Abstract
Background:
Maintenance chemotherapy is not routinely used in gastrointestinal (GI) cancers. Capecitabine is an oral formulation that is enzymatically converted to 5-fluorouracil preferentially in tumor tissue. We hypothesize that capecitabine could be used as a long-term maintenance therapy to improve outcomes in patients with high-risk GI cancers following standard chemotherapy regimens.
Methods:
We conducted a retrospective study to assess the toxicity of maintenance capecitabine in 28 patients with a variety of advanced GI malignancies. Capecitabine 1,000 mg twice daily without interruption was used for the first 11 patients. The dose was reduced to 1,000 mg twice daily 5 days per week in 8 patients who developed hand-foot syndrome. The remaining patients began treatment on the same abbreviated schedule. All documented clinical adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (v3.0, 2003).
Results:
Main toxicities were grade 1/2 fatigue and hand-foot syndrome. Only one grade 3 toxicity was observed and no grade 4 toxicities were seen. We also observed a significant increase in red blood cell mean corpuscular volume in participants, which may have potential use as a biomarker to monitor therapeutic response.
Conclusions:
Fixed therapeutic doses of oral capecitabine 1,000 mg twice daily, 5 days on, 2 days off, can be administered chronically with a high level of safety and should be explored in larger prospective studies to demonstrate efficacy in GI malignancies, especially pancreatic and metastatic colorectal cancers.
While an accepted modality in other cancer types, maintenance therapy in gastrointestinal (GI) cancers is not routinely delivered. Current standards are to administer a set number of chemotherapy cycles in the adjuvant setting followed by observation until disease progression. In the setting of metastatic colon cancer, combination therapy incorporating newer cytotoxic and biologic agents has changed practice in the past several years, extending the median survival of patients with metastatic disease to beyond 20 months. Advancements in neoadjuvant and adjuvant chemotherapy regimens and improvements in surgical techniques for liver and lung metastasectomy have offered patients with metastatic colon cancer the possibility of cure. Yet, to date, no therapeutic approaches have been defined for these patients after potentially curative surgeries and adjuvant chemotherapy. Given patient preferences for more tolerable regimens and improved quality of life, treatment approaches have changed from continuous high-dose aggressive therapy until disease progression to either chemotherapy-free intervals or reduced-dose, less-toxic maintenance regimens.1,2
The concept of maintenance therapy in gastrointestinal cancers has been explored using infusional 5-fluorouracil (5-FU), gemcitabine, erlotinib, marimastat, and oral S-1 in small clinical trials with positive results.3–8 Substantial evidence suggests that chronically administered intravenous 5-FU is safe and has antitumor activity against epithelial malignancies involved in gastrointestinal, breast, and head and neck malignancies.9 In the OPTIMOX2 study, it was shown that in terms of progression-free survival, maintenance therapy with leucovorin/5-FU was superior to a chemotherapy-free interval after FOLFOX7 administration.10
While continuous-infusion 5-FU is cumbersome when used as a maintenance agent, oral 5-FU derivatives are certainly more feasible options. Long-term maintenance therapy with 2 years of the oral 5-FU derivative UFT (uracil and tegafur) has been studied in stage I adenocarcinoma of the lung and shown overall survival benefits with only 2% of patients developing grade 3 toxic effects.11 In another study, UFT was used as adjuvant therapy for 12 months in serosa-positive gastric cancer.12 Though the study failed to show any survival benefit, only 2% of patients studied experienced grade 4 toxicity. Compared to other oral 5-FU derivatives such as S-1 and UFT, capecitabine is approved by the US Food and Drug Administration (FDA) and has been extensively studied in GI and breast malignancies. Capecitabine has been shown to be a safe and efficacious alternative to bolus 5-FU for metastatic colorectal cancer in phase III clinical trials.13,14 However, the safety profile and efficacy of capecitabine as a long-term maintenance therapy in GI malignancies has yet to be evaluated.15
Capecitabine is an oral 5-FU prodrug that is modified via a different metabolic pathway compared to other oral 5-FU derivatives. Capecitabine is absorbed intact through the gastrointestinal mucosa and is metabolized in the liver to 5′-deoxy-5-fluorocytidine (5′-DFCR), then to 5′-deoxy-5-fluorouridine (5′-DFUR). Subsequently, 5′-DFUR is converted to 5-FU by the enzyme thymidine phosphorylase (TP) at the tissue level. TP is known to be present in significantly higher concentrations in cancer cells than in plasma or surrounding normal tissue, thus generating a greater effect at the level of the tumor and sparing many of the side effects seen from the systemic activity of 5-FU.16 Compared to intravenous 5-FU, capecitabine is associated with a lower incidence and severity of diarrhea, stomatitis, nausea, and neutropenia but an increased rate of hand-foot syndrome.
