Abstract
Our observation that male rats innate fear response differed with hormonal status, as well as the higher prevalence of fear and anxiety disorders in human females led to the current investigation of the impact of phases of the estrous cycle on innate fear responding. Female rats in different phases of the cycle were exposed to an innate fear-inducing stimulus (2,5-dihydro- 2,4,5-trimethylthiazoline, TMT odor) and monitored for changes in behavior and brain activation. Behavioral data showed freezing responses to TMT were significantly enhanced during estrus as compared to other phases of the cycle. This data was supported by significant increases in pixel intensity in cortical and sub-cortical regions in estrus compared to proestrus and diestrus.
Imaging results demonstrated significant increases in brain activation in the somatosensory and insular cortices when comparing estrus to diestrus. There were significant increases in neural activity in the BNST and septum in estrus as compared to proestrus. Additionally, the hippocampus, hypothalamus, olfactory system, and cingulate cortex show significant increases in the estrus phase when compared to both diestrus and proestrus. Taken together, these results suggest that the female's hormonal status may be correlated with alterations in both neuronal and behavioral indices of fear.
Keywords: Innate Fear, Trimethylthiazoline (TMT), Estrus cycle, Estrogen
1. Introduction
Anxiety disorders affect more than 40 million individuals in the United States each year and have a yearly economic cost of $50 billion [26]. Studies suggest that females have a higher prevalence of anxiety disorders and phobias than their male counterparts [28]. In fact, the national comorbidity survey reports that women have a phobia prevalence rate of 15.7%, compared to a rate of 6.7% for men [30].
Phobias and other anxiety disorders have been linked to alterations in both conditioned and innate fear responses [46]. Although the literature is replete with studies on conditioned fear much less is known about innate fear and the possible impact of gender. Reports examining sex differences in stress responsivity demonstrate higher levels and enhanced release of the stress hormones adrenocorticotrophin (ACTH) and corticosterone in female rodents responding to stressors [6,12]. In addition to general stressors, female rodents have been shown to exhibit more defensive behaviors than males when exposed to predator induced stressors [3,4,29,31], suggesting a link to hormonal status. The possible role of alterations in gonadal hormones like estrogen and progesterone on female fear behavior appears complex and multidimensional. For example, while some rodent studies show that estrogen administration increases in anxiety-like behaviors [40] others report anxiolytic-like effects [56] and still others show no significant effect [38]. Much work needs to be done to elucidate the possible mechanisms and brain regions involved in these possible conflicting reports. Functional imaging provides an innovative way to view the neural correlates of a stimulus. When using fearful face recognition as an fMRI stimulus, women show a different pattern of activation in the left amygdala and other regions such as the prefrontal cortex. In fact, the female fear recognition response was characterized by increased habituation in the amygdala followed by increased activity in the hippocampus in human studies [8]. Independent of the current lack of consensus in this field, the important impact of hormones on psychiatric disorders in women have been supported by reports showing that the occurrence of mood disorders is greatest during times when gonadal hormones are in flux [45,58].
Recently several models of predation stress have been utilized to interrogate the neural mechanisms underlying innate fear. Behaviorally, 2,4,5-trimethylthiazoline (TMT), a molecule produced by the gland secretions of the red fox [55] has been shown to elicit fearful behaviors in rodents, including freezing [16], defensive burying [25], and avoidance [7]. With this in mind, the current study was designed to assess the influence of the estrus cycle in modulating innate fear response. We have examined both behaviorally and with functional MRI the TMT induced-innate fear response of female rats (at different phases of the cycle). We hypothesize that changes in phases of the cycle will correlate to heightened behavioral and neuronal responses to predator stress.
2. Materials and Methods
2.1. Animals
Sexually mature female Sprague-Dawley rats (250–300 g) were obtained from Harlan Sprague–Dawley Laboratories (Indianapolis, IN). Animals were housed in Plexiglas cages (two to a cage) and maintained in ambient temperature (22–24 °C) on a 12-h light: 12-h dark schedule (lights on at 09:00 h). Food and water were provided ad lib. All animals were acquired and cared for in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals (#80–23, Revised 1996). These studies were approved by the IACUC Committee of the University of Massachusetts Medical School.
2.2 Acclimation procedure
For imaging experiments, animals were acclimated to the restraint device for 3 days according to a previously published procedure [10]. Briefly, animals were lightly anesthetized with 2% isoflurane secured in a dual coil rodent restrainer developed for fMRI (Insight Neuroimaging Systems LLC, Worcester, MA). A plastic semicircular headpiece with blunted ear supports that fit into the ear canals was positioned over the ears. Lidocaine paste (2%) was added to points of mechanical restraint, e.g., bridge of the nose and ear canals to minimize any pain or discomfort during the study. The head was placed into the cylindrical head holder with the animals' incisors secured over a bite bar and the ears were positioned inside the head holder. The body of the animal was placed in a customfitted cylindrical body tube. The body restrainer isolates all of the body movement from the head restrainer and minimizes motion artifact [32], while allowing for unrestricted respiration. The head holder and body tube were subsequently placed in a black opaque tube “mock scanner” with a tape recording of scanner noises. Scanner noises were identical to the precise imaging protocol to which rats would later be exposed during the experimental imaging protocol.
