Abstract
Purpose
Autoimmune phenomena during immunotherapy are associated with favorable outcomes for patients with metastatic renal cell carcinoma (RCC). We have reported improved survival for Stage IV RCC patients carrying autoimmunity-associated HLA class II haplotypes. We propose that the clinical benefit is mediated by products of other autoimmunity-associated genes linked to these haplotypes. One candidate gene is complement C4, which replicates as part of the RCCX module, can be present in multiple copies, and exists as C4A and C4B isoforms. Deficiencies of either isoform are associated with autoimmunity. The objective of this study was to test the hypothesis that C4A or C4B deficiency predicts improved survival for patients with RCC.
Materials and Methods
Total C4 copy number was determined by simultaneous amplification of RP1 and TNXA/RP2 to quantitate RCCX modules. C4A and C4B alleles were distinguished by PshAI RFLP.
Results
Genetic complotypes were determined for 61 patients. Individuals with a solitary copy of either C4 isoform experienced longer survival. The median survival from the diagnosis of metastatic disease for patients with a solitary copy of C4A or C4B was 7.75 years vs. 1.25 years for the comparison group (p = 0.001), and was independent of the benefit derived from autoimmune class II genotypes.
Conclusions
We conclude that improved survival is seen for RCC patients with C4A or C4B deficiency, treated with cytokine therapy with/without surgery. These data support our hypothesis that RCC patients with autoimmune genotypes have favorable outcomes resulting from autoimmune mechanisms directed to the tumor.
Keywords: Renal cell carcinoma, complement, C4, autoimmunity
INTRODUCTION
Metastatic renal cell carcinoma (RCC) is generally resistant to most forms of systemic therapy. There is, however, a small subset of RCC patients who respond to immunotherapy, and some of these patients are cured.1 A fascinating correlate of clinical response to immunotherapy in this disease is the development of autoimmune phenomena, described in RCC patients treated with virtually all immunotherapeutics, but most commonly reported with interferon-α, interleukin-2, and CTLA-4 antibody.2, 3 The spectrum of autoimmune events ranges from mild arthralgias to Hashimoto-like thyroid failure to potentially life-threatening colitis and hypophysitis. The well-documented correlation of these autoimmune phenomena with response and survival in RCC has led to speculation that anti-self mechanisms activated by the immunotherapeutic drugs are similarly directed to the metastatic tumor, resulting in tumoricidal effects. This concept also predicts that RCC patients who are genetically predisposed to autoimmunity are more likely to respond to immunotherapy and enjoy prolonged survival. Supporting this paradigm, we have reported significantly improved survival for stage IV RCC patients, treated with immunotherapy, who carry autoimmunity-associated HLA class II haplotypes.4 Mechanistically, there are two explanations for our findings: the first is optimal tumor antigen presentation by these class II molecules; the second is genetic linkage of the true effector mechanism to these class II haplotypes. In exploring the second explanation, we have proposed the complement component C4 as a potential mediator of the observed favorable clinical outcomes.
The C4 gene, located on chromosome 6 in the mid-MHC region, telomeric to the HLA class II loci, exists as a component of the four-gene RCCX module (RP1/2, C4, CYP21A/B, TNXA/B). The majority of individuals carries one, two, or three RCCX modules per chromosome 6, and thus may have from two to six copies of C4 per diploid genome.5 Adding to the diversity of C4 genotypes in the population are the two functionally distinct C4A and C4B isoforms. Proteolytic activation of the C4 protein, whether initiated by the classical or lectin activation pathway, leads to the generation of various anaphylatoxins, and, ultimately, to formation of the membrane attack complex which participates in damage to target cells and pathogens.6
The paradoxical association of a relative or absolute deficiency of either C4 isoform with human autoimmune disease is well documented.7, 8 Our theory of an association of autoimmunity-associated genotypes with improved outcomes for RCC patients predicts a beneficial effect from C4 deficiency. In the present study, we test the hypothesis that a low copy number of C4A or C4B predicts improved survival for RCC patients with Stage IV disease.
