Abstract
Genistein is a phytoestrogen found at a high level in soybeans. In vitro and in vivo studies showed that high concentrations of genistein caused toxic effects. This study was designed to test the feasibility of zebrafish embryos for evaluating developmental toxicity and estrogenic potential of high genistein concentrations. The zebrafish embryos at 24 h post-fertilization were exposed to genistein (1 × 10−4 M, 0.5 × 10−4 M, 0.25 × 10−4 M) or vehicle (ethanol, 0.1%) for 60 h. Genistein-treated embryos showed decreased heart rates, retarded hatching times, decreased body length, and increased mortality in a dose-dependent manner. After 0.25 × 10−4 M genistein treatment, malformations of survived embryos such as pericardial edema, yolk sac edema, and spinal kyphosis were also observed. TUNEL assay results showed apoptotic DNA fragments in brain. This study also confirmed the estrogenic potential of genistein by EGFP expression in the brain of the mosaic reporter zebrafish embryos. This study first demonstrated that high concentrations of genistein caused a teratogenic effect on zebrafish embryos and confirmed the estrogenic potential of genistein in mosaic reporter zebrafish embryos.
Keywords: Brain aromatase, Developmental toxicity, EGFP, Genistein, Zebrafish embryo
Introduction
Endocrine-active compounds (EACs) threaten ecosystems by disrupting normal endocrine function, which can lead to reproductive failure in humans and wildlife (Guillette et al. 1994; Jobling et al. 2002). EACs include synthetic estrogen, xeno-estrogen, and phytoestrogen. Synthetic estrogens are pharmaceutical chemicals designed to mimic the action of natural estrogens. Xeno-estrogens are defined as environmental and industrial pollutants. Phytoestrogens are a class of compounds found in a variety of plants (Legler et al. 2000; Ingham et al. 2004).
Genistein (4′,5,7-trihydroxyisoflavone) is a phytoestrogen found in high levels in soybeans (Messina et al. 1994). Genistein has demonstrated anti-proliferative and anti-differentiation effects in many neoplastic cells, and it's prophylactic use has been studied with regards to cancer (Kanatani et al. 1993; Wei et al. 1993; Su et al. 2000; Myoung et al. 2003). High concentrations of genistein have been shown to cause apoptosis and necrosis in testis cells (Kumi-Diaka et al. 1999), primary cortical neuron cultures from Spraugue-Dawley rats (Linford et al. 2001), human thymocytes (McCabe and Orrenius 1993), and human lymphoblastoid cells (Morris et al. 1998). In addition, in vivo studies showed that genistein causes developmental and growth toxicity, myelotoxicity, and brain apoptosis (Choi and Lee 2004; Ingham et al. 2004; Guo et al. 2005).
Estrogen replacement therapy (ERT) is widely used to treat diseases such as Alzheimer's disease, osteoporosis, and cardiovascular disease, that are caused by low levels of estrogen (Kuller 1996; Spritzerm and Wender 2007). Isoflavones that have estrogenic-like effects have been viewed as alternatives to estrogen because long-term usage of ERT increases the risk of breast cancer. In this case, a high dose of isoflavones is needed to be useful for a therapeutic effect, owing to their low efficacy, only about 1–2% of that of ERT (Jin et al. 2007).
Recently, zebrafish embryos have become an exceptional model system for assessing the toxicity of chemicals (Fraysse et al. 2006; Kammann et al. 2006). As the development of zebrafish embryos is rapid and the embryos remain transparent throughout most of embryogenesis, we can easily observe abnormal morphologic changes caused by toxicants. Most of all, zebrafish embryos are very sensitive to toxic chemicals. Previously, we have established xenoestrogen-responsive mosaic zebrafish, in which a zebrafish brain aromatase (AroB) promoter-driven enhanced green fluorescent protein (EGFP) reporter gene was introduced to test estrogenic potential of chemicals. Expression of AroB is most prominent in radial glial cells (Menuet et al. 2003, 2005) and its expression is up-regulated by estrogens (Kazeto et al. 2004). In this model, EGFP was used as a reporter gene. In the presence of xenoestrogens, mosaic reporter zebrafish embryos express EGFP in the brain specifically.
This study was designed to test the feasibility of zebrafish embryos for evaluating developmental toxicity and estrogenic potential of high genistein concentrations.
Materials and methods
Chemicals
We obtained Genistein (Sigma, St. Louis, MO) from Veterinary Public Health Laboratory (Seoul National University, Korea) and prepared 20 mM Genistein stock solutions in absolute ethanol. Treatment doses were made from dilution of these stock solutions in Ringer's solution.
