intensity. By exploiting the inherent reducing environment of cells, disulfide compounds, Gd(III)-DOTA-SS-Arg8 and Gd(III)-DTPA-SS-Arg8, are cleaved from their cell penetrating peptide transduction domains upon cell internalization. In order to assess the ability of an incorporated disulfide linkage to increase cell retention and maintain MR image contrast over an extended time period, NIH/3T3 cells were incubated with 1.0 mM of 1-4 (Figure 9A). This reaction prolongs the cell-associated lifetime of the chelated Gd(III) by cleaving it from the cell transduction domain.
Figure 9.
T1-weighted MR images of NIH/3T3 cells incubated with complexes 1-4. The ‘No Leach’ rows are the cell populations that were not allowed to leach. The ‘Leach’ rows are the cell populations that were allowed to leach for 4 h with washes of DPBS. The control cells were not incubated with contrast agent but were harvested and packed following the same procedure. (a) Cells incubated with Gd(III)-DOTA-Arg8 or Gd(III)-DTPA-Arg8 (1 and 2). (b) Cells incubated with Gd(III)-DOTA-SS-Arg8 or Gd(III)-DTPA-SS-Arg8 (3 and 4).
MR images were acquired of cells incubated with complexes 1-4. Cells with 1 and 2 exhibit a significantly brighter signal than control cells. However, after repeated washing the cells have a significantly lower intensity in the MR image. This loss of image contrast (due to leaching of the contrast agent) is much less in the case of the cells incubated with complexes 3 and 4 (Figure 9B). These agents can be used to ectopically label cell populations and provide long-term contrast enhancement for cell tracking and lineage analysis using MR imaging.
Receptor-Targeted Contrast Agents
An efficient method for specific delivery of contrast agents involves conjugation to molecular targeting platforms such as monoclonal antibodies41 and other molecules that target cell receptors.42,43 An early example of receptor-targeted contrast agents involves the use of DNA as a molecular scaffold that electrostatically binds polylysine conjugated to Gd(III)-DTPA chelates.44 This class of agents is capable of both transfecting genes into cells and enhancing the contrast of the targeted cells for MR imaging. DNA is used both to encode a marker gene and as a molecular scaffold that electrostatically binds polylysine conjugated to transferrin and Gd(III)-modified polylysine. When cells displaying the transferrin receptor are treated with these conjugates, high levels of gene expression are observed, higher than with control conjugates composed only of transferrin, polylysine and DNA. The treated cells show specific MRI contrast enhancement that does not require expression of the marker gene.
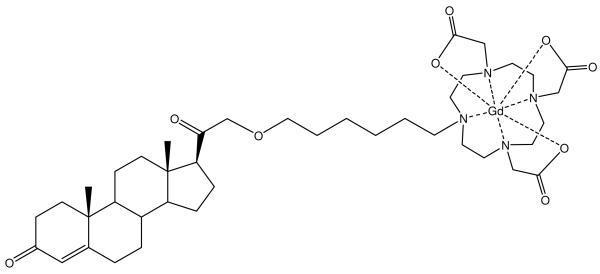
We have developed several targeted small-molecule steroid-conjugated Gd(III) chelates specific for hormone-receptor positive cells.45 The expression of progesterone receptor (PR) is a critical component in the diagnosis of human breast cancers and is typically measured by tissue biopsy or radioisotope injection. The recent development of progesterone-modified MRI contrast agents provides a non-invasive diagnostic tool to determine PR levels (Figure 7). In vitro results show that progesterone contrast agents interact favorably with progesterone receptors, accumulate in breast cancer cells, and enhance MRI contrast intensity. With the success of these in vitro results, research is underway for use of these agents in vivo.
Figure 7.
A progesterone Gd(III) chelate conjugate that is designed to image progesterone receptors for early detection of hormone related cancers.
Multimeric Conjugates for Signal Amplification
A number of approaches have been exploited to increase the signal intensity of MR agents by attachment to large molecules, proteins and particles.46-52 As a result of their larger size and numerous functional groups, additional capabilities can be included such as targeting and the ability to prepare multimodal probes. Not surprisingly, the biodistribution of these conjugates is very different than observed for small molecule agents.
