Abstract
Background
Quantification of serum tumor markers plays an important role in determining whether patients treated for cancer require further therapy. Whereas large-scale proteomic efforts aim to identify novel tumor markers to facilitate early detection, optimization of methods for quantifying known tumor markers offers another approach to improving management of malignancies. For example, immunoassays used in clinical practice to measure established tumor markers suffer from potential interference from endogenous immunoglobulins and imperfect concordance across platforms – problems that also plague many other immunoassays. To address these important limitations, this study used peptide immunoaffinity enrichment in concert with liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify thyroglobulin, a well-characterized tumor marker.
Methods
First, we identified three peptides in tryptic digests of thyroglobulin that were detected at low concentrations by tandem mass spectrometry. We then raised polyclonal antibodies to those peptides. The antibodies were used to extract the three corresponding peptides from tryptic digests of human serum. Each endogenous peptide was then quantified with LC-MS/MS and multiple reaction monitoring, using external calibrators.
Results
The detection limit for endogenous thyroglobulin in serum was 2.6 μg/L (4 pmol/L). Direct comparison with immunoassay revealed good correlation (r2 = 0.81).
Conclusions
Immunoaffinity peptide enrichment-tandem mass spectrometry can detect tryptic peptides of thyroglobulin at picomolar concentrations, while also digesting the endogenous immunoglobulins that can potentially interfere with traditional immunoassays. Our observations suggest a general analytical strategy for using immunoaffinity isolation together with tandem mass spectrometry to quantify tumor antigens and other low-abundance proteins in human serum.
INTRODUCTION
There is intense interest in using proteomics to discover novel biomarkers for the diagnosis and prognosis of disease. However, validation has proved to be a major stumbling block (1). While the final aim of biomarker discovery efforts is the development of a novel quantitative clinical assay, current immunoassay methodologies suffer from well-known limitations. Successful clinical validation of new disease markers may be hampered by these limitations when tested in diverse patient populations. Serum thyroglobulin (Tg), which is already used in conjunction with imaging to determine the clinical management of patients following treatment of thyroid carcinoma, represents one of the best-validated serum tumor markers in clinical chemistry (2–5). It is also an example of a biomarker whose clinical utility is hampered by limitations in analytical methods.
Several studies have meticulously characterized the problems associated with the quantification of serum thyroglobulin, problems that are shared by many other immunoassays. For example, approximately 10% of patients have antibodies to Tg that could potentially interfere with immunoassays (6–9). The prevalence of Tg autoantibodies increases to 25% in patients with differentiated thyroid carcinoma, although the reasons for this are incompletely understood (10–12). In addition, 3% of all patients have non-specific heterophilic interfering antibodies that bind to reagent immunoglobulins, typically causing false-positive results in sandwich immunometric assays. Heterophilic antibody blocking reagents can reduce the number of false-positives 30-fold, but a number of subjects continue to have false-positive results (13). Commercial serum thyroglobulin assays also suffer from a lack of concordance across platforms, likely due to significant variability of post-translational modification of Tg in vivo (14–16). Even with an international reference material available, results for patient samples are often substantially different with different assays – an issue common to other immunoassays (17–19).
To test a general approach to detecting low-abundance proteins in serum that avoids the limitations inherent to immunoassays, we took advantage of the power of mass spectrometry (MS) to detect specific peptides in complex mixtures. Previous attempts to use MS to quantify low-abundance endogenous serum proteins include the direct detection of peptides from trypsin-digested serum with (20) and without (21) serum fractionation. However, only nanomolar concentrations of endogenous protein were detectable. We therefore tested a method developed by Anderson et al. (22), termed “stable isotope standards and capture by anti-peptide antibodies.” The approach combines immunoaffinity peptide purification with tandem mass spectrometry. As a test protein, we selected thyroglobulin.