2.3 Estrus cycle assessment
Estrus cycle was determined by virginal smear examination [9]. Female in diestrus, proestrus and estrus phases were used in this experiment.
The vagina presents cyclic changes according to hormonal fluctuations, so estrus cycle can be determined by virginal smear examination [9]. Epithelial cells exfoliated from the vaginal wall can be collected onto a swab, smeared onto a slide, and examined under the microscope; the presence of cornified cells is indicative of estrus. In this study, all rats had vaginal smears to determine estrus cycle stage. This involved sampling the cells of the vaginal canal with sterile saline using plastic pipette. The recovered solution containing cells were placed on microscope slides. Subsequent cell cytology was examined under low or medium-power with a light microscope. Cell descriptions, as described by Sharp and LaRegina [49], were used to classify rats as being in diestrus, proestrus, or estrus.
Usually in diestrus phase, there is an abrupt decrease in superficial epithelial cell numbers and a marked increase in basal and parabasal epithelial cell numbers, many neutrophils are present at first with a marked decrease in 1-2 days, red cells may or may not be present. Simply, there were a variety of cell types along with leukocytes In diestrus. In proestrus, epithelial cells are all non-cornified basal, parabasal, and intermediate cells gradually decreasing in number, superficial epithelial cells appear by 2nd or 3rd day and increase in number over time. Erythrocytes are numerous and gradually decrease in number. Leukocytes disappear by the last day or two of proestrus and large and small intermediate cells along with red cells predominate.
Specifically, in proestrus the majorities of cells were large, round and nucleated. In estrus phase, epithelial cells are mostly large flat angular cornified epithelial cells that become wrinkled and irregular as estrus progresses. Red cells may be observed micro scopically throughout estrus, leukocytes appear on the last day or two of estrus. Large numbers of leukocytes indicate the end of estrus. In estrus the majority of cells were cornified.
2.4 Behavioral assessment
Rats were tested to assess their behavioral response to each scent and control prior to imaging studies. Briefly, a separate group of female rats (n = 8 per group) were removed from their home cage between 9:00 - 11:00 am EST (to minimize the impact of circadian rhythms) and allowed to habituate to the environment (plastic container) for two 10 minute sessions. The following day, the rats were placed in the same environment (Plexiglass cage, 40 cm in length, 20 cm in breadth, 20 cm in height) and 100 ul of either water (no odor), lemon scent, and TMT odor (Phero Tech Inc., British Columbia, Canada) were presented in this order. Between each scent the animal was allowed to return to its home cage for a resting period of 1o minutes. This quantity of TMT has been used in past behavioral and imaging experiments to examine innate fear responses in our laboratory [10].
The animal remained in each condition for 5 min. Each test session was scored as well as videotaped for future analysis. The videotapes were then blindly scored for fear behaviors, particularly freezing behavior. The freezing response consisted of cessation of all movement except those necessary for breathing [23].
2.5 Magnetic Resonance Imaging
All images were acquired using a 4.7T/40 cm horizontal magnet equipped with a Bruker BioSpec console and a 20 Gauss/cm magnetic field gradient insert (inner diameter, 12 cm) capable of a 120 μs rise time (Bruker, Billerica, MA U.S.A). High resolution multislice anatomical images for each subject were obtained using a fast spin-echo pulse sequence (RARE, or rapid acquisition relaxation enhanced) with relaxation time (TR) = 2.0s, effective excitation time (TE =12 ms), Matrix = 256 × 256, field of view (FOV) = 3.0cm × 3.0 cm, eighteen 1.0-mm slices. Subtraction of these data sets confirmed there was no significant movement of the animal over the imaging session. Subsequent functional imaging was performed at a resolution of 642 × 18 slices with the same FOV and slice thickness with RARE sequence of TR = 2.5s, TE = 7 ms,16 echo train length, average =2, Number of repetitions=30, total acquisition time=10 minutes. The first 10 repetitions were used for acclimation and control. Following baseline data acquisition, the stimulus (TMT, British Colombia, Canada) was introduced and acquisition continued for an additional 20 repetitions.
2.5 Data processing
Following the functional sequences repeated anatomy images were collected and compared to the initial anatomy image sets. Motion artifact was assessed by: 1) subtraction of anatomical data across the imaging session, 2) qualitative analysis of time series movies looking for voxel displacement, and 3) analysis of raw data time series for course spikes. The time series movies correlated with course spike activity. The multiple data sets collected from these imaging sessions showed very little motion artifact using these criteria. On the rare occasion there would be a course spike usually caused by movement of the mouth such as in swallowing. The data for these images were excluded.
Each subject was registered or aligned to a fully segmented rat brain atlas that delineates more than 1200 distinct anatomical subvolumes within the brain based on 2D atlas textbooks [42,52]. These detailed regions are collected into some major regions of the brain, e.g. amygdaloid complex, cerebrum, hippocampus, BNST, etc. The anatomy volumes were aligned to the atlas volume using interactive manual registration. The affine registration involved translation, rotation, and scaling in all 3 dimensions, independently.