Zebrafish maintenance and eggs collection
Adult zebrafish were raised and maintained on a 14:10 h light:dark cycle at 28.5°C, and bred in tanks as described previously (Westerfield 1995). Mature fish were fed twice daily a combination of Freshwater Aquarium Flake food (TetraWerke, Melle, Germany) and live brine shrimp (San Francisco Bay Brand, Inc., Newark, CA). The care and treatment of the animals were conducted in accordance with the guidelines established by the Seoul National University Institutional Animal Care and Use Committee (Approval No. SNU-050418-2). Fertilized eggs were obtained from naturally reproducing adult zebrafish bred in our laboratory as described previously (Westerfield 1995). Eggs were collected, pooled, rinsed, and placed into clean Ringer's solution.
Effects of genistein on the development of zebrafish embryos
The zebrafish embryos at 24 h post-fertilization were transferred 12-well plate and exposed to 3 ml of genistein (1 × 10−4 M, 0.5 × 10−4 M, 0.25 × 10−4 M) or vehicle (ethanol, 0.1%) for 60 h. Genistein was dissolved in 100% ethanol and the final ethanol concentration for each treatment was 0.1%. Each treatment group had 60 embryos that were divided into six plates. For each treatment, half of the exposure solution was renewed every day. Per-minute heart rates of genistein-treated embryos 30 h after treatment, the number of hatched embryos, and body length at 37 h after treatment were examined under a stereomicroscope (SZ-PT, OLYMPUS, Japan). Sixty hours after genistein treatment, the mortality and malformation of embryos were determined, and live embryos were fixed in 10% phosphate-buffered formalin for histopathological examination.
To determine whether the toxicity observed in genistein-treated embryos was related to the negative effects on heart rate, zebrafish embryos were exposed to beta-adrenergic receptor agonist isoproterenol (Sigma, St. Louis, MO; Vehicle, 1 × 10−6 M, 10 × 10−6 M, 100 × 10−6 M) in the presence of genistein (0.5 × 10−4 M). Experimental methods were the same as described above.
Histopathological Examination
Whole embryos were fixed in 10% phosphate-buffered formalin for 24 h with slight shaking. Fixed embryos were dehydrated in an alcohol-xylene series, and embedded in paraffin wax. From each block, 2 μm sections were prepared and stained with Hematoxylin and eosin (HE) for histopathological examination.
TUNEL Assay
To test for apoptosis in genistein-treated embryos, TUNEL assay (terminal deoxynucleotide transferase-mediated dUTP nick-end labeling, In Situ Cell Death Detection Kit, PDO, Roche) was carried out according to the kit manufacturer's instruction. Briefly, 2 μm sections from a paraffin wax-embedded block were deparaffinized and permeabilized by incubation in proteinase K solution (20 μg/ml) for 30 min at 37°C. After two rinses in phosphate-buffered saline (PBS), slides were incubated for 60 min at 37°C in labeling solutions consisting of TdT and fluorescein-conjugated deoxynucleotides in buffer. After three PBS washes, the slides were incubated for 30 min at 37°C in converter-POD (anti-fluorescein peroxidase) solution. Excess converter-POD solution was removed by three PBS rinses, and apoptotic DNA fragments were visualized by incubation in DAB substrates.
Plasmid Construction
A zebrafish brain aromatase-regulated reporter plasmid, pzfAroB-EGFP, was constructed by linking the proximal promoter region of the zebrafish brain aromatase gene with the enhanced green fluorescent protein (EGFP) reporter gene. A proximal promoter region of the zebrafish brain aromatase gene was amplified by PCR from zebrafish genomic DNA using a specific primer set, zfAroB-F (5′-CCGCTCGAGGTTCAAAGCCCTCCCAAATA-3′) and zfAroB-R (5′-CGGGATCCCCGTCCTCAGGCTTCCATCAT-3′) containing a restriction-digested adaptor (underlined) corresponding to XhoI and BamHI sites, respectively (Kazeto et al. 2001; Menuet et al. 2005). The gene amplification reaction conditions were as follows: first denaturation at 94°C for 3 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 45 s; and final extension at a cycle of 72°C for 7 min. The PCR product double digested by XhoI and BamHI was subcloned upstream of the EGFP gene in the XhoI/BamHI restriction sites of the T2KXIG vector (Kawakami et al. 2004).
Microinjection of pzfAroB-EGFP and transposase mRNA
Single-cell fertilized zebrafish embryos were microinjected with pzfAroB-EGFP and transposase mRNA. Transposase mRNA was synthesized in vitro using transposase cDNA (Kawakami et al. 2004) and an mMESSAGE mMACHINE SP6 Kit (Ambion Inc.). The embryos were transferred to agarose ramps, and then injected with a DNA/RNA solution containing 25 ng/μl each of pzfAroB-EGFP circular DNA and transposase mRNA using a micropipette secured in a micromanipulator (World Precision Instruments Inc., Sarasota, FL).
Genistein-induced EGFP expression in mosaic reporter zebrafish embryos
Two hours after fertilization, the injected embryos were transferred to Petri dishes and exposed to genistein (10−6 M) or ethanol alone (0.1%) for 4 days. For each treatment, half of the exposure solution was renewed every day. After genistein exposure, the larvae were transferred to Ringer's solution, as described by Westerfield, and monitored for EGFP expression using an Olympus IX70 microscope equipped with a NIBA2 filter (λex = 470–490 nm; λem = 510–550 nm).