The FDA-approved dosage of oral capecitabine for both metastatic colorectal and breast cancer is 2,500 mg/m2 divided into two equal daily doses for the first 2 weeks of a 3-week cycle. The original study by Van Cutsem divided the study participants into three capecitabine treatment groups based on three prior phase I studies showing the maximum tolerated dose for each dosing regimen.17 Arm A was continuous monotherapy with capecitabine 1,331 mg/m2/day divided into two equal doses per day. Arm B was intermittent monotherapy with capecitabine at 2,510 mg/m2/day divided into two equal daily doses on the intermittent schedule now approved by the FDA. Arm C patients received intermittent therapy with capecitabine 1,657 mg/m2/day combined with oral leucovorin 60 mg/day, with both drugs divided into equal twice-daily doses.
No significant difference in activity was observed among the three regimens in terms of response rates. As for tolerability and safety, there were fewer grade 3 adverse events and no grade 4 adverse events in the continuous monotherapy arm (A), compared to arms B and C. Of the grade 3 diarrhea events, those occurring in arm A did so at a significantly later time than those in arms B and C. The cumulative capecitabine dose was highest in the intermittent monotherapy arm (B).
Since capecitabine’s antitumor effect in human xenograph models is dependent on total dose instead of dosing schedule, the intermittent monotherapy schedule that would provide the highest cumulative dose was further investigated. Subsequent studies led to the current approved regimen of capecitabine.17 In actual practice, this FDA-approved dosing schedule is rarely used, due to intolerable, dose-limiting hand-foot syndrome. Given the similar efficacy and superior safety profile of continuous dosing, we would argue that such dosing (arm A) warrants further investigation as a maintenance regimen.
For simplicity of patient use in maintenance therapy, a continuous fixed dose of capecitabine was chosen. Body surface area (BSA) was not incorporated in the dosing schedule, as its validity in humans has not been evaluated. The importance of BSA in allometric scaling was first described as a means to extrapolate dosing in lower mammals, such as rodents, to dosing in humans.18 The concept of dosing based on BSA was then carried through to phase II and III studies of humans without scientific evaluation of its validity. While studies have yet to be performed evaluating fixed dosing of capecitabine, other chemotherapeutics such as irinotecan, paclitaxel, and epirubicin have been investigated.19–21 When examined, no difference was observed in severity of toxicity or in response to therapy based on body size or BSA. Based on the lack of scientific evidence to support BSA-based dosing and the inherent risk of medical errors due to potential miscalculations, we elected to use a regimen of fixed-dose capecitabine 1,000 mg twice daily. It was felt that this dose and schedule represented the simplest regimen to administer that approximated the dosing used in arm A of the Van Cutsem study.
The use of biomarkers in oncology is a rapidly developing field. There is great interest in finding ways to detect malignancy before it becomes clinically evident and to monitor tumor response to treatment. Several such biomarkers, such as prostate specific antigen (PSA), carcinoembryonic antigen (CEA), and alpha-fetoprotein (AFP), have improved our ability to screen for cancer as well as to follow the disease course. Yet these measures are by no means perfect and the search for a biomarker that can accurately gauge biologic effect continues. In our analysis, we retrospectively evaluated patients’ basic laboratory tests looking for abnormalities that might plausibly be attributed to the effects of capecitabine. Of particular interest, previous studies have noted an increase in mean corpuscular volume (MCV) in patients taking capecitabine.22,23
We conducted this retrospective study to determine the safety profile of chronic fixed low-dose capecitabine as a maintenance adjuvant therapy in patients with high-risk GI malignancies. The purpose of this study was to evaluate the toxicity of such a regimen in patients with advanced GI malignancies, including cancers of the pancreas, stomach, gallbladder, bile ducts, and metastatic colorectal cancer.
PATIENTS AND METHODS
Patient medical records were used to obtain the data analyzed in this study. Records of patients who received the above-described capecitabine maintenance regimen at the Lombardi Comprehensive Cancer Center in Washington, DC, from March 2000 to July 2008 were reviewed. Given the retrospective nature of this study and the fact that no actual patient contact was required, the requirement for informed consent was waived by the Institutional Review Board.