The matrices that transformed the subject's anatomy volume to the atlas space were used to embed each slice within the atlas. All transformed pixel locations of the anatomy images were tagged with the segmented atlas major and minor regions creating a fully segmented representation of each subject. The inverse transformation matrix [Ti]-1for each subject (i) was also calculated. An interactive GUI facilitated these alignments [59].
Unpaired t-test statistics were performed on each individual subject within their original coordinate system. The control period was repetitions 2 to 9. The stimulation was repetition 12 to 19 when stimulus was presented. The t-test used a 95% confidence level, two-tailed distributions and heteroscedastic variance assumptions. These analysis settings provided conservative estimates for significance. Those pixels deemed statistically significant, retained their percent change values, (stimulation mean minus control mean) relative to control mean. All other pixel values were set to zero.
The segmented atlas was cropped and rendered onto 18 slices of 256×256 resolution corresponding to the 30×30mm FOV of the subjects. This cropped atlas served as the segmented composite with coordinates for row, column, and slice. For each group, by taking the average of activated number of pixels (Nact) within each ROI, and presenting the Nact pixels with the highest BOLD change, a group statistical composite was created.
The segmented atlas was cropped and rendered onto 18 slices of 2562 resolution corresponding to the FOV of the subjects. This cropped atlas served as the segmented composite with coordinates for row, column, and slice. A statistical composite was created for each group. The individual analyses were summed within groups.
The BOLD response maps of the composite were somewhat broader in their spatial coverage than an individual subject. Consequently, the magnitudes of the statistical values were commensurately reduced. This outcome was a natural consequence of the inability to align each subject within a pixel resolution. However, the subjects were aligned very well at the resolution of the regions of interest (ROIs). Regions of interest were chosen a priori based on previous imaging studies done with TMT and other regions commonly cited to be involved in the innate fear response, which will be further described in the discussion portion of this paper.
3. Results
Figure 1 summarizes behavioral responses to fear-inducing (TMT) odor, neutral (lemon), and no stimulus as monitored by changes in freezing behavior in different phases of the estrus cycle. Time spent freezing, defined as an animal remaining motionless for at least 3 seconds was significantly enhanced in the estrus phase (F=41.3, p=0.03) compared to both the proestrus and diestrus phases during TMT presentation. There were no significant differences across estrus cycle during control scent presentation (lemon and no odor, p>0.05). This behavioral increase in fear responding was also monitored for corresponding changes in brain activation (see below).
Figure 1. Behavioral response to TMT and lemon scent across the estrus cycle.
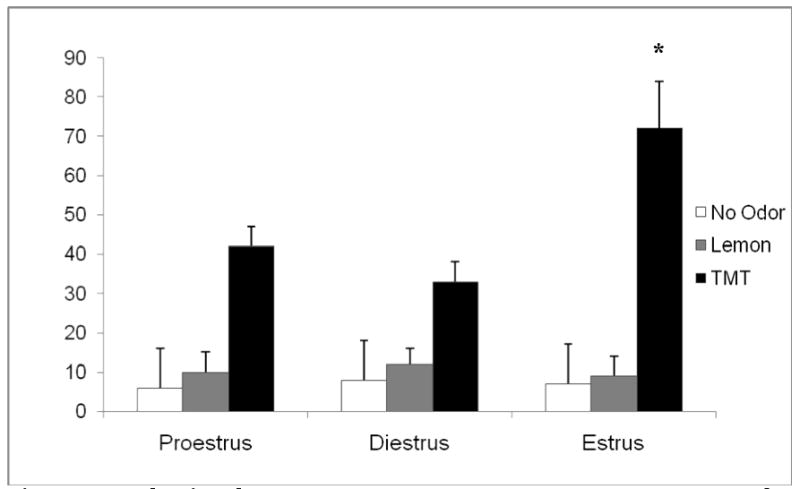
Error bars represent SEM. Time spent freezing, defined as an animal remaining motionless for at least 3 seconds was recorded. The estrus rat froze significantly longer than both the proestrus and diestrus phases (p=0.03). There were no significant differences between estrus phases in response to lemon scent (p>0.05). Additionally there were no significant differences between phases in freezing in response when no scent stimulus was presented (p>0.05).
Figure 2 depicts TMT odor-induced brain activation maps (2D) of positive BOLD signal response across the estrus cycle. Each subject was registered or aligned to a fully segmented rat brain atlas using MIVA (Medical Image Visualization and Analysis). The activated voxels (based on a t-test, thresholded at p <0.05) are overlaid onto the corresponding anatomy (2D) atlas. Independent of the phase of the cycle all animals exhibited regional changes in brain activation in cortical and sub-cortical regions in response to TMT olfactory cue.
Figure 2. TMT-elicited brain activation maps (2D) of positive BOLD signal response across the estrus cycle.