Statistical analysis
The differences between experimental and control groups were assayed for significance by Duncan's Multiple Range Test (SAS ver. 9.1; SAS Institute Inc., Cary, NC, USA). P-values < 0.05 were considered significant.
Results
Developmental toxicity of genistein on zebrafish embryos
Per-minute heart rates of genistein-treated embryos 30 h after treatment decreased in a dose-dependent manner (vehicle: 127.62 ± 6.34, 0.25 × 10−4 M: 97.12 ± 12.33, 0.5 × 10−4 M: 86.87 ± 9.62, 1 × 10−4 M: 53.00 ± 2.72 beats/min) (Fig. 1A). Although about 90% of the 10 vehicle-treated embryos in each plate (8.83 ± 1.94 embryos) hatched 37 h after treatment; genistein treatment decreased the number of hatched embryos (0.25 × 10−4 M: 4.16 ± 1.47, 0.5 × 10−4 M: 1.16 ± 1.47 embryos). None of the ten 1 × 10−4 M genistein-treated embryos in each plate hatched (Fig. 1B). The body length of vehicle-treated embryos was 3.43 ± 0.27 mm, but those of genistein-treated embryos decreased in a dose-dependent manner (0.25 × 10−4 M: 3.00 ± 0.10, 0.5 × 10−4 M: 2.72 ± 0.13 mm) (Fig. 1C and Fig. 2A). Because the embryos treated with the highest concentration of genistein did not hatch, we could not measure the body length of these embryos. Sixty hours after genistein treatment, all embryos survived in the vehicle-treated group, but none of the 0.5 × 10−4 M and 1 × 10−4 M genistein-treated embryos survived. Moreover, only 6.16 ± 2.48 embryos survived in the 0.25 × 10−4 M genistein-treated group (Fig. 1D). We also observed physical malformations in embryos surviving 0.25 × 10−4 M genistein treatment such as pericardial edema, yolk sac edema, and spinal kyphosis (Fig. 2B and 2C).
Figure 1.
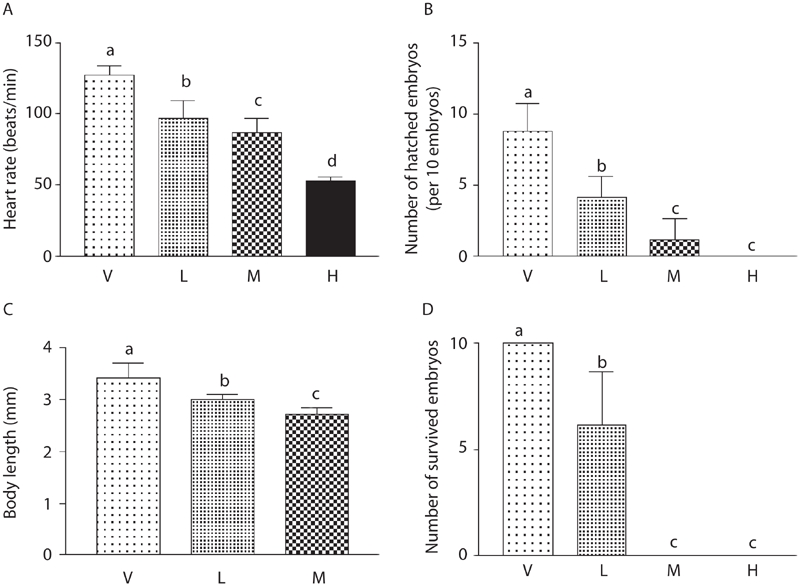
Developmental toxicity of genistein on zebrafish embryos. The zebrafish embryos at 24 h post-fertilization were exposed to genistein (1 × 10−4 M, 0.5 × 10−4 M, 0.25 × 10−4 M) or vehicle (ethanol, 0.1%) for 60 h. (A) Heart rates of genistein-treated embryos at 30 h after treatment decreased in dose-dependent manner. (B) Although about 90% of vehicle-treated embryos hatched at 37 h after treatment, genistein treatment decreased the number of hatched embryos. (C) Body lengths of genistein-treated embryos at 37 h after treatment decreased in a dose-dependent manner. (D) At 60 h after genistein treatment, all embryos survived in the vehicle-treated group, but none of the 0.5 × 10−4 M and 1 × 10−4 M genistein-treated embryos survived, and about six embryos survived in the 0.25 × 10−4 M genistein-treated group [genistein concentration: (V) vehicle, (H) 1 × 10−4 M, (M) 0.5 × 10−4 M, (L) 0.25 × 10−4 M]. Data are expressed as mean ± SD. Values of each group with identical letters in each panel were not significantly different (p > 0.05).
Figure 2.