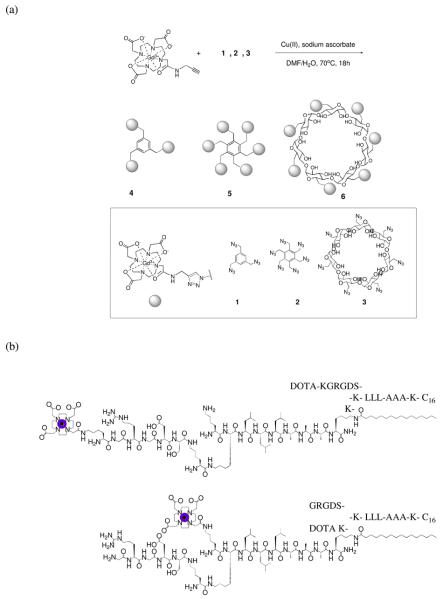
We have prepared a series of low molecular weight, mono-dispersed MR contrast agents with multiple Gd(III) complexes (Figure 10a).52 Click chemistry was employed to generate three pre-templated macromolecular architectures with multiple Gd(III) chelates and relatively rigid linkers. The relaxivities of the agents range from 17 to 85 mM−1s−1 per molecule. Cellular uptake is concentration dependent and cell viability is excellent. MR images of cell pellets reveal a marked increase in observed signal intensity.
Figure 10.
(a) Synthetic scheme of multimeric MR contrast agents via click chemistry. (b) The basic structure of labeled PAs containing three sections: a head-group, body, and an alkyl tail. The head-group is composed of an epitope for specific cell interaction and is presented on the outside of the supramolecular fibers.
The need for new biomaterials for tissue engineering and drug delivery applications has incorporated the use of self-assembling peptide amphiphiles (PAs).53 The resulting nanofiber networks produced from self-assembling PAs can be used as biomaterial scaffolds where specificity is imparted by the incorporation of peptide-epitopes. Labeling these materials with MR agents allows for long-term, in vivo fate mapping in whole animals. The PA derivatives were doped into various epitope bearing PA solutions and upon gelling resulted in a homogeneous biomaterial that is detectable by MR imaging (Figure 10b).50,51
A second approach to preparing biomaterials for tissue engineering combines biosynthetic and chemical methods to create high-contrast, multivalent, protein polymer-based MR contrast agents for in vivo applications. The labeled biomaterials are a family of multivalent, biomacromolecular, genetically engineered proteins with evenly spaced lysines that are derivatized with Gd(III) chelates.54 The protein length and repeating amino acid sequence are genetically specified and conjugates with an average of 8–9 Gd(III) chelators per protein were prepared. These multivalent CAs reproducibly have a relaxivity of 7.3 mM-1 s-1 per Gd(III) and 62.6 mM-1 s-1 per molecule. These derivatives can be incorporated into biomaterial hydrogels via chemical cross-linking of the remaining free lysines and provide a contrast enhancement for the in vivo evaluation of tissue engineering scaffolds.
Concluding Remarks
While advancements in technology have made MR imaging a modality of choice, the development of highly sensitive and multi-purpose MR contrast agents has begun to accelerate. The agents of today have numerous capabilities that were not available 10 years ago and include the ability to monitor in vivo gene expression and a number of metabolic processes. For example, the detection of biochemical events such as changes in ion concentration and enzyme activity has been realized.
In the last few years, significant strides have been made in the design and synthesis of cell-penetrating and receptor-targeted MR imaging agents. It is expected that these agents will allow for the realization of the full potential of MR imaging technology to provide a means to obtain three-dimensional images of opaque organisms that can report on physiological and metabolic processes.
Acknowledgements
We thank Dr. Amanda Eckermann for helpful discussions. This work was supported by the National Institutes of Health/NIBIB grant 1 R01 EB005866-01 and the Nanomaterials for Cancer Diagnostics and Therapeutics under Grant 5 U54 CA1193 41-02.
Biographies
Jody L. Major received her B.S. in Biochemistry in 2001 at the University of California, Santa Barbara. She pursued her Ph.D. degree at Northwestern University that was awarded in 2008 under the supervision of Professor Thomas J. Meade. Her work focused on the development of Zn(II)-activated contrast agents. She is currently a post-doctoral fellow at the University of California, San Diego. Her research interests are in the interface of chemistry and biology for the development of diagnostic probes and medicinal therapies.
Thomas Meade, Ph.D., Received BS in Chemistry, a Masters in Biochemistry and PhD in inorganic chemistry. After completing a NIH Postdoctoral fellowship at Harvard Medical School he was a postdoctoral fellow at the California Institute of Technology. In 1991 he joined the Division of Biology and the Beckman Institute at Caltech. In 2002 he moved to Northwestern University and is currently the Eileen Foell Professor of Chemistry, Biochemistry and Molecular and Cell Biology. His research focuses on bioinorganic coordination chemistry and its application in research that includes biological molecular imaging, electron transfer processes and the development of electronic biosensors for the detection of DNA and proteins and has founded three biotech companies, Clinical MicroSensors, Metaprobe and Ohmx.