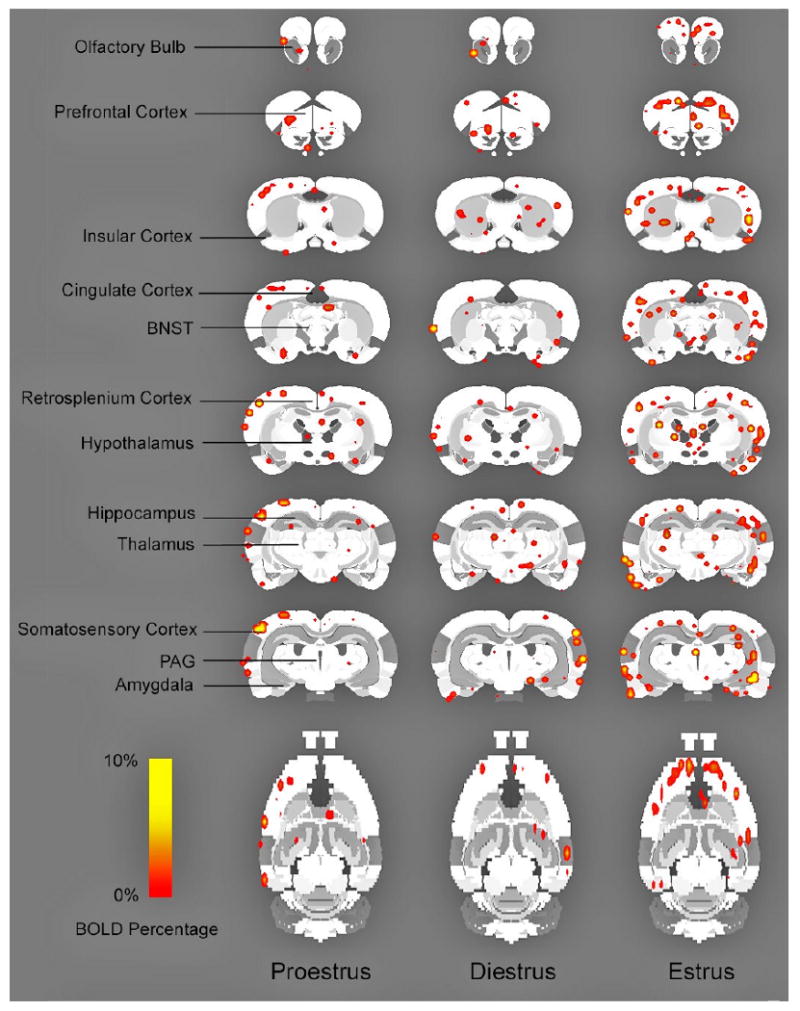
Each subject was registered or aligned to a fully segmented rat brain atlas using MIVA (Medical Image Visualization and Analysis). Activated voxels (based on a t-test, thresholded at p <0.05) are overlaid onto the corresponding segmented atlas (2D).
Figure 3 summarizes the number of activated positive pixels across the estrus cycle in the olfactory cortex and other cortical brain regions in response to TMT odor. Significant differences in positive signal intensity were observed after TMT exposure in several cortical brain regions including the olfactory cortex (F=6.674, p=0.009), somatosensory cortex (F=4.193, =0.037), insular cortex (F=6.964, p=0.008) and cingulate cortex (F=5.409, p=0.018). Post-hoc tests showed that signal intensity was significantly increased in the estrus compared to proestrus phase of the cycle in the olfactory cortex (0.009) and cingulate cortex (p=0.039). When comparing estrus to diestrus significant changes in pixel intensity were observed in the olfactory cortex (p=0.041), somatosensory cortex (p=0.034), insular cortex (p=0.007), and the cingulate cortex (p=0.024) compared to estrus.
Figure 3. Average number of significantly activated voxels across the estrus cycle in cortical areas.
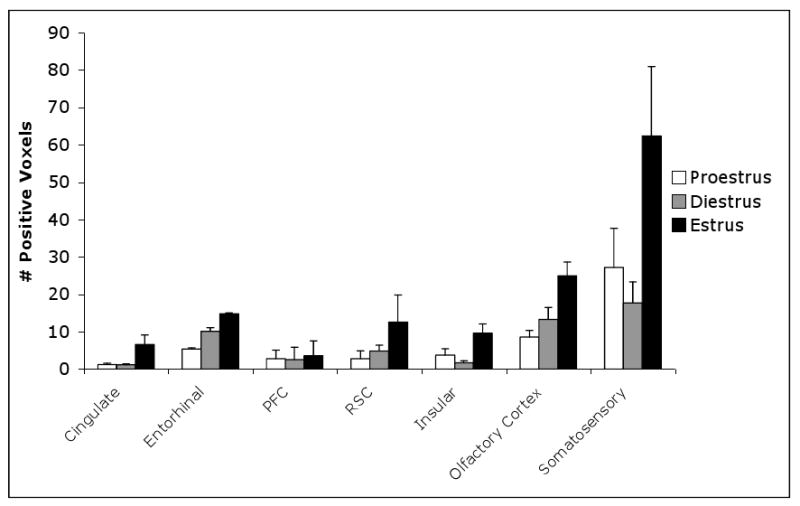
Error bars represent SEM (PFC, prefrontal cortex, RSC, retrosplenial cortex). The somatosensory cortex, insular cortex exhibits significant difference between diestrus and estrus (shown by *). The olfactory system and cingulate cortex exhibit significant differences between estrus and both pro and diestrus (represented by **).