PATIENTS AND METHODS
Patients
The study was approved by the M. D. Anderson Cancer Center (MDACC) IRB and conducted according to HIPAA guidelines. Informed consent was obtained from all individuals. The study population was derived from the same 80 patient cohort included in our previous report4, of which 61 individuals had sufficient DNA remaining to complete the present study.
Determination of the C4 copy number
The total C4 copy number was determined for each patient by quantification of RCCX modules as described by Chung et al.9, with minor modification. This methodology utilizes simultaneous amplification of RP1 and a DNA fragment at the TNXA-RP2 junction. Each reaction included 200 ng of genomic DNA, 2.5 mM MgCl2, 600 nM RPa (forward primer for RP1), 600 nM RPb (reverse primer for RP1 and TNXA-RP2), and 100 nM TNXc (forward primer for TNXA-RP2). Primer sequences were: RPa, 5′CAAGAGAGGAGGCCTATCTTACCTGG3′; RPb, 5′GCTCAAGCTGTGAGGAGAACT3′; TNXc, 5′TATCACAGGCTCTGGCCCCA3′ Conditions were 94°C 5 minutes, 1 cycle; 94°C 45 sec, 59°C 45 sec, 72°C 60 sec, 32 cycles; 72°C 10 min, 1 cycle. PCR products of 1.2 kb (RP1) and 1.0 kb (TNXA-RP2) were digitally imaged and quantitated using Kodak Image Station 4000R (Eastman Kodak, Rochester, NY). The RP1 band was given a fixed value of two copies, and the TNXA-RP2 copy number was determined according to its ratio to RP1. The total C4 copy number was calculated as the sum of these two quantities.
Determination of the C4A:C4B allelic ratio
The methodology for determining the allelic ratio of the C4 isoforms was again based on the methods of Chung et al.9 A 1110 base pair (bp) fragment of the C4 gene was amplified with the following primers: Forward, 5′GCTCACAGCCTTTGTGTTGAA3′; Reverse, 5′TTGGGTACTGCGGAATCCCC3′. Conditions were 94°C 5 minutes, 1 cycle; 94°C 30 sec, 58°C 45 sec, 72°C 60 sec, 34 cycles; 72°C 7 min, 1 cycle. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA), and eluted in a volume of 50 ul. This purified product was subjected to one additional round of amplification using 32P-end-labeled reverse primer as follows: 94°C 5 minutes, 58°C 5 minutes, 72°C 10 minutes, 1 cycle. The labeled product was again purified as above and eluted in a volume of 30 ul. One half of the product (15 ul) was digested overnight with PshAI. Restriction products were separated by agarose gel electrophoresis, transferred to a nylon membrane, and subjected to autoradiography from which digital images were prepared. Bands representing C4A (970 bp) and C4B (1100 bp) were quantitated by ImageQuant Software (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
Survival was calculated from the diagnosis of metastatic disease. Survival curves were estimated using the Kaplan-Meier method. Log-rank test was used to compare survival curves of patients with different copy numbers of genes. Cox proportional hazards regression modeling was used to fit univariate and multivariate survival models and to identify the final model. All tests were two-sided and p-values of 0.05 or less were considered statistically significant. Statistical analysis was carried out using SAS version 9 (SAS Institute, Cary, NC) and S-Plus 7 (Insightful Inc., Seattle, WA).
RESULTS
Study population
C4 genotyping was performed for 61 patients with metastatic RCC (Table I). The study subjects included 16 females and 45 males, reflecting the gender distribution of this malignancy. All patients had been treated with interferon-alpha, interferon-gamma, and/or interleukin-2 on phase II clinical trials conducted between the years 1990 and 1997. The study population was selected to represent four distinct clinical groups based on outcome to treatment. Group 1 consisted of seven individuals with metastatic RCC who achieved a major response and disease-free status, and who have remained disease-free for more than seven years without further intervention. Group 2 consisted of 11 patients who achieved major responses but, unlike Group 1, suffered relapses requiring further medical or surgical intervention. Group 3 included 10 patients who did not experience significant tumor reduction as a consequence of cytokine therapy, but, regardless, enjoyed prolonged survival of greater than 5 years. Group 4 was composed of 33 patients whose disease progressed through therapy and who ultimately succumbed to metastatic disease.