References
- 1.Raymond KN, Pierre VC. Next Generation, High Relaxivity Gadolinium MRI Agents. Bioconjugate Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 2.Lauterbur PC. Image Formation by Incduced Local Interactions: Examples Employing Nuclear Paramagnetic Resonance. Nature. 1973;242:190–191. [PubMed] [Google Scholar]
- 3.Merbach A, Toth E. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging. John Wiley & Sons, Ltd.; New York: 2001. [Google Scholar]
- 4.Caravan P, Ellison JJ, McMurry TJ, Laufer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 5.Solomon I. Relaxation processes in a system of two spins. Physics Reviews. 1955;99:559–565. [Google Scholar]
- 6.Bloembergen N, Morgan LO. Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation. J. Chem. Phys. 1961;34:842–850. [Google Scholar]
- 7.Caravan P. Strategies for Increasing the Sensitivity of Gadolinium Based MRI Contrast Agents. Chem. Soc. Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 8.Moats RA, Fraser SE, Meade TJ. A “smart” magnetic resonance imaging agent that reports on specific enzymic activity. Angew. Chem. Int. Ed. 1997;36:726–728. [Google Scholar]
- 9.Alauddin MM, Louie AY, Shahinian A, Meade TJ, Conti PS. Receptor mediated uptake of a radiolabeled contrast agent sensitive to β-galactosidase activity. Nuclear Medicine and Biology. 2003;30:261–265. doi: 10.1016/s0969-8051(02)00392-x. [DOI] [PubMed] [Google Scholar]
- 10.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. In vivo visualization of gene expression using magnetic resonance imaging. 2000;18:321–325. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 11.Urbanczyk-Pearson LM, Femia FJ, Smith J, Parigi G, Duimstra JA, Eckermann AL, Luchinat C, Meade TJ. Mechanistic Investigation of β-Galactosidase-Activated MR Contrast Agents. Inorg. Chem. 2008;47:56–68. doi: 10.1021/ic700888w. [DOI] [PubMed] [Google Scholar]
- 12.Urbanczyk-Pearson LM, Meade TJ. Preparation of magnetic resonance contrast agents activated by β-galactosidase. Nature Protocols. 2008;3:341–350. doi: 10.1038/nprot.2007.529. [DOI] [PubMed] [Google Scholar]
- 13.Li WH, Parigi G, Fragai M, Luchinat C, Meade TJ. Mechanistic Studies of a Calcium-Dependent MRI Contrast Agent. Inorg. Chem. 2002;41:4018–24. doi: 10.1021/ic0200390. [DOI] [PubMed] [Google Scholar]
- 14.Li W-H, Fraser SE, Meade TJ. A Calcium-Sensitive Magnetic Resonance Imaging Contrast Agent. J. Am. Chem. Soc. 1999;121:1413–1414. [Google Scholar]
- 15.Major JL, Boiteau RM, Meade TJ. Investigation into the Mechanism of Zn(II)-Activated MR Imaging Contrast Agents. Inorg. Chem. 2008;47:10788–10795. doi: 10.1021/ic801458u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Major JL, Parigi G, Luchinat C, Meade TJ. The Synthesis and In Vitro Testing of a Zinc-Activated MRI Contrast Agent. Proc. Natl. Acad. Sci. 2007;104:13881–13886. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aime S, Barge A, Botta M, Howard JAK, Kataky R, Lowe MP, Moloney JM, Parker D, de Sousa AS. Dependence of the relaxivity and luminescence of gadolinium and europium amino-acid complexes on hydrogencarbonate and pH. Chem. Commun. 1999:1047–1048. [Google Scholar]
- 18.Zhang S, Wu K, Sherry AD. A Novel pH-Sensitive MRI Contrast Agent. Angew. Chem. Int. Ed. 1999;38:3192–3194. [PubMed] [Google Scholar]
- 19.Nivorozhkin AL, Kolodziej AF, Caravan P, Greenfield MT, Lauffer RB, McMurry TJ. Enzyme-activated Gd3+ magnetic resonance imaging contrast agents with a prominent receptor-induced magnetization enhancement. Angew. Chem. Int. Ed. 2001;40:2903–2906. doi: 10.1002/1521-3773(20010803)40:15<2903::AID-ANIE2903>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Malloy CR, Sherry AD. MRI Thermometry Based on PARACEST Agents. J. Am. Chem. Soc. 2005;127:17572–17573. doi: 10.1021/ja053799t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aime S, Botta M, Gianolio E, Terreno E. A p(O2)-responsive MRI contrast agent based on the redox switch of manganese(II/III)-porphyrin complexes. 2000;39:747–750. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Sun PZ, Schoening ZB, Jasanoff A. In vivo oxygen detection using exogenous hemoglobin as a contrast agent in magnetic resonance microscopy. Magn. Res. Med. 2003;49:609–614. doi: 10.1002/mrm.10405. [DOI] [PubMed] [Google Scholar]