Developmental toxicity of genistein on zebrafish embryos. The zebrafish embryos at 24 h post-fertilization were exposed to genistein or vehicle for 60 h. (A) The body lengths of the genistein-treated embryos decreased in dose-dependent manner [genistein concentration: (V) vehicle, (L) 0.25 × 10−4 M, (M) 0.5 × 10−4 M]. (B, C) Malformations of embryos that survived after 0.25 × 10−4 M genistein treatment such as (1) pericardial edema, (2) yolk sac edema, and (3) spinal kyphosis. Bar = 1 mm.
10 × 10−6 M and 100 × 10−6 M isoproterenol increased heart rate in the presence of 0.5 × 10−4 M genistein (vehicle: 126.00 ± 7.21, genistein only: 85.00 ± 5.56, 1 × 10−6 M isoproterenol: 86.00 ± 2.00, 10 × 10−6 M isoproterenol: 98.00 ± 6.24, 100 × 10−6 M isoproterenol: 97.66 ± 7.02 beats/min) (Fig. 3). However, it could not recover genisterin-induced retarded hatching time, decreased body length, and mortality (data not shown).
Figure 3.
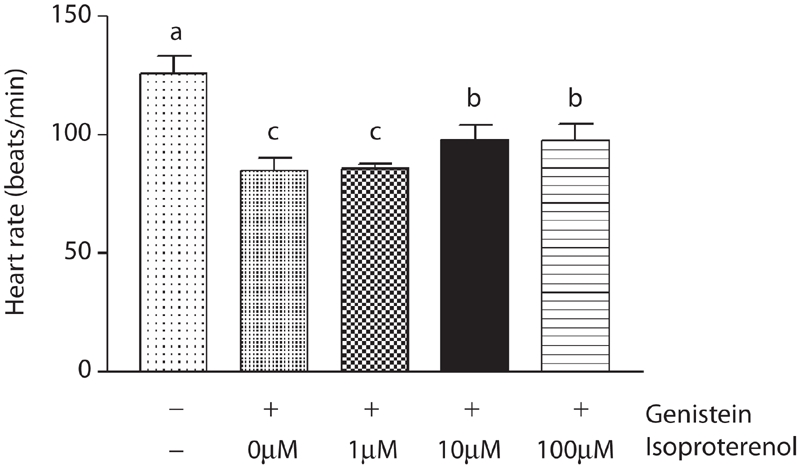
Effect of isoproterenol in the presence of genistein on heart rate in zebrafish embryos. The zebrafish embryos at 24 h post-fertilization were exposed to isoproterenol (Vehicle, 1 × 10−6 M, 10 × 10−6 M, 100 × 10−6 M) in the presence of genistein (0.5 × 10−4 M). In the presence of genistein, isoproterenol (10 × 10−6 M, 100 × 10−6 M) increased heart rate significantly at 30 h after treatment. Data are expressed as mean ± SD. Values of each group with identical letters in each panel were not significantly different (p > 0.05).
Histopathological examination and TUNEL assay
Each group of 20 of the zebrafish embryos surviving 0.25 × 10−4 M genistein or vehicle treatments were prepared for histopathological examination. Histopathological examination revealed granular degeneration of myocytes in skeletal muscle in addition to loss and apoptosis of neural cells in brain in the all of the 0.25 × 10−4 M genistein-treated embryos (Fig. 4). To determine whether neural cell damage was related to apoptosis, we conducted TUNEL analysis using the same tissue samples. TUNEL assay results showed apoptotic DNA fragments in the brain and spinal cord of 0.25 × 10−4 M genistein-treated zebrafish embryos (Fig.5).
Figure 4.
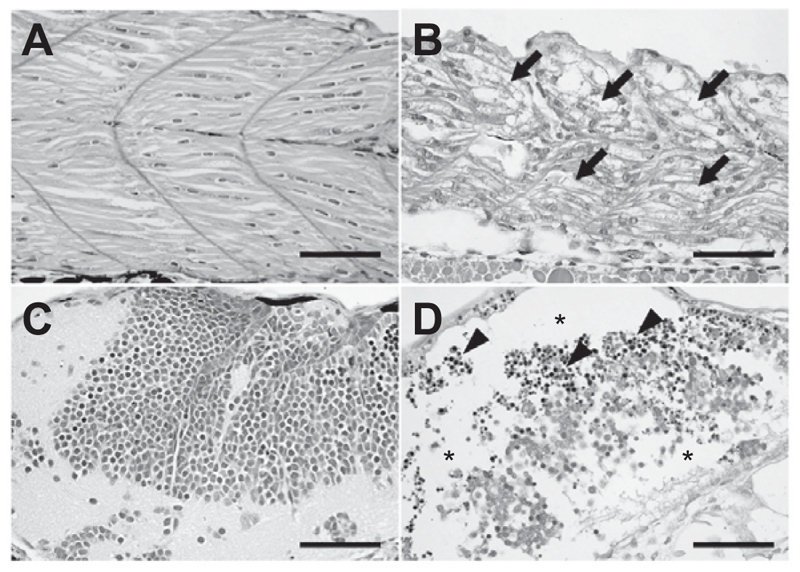
Histopathological examination. The zebrafish embryos at 24 h post-fertilization were exposed to genistein or vehicle for 60 h. The zebrafish embryos that survived after 0.25 × 10−4 M genistein or vehicle treatments were prepared for histopathological examination. Histopathological examination revealed that (arrows) granular degeneration of (B) myocytes in skeletal muscle in addition to (asterisks) loss and (arrow heads) apoptosis of (D) neural cells in the brain of the 0.25 × 10−4 M genistein-treated group. (A and C) vehicle-treated zebrafish embryos, (B and D) genistein-treated zebrafish embryos. Bar = 50 μm.