All reported patients had histologically confirmed advanced or metastatic pancreatic, gallbladder, bile duct, gastric, or colorectal cancer. All patients had previously received accepted chemotherapy regimens in either the adjuvant or metastatic setting, radiotherapy, surgical resection of the primary tumor, and/or metastasectomy as indicated for their disease status. Patients reported in this study had no evidence of disease or had stable disease at the time maintenance therapy was initiated. All patients with colorectal cancer had stage IV disease with metastasis to the liver, lungs, adrenal gland, pelvis, abdominal lymph nodes, uterus, or ovaries. Eight of eleven patients with metastatic colorectal cancer had complete resection of the metastases and were without any evidence of disease before starting the capecitabine therapy. Three patients with metastatic colorectal cancer to liver, lungs, and bone did not have complete resection of metastases, but their disease was considered stable at the time maintenance therapy with capecitabine was initiated. The end point of the capecitabine treatment was disease progression.
A regimen of capecitabine 1,000 mg, twice daily, 7 days per week was used in the first 11 patients. Eight of these patients eventually developed hand-foot syndrome at 8 to 12 weeks of therapy. As a result, the capecitabine regimen for these 8 patients was modified to 1,000 mg twice daily, Monday through Friday, with Saturday and Sunday off. Likewise, due to the observed toxicities in the initial patients, the remaining patients began treatment with capecitabine at the reduced dosage schedule, except for one person who was started at 500 mg twice daily due to a prior history of colitis while receiving concurrent capecitabine and radiotherapy. The dose level for three of the patients who started capecitabine at 1,000 mg bid, 5 days on, 2 days off, was subsequently reduced to 500 mg twice daily at the same schedule due to grade 2 hand-foot syndrome at 1, 2, and 5 months, respectively.
All patients underwent a comprehensive medical evaluation prior to chemotherapy, including medical history, physical examination, complete blood cell counts, comprehensive metabolic panels (including liver function tests), and tumor staging according to pathology reports and computed tomography (CT) studies. All patients were followed for adverse events and disease progression with detailed histories, physical examinations, and laboratory and radiographic testing as clinically indicated. All documented clinical adverse events were graded in accordance with the Common Terminology Criteria for Adverse Events (v3.0, 2003) as implemented by the Cancer Therapy Evaluation Program of the National Cancer Institute. Grade 1 to 2 toxicities were classified as mild to moderate and grade 3 to 4 as severe. Hand-foot syndrome was classified as grade 1, painless mild skin changes; grade 2, pain or skin changes including peeling, blisters, bleeding, edema not affecting daily function; grade 3, painful skin changes affecting daily function.
Statistical Methods
Adverse events were tabulated in grade categories of 1 and 2 vs. 3 and 4. Differences in toxicities and mean corpuscular volume (MCV) elevation were compared between younger (< 65 years) and older (≥ 65 years) patients, between genders, and between high (≥ 1.725) and low (< 1.725) body surface area (BSA) using Fisher’s exact test to accommodate small sample sizes. Means and 95% confidence intervals (95% CI) were calculated assuming a normal distribution for MCV and hemoglobin measurements. Percent change was tested with a one-sample t-test for the percent change of MCV and hemoglobin level between baseline and peak MCV on treatment.
RESULTS
Patient Population
Data from 28 patients were analyzed in this retrospective study. The earliest record included in the analysis is of a patient who began treatment on 1/31/2003; the most recent started treatment on 1/13/2006. Patients were being treated for a variety of GI malignancies, including pancreatic cancer (n = 10), cancer of the gallbladder and bile ducts (n = 4), gastric cancer (n = 3), or metastatic colorectal cancer (n = 11). Ten patients were over 65 years old. All patients had Eastern Cooperative Oncology Group performance status 0 to 1. Patient characteristics and previous chemotherapy regimens are summarized in Tables 1 and 2, respectively.
Table 1.