METHODS AND MATERIALS
Peptide isolation and identification
Purified human thyroglobulin (Cortex Biochem, San Leandro, CA; Swiss-Prot accession number P01266) was digested with three different digestion protocols (see details in Supplemental Methods). For Digestion 1, Tg was denatured with urea, reduced, alkylated, and digested with trypsin. Digestion 2 used the same conditions as Digestion 1 without reduction and alkylation. Digestion 3 used the same conditions as Digestion 1 except that Tg was supplemented with normal human serum (Tg:serum protein ratio of ~1:60). The resulting peptides from each digestion were purified using solid phase extraction and identified using two complementary techniques: i) microelectrospray ionization liquid chromatography-tandem mass spectrometry (LC-MS/MS) on a linear ion trap mass analyzer and ii) off-line HPLC-plate spotting followed by matrix assisted laser desorption ionization-tandem time of flight mass spectrometry (MALDI-TOF-TOF). Database searching was performed as described in the Supplemental Methods. The peptides used below were unique in the translated non-redundant human genome database by BLAST searching performed as described in Supplemental Methods.
Internal standard peptide
Stable-isotope labeled internal standard peptide (VIFDANAPV*AVR, where V* represents 13C5,14N-labeled valine) was synthesized, analyzed for purity, and analyzed for amino acid content by Anaspec (San Jose, CA). Subsequent calculations of concentration were based on the purity assessment and amino acid analysis. Isotopic purity of the peptide was 99.9%.
Quantification of international Tg reference material
After reduction and alkylation, triplicate dilution series of a certified reference preparation of Tg (BCR-457; Sigma) were digested for 2 h at 37°C using 0.5 μg sequencing grade modified trypsin in 0.1% Rapigest (Waters Corp.). Peptides were then lyophilized and reconstituted in 5% acetonitrile, 0.1% formic acid in water. Suspended peptides were mixed with an equal volume of isotope-labeled peptide (15 nmol/L) and analyzed with LC-MS/MS. To determine the concentration of BCR-457, data from three independent experiments were fit using the relation y=Axn, which was used to calculate the dilution necessary to achieve a response of 1.0 (i.e. solving for the dilution (x) where the response (y) equals 1.0).
Antiserum preparation
Based on the MS/MS analysis of tryptic digests of Tg (see Results), we selected 3 peptides for antiserum production (VIFDANAPVAVR, Peptide 1; LGDQEFIK, Peptide 2; and FPLGESFLVAK, Peptide 3; EZBiolab, Westfield, IN). Peptides (2mg) were each conjugated to 10 mg keyhole limpet hemocyanin (KLH; Calbiochem) using the glutaraldehyde method. Peptide 1 was also conjugated to KLH using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDAC; Sigma), according to the manufacturer’s instructions. The efficiency of conjugation was >65% for each reaction as assessed by MS/MS quantification of peptide that passed through a 10 kDa MWCO Microcon filter (Milipore). The peptide-KLH conjugates were combined and used to immunize two New Zealand white rabbits (Standard protocol; Pacific Immunology, Ramona, CA).
Peptide-conjugate beads, affinity purification of antibody, and anti-peptide paramagnetic beads
We conjugated the peptides to bovine serum albumin (BSA) using the same procedures as for KLH. Peptide-BSA conjugates were then covalently bound to CNBr-activated Sepharose 4B (0.32 g; GE Lifesciences) according to the manufacturer’s instructions. Analysis of the protein concentration of the supernatant confirmed >63% conjugate binding to the beads (Coomassie Plus; Pierce). Peptide-BSA Sepharose conjugate was incubated with 10 mL rabbit serum (16 h, 4°C). The beads were washed with PBS and the antibodies eluted in 870 μL fractions with 200 mmol/L glycine, pH 2.80, into tubes containing 27 μL 3 mol/L Tris, pH 8.6, and 100 μL 3 mol/L KCl. Peak fractions were pooled. The yield was 2–6 mg per 10 mL rabbit serum. The affinity-purified antibody was covalently bound to tosyl-activated Dynal M-280 beads (Invitrogen) according to the manufacturer’s instructions. Reactions resulted in 2.3 ± 0.12 (SD) pmol binding sites/μL paramagnetic beads (2 × 109 beads/μL).