Figure 4 depicts the number of activated positive pixels across the estrus cycle in sub-cortical brain regions in response to TMT odor. Significant differences in positive signal intensity were observed after TMT exposure in several sub-cortical brain regions including hippocampus (F=6.863, p= 0.008), hypothalamus (F=5.004, p=0.023), BNST (F=5.647, p=0.016), and septum (F=3.7388, p=0.0001). Post hoc analysis showed significantly enhanced activation in estrus compared proestrus in the hippocampus (p=0.01), hypothalamus (p=0.031), BNST (p=0.034), and septum (p=0.001). While significantly positive pixels were observed in estrus compared to the diestrus phase of the cycle in the following sub-cortical brain regions namely the hippocampus (p=0.024), hypothalamus (p=0.045), and septum (p=0.001).
Figure 4. Average number of significantly activated voxels across the estrus cycle in sub-cortical areas.
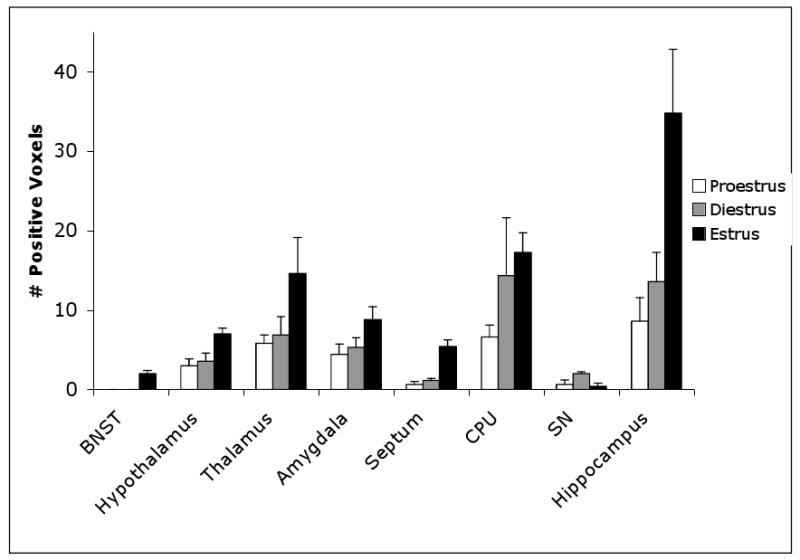
Error bars represent SEM. (BNST, Bed nucleus stria terminalis; CPU- Caudate putamen, SN, Substantia nigra) The hypothalamus, hippocampus, BNST, and septum exhibit significant differences between the proestrus and estrus phases (*).
Both cortical and sub-cortical sites were uniquely activated in different phases of the cycle in response to TMT odor. These data suggest that the phase of the cycle may facilitate a selective neuronal and behavioral response to emotionally relevant stimuli.
Figure 5 shows a time course exhibiting BOLD signal change over time in response to TMT presentation. This data demonstrates there were no significant differences between estrus phases during baseline fMRI imaging (p>0.05).
Figure 5. Time course showing signal change across estrus phases for the olfactory cortex.
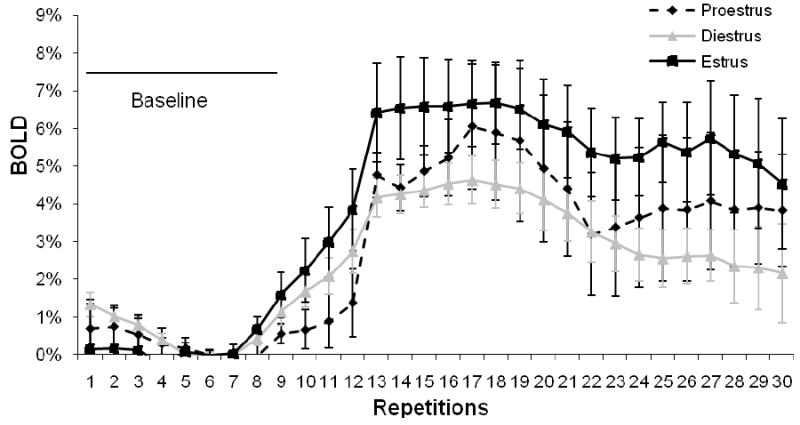
Error bars represent SEM. This figure demonstrates that there were no significant differences (p>0.05) in BOLD response during baseline imaging where no scent was presented.