Patient characteristics
| Patient | Age at treatment start | Stage | Gender | BSA (m2) | Duration of therapy (weeks) | MCV peak (fL) |
|---|---|---|---|---|---|---|
| MCRC | ||||||
| 1 | 44 | 4 | M | 1.87 | 23 | 91.3 |
| 2 | 49 | 4 | M | 1.73 | 6.7 | 93 |
| 3 | 52 | 4 | F | 1.84 | 93.1 | 104.9 |
| 4 | 74 | 4 | F | 1.51 | 13.5 | 102.8 |
| 5 | 62 | 4 | F | 1.71 | 97 | 102.8 |
| 6 | 60 | 4 | M | 1.97 | 38 | 96.1 |
| 7 | 52 | 4 | F | 1.7 | 16 | 113.3 |
| 8 | 51 | 4 | M | 2.1 | 12.9 | 93.7 |
| 9 | 60 | 4 | M | 2.04 | 56.4 | 105.7 |
| 10 | 57 | 4 | F | 1.43 | 78 | 119.5 |
| 11 | 52 | 4 | M | 2.15 | 33.9 | 107.1 |
| Pancreatic | ||||||
| 12 | 71 | 1B | F | 1.79 | 111.9 | 110.5 |
| 13 | 72 | 3 | F | 1.42 | 163 | — |
| 14 | 58 | 3 | F | 1.6 | 47 | — |
| 15 | 66 | 2B | M | 1.88 | 91.4 | 128.3 |
| 16 | 54 | 2B | F | 1.59 | 42 | 100 |
| 17 | 77 | 1B | M | 1.76 | 57 | 94.8 |
| 18 | 70 | 3 | M | 1.83 | 22.4 | 107.4 |
| 19 | 81 | 4 | F | 1.47 | 35 | 109.3 |
| 20 | 38 | 1A | F | 1.57 | 63 | 110.9 |
| 21 | 43 | 2B | M | 2.05 | 97.6 | 94.8 |
| Biliary | ||||||
| 22 | 41 | 2B | M | 1.71 | 10.7 | 90.1 |
| 23 | 53 | 4 | F | 1.48 | 56 | 105.4 |
| 24 | 68 | 1B | F | 1.73 | 76.9 | 99.7 |
| 25 | 42 | 4 | F | 1.51 | 18.6 | 95.3 |
| Gastric | ||||||
| 26 | 79 | 4 | M | 1.51 | 10 | 98.5 |
| 27 | 57 | 4 | M | 2.14 | 13 | — |
| 28 | 60 | 4 | M | 1.83 | 9.4 | 104.5 |
Abbreviations: BSA = body surface area; MCV = mean corpuscular volume; MCRC = metastatic colorectal cancer
Table 2.
Previous systemic chemotherapy regimens
| Patient | Diagnosis | Previous systemic chemotherapy |
|---|---|---|
| 1 | MCRC | FOLFOX, bevacizumab |
| 2 | MCRC | XELIRI, bevacizumab |
| 3 | MCRC | FOLFIRI, XELOX |
| 4 | MCRC | FOLFOX, capecitabine |
| 5 | MCRC | FOLFIRI, bevacizumab |
| 6 | MCRC | FOLFIRI, bevacizumab |
| 7 | MCRC | FOLFIRI, bevacizumab |
| 8 | MCRC | IFL, FOLFOX, imatinib, paclitaxel, cetuximab, bevacizumab |
| 9 | MCRC | FOLFOX, XELIRI, bevacizumab |
| 10 | MCRC | FOLFIRI, XELOX |
| 11 | MCRC | FOLFOX, XELIRI, bevacizumab |
| 12 | Pancreatic | Gemcitabine, capecitabine with radiation |
| 13 | Pancreatic | Capecitabine with radiation, gemcitabine |
| 14 | Pancreatic | Capecitabine with radiation, gemcitabine, bevacizumab |
| 15 | Pancreatic | Gemcitabine, capecitabine with radiation |
| 16 | Pancreatic | Capecitabine with radiation, gemcitabine |
| 17 | Pancreatic | Gemcitabine |
| 18 | Pancreatic | Capecitabine with radiation, gemcitabine |
| 19 | Pancreatic | Gemcitabine, capecitabine with radiation |
| 20 | Pancreatic | Gemcitabine, capecitabine with radiation |
| 21 | Pancreatic | Gemcitabine |
| 22 | Biliary | FOLFOX, bevacizumab |
| 23 | Biliary | Gemcitabine, capecitabine, FOLFOX, bevacizumab |
| 24 | Biliary | None |
| 25 | Biliary | None |
| 26 | Gastric | Etoposide, cisplatin, 5-FU |
| 27 | Gastric | Epirubicin, oxaliplatin, capecitabine |
| 28 | Gastric | Capecitabine, oxaliplatin |
Abbreviations: MCRC = metastatic colorectal cancer; FOLFOX = folinic acid (leucovorin)/5-fluorouracil (5-FU)/oxaliplatin; XELIRI = capecitabine/irinotecan; FOLFIRI = folinic acid (leucovorin)/5-FU/irinotecan; IFL = irinotecan/folinic acid/5-FU; XELOX = capecitabine/oxaliplatin
Safety Profile
The most frequent treatment-related adverse events are listed in Table 3. The most common treatment-emergent side effect reported was hand-foot syndrome in 20 patients (71%), only one of whom experienced grade 3 toxicity. No other grade 3 or 4 toxicities were observed. Fatigue, diarrhea, anorexia, and neutropenia were the next most common adverse events in 16 (57%), 6 (21%), 4 (14%), and 3 (11%) patients, respectively. Laboratory abnormalities associated with long-term capecitabine therapy were limited to grade 1 and 2 neutropenia, anemia, and hyperbilirubinemia (Table 3). The incidence of toxicity did not differ significantly by age group (< 65 vs. ≥65), gender, or BSA (< 1.725 vs. ≥ 1.725) (Table 4).