Affinity purification of thyroglobulin peptides
Serum (100 μL) was diluted with 400 μL 100 mmol/L NH4HCO3, 0.005% Tween 20, and reduced with 5 mmol/L DTT (60 min, 37°C, with rotation). Samples were alkylated in the dark with 15 mmol/L iodoacetamide (15–120 min) and digested in two steps: first with 4.5 μg trypsin (4 h, 37°C, with rotation); and second with 4.5 μg trypsin (16 h, 37°C, with rotation). We added 20 μL of beads (46 pmol of peptide binding sites) and 10 μL of internal standard peptide (15 nmol/L), followed by incubation for 16 h at 4°C. The beads were then washed twice with 500 μL 100 mmol/L ammonium acetate and twice with 500 μL water. Bound peptides were eluted with 15 μL 2% acetic acid (1–2 h, room temperature, with rotation). Human serum calibrators with known Tg concentrations were processed in parallel with clinical specimens (0, 2, 5, 20 μg/L).
Liquid chromatography-tandem mass spectrometry
Complete method details are provided in Supplemental Methods. Peptides were loaded onto a peptide trapping column and washed with 2% acetonitrile, 0.1% formic acid in water before being eluted onto a 0.15 mm diameter C18 analytical column with a linear gradient to 34.3% acetonitrile, 0.1% formic acid over 8 min at 1 μL/min. Peptides were analyzed in multiple reaction monitoring mode (MRM) with an Applied Biosystems API 4000 QTRAP using the m/z transitions 636.4/1059.6 (precursor+2/y10), 636.4/541.3 (precursor+2/y5) for peptide 1, 475.3/836.4 (precursor+2/y7), 475.3/779.4 (precursor+2/y6) for peptide 2, 604.3/850.5 (precursor+2/y8), 604.3/963.5 (precursor+2/y9) for peptide 3, and 639.8/1065.6 (precursor+2/y10), 639.4/547.3 (precursor+2/y5) for the internal standard peptide. Endogenous peptide peak areas were normalized to the peak area of the internal standard peptide and averaged for duplicate injections. Normalized endogenous peptide peak areas were then converted to Tg concentrations using a calibration curve generated from external human serum calibrators as illustrated in Supplemental Figure 1.
Human samples
All human samples were used in accordance with guidelines established by the Human Subjects Committee of the University of Washington.
RESULTS
Selecting Tg peptides for LC-MS/MS analysis
We analyzed tryptic digests of Tg with and without serum supplementation by tandem mass spectrometers interfaced with different ionization systems to determine which Tg peptides could best be detected and quantified by this approach. Data for the 15 most abundant peptides are presented in Supplemental Table 1. We immunized two New Zealand white rabbits with the three peptides that were detected most reliably by both ESI-ion trap and MALDI-TOF/TOF instruments. We included the fourth-most abundant peptide from Digestion 1 (FPLGESFLVAK) rather than the third-most abundant one (SHGQDSPAVYLK), because the latter was not well recovered when Tg was digested without reduction and alkylation or in the presence of serum proteins. Rather than generating three individual polyclonal preparations, we separately conjugated the peptides to KLH and immunized the rabbits with the pooled conjugates. The most abundant peptide across platforms and digestions (VIFDANAPVAVR, peptide 1) was chosen as the internal standard peptide, and a stable isotope-labeled peptide was synthesized.