4. Discussion
The current study was performed to assess the impact of estrus cycle phase on female's response to an innate fear-evoking stimulus. Behavioral studies assessing olfactory-induced freezing responses to TMT clearly demonstrate enhanced fear-induced response in estrus versus other phases of the cycle. Additionally, freezing was not significantly altered during different estrus phases in response to control stimuli suggesting that the animals exhibit a more appropriate response following. Since the estrus phase, is the period of female sexually receptivity, these data suggest that females in this phase of the cycle maybe more perceptive to olfactory stimuli warning the organism of possible impending danger. Because predation odor elicits relevant defensive behavioral responses in rodents, it is not surprising that these responses are plastic and subject to factors such as context [5] and physiological variables like age, sex, and reproductive status [27, 43]. In addition to the behavioral responses, neuronal activation in the olfactory system is significantly enhanced in estrus supporting the phenomenon that olfactory sensitivity may be modulated by reproductive state as observed in rodents [48] and humans [44]. In a human study using n-butanol to address the influence of fluctuating hormones on olfaction researchers observed thresholds were lower in midcycle as opposed to during menses, when this pattern switches [44]. We speculate that during sexually receptivity stage, it would be evolutionarily advantageous for females to become more aware of relevant olfactory stimuli necessary for both survival and procreation (i.e. pheromones and predator odor). Since we only monitored each experimental condition for freezing behavior due to its relevance to the defense response, we are unsure if olfactory sensitivity to other odors changed with other phases of the estrus cycle. This would be an interesting question to examine in future studies.
Since several studies have shown that hormonal alterations across the menstrual cycle may be associated with significant changes in brain activity [11,14,17,24], this study was designed to explore the neural components associated with exposure to a fear-inducing stimulus across the esrus cycle. Past reports exploring the effects of estrous status on mood and anxiety related behaviors have not arrived at a clear consensus. Some studies suggest that proestrus is period when females show reduced immobility on the forced swim test [19], inceased latencies in burying an electrified prod [20], and spend more time in the open arms of the elevated plus maze than do diestrus females or males [21,37,58]. Other studies show that females in proestrus and estrus show increased anxiety on elevated plus maze, additionally when estrogen and progesterone are given to ovariectomized rats, they exhibit decreased anxity on this task [39].
In accordance with past studies, several brain regions implicated in the neural response to TMT (in male rats) [10] were activated in response to the fear-eliciting odor in females. Namely, the enhanced defensive fear behaviors exhibited by female rats were accompanied by brain activation in both cortical and sub-cortical sites [13]. Past studies in our laboratory [10] using manganese–enhanced MRI (MEMRI) in males exposed to TMT show enhanced uptake in the cingulate cortex, thalamus, hypothalamus, hippocampus, and amygdala. The current report shows that the brain regions participating in innate fear are similar in both males and females exposed to TMT. However, in the female studies we found neuronal activation alterations in another cortical region namely the insular cortex.
Other studies have found changes in the insular cortex when processing negative emotional stimuli [34,36] and or the reported action of estrogen in modulating arousal/sympathetic tone in the insular cortex to [47]. Outside of the insular cortex the septum is another region that did not appear as critical using the MEMRI method. In the current data the septum shows enhanced activation in estrus compared to the other stages of the cycle. The septum, a subregion of the basal ganglia, has been implicated in threat assessment in mammals. More specifically, recent observations demonstrate that this region is critical to TMT–induced fear behaviors[15].
Taken together, these data support TMT enhanced behavioral and neuronal activation in estrus females as opposed to other stages of the estrus cycle. We speculate that the influence of hormonal status on the observed emotional responding may be via complex interactions between ovarian hormones and the cortico-limbic circuitry. For example, in the hippocampus a brain region critical to the expression of certain types of defensive behavior [4,53] and implicated in fear conditioning [1,2] has been shown to contain large numbers of estrogen receptors [18]. In addition, estrogen may function at these sites critical to fear expression in an indirect manner. Estrogen down-regulates 5-HT1A receptor in the limbic system and up-regulates the levels of 5-HT2A receptor in the cortex of both female rats and pre-menopausal women [41,51]. These receptors have opposite effects on excitation in the bed nucleus of the stria terminals (BNST), another important area in the anxiety and unconditioned fear response [35,50]. The differential effects of estrogen in several of these critical brain regions may modulate the response to different evolutionarily relevant olfactory cues associated with survival and procreation.
In conclusion, greater responsiveness to fear both behaviorally and neuronally in estrus may indicate that fluctuations in gonadal hormones that occur with the estrus cycle may influence females in an evolutionary distinct manner. Further research elucidating the exact mechanisms involved in this response can have both scientific and clinical relevance.
Acknowledgments
This publication was made possible by Grant Number R01 MH067096-01 from the National Institute of Mental Health. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wei Chen, Email: Wei.chen@umassmed.edu.
Jessica Shields, Email: Jessica.shields@umassmed.edu.
Wei Huang, Email: Wei.huang2@umassmed.edu.