- 23.Yoo B, Pagel MD. An overview of responsive MRI contrast agents for molecular imaging. Frontiers in Bioscience. 2008;13:1733–1752. doi: 10.2741/2796. [DOI] [PubMed] [Google Scholar]
- 24.Louie AY, Duimstra JA, Meade TJ. In: BRAIN MAPPING: The Methods. Mazziota J, Toga A, editors. Elsevier; 2002. pp. 819–828. [Google Scholar]
- 25.Duimstra JA, Femia FJ, Meade TJ. A Gadolinium Chelate for Detection of β-Glucuronidase: A Self-Immolative Approach. J. Am. Chem. Soc. 2005;127:12847–12855. doi: 10.1021/ja042162r. [DOI] [PubMed] [Google Scholar]
- 26.Dhingra K, Fouskova P, Angelowski G, Maier ME, Logothetis NK, Toth E. Towards Extracellular Ca2+ Sensing by MRI: Synthesis and Calcium-Dependent 1H and 17O Relaxation Studies of Two Novel Bismacrocyclic Gd3+ Complexes. J. Biol. Inorg. Chem. 2007 doi: 10.1007/s00775-007-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra A, Fouskova P, Angelowski G, Balogna E, Mishra AK, Logothetis NK, Toth E. Facile Synthesis and Relaxation Properties of Novel Bispolyazamacrocyclic Gd3+ Complexes: An Attempt Towards Calcium-Sensitive MRI Contrast Agents. Inorg. Chem. 2008;47:1370–1381. doi: 10.1021/ic7017456. [DOI] [PubMed] [Google Scholar]
- 28.Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: A Multipurpose Trace Element. Arch. Toxicol. 2006;80:1–9. doi: 10.1007/s00204-005-0009-5. [DOI] [PubMed] [Google Scholar]
- 29.Frederickson CJ, Cuajungco MP, Frederickson CJ. Is Zinc the Link Between Compromises of Brain Perfusion (Excitotoxicity) and Alzheimer's Disease? J. Alzheimers Dis. 2005;8:155–160. doi: 10.3233/jad-2005-8208. [DOI] [PubMed] [Google Scholar]
- 30.Takeda A. Movement of Zinc and Its Functional Significance in the Brain. Brain Res. Rev. 2000;34:137–48. doi: 10.1016/s0165-0173(00)00044-8. [DOI] [PubMed] [Google Scholar]
- 31.Vallee BL, Falchuk KH. The Biochemical Basis of Zinc Physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 32.Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of Zinc in the Central Nervous System: The Zinc-Containing Neuron. J. Nutr. 2000;130:1471S–1483S. doi: 10.1093/jn/130.5.1471S. [DOI] [PubMed] [Google Scholar]
- 33.Hanaoka K, Kikuchi K, Urano Y, Narazaki M, Yokawa T, Sakamoto S, Yamaguchi K, Nagano T. Design and Synthesis of a Novel Magnetic Resonance Imaging Contrast Agent for Selective Sensing of Zinc Ion. Chem. Biol. 2002;9:1027–1032. doi: 10.1016/s1074-5521(02)00216-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X.-a., Lovejoy KS, Jasanoff A, Lippard SJ. Water-Soluble Porphyrins as a Dual-Function Molecular Imaging Platform for MRI and Fluorescence Zinc Sensing. Proc. Natl. Acad. Sci. 2007;104:10780–10785. doi: 10.1073/pnas.0702393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frullano L, Tejerina B, Meade TJ. Synthesis and characterization of a doxorubicin-Gd(III) contrast agent conjugate: A new approach toward prodrug-procontrast complexes. Inorg. Chem. 2006;45:8489–8491. doi: 10.1021/ic0612045. [DOI] [PubMed] [Google Scholar]
- 36.Bhorade R, Weissleder R, Nakakoshi T, Moore A, Tung C-H. Macrocyclic Chelators with Paramagnetic Cations are Internalized into Mammalian Cells via a HIV-Tat Derived Membrane Translocation Peptide. Bioconjugate Chem. 2000;11:301–305. doi: 10.1021/bc990168d. [DOI] [PubMed] [Google Scholar]