Table I.
Characteristics of study population by group
| Group 1 Disease-Free n = 7 | Group 2 Response/Relapse n = 11 | Group 3 Stable Disease n = 10 | Group 4 Disease Progression n = 33 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Gender | Male | 5 | (71.4) | 7 | (63.6) | 6 | (60.0) | 27 | (81.8) |
| Female | 2 | (28.6) | 4 | (36.4) | 4 | (40.0) | 6 | (18.2) | |
| Race | Caucasian | 7 | (100.0) | 8 | (72.7) | 8 | (80.0) | 29 | (87.8) |
| Hispanic | 0 | (0.0) | 3 | (27.3) | 1 | (10.0) | 2 | (6.1) | |
| Black | 0 | (0.0) | 0 | (0.0) | 1 | (10.0) | 2 | (6.1) | |
| Stage IV Age (yrs) | Median | 52 | 47 | 61.5 | 50 | ||||
| Range | 40–70 | 40–70 | 35–74 | 30–65 | |||||
| No. disease sites | 1 | 4 | (57.1) | 10 | (90.9) | 6 | (60.0) | 14 | (42.4) |
| 2 | 3 | (42.9) | 0 | (0.0) | 4 | (40.0) | 13 | (39.4) | |
| ≥3 | 0 | (0.0) | 1 | (9.1) | 0 | (0.0) | 6 | (18.2) | |
| Lung, lymph node only | yes | 4 | (57.1) | 8 | (72.7) | 3 | (30.0) | 15 | (45.5) |
| no | 3 | (42.9) | 3 | (27.3) | 7 | (70.0) | 18 | (54.5) | |
| Treatment | IFN alone | 3 | (42.9) | 3 | (27.3) | 7 | (70.0) | 2 | (6.1) |
| IL-2 alone | 0 | (0.0) | 0 | (0.0) | 0 | (8.3) | 2 | (6.1) | |
| IL-2 + IFN | 4 | (57.1) | 8 | (72.7) | 3 | (30.0) | 26 | (78.8) | |
| IL2 + TNF | 0 | (0.0) | 0 | (0.0) | 0 | (0.0) | 3 | (9.0) | |
| Stage IV Surv (mos) | Median | 137 | 55 | 124 | 14.5 | ||||
| Range | 94+–167+ | 4–162+ | 62–184+ | 4–57 | |||||
Establishment of C4 complotypes
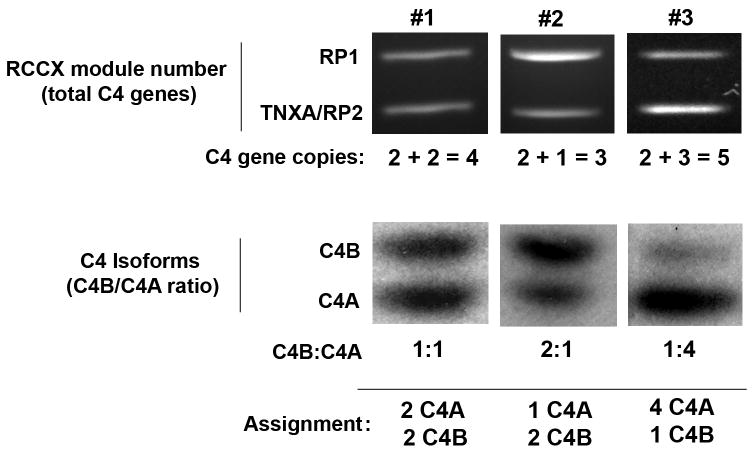
Determination of the complotype for each patient was a two-step process. First, the total copy number of C4 genes was established by enumerating RCCX modules. The first module on each chromosome 6 is initiated by the RP1 allele; subsequent modules begin with RP2 (Figure 1). RCCX quantitation was based on simultaneous PCR amplification of a fragment of RP1 and the junction of TNXA/RP2. Because the diploid genome contains exactly two RP1 genes, the RP1 PCR product was assigned a value of two gene copies. The TNXA/RP2 band, representing the additional RCCX modules, was quantitated relative to the two-copy RP1 band. Step two of the complotyping process established the copy number of the individual C4A and C4B isoforms by restriction fragment length polymorphism analysis. The isoform ratio was adjusted to the total C4 copy number to establish the total number of alleles of each isoform. An example of this calculation is demonstrated in Figure 2.