Isotope dilution quantification of thyroglobulin in the certified reference material
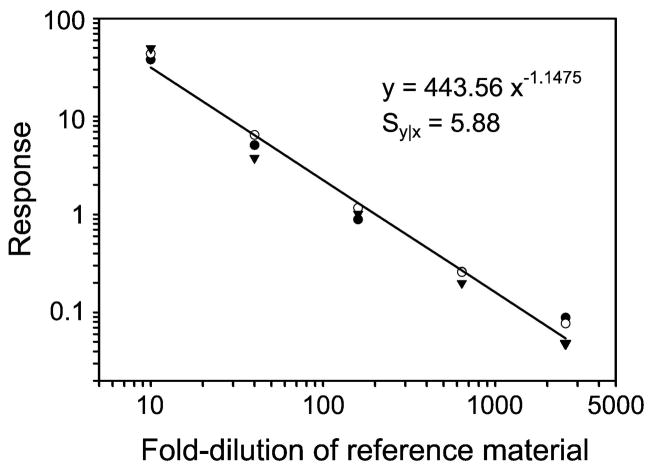
The international certified reference material BCR-457, which is used to provide traceable commercial immunoassays, was generated in 1992 from pooled human thyroglobulin from cadaveric thyroid tissue (23). However, the concentration of thyroglobulin was quantified using the Lowry method, which has been shown to be less precise than direct detection (24). We therefore quantified the amount of thyroglobulin in the preparation by isotope dilution, using a stable isotope-labeled synthetic peptide (peptide 1). Linear dilutions of a thyroglobulin–internal standard peptide mixture were digested, and the peptide mixture was supplemented with known amounts of internal standard peptide (Figure 1). This approach indicated that the concentration of thyroglobulin in BCR-457 was 317 ± 7 mg/L (mean ± SD; N=3), which is very similar to the previously assigned value of 324 ± 18 mg/L.
Figure 1. Quantification of international certified reference material (BCR-457) by isotope dilution MS/MS.
Triplicate dilutions of the certified reference material were digested with trypsin. The concentration of peptide 1 was determined in the tryptic digests from the ratio of endogenous to internal standard peptide. Each replicate dilution series is represented with different symbols. The curve representing the average of the data points was fit to the equation y = Axn, which was used to determine the dilution necessary to achieve a response of 1.0. Results represent those observed in three independent experiments.
Optimization of digestion for quantification by LC-MS/MS
Thyroglobulin is a large macromolecule consisting of two 330 kDa monomers. We first attempted to detect Tg after using size-exclusion chromatography or ultrafiltration to separate it from other serum proteins prior to trypsin digestion. Unfortunately, enrichment of Tg using size separation was ineffective, resulting in a limit of detection more than 1,000-fold higher than the clinically desired limit of detection. We therefore optimized the digestion of whole serum for the immunoaffinity enrichment of Tg peptides. One important source of variability in proteomic analyses is partial proteolytic digestion of complex biological material. High concentrations of chaotropic salts are commonly used to denature proteins and help facilitate more complete tryptic digestion (25). Although the denaturants are generally removed by solid-phase extraction after digestion, their use could increase variability due to differential recovery of peptide and additional specimen manipulation. As an alternate approach, we tested different concentrations of Tween 20, a nonionic detergent that helps denature proteins and may also improve stringency in the immunoaffinity purification step. Reduction and alkylation of 20% serum made the solution cloudy, but adding trypsin always cleared it when Tween 20 was present (0.005%, w:v). To promote proteolysis, we therefore included Tween rather than chaotropic salts in the serum digestion step. Sequencing-grade modified trypsin, which is usually used at much higher concentrations than in our studies, is expensive. We therefore investigated other commercial preparations of modified trypsin at 135–17,000 units of enzyme specific activity per sample. However, only sequencing grade trypsin was able to clarify the 10 human samples tested. Importantly, at the relatively low concentrations of trypsin used, it is unlikely that these clarified samples were completely digested.