Table 3.
Frequencies of adverse events for all patients
| Adverse events | Grade | Total (%) | |
|---|---|---|---|
| 1/2 | 3/4 | ||
| Hand-foot syndrome | 19 | 1 | 20 (71) |
| Fatigue | 16 | 0 | 16 (57) |
| Diarrhea | 6 | 0 | 6 (21) |
| Anorexia | 4 | 0 | 4 (14) |
| Neutropenia | 3 | 0 | 3 (11) |
| Abdominal pain | 2 | 0 | 2 (7) |
| Elevated bilirubin | 2 | 0 | 2 (7) |
| Mucositis | 2 | 0 | 2 (7) |
| Anemia | 1 | 0 | 1 (4) |
| Nausea | 1 | 0 | 1 (4) |
| Vomiting | 1 | 0 | 1 (4) |
Table 4.
Incidence of adverse events by body surface area (BSA)
| Adverse events | BSA < 1.725 (m2) n =13 (%) | BSA ≥ 1.725 (m2) n = 15 (%) |
|---|---|---|
| Hand-foot syndrome | 8 (62) | 12 (80) |
| Fatigue | 8 (62) | 8 (53) |
| Diarrhea | 3 (23) | 3 (20) |
| Anorexia | 2 (15) | 2 (13) |
| Neutropenia | 2 (15) | 1 (7) |
| Abdominal pain | 1 (8) | 1 (7) |
| Elevated bilirubin | 2 (15) | 0 (0) |
| Mucositis | 1 (8) | 1 (7) |
| Anemia | 1 (8) | 0 (0) |
| Nausea | 1 (8) | 0 (0) |
| Vomiting | 1 (8) | 0 (0) |
| ↑ Mean corpuscular volume | 9 (75) | 7 (50) |
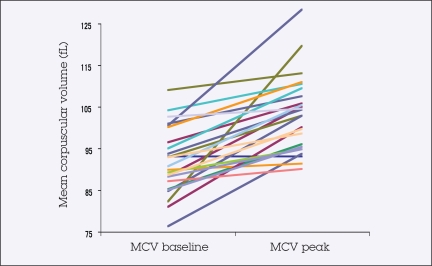
A significant rise in MCV in the absence of progressive anemia was noted in the majority of patients treated. MCV data, available for 26 of 28 patients, revealed an average peak increase of 12.9% over baseline on treatment (P < .0001; 95% CI, 8.9%–16.9%). The mean peak MCV level for all patients during therapy was 103.4 fL (Figure 1). Peak MCV on treatment occurred at a mean of 189.6 days (range, 125.9–253.3). Hemoglobin level did not reduce with increased MCV, but actually showed an average increase of 4.3% (range, 2.2%–10.7%) between baseline and peak MCV, which was not significant (P = .19). Sixteen patients (62%; range, 41%–80%) developed elevated MCV levels, defined as ≥ 100 fL. Elevation in MCV did not differ significantly by age or BSA, but it did differ by gender, with 38% of men compared to 79% of women experiencing elevations (P = .04) (Tables 1 and 5).
Figure 1.
Change in mean corpuscular volume (MCV) with capecitabine treatment
Table 5.