Figure 1.
Schematic diagram of the RCCX module. Each chromosome 6 can carry one, two, three, or (rarely) four consecutive modules. The RP gene of the first module is always the RP1 allele; if additional RCCX modules are present, they are initiated by RP2. Similarly, the final RCCX module ends with TNXB, whereas the others carry TNXA. Components of the RCCX modules are not drawn to scale.
Figure 2.
Methodology for C4 complotyping. Quantitation of RCCX modules yields the total C4 copy number (top panel). After determining C4B:C4A ratios (bottom panel), the complete complotype can be ascertained. Data are shown for three individual patients.
C4 complotype and survival
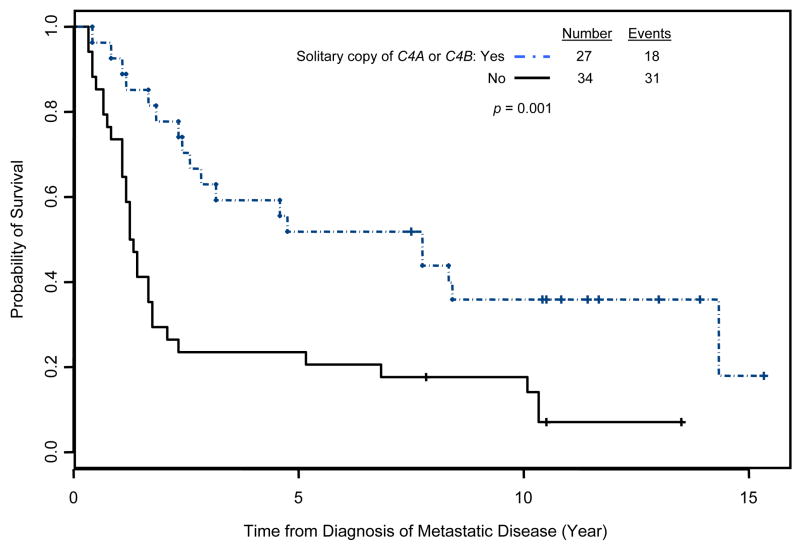
Our hypothesis predicted that C4 deficiency, whether manifested as a low total copy number or a deficiency of a given isoform, would be associated with improved outcomes. As shown in Table II, this was indeed the case. Four copies of C4 is the most common gene dose in the general population. RCC patients with C4 deficiency, defined as fewer than four copies, survived longer than those with four or more copies (7.75 vs. 1.67 years, p = 0.051). With regard to C4 isoform number, two copies of each is the norm. Preliminary analysis suggested that patients with exactly one copy of either C4A or C4B had prolonged survival. Because the survival times were similar, patients with a solitary copy of either isoform were combined into a single group for the final analysis. The median survival from the diagnosis of metastatic disease for patients with a solitary copy of C4A or C4B was 7.75 years vs. 1.25 years for the comparison group (p = 0.001, Fig 3). Similar results were found when survival was calculated from the initiation of treatment, with an identical p-value of 0.001 (data not shown).
Table II.