Optimizing Tg quantification by immunoaffinity peptide enrichment and LC-MS/MS
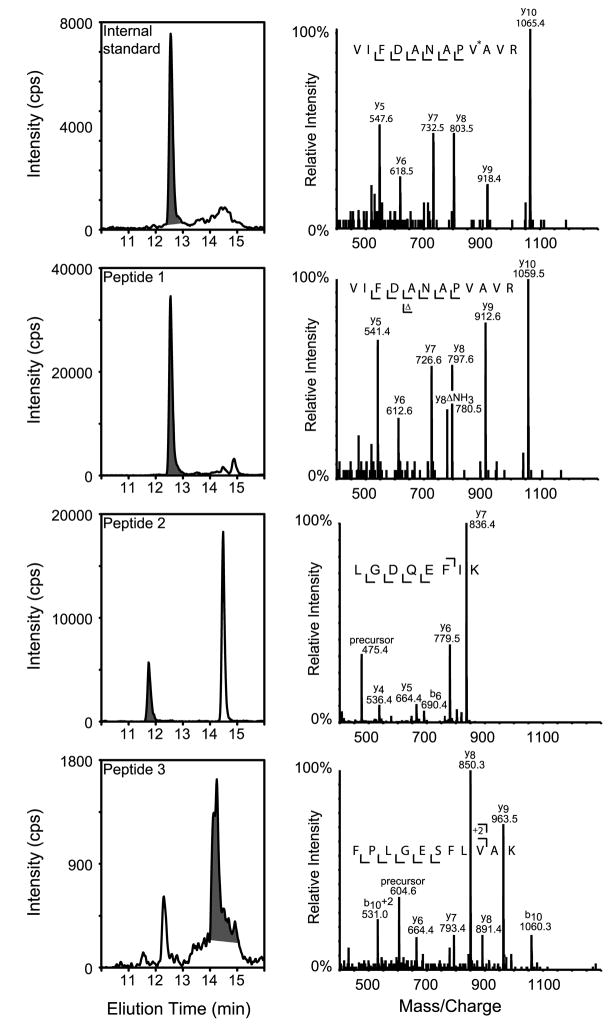
Tryptic digests of reduced and alkylated serum were incubated with anti-Tg peptide antibodies bound to paramagnetic beads. After washing, bound peptides were eluted from the beads and bound to a C18 trapping column. The column was then washed, and the peptides were eluted and resolved on an analytical column with an acetonitrile gradient. Figure 2 illustrates the ion current chromatogram for the internal standard and endogenous peptide MRM transitions. Peptides 1 and 2 elute earlier than peptide 3 and have a narrower peak width. Tandem mass spectra were collected in parallel with the MRM transitions, using the linear ion trap of the 4000 Q-TRAP mass spectrometer (Figure 2, right panels). Interpretable MS/MS spectra were acquired for each peptide in samples containing as little as 100 μg/L of endogenous Tg.
Figure 2. Chromatography and tandem mass spectrometry of immunoaffinity purified thyroglobulin peptides.
Immunoaffinity purified peptides were first loaded onto a peptide-trapping column before being resolved with a C18 analytical column, ionized using micro-ESI, and analyzed using multiple reaction monitoring (MRM). Elution profiles of peptides purified from a digest of human serum are shown (left panels). Fragment ion spectra were collected in the linear ion trap of the mass spectrometer at the same time as MRM data. They are shown for each peptide (right panels). V* represents a stable isotope-labeled valine residue.
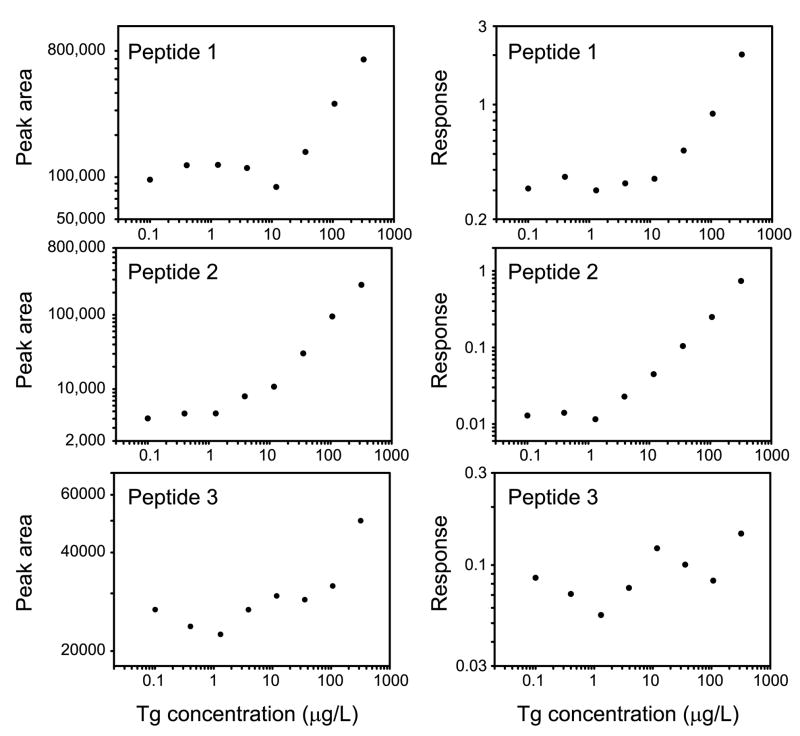
To determine which of the three peptides performed optimally in the assay, we tested human serum containing 319 μg/L endogenous thyroglobulin diluted serially with serum lacking Tg. The three peptides exhibited different behaviors (Figure 3). Each of the peptides had a marked background signal resulting from peptide that populated antigen-binding sites after affinity purification of the antibody. However, peptide 2 performed optimally with a minimal detectable concentration of 3.9 μg/L in this experiment. Peptide 1 had a higher background signal and had a minimal