Figure 5.
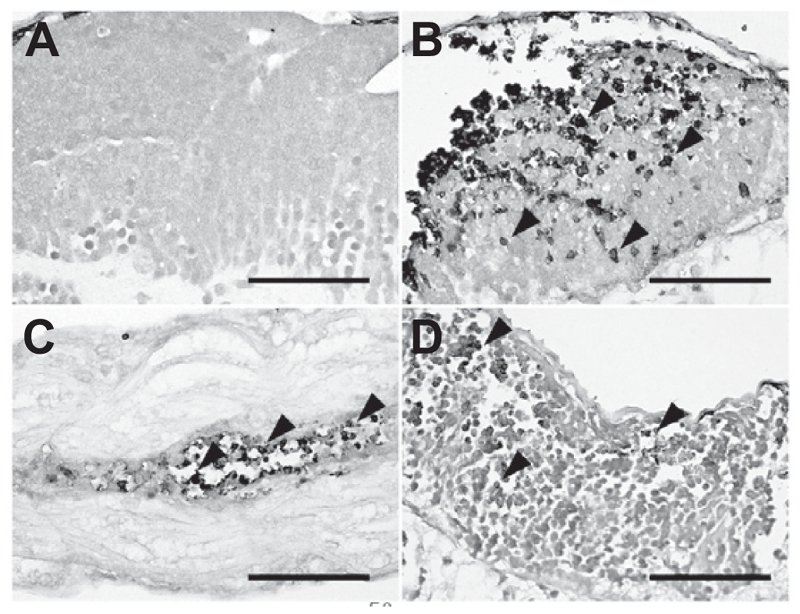
TUNEL assay. To determine whether neural cell damages were related to apoptosis, we conducted a TUNEL assay using the same tissue samples. TUNEL assay results showed apoptotic DNA fragments in (B and D) the brain and (C) spinal cord of 0.25 × 10−4 M genistein-treated zebrafish embryos. (A) vehicle treated zebrafish embryos, (B–D) genistein-treated zebrafish embryos. Bar = 50 μm.
AroB reporter gene expression in response to genistein exposure
To confirm the estrogenic potential of genistein, we used mosaic reporter zebrafish embryos that contained a zebrafish brain aromatase promoter-driven EGFP reporter gene, pzfAr-oB-EGFP. Mosaic reporter zebrafish embryos were exposed to genistein (E2) (10−6 M) or vehicle (ethanol alone, 0.1%) for 4 days. Although EGFP expression was not observed in vehicle-treated embryos, genistein-treated embryos exhibited EGFP expression in the telencephalon of the brain (Fig. 6).
Figure 6.
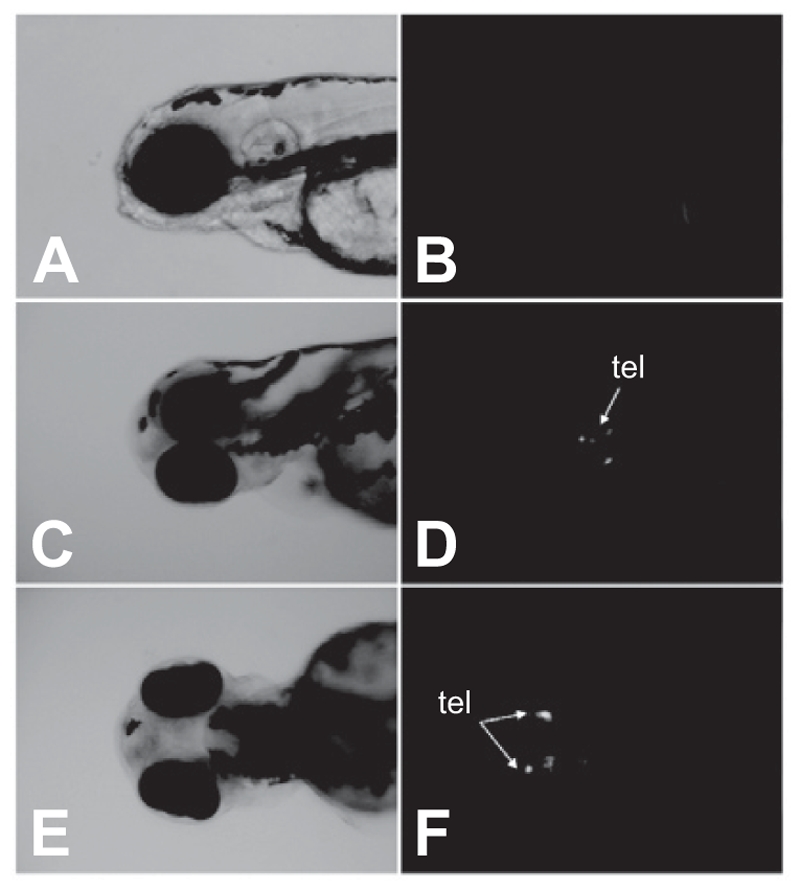
EGFP expression in response to genistein exposure. pzfAroB-EGFP injected embryos were exposed to genistein (10−6 M) at 2 hpf for 4 days. EGFP expression was localized in the telencephalon (tel) located in the forebrain. (A and B) vehicle-treated zebrafish embryos, (C–F) genistein-treated zebrafish embryos. (A, C, E: light microscope images, B, D, F: fluorescence microscope images).