Changes in mean corpuscular volume (MCV) and hemoglobin (Hgb) during treatment*
| Blood measurements | No. patients | Mean (95% CI) |
|---|---|---|
| MCV at start (fL) | 26 | 91.9 (88.8–95.0) |
| MCV peak on treatment (fL) | 26 | 103.4 (99.8–107.0) |
| Time to MCV peak volume on treatment (days) | 26 | 189.6 (125.9–253.3) |
| Hgb at start (g/dL) | 26 | 12.2 (11.8–12.7) |
| Hgb at MCV peak (g/dL) | 25 | 12.7 (12.0–13.4) |
| % change in Hgb between peak and start | 25 | 4.3 (−2.2–10.7) |
The Pearson Correlation Coefficient is −0.031 (P = .88) between MCV peak on treatment and hemoglobin at MCV peak and −0.015 (P = .94) for percent changes in MCV and Hgb.
Dose Modifications
Dose reduction was required in 11 of 28 patients (32%) receiving capecitabine maintenance therapy, as detailed in the Patients and Methods section. The most common adverse events that contributed to dose reductions were hand-foot syndrome (67%), fatigue (22%), and abdominal pain (11%).
Patient Outcomes
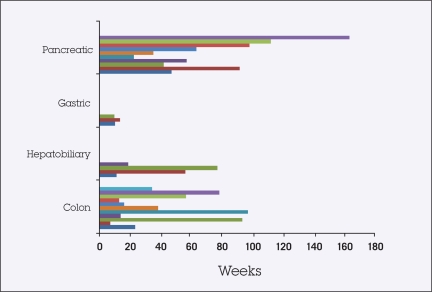
The median duration of therapy was 45 weeks (range, 6.7–111.8 weeks), with 13 patients (6 of 10 with pancreatic cancer, 5 of 11 with metastatic colorectal, 0 of 3 with gastric cancer, and 2 of 4 with gallbladder and bile duct cancer) continuing maintenance capecitabine treatment without any evidence of disease progression at the completion of this analysis (Figure 2). Capecitabine therapy was stopped in 13 patients due to disease progression. One patient with stable disease was switched to a different treatment regimen due to the availability of other clinical trials at the time. One patient was hospitalized for pneumonitis due to prior radiotherapy and discontinued treatment. No patients were withdrawn from therapy due to adverse events. There were no patient deaths while maintained on capecitabine therapy.
Figure 2.
Duration of treatment
DISCUSSION
The most common dose-limiting adverse effects reported to be associated with capecitabine monotherapy are hyperbilirubinemia, diarrhea, and hand-foot syndrome. Myelosuppression, fatigue/weakness, abdominal pain, and nausea have also been reported. Compared with bolus 5-FU, capecitabine is associated with more hand-foot syndrome but less stomatitis, alopecia, diarrhea, nausea, and neutropenia requiring medical intervention.24 These toxicities have been reported in a large number of clinical trials involving breast and colorectal cancer. Our study confirms the above adverse-effect profile. Yet by using fixed lower doses of capecitabine in a continuous manner, we were able to limit grade 3/4 toxicities significantly. The regimen was well tolerated with no withdrawals due to adverse events. This is in contrast to standard-dose capecitabine, which is associated with a 13% discontinuance rate due to side effects.24
The main toxicities in this study were grade 1/2 fatigue and hand-foot syndrome. Only one grade 3 toxicity (hand-foot syndrome) was observed. In contrast, the incidence of grade 3 hand-foot syndrome with standard dosing of capecitabine ranges from 16% to 44%.14,17 In addition, this fixed-dosing maintenance regimen was well tolerated among patients older than 65 years. There was no difference in frequency of adverse events based on gender or BSA. This result is supportive of our argument that BSA calculation is less important, if not entirely unnecessary, when administering this maintenance regimen of capecitabine.
In our series, patients with pancreatic and metastatic colorectal cancer were noted to have longer progression-free survival with capecitabine treatment in comparison to other GI malignancies. However, given the diverse study population, small sample size, and retrospective nature of the analysis, no conclusions on therapeutic response can be drawn. Larger, prospective studies are needed to assess the time to progression with this fixed-dose maintenance regimen.
Mean corpuscular volume is a measure of average red blood cell volume, which is dependent on the production of DNA and proteins during cell maturation, which in turn is controlled by a multitude of essential enzymatic reactions. The elevation in MCV observed in this study has also been reported in patients on standard-dose capecitabine regimens either as monotherapy or in combination with other chemotherapeutics.22,23 In one study, 154 patients with various advanced-stage cancers being treated with capecitabine-containing regimens at a dose of 2,500 mg/m2 divided into two daily doses following the 2 weeks on, 1 week off schedule were followed over 9 weeks. A statistically significant increase in MCV was observed up to 92.2 fL. Hemoglobin levels did not change over that time period and a subset of patients observed prospectively (n = 39) were not found to have vitamin B12, folic acid, or homocysteine deficiencies. When comparing the rise of MCV with response to capecitabine, there was a statistically significant difference in rise of MCV between patients who responded to therapy and those who progressed on therapy.