Summary of complotyping data.
| N (%) | Median survival in years from Stage IV diagnosis (95%CI) | p* | |
|---|---|---|---|
| Total C4 copy number | |||
| ≥ 4 | 46 (75.4) | 1.67 (1.25, 2.83) | |
| < 4 | 15 (24.6) | 7.75 (2.58, NA) | 0.051 |
| Solitary isoform copy | |||
| No | 34 (55.7) | 1.25 (1.17, 1.75) | |
| Yes | 27 (44.3) | 7.75 (2.83, NA) | 0.001 |
Log-rank test. NA, not applicable; CI, confidence intervals
Figure 3.
Improved survival for RCC patients with a solitary copy of either C4A or C4B. Survival is measured from the diagnosis of Stage IV disease.
A Cox proportional hazards model was used to carry out multivariate survival analysis with the total C4 gene copy number and C4 isoform data included in the model. The presence of a solitary isoform copy remained significant after adjusting for the total copy number; conversely, total copy number lost its borderline significant p-value (data not shown). Being the dominant factor associated with survival, isoform copy number was used for all additional analyses.
C4A/C4B Isoform Copy Number and Response to Immunotherapy
The association of isoform copy number with survival begged the question of whether this effect was mediated by therapeutic response, i.e., whether C4 isoform deficiency in some way improved sensitivity to immunotherapy, which then influenced survival. On first glance at the data in Table III, one might come to this conclusion. Rates of isoform deficiency differed significantly among the four clinical groups (p = 0.036, Fisher’s exact test), with the highest rates found in the patients with the best response (85.7%, Group 1) and the lowest rates in patients whose disease progressed through therapy (30.3%, Group 4). In contrast, however, the two intermediate groups displayed the opposite pattern. Group 3 patients, who lacked clinical response to immunotherapy but survived for years with longstanding stable disease, had a higher rate of isoform deficiency (60.0%) than Group 2 patients (45.5%) who responded to treatment, albeit transiently and/or incompletely. Thus, it appears that the influence of C4 isoform deficiency on survival is not entirely a function of major clinical therapeutic response.
Table III.
Association of isoform complotype with clinical group
| Number | Median survival in years from Stage IV diagnosis (range) | Number with solitary copy of either C4 isoform (%) | ||
|---|---|---|---|---|
| Group | 1 | 7 | 11.4+ (7.8+–13.9+) | 6 (85.7) |
| 2 | 11 | 4.6 (0.3–13.5+) | 5 (45.5) | |
| 3 | 10 | 10.3 (5.2–15.3+) | 6 (60.0) | |
| 4 | 33 | 1.3 (0.3–4.8) | 10 (30.3) |
C4A/C4B Isoform Copy Number and HLA Class II Haplotype
Previous data from our laboratory demonstrated that in this same patient population, improved survival was associated with the presence of components of either of two autoimmunity-associated HLA class II haplotypes: DRB1*0301/DQA1*0501/DQB1*0201 and DRB1*1501/DQA1*0102/DQB1*0602. The strongest survival benefit was associated with any combination of the two DQA1 alleles (*0102/*0501, *0501/*0501, or *0102/*0102).4 Because the C4 gene, in the mid-MHC region, is in the vicinity of the HLA class II locus, multivariate analysis was performed to determine if the survival benefit provided by a relative C4 isoform deficiency was independent of the presence of these autoimmune class II haplotypes. For the patient cohort included in the present study, the presence of two of the above DQA1 alleles was associated with improved survival from the diagnosis of metastatic disease (p = 0.035). However, when DQA1 and C4 isoform deficiency were included in multivariate analysis, the Cox proportional hazard model showed only C4 deficiency to be included in the final model, i.e., the copy number of C4 isoforms is a more dominant predictor of survival than the DQA1 genotype (Table IV).
Table IV.