- 37.Futaki S, Goto S, Sugiura Y. Membrane Permeability Commonly Shared Among Arginine-Rich Peptides. J. Mol. Recog. 2003;16:260–264. doi: 10.1002/jmr.635. [DOI] [PubMed] [Google Scholar]
- 38.Allen MJ, Meade TJ. Synthesis and Visualization of a Membrane-Permeable MRI Contrast Agent. J. Biol. Inorg. Chem. 2003;8:746–50. doi: 10.1007/s00775-003-0475-2. [DOI] [PubMed] [Google Scholar]
- 39.Allen MJ, MacRenaris KW, Venkatasubramanian PN, Meade TJ. Cellular delivery of MRI contrast agents. Chem. Biol. 2004;11:301–7. doi: 10.1016/j.chembiol.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Endres PJ, MacRenaris KW, Vogt S, Allen MJ, Meade TJ. Quantitative Imaging of Cell-Permeable Magnetic Resonance Contrast Agents Using X-Ray Fluorescence. Mol. Imaging. 2006;5:485–497. [PubMed] [Google Scholar]
- 41.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic Resonance Molecular Imaging of the Her-2/Neu Receptor. Cancer Res. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 42.Gustafsson B, Youens S, Louie AY. Development of Contrast Agents to Macrophage Scavenger Receptors for MRI of Vascular Inflammation. Bioconjugate Chem. 2006;17:538–547. doi: 10.1021/bc060018k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zigelboim I, Offen D, Melamed E, Panet H, Rehavi M, Cohen Y. Synthesis, Binding Affinity, and Relaxivity of Target-Specific MRI Contrast Agents. J. Incl. Phenom. Macro. 2007;59:323–329. [Google Scholar]
- 44.Kayyem JF, Kumar RM, Fraser SE, Meade TJ. Receptor-Targeted Co-Transport of DNA and Magnetic Resonance Contrast Agents. Chem. Biol. 1995;2:615–620. doi: 10.1016/1074-5521(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Burdette JE, MacRenaris KW, Mustafi D, Woodruff TK, Meade TJ. Rational Design, Synthesis, and Biological Evaluation of Progesterone-Modified MRI Contrast Agents. Chem. Biol. 2007;14:824–834. doi: 10.1016/j.chembiol.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. High Relaxivity Gadolinium Hydroxypyridonate-Viral Capsid Conjugates: Nanosized MRI Contrast Agents. J. Am. Chem. Soc. 2008;130:2546–2552. doi: 10.1021/ja0765363. [DOI] [PubMed] [Google Scholar]
- 47.Frullano L, Meade TJ. Multimodal MRI contrast agents. J. Biol. Inorg. Chem. 2007;12:939–949. doi: 10.1007/s00775-007-0265-3. [DOI] [PubMed] [Google Scholar]
- 48.Pan D, Caruthers SD, Hu G, Senpan A, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM. Ligand-Directed Nanobialys as Theranostic Agent for Drug Delivery and Manganese-Based Magnetic Resonance Imaging of Vascular Targets. J. Am. Chem. Soc. 2008;130:9186–9187. doi: 10.1021/ja801482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hueber MM, Staubli AB, Kustedjo K, Gray MHB, Shih J, Fraser SE, Jacobs RE, Meade TJ. Fluorescently Detectable Magnetic Resonance Imaging Agents. Bioconjugate Chem. 1998;9:242–249. doi: 10.1021/bc970153k. [DOI] [PubMed] [Google Scholar]
- 50.Bull SR, Guler MO, Bras RE, Meade TJ, Stupp SI. Self-assembled peptide amphiphile nanofibers conjugated to MRI contrast agents. Nano Lett. 2005;5:1–4. doi: 10.1021/nl0484898. [DOI] [PubMed] [Google Scholar]
- 51.Bull SR, Guler MO, Bras RE, Venkatasubramanian PN, Stupp SI, Meade TJ. Magnetic resonance imaging of self-assembled biomaterial scaffolds. Bioconjugate Chem. 2005;16:1343–8. doi: 10.1021/bc050153h. [DOI] [PubMed] [Google Scholar]
- 52.Song Y, Kohlmeir EK, Meade TJ. Synthesis of Multimeric MR Contrast Agents for Cellular Imaging. J. Am. Chem. Soc. 2008;130:6662–6663. doi: 10.1021/ja0777990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc. Natl. Acad. Sci. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karfeld LS, Bull SR, Davis NE, Meade TJ, Barron AE. Use of a Genetically Engineered Protein for the Design of a Multivalent MRI Contrast Agent. Bioconjugate Chem. 2007;18:1697–1700. doi: 10.1021/bc700149u. [DOI] [PMC free article] [PubMed] [Google Scholar]