Capecitabine’s cytotoxic effect is mainly due to inhibition of thymidylate synthase (TS). This is mediated by the 5-FU metabolite 5-fluoro-2′-deoxyuridine 5′-monophosphate (FdUMP), which blocks the production of thymidylate (dTMP), an essential enzyme for DNA synthesis in all cells. This blockade occurs by formation of a ternary complex with TS and 5,10-methylenetetrahydrofolate (CH2THF).24 The same blockade that leads to cell death and apoptosis at the level of the tumor is also hypothesized to be the cause of the elevation in MCV of red blood cells.22 Thymidylate synthase plays an essential role in red blood cell DNA synthesis downstream of the effects of folate, vitamin B12, and homocysteine. When DNA synthesis is slowed by lack of thymidylate due to TS blockade, the prolonged cell cycle allows time for excess synthesis of RNA and other cytoplasmic components, such as hemoglobin. Thus, it was hypothesized that inhibition of TS in erythroid precursor cells was the cause of elevated MCV levels in patients treated with capecitabine.22
The fact that such inhibition was seen in the participants of this study, who received significantly lower doses of capecitabine than the standard FDA-approved regimen, raises the question of whether MCV can be used as a surrogate marker of tumor response. It cannot be concluded from this analysis that MCV elevation is a marker of therapeutic response. Yet the fact that a change in MCV was significantly associated with response rate in prior studies coupled with the fact that we observed the same MCV elevations now warrants future prospective studies using MCV as a surrogate marker in patients treated with maintenance capecitabine therapy.
In conclusion, fixed low-dose oral capecitabine maintenance therapy using a schedule of 1,000 mg twice daily, 5 days on, 2 days off is safe and well tolerated. This regimen should be further explored in larger prospective studies to demonstrate efficacy in high-risk GI cancer populations, especially pancreatic and metastatic colorectal cancer, as a maintenance therapy.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Pfeiffer P, Mortensen JP, Bjerregaard B, et al. Patient preference for oral or intravenous chemotherapy: a randomized cross-over trial comparing capecitabine and Nordic fluorouracil/leucovorin in patients with colorectal cancer. Eur J Cancer. 2006;42:2738–2743. doi: 10.1016/j.ejca.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 2.Petrioli R, Paolelli L, Marsili S, et al. FOLFOX-4 stop and go and capecitabine maintenance chemotherapy in the treatment of metastatic colorectal cancer. Oncology. 2006;70:345–350. doi: 10.1159/000098107. [DOI] [PubMed] [Google Scholar]
- 3.Buter J, Sinnige HA, Sleijfer DT, et al. 5-Fluorouracil/leucovorin/interferon alpha-2a in patients with advanced colorectal cancer: effects of maintenance therapy on remission duration. Cancer. 1995;75:1072–1076. doi: 10.1002/1097-0142(19950301)75:5<1072::aid-cncr2820750504>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 4.Satoh S, Kawashima K, Matsumoto S, et al. Retrospective evaluation of sequential outpatient chemotherapy for advanced gastric cancer. Chemotherapy. 2007;53:226–232. doi: 10.1159/000100865. [DOI] [PubMed] [Google Scholar]
- 5.Bramhall SR, Hallissey MT, Whiting J, et al. Marimastat as maintenance therapy for patients with advanced gastric cancer: a randomized trial. Br J Cancer. 2002;86:1864–1870. doi: 10.1038/sj.bjc.6600310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawano K, Inokuchi T, Fujihara T, et al. Usefulness of 5-FU high-dose continuous therapy at home in patients with recurrent gastric and colon cancer. Gan To Kagaku Ryoho. 1994;21(Suppl):427–432. Japanese. [PubMed] [Google Scholar]
- 7.Brunner TB, Tinkl D, Grabenbauer GG, et al. Maintenance chemotherapy after chemoradiation improves survival of patients with locally advanced pancreatic carcinoma: a retrospective analysis of prospectively recruited patients. Strahlenther Onkol. 2006;182:210–215. doi: 10.1007/s00066-006-1524-x. [DOI] [PubMed] [Google Scholar]