Univariate and multivariate Cox proportional hazards regression models for survival from diagnosis of stage IV disease, incorporating the presence of autoimmune DQA1 alleles and solitary copies of the genes for C4A or C4B
| Univariate Model |
Multivariate Model |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | n | HR | 95% LL | 95% UL | p-value | HR | 95% LL | 95% UL | p-value |
| DQA1 alleles: both autoimmune | |||||||||
| No | 46 | 1.00 | 1.00 | ||||||
| Yes | 15 | 0.440 | 0.205 | 0.944 | 0.035 | 0.591 | 0.267 | 1.309 | 0.19 |
| Solitary copy of C4A or C4B | |||||||||
| No | 34 | 1.00 | 1.00 | ||||||
| Yes | 27 | 0.373 | 0.205 | 0.681 | 0.0013 | 0.428 | 0.229 | 0.800 | 0.0078 |
Abbreviations: HR, hazard ratio; 95% LL, lower limit of the 95% confidence interval; 95% UL, upper limit of the 95% confidence interval
DISCUSSION
Autoimmunity is a poorly understood dysregulation of self-tolerance. There is a clear genetic component, particularly with loci in the mid-MHC region of chromosome 6. We have previously reported that autoimmunity-associated HLA class II haplotypes are associated with improved outcomes for RCC patients after treatment with cytokine therapy. We now demonstrate an even stronger and independent influence associated with relative genetic deficiencies of C4A and C4B, established correlates of autoimmune disease. It is notable that the survival benefit provided by complement deficiency extended to patients who did not respond to immunotherapy, in contrast to the HLA class II effect which appeared to be limited to responding patients. This would indicate that the survival benefit derived from complement deficiency may not depend on a major reduction of tumor burden, but instead, may result from tumor growth inhibition. Whether the influence of C4 isoform deficiency on survival requires the presence of exogenous immune stimulation is an important question, but cannot be answered in the present study as all patients had received immunotherapy.
Intuitively, one would anticipate that the roles for C4 isoform deficiency are similar in autoimmune tissue destruction and in control of metastatic renal cancer. Unfortunately, mechanisms by which C4 deficiency contributes to autoimmunity are poorly understood and difficult to apply to the oncologic setting. One leading theory is based on the requirement for C4 for the solubilization and clearance of immune complexes, which otherwise circulate for prolonged periods of time and deposit in healthy tissues.10 A second theory, based primarily on animal models, derives from data suggesting that complement, bound to specific receptors on B-cells, is required for the induction and maintenance of self-tolerance, possibly by delivery of self-antigens to auto-reactive B-cells, which subsequently undergo deletion.11, 12 These theories are not mutually exclusive and both mechanisms could result in tumor damage, whether through the deposition of immune complexes or targeting by auto-reactive B-cells.
It is interesting to note that overt autoimmunity is overwhelmingly a disease of women, and the incidence of RCC in women is substantially lower than in men. The prevalence of overt autoimmunity has not been reported for patients with RCC. However, we predict that a clinically relevant renal cancer will develop only with difficulty in the presence of active autoimmune disease, and that the prevalence of rheumatologic disorders in the RCC population is likely to be lower than in the general population. In keeping with this prediction, none of the patients in the study cohort carried the diagnosis of an autoimmune disease.
A shortcoming of our study is the use of gene copy number as a surrogate for C4 protein levels. Although the major determinant of circulating C4 levels is, in fact, the gene copy number, the correlation is not exact, and other factors influence protein levels.5 Quantitation of circulating C4 protein and its isoforms is most commonly carried out by an immunofixation assay that performs best with fresh samples collected in EDTA.13 These requirements preclude protein complotyping of our present study cohort, the majority of whom are deceased. However, studies are currently being designed for prospective C4 protein determination for Stage IV RCC patients. We will also examine levels in early stage RCC patients to determine if there is a beneficial effect of relative C4 isoform deficiency in this patient subgroup as well. Additionally, it would be worthwhile to look for similar C4 deficiencies in long-surviving melanoma patients, another clinical group for which autoimmune phenomena are associated with therapeutic response.14
In conclusion, a body of data is accumulating from numerous studies supporting a role for autoimmune mechanisms in the control of advanced RCC. As the mechanisms of human autoimmunity are gradually deciphered, these findings should translate into improved clinical management of this malignancy.