detectable concentration of 35 μg/L. The peak area of peptide 3 demonstrated greater variability in its signal-to-noise ratio and the minimal detectable concentration of Tg was 319 μg/L. Internal standard peptide was added after digestion to control for variability in the volume of immunoglobulin-coated beads added, variability in sample volume injected, and mass spectrometer performance. After normalization to the internal standard peptide, the responses of peptides 1 and 2 had similar characteristics to their respective ion current peak areas, but the response of peptide 3 was still more erratic (Figure 3).
Figure 3. LC-MS/MS response of endogenous thyroglobulin peptides vs. concentration.
Shown are the ion current peak areas (left panels) and responses (peak area of endogenous/peak area internal standard peptide, right panels) for each peptide detected in a dilution series of a human serum sample containing 319 μg/LTg. The internal standard peptide is chemically identical to Peptide 1 and differs only in mass.
Because of its superior performance in the LC-ESI-MS/MS analysis, we used peptide 2 to quantify Tg in subsequent human serum specimens. Peptide response was calculated as the peak area of analyte divided by the peak area of the surrogate internal standard isotope-labeled peptide, which was chemically identical to Peptide 1 rather than Peptide 2. External human serum calibrators were analyzed in parallel with other specimens (0, 2, 5, 20 μg/L). Performance characteristics of the assay are presented in Table 1. Intraassay imprecision was 13.7% at 22.3 μg/L, 22.5% at 5.5 μg/L, and 21.4% at 3.8 μg/L (6 pmol/L). Interassay precision was 17.4% at 6.0 μg/L. The lower limit of detection, defined as the concentration corresponding to mean + 2 SD of the zero calibrator, was 2.6 μg/L (4 pmol/L), which is equivalent to 0.8 femtomoles of peptide injected on column. At 3.8 μg/L the mean signal-to-noise ratio was 13.8. Recovery of Tg added at 21.6 μg/L to five different human serum samples lacking Tg ranged from 85.0 to 99.6% (mean ± SD 93.0 ± 5.4%). Calculated using peak area, mean carryover was 0.8% ± 0.8% (SD).
Table 1.
Performance characteristics.