Works Cited
- 1.Antoniadis EA, McDonald RJ. Amygdala, hippocampus and discriminative fear conditioning in context. Behav Brain Res. 2000;108:1–19. doi: 10.1016/s0166-4328(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman DM, Yee BK, Lemaire M, Jarrard L, Iversen SD. Contextual fear conditioning is disrupted by lesions of the subcortical, but not entorhinal, connections to the hippocampus. Exp Brain Res. 2001;141:304–311. doi: 10.1007/s002210100869. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard RJ, Blanchard DC, Rodgers J, Weiss SM. The characterization and modeling of antipredator defensive behavior. Pharmacol Biochem Behav. 1991;40:819–28. doi: 10.1016/0091-3057(91)90092-g. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard RJ, Blanchard DC. Effects of hippocampal lesions on the rat's reaction to a cat. J Comp Physiol Psychol. 1972;78:77–82. doi: 10.1037/h0032176. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard, Yang, Li, Gervacio, Blanchard Cue and context conditioning of defensive behaviors to cat odor stimuli. Neurosci Biobehav Rev. 2001;25:587–95. doi: 10.1016/s0149-7634(01)00043-4. [DOI] [PubMed] [Google Scholar]
- 6.Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor mediated function in female rats. Endocrinology. 1992;131:1261–9. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- 7.Burwash MD, Tobin ME, Woolhouse AD, Sullivan TP. Laboratory evaluation of predator odors for roof rats. J Chem Ecology. 1998;24:49–66. [Google Scholar]
- 8.Campbell R, Elgar K, Kuntsi J, Akers R, Terstegge J, Coleman M, Skuse D. The classification of ‘fear’ from faces is associated with face recognition skill in women. Neuropsychologia. 2002;40:575–84. doi: 10.1016/s0028-3932(01)00164-6. [DOI] [PubMed] [Google Scholar]
- 9.Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mous by the appearance of the vagina. Biology of Reproduction. 1973;8:191–4. doi: 10.1093/biolreprod/8.4.491. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Tenney J, Kulkarni P, King JA. Imaging unconditioned fear response with manganese-enhanced MRI. Neuroimage. 2007;37:221–9. doi: 10.1016/j.neuroimage.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Murphy DG. Physiological variation in estradiol and brain function: a functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm Behav. 2008;53:503–8. doi: 10.1016/j.yhbeh.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Critchlow V, Liebelt RA, Bar-Sela M, Mountcastle W, Lipscomb HS. Sex difference in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–15. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 13.Dielenberg RA, McGregor IS. Defensive behavior I rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25:597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich T, Krings T, Neulen J, Willmes K, Erberich S, Thron A, Sturm W. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. Neuroimage. 2001;13:425–32. doi: 10.1006/nimg.2001.0703. [DOI] [PubMed] [Google Scholar]
- 15.Endres T, Fendt M. Inactivation of the lateral septum blocks fox odor-induced fear behavior. Neuroreport. 2008;19:667–70. doi: 10.1097/WNR.0b013e3282fb78d9. [DOI] [PubMed] [Google Scholar]
- 16.Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS. TMT-induced autonomic and behavioral changes and the neural basis of its processing. Neurosci Biobehav Rev. 2005;29:1145–56. doi: 10.1016/j.neubiorev.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez G, Weis S, Stoffel-Wagner B, Tendolkar I, Reuber M, Beyenburg S, Klaver P, Fell J, de Greiff A, Ruhlmann J, Reul J, Elger CE. Menstrual cycle-dependent neural plasticity in the adult human brain is hormone, task, and region specific. J Neurosci. 2003;23:3790–5. doi: 10.1523/JNEUROSCI.23-09-03790.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sci. 1998;21:311–27. doi: 10.1017/s0140525x98001216. [DOI] [PubMed] [Google Scholar]
- 19.Frye CA, Wawrzycki J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm Behav. 2003;44:319–26. doi: 10.1016/s0018-506x(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 20.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav. 2000;67:587–96. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 21.Galeeva A, Tuohimaa P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav Brain Res. 2001;119:41–7. doi: 10.1016/s0166-4328(00)00341-7. [DOI] [PubMed] [Google Scholar]
- 22.Galea LA, McEwen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow vowles. Neuroscience. 1999;89:955–64. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- 23.Godsil BP, Quinn JJ, Fanselow MS. Body temperature as a conditional response measure for pavlovian fear conditioning. Learn & Mem. 2000;7:353–6. doi: 10.1101/lm.32800. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, Makris N. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–16. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes MM, Galea LA. Defensive behavior and hippocampal cell proliferation: differential modulation by naltrexone during stress. Behav Neuroscie. 2002;116:160–8. [PubMed] [Google Scholar]
- 26.Insel TR. Assessing the Economic costs of serious mental illness. Am J Psychiatry. 2008;165:663–5. doi: 10.1176/appi.ajp.2008.08030366. [DOI] [PubMed] [Google Scholar]
- 27.Jedrzejewski W, Jedrzejewska B. Effect of a predator's visit on the spatial distribution of bank voles: experiments with weasels. Can J Zool. 1990;68:660–6. [Google Scholar]
- 28.Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry. 2001;158:11–20. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- 29.Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotnin. Brain Res. 1986;382:416–21. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- 30.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 31.Klein SL, Lambert KG, Durr D, Schaefer RE. Influence of environmental enrichment and sex on predator stress response in rats. Physiol Behav. 1994;56:291–7. doi: 10.1016/0031-9384(94)90197-x. [DOI] [PubMed] [Google Scholar]