Acknowledgments
The authors are indebted to Dr. C. Yung Yu and Dr. Joann Moulds for their assistance in the generation of preliminary data leading to this study, and for helpful discussions regarding C4 analysis. The outstanding technical work of Carolyn Cooke and Marilyn Johnson is greatly appreciated. Additionally, we would like to thank the many individuals who have generously provided philanthropic support as memorials to loved ones who have succumbed to kidney cancer. This work was supported by NIH CA111369 (JAE). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
References
- 1.Figlin RA. Renal cell carcinoma: management of advanced disease. J Urol. 1999;161:381. doi: 10.1016/s0022-5347(01)61897-4. [DOI] [PubMed] [Google Scholar]
- 2.Franzke A, Peest D, Probst-Kepper M, Buer J, Kirchner GI, Brabant G, et al. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J Clin Oncol. 1999;17:529. doi: 10.1200/JCO.1999.17.2.529. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellerhorst JA, Hildebrand WH, Cavett JW, Fernandez-Vina MA, Hodges S, Poindexter N, et al. Heterozygosity or homozygosity for two HLA class II haplotypes predict favorable outcomes for renal cell carcinoma treated with cytokine therapy. J Urol. 2003;169:2084. doi: 10.1097/01.ju.0000065810.80617.f4. [DOI] [PubMed] [Google Scholar]
- 5.Blanchong CA, Chung EK, Rupert KL, Yang Y, Yang Z, Zhou B, et al. Genetic, structural and functional diversities of human complement components C4A and C4B and their mouse homologues, Slp and C4. Int Immunopharmacol. 2001;1:365. doi: 10.1016/s1567-5769(01)00019-4. [DOI] [PubMed] [Google Scholar]
- 6.Isenman DE. The Complement FactsBook. In: Morley BJ, Walport MJ, editors. C4. San Diego: Academic Press; 2000. pp. 95–103. [Google Scholar]
- 7.Yang Y, Chung EK, Zhou B, Lhotta K, Hebert LA, Birmingham DJ, et al. The intricate role of complement component C4 in human systemic lupus erythematosus. Curr Dir Autoimmun. 2004;7:98. doi: 10.1159/000075689. [DOI] [PubMed] [Google Scholar]
- 8.Gilliam BE, Wolff AE, Moore TL. Partial C4 deficiency in juvenile idiopathic arthritis patients. J Clin Rheumatol. 2007;13:256. doi: 10.1097/RHU.0b013e318156b9e3. [DOI] [PubMed] [Google Scholar]
- 9.Chung EK, Yang Y, Rupert KL, Jones KN, Rennebohm RM, Blanchong CA, et al. Determining the one, two, three, or four long and short loci of human complement C4 in a major histocompatibility complex haplotype encoding C4A or C4B proteins. Am J Hum Genet. 2002;71:810. doi: 10.1086/342778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis MJ, Botto M. Complement deficiencies in humans and animals: Links to autoimmunity. Autoimmunity. 2006;39:367. doi: 10.1080/08916930600739233. [DOI] [PubMed] [Google Scholar]
- 11.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot AM, et al. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 12.Carroll MC. A protective role for innate immunity in systemic lupus erythematosus. Nat Rev Immunol. 2004;4:825. doi: 10.1038/nri1456. [DOI] [PubMed] [Google Scholar]
- 13.Mauff G, Luther B, Schneider PM, Rittner C, Stradmann-Bellinghausen B, Dawkins R, et al. Reference typing report for complement component C4. Exp Clin Immunogenet. 1998;15:249. doi: 10.1159/000019079. [DOI] [PubMed] [Google Scholar]
- 14.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]