| Tg Concentration (μg/L) | Result | |
|---|---|---|
| Intra-assay Imprecision | 22.3 | 13.7% |
| 5.5 | 22.5% | |
| 3.8 | 21.4% | |
| Inter-assay Imprecision | 6.0 | 17.4% |
| Lower Limit of Detection | 2.6 μg/L | |
| Signal-to-Noise Ratio | 3.8 | 13.8 |
| Recovery | 21.6 | 85.0 – 99.6% |
| Carryover | 0.8% |
Correlation of Tg quantification by immunoassay and mass spectrometry
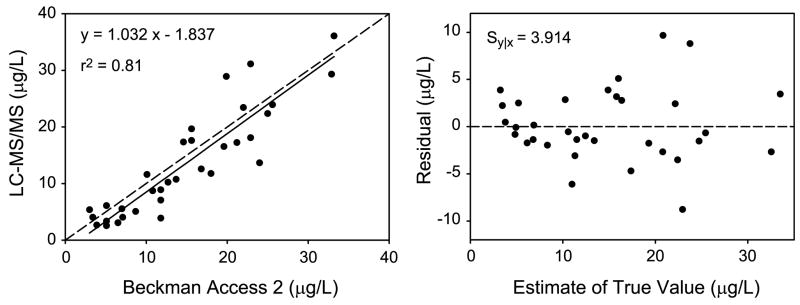
To determine how well the results of the LC-ESI-MS/MS assay correlated with those from a clinical immunoassay for Tg, we analyzed 33 samples previously quantified by the Beckman Access 2 assay. The range of Tg concentrations determined by the immunoassay was 3.0 to 33.2 μg/L. The Deming regression equation had a slope (95% confidence interval) of 1.03 (0.85—1.22) and an intercept of −1.84 (−4.93 to 1.26). The correlation (r2) was 0.81, and the standard error of the residuals (Sy|x) was 3.91 (Figure 4).
Figure 4. Correlation of Tg quantification by immunoaffinity enrichment-LC-MS/MS and an immunoassay.
Results using immunoaffinity purification of peptides from tryptic digests with external calibration were compared with a standard clinical immunoassay for Tg in serum (left). The Deming regression equation (Analyse-It; Leeds, England, UK) is shown with the Pearson correlation coefficient (r2). The residuals of each data point from the regression line are also shown (right) with the standard error of the residuals (Sy|x). The estimate of the true value was calculated using the ratio of the standard deviations of each method with Analyse-It.
DISCUSSION
Serum Tg has long been a thorn in the side of clinical tumor marker immunoassays because of its low concentration and common interferences (8, 10, 11, 13, 26). Therefore, we used Tg as the analyte in this study, which tested peptide immunoaffinity purification/mass spectrometry to measure an endogenous, clinically important low-abundance protein in human serum, a complex biological matrix. We were able to reliably detect 2.6 μg/L (4 pmol/L) of endogenous Tg, which is well within the range of 2.3–139 μg/L observed in euthyroid subjects (27). Our results demonstrate a marked improvement in limit of detection over previously published MS approaches. For example, MS has been used to quantify peptides derived from tryptic digests of proteins in human serum, including Zn-α2 glycoprotein (21), which was detected with a limit of detection of 90 nmol/L (~3 mg/L). A study that fractionated serum to quantify C-reactive protein had a limit of detection of 7 nmol/L (20). Whiteaker et al. used stable isotope standards and capture by anti-peptide antibodies to detect endogenous fibulin-2 in mouse plasma with a limit of detection of 380 pmol/L (28).
Quantifying Tg by peptide capture and LC-MS/MS performed well in comparison with the immunoassay we used, even in the low picomolar range in serum. Peptide immunoaffinity purification/mass spectrometry (22) has potential advantages over immunoassays. For example, it is in principle straightforward to multiplex the assay by including additional anti-peptide antibodies and isotope-labeled peptides. Moreover, because of the power of MS/MS and MRM to detect peptides in complex mixtures, it is not necessary to develop highly specific antibodies that react only with the peptides or proteins of interest. Indeed, in this experiment, the rabbit antiserum employed contained antibodies directed against all three Tg peptides. Finally, because our protocol digests endogenous immunoglobulins that can potentially interfere with immunometric measurements, it should avoid many potential limitations of conventional immunoassays (8, 13, 26).
The peptide immunoaffinity enrichment strategy offers other advantages. For example, many investigators have attempted to decrease the limit of detection of MS analysis by immunodepleting abundant proteins. However, removing immunoglobulins could also deplete the analyte of interest in patients with autoantibodies. It is well known that many proteins strongly interact with albumin (29), so removal of this single-most abundant serum protein could also produce erroneously low measurements. By directly detecting analyte in reference to a stable isotope-labeled internal standard peptide, mass spectrometry has the advantage of being more easily standardized across laboratories (18), provided a reference preparation is available, as is the case for Tg (23). The improvements in low-abundance serum protein quantification presented here could help provide an alternative diagnostic approach to the methods that are traditionally plagued by interference and lack of standardization (7, 19, 26).