Discussion
In this study, we first demonstrated that high genistein concentrations caused developmental toxicity in the form of decreased heart rate, retarded hatching time, decreased body length, and mortality in zebrafish embryos. Cardiac Ca2+ channel is activated by beta-adrenergic receptor via protein kinase A-dependant phosphorylation of the channel (Hool 2001). Genistein is a tyrosine kinase inhibitor which can interfere with the activity of several ionic channels either by altering the modulatory phosphorylating process or by direct binding (Chiang et al. 1996). In human atrial fibers, rabbit pacemaker cells, and guinea pig cardiomyocytes, negative chronotropic action of genistein is likely due to a direct blocking of Ca2+ channel, instead of inhibition of tyrosine kinase activity (Chiang et al. 1996; Ma et al. 2002; Altomare et al. 2006). In our results, decreased heart rate is thought to be caused by genistein via direct blocking of Ca2+ channel. Genistein administration in neonatal rats from birth to postnatal day 21 resulted in decreased body weight (Lewis et al. 2003). Furthermore, a high concentration of genistein-treated minnows grew more slowly than control minnows, and Xenopus embryos failed to gastrulate at all within the 48 h period in response to genistein exposure (Ingham et al. 2004). Also, when mouse blastocysts were incubated in medium containing genistein, cell proliferation and growth were inhibited (Chan et al. 2007). These developmental toxicities shown in other reports agreed well with our results. Studies using several cell systems suggested that the mechanism of genistein-induced growth inhibition includes: regulation of cell cycle checkpoints, modulation of the transforming growth factor beta-1 signaling pathway, antiangiogenesis, and antioxidant activities (Kim et al. 1998). Genistein has been shown to inhibit protein tyrosine kinase and topoisomerase-II activity, tumor cell proliferation and differentiation (Akiyama et al. 1987; Markovits et al. 1989), and may inhibit embryonic development by altering mitochondria structure and function (Bogoliubova et al. 1999).
Checking the number of hatched embryos 37 h after treatment showed almost 100% of vehicle-treated embryos were hatched and the genistein treatment showed dose-dependent inhibition of hatching rates at this time. Because the 1 × 10−4 M genistein-treated embryos did not hatch during all experimental periods, we did not measure the body length of the 1 × 10−4 M genistein-treated embryos.
To determine whether the toxicity induced by genistein was related to the negative effects on heart rate, zebrafish embryos were exposed to isoproterenol in the presence of genistein. Although isoproterenol (10 × 10−6 M and 100 × 10−6 M) increased heart rate significantly in the presence of genistein, it could not recover genistein-induced retarded hatching time, decreased body length, and mortality. Genistein-induced toxicity may be induced not by decreased heart rate completely but by other mechanisms. However, because the zebrafish embryo heart at 24 h post-fertilization doesn't respond to adrenergic stimulation completely, isoproterenol could not recover decreased heart rate until the zebrafish embryo heart responded to adrenergic stimulation completely (Milan et al. 2003; Schwerte et al. 2006).
The chronic oral treatment of rats with high doses of genistein increased lactate dehydrogenase (LDH) activity and loss of mitochondria function in rat brain tissue homogenates. Furthermore, DNA fragmentation was detected in these brain tissue homogenates (Choi and Lee 2004). In our case, we also observed apoptotic DNA fragments in the brain and spinal cord of genistein-treated embryos via TUNEL assay. This result showed that a zebrafish system can represent a specific toxic response in a genistein-treated mammalian system, and might be used to test developmental toxicity of endocrine disruptors. We also observed granular degeneration of myocytes. The morphologic changes such as kyphosis observed in genistein-treated embryos might be caused by degeneration of myocytes and neural cell apoptosis.
In addition to offering sensitivity for toxic chemicals and transparency, zebrafish embryos provide a model system that mimics embryos under intrauterine conditions. Genistein in soybean-consuming pregnant women may affect embryo development. Teratogenic effects of genistein observed in this study emphasize the risk of genistein to embryos under intrauterine conditions.