- 8.Iannitti D, Dipetrillo T, Akerman P, et al. Erlotinib and chemoradiation followed by maintenance erlotinib for locally advanced pancreatic cancer: a phase I study. Am J Clin Oncol. 2005;28:570–575. doi: 10.1097/01.coc.0000184682.51193.00. [DOI] [PubMed] [Google Scholar]
- 9.Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. The Oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- 10.Maindrault-Goebel F, Lledo G, Chibaudel B, et al. OPTIMOX2, a large, randomized phase II study of maintenance therapy or chemotherapy-free intervals (CFI) after FOLFOX in patients with metastatic colorectal cancer (MCRC). A GERCOR study. 2006 ASCO Annual meeting Proceedings. J Clin Oncol. 2006;24(18S) (abstr 3504) [Google Scholar]
- 11.Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–1721. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 12.Miyashiro I, Furukawa H, Sasako M, et al. Gastric Cancer Surgical Study Group, Japan Clinical Oncology Group No survival benefit with adjuvant chemotherapy for serosa-positive gastric cancer: Randomized trial of adjuvant chemotherapy with cisplatin followed by oral fluorouracil in serosa-positive gastric cancer. Japan Clinical Oncology Group 9206-2. 2005 ASCO Gastrointestinal Cancers Symposium. (abstr 4)
- 13.Cassidy J, Twelves C, Van Cutsem E, et al. Capecitabine Colorectal Cancer Study Group First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol. 2002;13:566–575. doi: 10.1093/annonc/mdf089. [DOI] [PubMed] [Google Scholar]
- 14.Van Cutsem E, Twelves C, Cassidy J, et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J Clin Oncol. 2001;19:4097–4106. doi: 10.1200/JCO.2001.19.21.4097. [DOI] [PubMed] [Google Scholar]
- 15.Marshall J. Optimum use of biologics and role of maintenance therapy in colon cancer. Semin Oncol. 2006;33(suppl 11):S33–S35. doi: 10.1053/j.seminoncol.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Verweij J. Rational design of new tumor activated cytotoxic agents. Oncology. 1999;57(suppl):9–15. doi: 10.1159/000055263. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Findlay M, Osterwalder B, et al. Capecitabine, an oral fluoropyrimidine carbamate with substantial activity in advanced colorectal cancer: results of a randomized phase II study. J Clin Oncol. 2000;18:1337–1345. doi: 10.1200/JCO.2000.18.6.1337. [DOI] [PubMed] [Google Scholar]
- 18.Ratain M. Body-surface area as a basis for dosing of anticancer agents: science, myth, or habit? J Clin Oncol. 1998;16:2297–2298. doi: 10.1200/JCO.1998.16.7.2297. [DOI] [PubMed] [Google Scholar]
- 19.de Jong FA, Mathijssen RHJ, Zie R, et al. Flat-fixed dosing of irinotecan: influence on pharmacokinetic and pharmacodynamic variability. Clin Cancer Res. 2004;10:4068–4071. doi: 10.1158/1078-0432.CCR-03-0591. [DOI] [PubMed] [Google Scholar]
- 20.Miller AA, Rosner GL, Egorin MJ, et al. Prospective evaluation of body surface area as a determinant of paclitaxel pharmacokinetics and pharmacodynamics in women with solid tumors: Cancer and Leukemia Group B Study 9763. Clin Cancer Res. 2004;10:8325–8331. doi: 10.1158/1078-0432.CCR-04-1078. [DOI] [PubMed] [Google Scholar]
- 21.Gurney HP, Ackland S, Gebski V, et al. Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. J Clin Oncol. 1998;16:2299–2304. doi: 10.1200/JCO.1998.16.7.2299. [DOI] [PubMed] [Google Scholar]
- 22.Wenzel C, Mader RM, Steger GG, et al. Capecitabine treatment results in increased mean corpuscular volume of red blood cells in patients with advanced solid malignancies. Anti-Cancer Drugs. 2003;14:119–123. doi: 10.1097/00001813-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Karvellas CJ, Sawyer M, Hamilton M, et al. Effect of capecitabine on mean corpuscular volume in patients with metastatic breast cancer. Am J Clin Onc. 2004;27:364–368. doi: 10.1097/01.coc.0000071464.83271.08. [DOI] [PubMed] [Google Scholar]
- 24.Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27:23–44. doi: 10.1016/j.clinthera.2005.01.005. [DOI] [PubMed] [Google Scholar]