- 32.Lahti K, Ferris CF, Li F, Sotak CH, King JA. Imaging brian activation in conscious animals using functional MRI. J Neurosci Methods. 1998;82:75–82. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 33.Le Doux JE. Cognitive–Emotional Interactions-Listen to the Brain. In: Richard L, Lynn N, editors. Cognitive Neuroscience of Emotion. NY: Oxford University Press; 2000. pp. 129–155. [Google Scholar]
- 34.Lee BT, Seok JH, Lee BC, Cho SW, Yoon BJ, Lee KU, Chae JH, Choi IG, Ham BJ. Neural correlates of afffective processing in response to sad and angry facial stimuli in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:778–85. doi: 10.1016/j.pnpbp.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Levita L, Hammack SE, Mania I, Li XY, Davis M, Rainnie DG. 5-hydroxytryptamine1A-like receptor activation in the bed nucleus of the stria terminalis: electrophysiological and behavioral studies. Neuroscience. 2004;128:583–96. doi: 10.1016/j.neuroscience.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 36.Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter T. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disord. 2007;9:345–57. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- 37.Marcondes FK, Miguel KJ, Mello LL, Spadari-Bratfish RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Mota L, Estrada-Camarena E, Lopez-Rubalcava C, Contreras CM, Fernandez-Guasti A. Interaction of desipramine with steroid hormones on experimental anxiety. Psychoneuroendocrinology. 2000;68:279–84. doi: 10.1016/s0306-4530(99)00042-6. [DOI] [PubMed] [Google Scholar]
- 39.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–20. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 40.Morgan MA, Pfaff DW. Estrogen's effects on activity anxiety, and fear in two mouse strains. Behav Brain Res. 2002;132:85–93. doi: 10.1016/s0166-4328(01)00398-9. [DOI] [PubMed] [Google Scholar]
- 41.Moses-Kolko EL, Berga SL, Greer PJ, Smith G, Cidis Meltzer C, Drevets WC. Widespread increases of cortical serotonin type 2A receptor availability after hormone therapy in euthymic postmenopausal women. Fertil Steril. 2003;80:554–9. doi: 10.1016/s0015-0282(03)00973-7. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C, editors. The rat brain in stereotaxic coordinates. 3rd. San Diego: Academic Press; 1991. [Google Scholar]
- 43.Perrot-Sinal T, Ossenkopp KP, Kavaliers M. Influence of a natural stressor (predator odor) on locomotor activity in the meador vole: modulation by sex, reproductive condition, and gonadal hormones. 2000;25:259–76. doi: 10.1016/s0306-4530(99)00054-2. [DOI] [PubMed] [Google Scholar]
- 44.Purdon SE, Klien S, Flor-Henry P. Menstrual effects on asymmetrical olfactory acuity. J Int Neuropsychol Soc. 2001;7:703–9. doi: 10.1017/s1355617701766064. [DOI] [PubMed] [Google Scholar]
- 45.Rapkin AJ, Mikacich JA, Moatakef-Iman B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Report. 2002;4:419–428. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- 46.Rosen JB. The neurobiology of conditioned and unconditioned fear: A neurobehavioral system analysis of the amygdala. Behav and Cognitive Neurosci Rev. 2004;3:23–41. doi: 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- 47.Saleh TM, Connell BJ, Legge C, Cribb AE. Estrogen synthesis in the central nucleus of the amygdala following middle cerebral artery occlusion: role in modulating neurotransmission. Neuroscience. 2005;135:1141–53. doi: 10.1016/j.neuroscience.2005.06.061. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt C, Schmidt U. Changes of olfactory sensitivity during the estrus cycle in female laboratory mice. Chemical Senses. 1980;5:359–65. [Google Scholar]
- 49.Sharp PE, La Regina MC. The laboratory rat. CRC Press; 1998. [Google Scholar]
- 50.Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007m;37:1427–36. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 51.Summer BE, Fink G. Estrogen increases the density of 5-hyroxytryptamine(2A) receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol. 1995;54:15–20. doi: 10.1016/0960-0760(95)00075-b. [DOI] [PubMed] [Google Scholar]
- 52.Swanson LW. Brain Maps: Structure of the Rat Brain. 2nd. Elsevier; The Netherlands: 1998. [Google Scholar]
- 53.Takahashi LK, Nakashima BR, Hong H, Watanabe K. The smell of danger: A behavioral and neural analysis of predator odor-induced fear. Neuroscience and Biobehav Rev. 2005;29:1157–67. doi: 10.1016/j.neubiorev.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–11. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vernet-Maury E, Polack EH, Demael A. Structure-activity relationship of stress-inducing odorants in the rat. J Chem Ecol. 1984;10:1007–18. doi: 10.1007/BF00987509. [DOI] [PubMed] [Google Scholar]
- 56.Walf AA, Frye CA. Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects. Stress. 2007;10:251–60. doi: 10.1080/00958970701220416. [DOI] [PubMed] [Google Scholar]
- 57.Yonkers KA. Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? J Clin Psychiatry. 1997;58:62–7. [PubMed] [Google Scholar]
- 58.Zimmerberg B, Farley MJ. Sex differences in anxiety behavior in rats: role of gonadal hormones. Physiol Behav. 1993;54:1119–24. doi: 10.1016/0031-9384(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Sullivan JM, Wu Z, Huang W, Kinkar S. Registration and Visualization, A Framework for Multi-Modality Imaging Registration and Visualization. 8th US National Congress on Computational Mechanics; 2005. [Google Scholar]