EGFP was expressed in response to genistein (10−6 M) on mosaic reporter zebrafish embryos. EGFP expression was localized in the telencephalon of the brain, which was not related with areas of apoptosis induced by 1 × 10−4 M genistein. When determining the estrogenic potential of some chemicals, in vivo assays are necessary because they can reflect in vivo functions such as absorption, metabolism, and secretion. Recently, our laboratory has developed a transgenic reporter mosaic zebrafish system for the rapid screening of chemicals for estrogenic potential. In this study, we confirmed the estrogenic potential of genistein on our transgenic reporter mosaic zebrafish system, which might be used to detect endocrine disruptors effectively.
In summary, although low doses of genistein maybe beneficial to prevent some cancers, excessive genistein might cause teratogenic effects in exposed zebrafish embryos. We also confirmed the estrogenic potential of genistein on our mosaic reporter zebrafish embryos.
Acknowledgments
This work was supported by the Korea Research Foundation grants (KRF-005-E00077), the BK21 Program for Veterinary Science and the Research Institute for Veterinary Science, Seoul National University, Korea.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Altomare C, Tognati A, Bescond J, Ferroni A, Baruscotti M. Direct inhibition of the pacemaker (If) current in rabbit sinoatrial node cells by genistein. Br. J. Pharmacol. 2006;147:36–44. doi: 10.1038/sj.bjp.0706433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoliubova NA, Bogoliubova IO, Parfenov VN, Sekirina GG. Characteristic of structural and functional organization of two-cell mouse embryos exposed to inhibitors of cell proliferation. Tsitologiia. 1999;41:698–706. [PubMed] [Google Scholar]
- Chan WH, Lu HY, Shiao NH. Effect of genistein on mouse blastocyst development in vitro. Acta Pharmacol. Sin. 2007;28:238–245. doi: 10.1111/j.1745-7254.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- Chiang CE, Chen SA, Chang MS, Lin CI, Luk HN. Genistein directly inhibits L-type calcium currents but potentiates cAMP-dependent chloride currents in cardiomyocytes. Biochem. Biophys. Res. Commun. 1996;223:598–603. doi: 10.1006/bbrc.1996.0941. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Lee BH. Evidence for genistein mediated cytotoxicity and apoptosis in rat brain. Life Sci. 2004;75:499–509. doi: 10.1016/j.lfs.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Mons R, Garric J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006;63:253–267. doi: 10.1016/j.ecoenv.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Jr., Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ. Health Perspect. 1994;102:680–688. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TL, Germolec DR, Musgrove DL, Delclos KB, Newbold R R, Weis C, White KL., Jr. Myelotoxicity in genistein-, nonylphenol-, methoxychlor-, vinclozolin- or ethinyl estradiol-exposed F1 generations of Sprague-Dawley rats following developmental and adult exposures. Toxicology. 2005;211:207–219. doi: 10.1016/j.tox.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Hool LC. Hypoxia alters the sensitivity of the L-type Ca(2+) channel to alpha-adrenergic receptor stimulation in the presence of beta-adrenergic receptor stimulation. Circ. Res. 2001;88:1036–1043. doi: 10.1161/hh1001.090841. [DOI] [PubMed] [Google Scholar]
- Ingham RR, Gesualdi DA, Toth CR, Clotfelte ED. Effects of genistein on growth and development of aquatic vertebrates. Bull. Environ. Contam. Toxicol. 2004;72:625–631. doi: 10.1007/s00128-004-0289-0. [DOI] [PubMed] [Google Scholar]
- Jin Y, Wu H, Cohen EM, Wei J, Jin H, Prentice H, Wu JY. Genistein and daidzein induce neurotoxicity at high concentrations in primary rat neuronal cultures. J. Biomed. Sci. 2007;14:275–284. doi: 10.1007/s11373-006-9142-2. [DOI] [PubMed] [Google Scholar]
- Jobling S, Coey S, Whitmore JG, Kime DE, Van Look KJ, McAllister BG, Beresford N, Henshaw AC, Brighty G, Tyler CR, Sumpter JP. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 2002;67:515–524. doi: 10.1095/biolreprod67.2.515. [DOI] [PubMed] [Google Scholar]
- Kammann U, Vobach M, Wosniok W. Toxic effects of brominated indoles and phenols on zebrafish embryos. Arch. Environ. Contam. Toxicol. 2006;51:97–102. doi: 10.1007/s00244-005-0152-2. [DOI] [PubMed] [Google Scholar]
- Kanatani Y, Kasukabe T, Hozumi M, Motoyoshi K, Nagata N, Honma Y. Genistein exhibits preferential cytotoxicity to a leukemogenic variant but induces differentiation of a non-leukemogenic variant of the mouse monocytic leukemia Mm cell line. Leuk. Res. 1993;17:847–853. doi: 10.1016/0145-2126(93)90150-j. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Ijiri S, Place AR, Zohar Y, Trant JM. The 5′-flanking regions of CYP19A1 and CYP19A2 in zebrafish. Biochem. Biophys. Res. Commun. 2001;288:503–508. doi: 10.1006/bbrc.2001.5796. [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Place AR, Trant JM. Effects of endocrine disrupting chemicals on the expression of CYP19 genes in zebrafish (Danio rerio) juveniles. Aquat. Toxicol. 2004;69:25–34. doi: 10.1016/j.aquatox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Kim H, Peterson TG, Barnes S. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 1998;68:1418S–1425S. doi: 10.1093/ajcn/68.6.1418S. [DOI] [PubMed] [Google Scholar]
- Kuller LH. Hormone replacement therapy and its potential relationship to dementia. J. Am. Geriatr. Soc. 1996;44:878–880. doi: 10.1111/j.1532-5415.1996.tb03753.x. [DOI] [PubMed] [Google Scholar]
- Kumi-Diaka J, Nguyen V, Butler A. Cytotoxic potential of the phytochemical genistein isoflavone (4′,5′,7-trihydroxyisoflavone) and certain environmental chemical compounds on testicular cells. Biol. Cell. 1999;91:515–523. doi: 10.1016/s0248-4900(00)88208-8. [DOI] [PubMed] [Google Scholar]
- Legler J, Broekhof JLM, Brouwer A, Lanser PH, Murk AJ, van der Saag PT, Vethaak AD, Wester P, Zivkovic D, van der Burg B. A novel in vivo bioassay for (xeno-)estrogens using transgenic zebrafish. Environ. Sci. Technol. 2000;34:4439–4444. [Google Scholar]
- Lewis RW, Brooks N, Milburn GM, Soames A, Stone S, Hall M, Ashby J. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol. Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- Linford NJ, Yang Y, Cook DG, Dorsa DM. Neuronal apoptosis resulting from high doses of the isoflavone genistein: role for calcium and p42/44 mitogen-activated protein kinase. J. Pharmacol. Exp. Ther. 2001;299:67–75. [PubMed] [Google Scholar]
- Ma T, Liu S, Zhang WL, Zhao H, Yu SY, He RR. Effects of phytoestrogen genistein on electrophysiology of human atrial fibers. Acta Pharmacol. Sin. 2002;23:851–854. [PubMed] [Google Scholar]
- Markovits J, Linassier C, Fosse P, Couprie J, Pierre J, Jacquemin-Sablon A, Saucier JM, Le Pecq JB, Larsen AK. Inhibitory effects of the tyrosine kinase inhibitor genistein on mammalian DNA topoisomerase II. Cancer Res. 1989;49:5111–5117. [PubMed] [Google Scholar]
- McCabe MJ, Jr., Orrenius S. Genistein induces apoptosis in immature human thymocytes by inhibiting topoisomerase-II. Biochem. Biophys. Res. Commun. 1993;194:944–950. doi: 10.1006/bbrc.1993.1912. [DOI] [PubMed] [Google Scholar]
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F, Kah O. Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: Comparison with estrogen receptor alpha. J. Comp. Neurol. 2003;462:180–193. doi: 10.1002/cne.10726. [DOI] [PubMed] [Google Scholar]
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade I, Pakdel F, Kah O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005;485:304–320. doi: 10.1002/cne.20497. [DOI] [PubMed] [Google Scholar]
- Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1368. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- Morris SM, Chen JJ, Domon OE, McGarrity LJ, Bishop ME, Manjanatha MG, Casciano DA. p53, mutations, and apoptosis in genistein-exposed human lymphoblastoid cells. Mutat. Res. 1998;405:41–56. doi: 10.1016/s0027-5107(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Myoung H, Hong SP, Yun PY, Lee JH, Kim MJ. Anti-cancer effect of genistein in oral squamous cell carcinoma with respect to angiogenesis and in vitro invasion. Cancer Sci. 2003;94:215–220. doi: 10.1111/j.1349-7006.2003.tb01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerte T, Prem C, Mairösl A, Pelster B. Development of the sympathovagal balance in the cardiovascular system in zebrafish (Danio rerio) characterized by power spectrum and classical signal analysis. J. Exp. Biol. 2006;209:1093–1100. doi: 10.1242/jeb.02117. [DOI] [PubMed] [Google Scholar]
- Spritzerm PM, Wender MC. Hormone therapy in menopause: when not to use. Arq. Bras. Endocrinol. Metabol. 2007;51:1058–1063. doi: 10.1590/s0004-27302007000700006. [DOI] [PubMed] [Google Scholar]
- Su SJ, Yeh TM, Lei HY, Chow NH. The potential of soybean foods as a chemoprevention approach for human urinary tract cancer. Clin. Cancer Res. 2000;6:230–236. [PubMed] [Google Scholar]
- Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr. Cancer. 1993;20:1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR, USA: University of Oregon Press; 1995. [Google Scholar]