It is important to note several limitations of our studies. For example, 4 μg affinity purified polyclonal antibody was required for each sample and calibrator, which is 2–3 orders of magnitude more than what is commonly used in sandwich ELISA protocols (30). Additionally, small amounts of the peptides were noncovalently associated with the albumin-peptide conjugate and bound directly to the CNBr-activated beads used for affinity purification. Those peptides were slowly hydrolyzed from the solid phase, which led to a background signal. As a result, only peptide 2 of the three peptides tested allowed sufficient detection of endogenous thyroglobulin, owing to its lower background signal. In addition, it appears that peptide 3 elicited a weaker antibody response than the other peptides, as evidenced by its relatively high limit of detection. Using other strategies to generate affinity-purified, high avidity polyclonal antibodies might alleviate these problems, which could also be avoided by using monoclonal antibodies that do not require affinity purification. Even in the face of difficulties with two of the three peptides, however, we were able to demonstrate good intra-assay imprecision for peptide 2 at a concentration of 3.8 μg/L (6 pmol/L) in human serum.
Despite these limitations, this peptide affinity isolation strategy offers major advantages. It is relatively straightforward to generate polyclonal antibodies to candidate biomarkers, and cross-reacting antibodies can be used. Also, an internal standard automatically corrects for differences in analyte recovery and detection during sample work-up and analysis.
In conclusion, we employed Tg, a protein that is present at low concentrations in serum and commonly associated with interfering antibodies, to demonstrate the feasibility of using peptide affinity capture and MS/MS to quantify a well-characterized tumor marker. Further studies of this approach have the potential to provide new benchmark methods for quantifying proteins in clinical assays.
Supplementary Material
Acknowledgments
GRANT/FUNDING SUPPORT
This research was supported in part by grants from the National Institutes of Health (P30DK017047, P30ES07083, PO1HL030086; JWH). ANH was supported by a Pilot and Feasibility Award from the Clinical Nutrition Research Unit (P30DK035816), which also provided additional support (MHW, JOB). Mass spectrometric experiments were performed at the Clinical Mass Spectrometry Center, Department of Laboratory Medicine, and the Mass Spectrometry Core, Diabetes and Endocrinology Research Center, University of Washington.
The authors thank Matthew Champion, Jeff Whiteaker, and Mike MacCoss for helpful discussions.
Abbreviations
- Tg
thyroglobulin
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
Footnotes
FINANCIAL INTERESTS
The University of Washington has submitted a patent application using his methodology to quantify thyroglobulin in human serum.
References
- 1.Rifai N, Gillette MA, Carr SA. Protein Biomarker Discovery and Validation: The Long and Uncertain Path to Clinical Utility. Nat Biotechnol. 2006;24:971–83. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 2.Pacini F. Follow-up of Differentiated Thyroid Cancer. Eur J Nucl Med Mol Imaging. 2002;29:S492–6. doi: 10.1007/s00259-002-0847-9. [DOI] [PubMed] [Google Scholar]
- 3.Saghari M, Gholamrezanezhad A, Mirpour S, Eftekhari M, Takavar A, Fard-Esfahani A, et al. Efficacy of Radioiodine Therapy in the Treatment of Elevated Serum Thyroglobulin in Patients with Differentiated Thyroid Carcinoma and Negative Whole-Body Iodine Scan. Nucl Med Commun. 2006;27:567–72. doi: 10.1097/00006231-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Whitley RJ, Ain KB. Thyroglobulin: A Specific Serum Marker for the Management of Thyroid Carcinoma. Clin Lab Med. 2004;24:29–47. doi: 10.1016/j.cll.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Kloos RT, Mazzaferri EL. A Single Recombinant Human Thyrotropin-Stimulated Serum Thyroglobulin Measurement Predicts Differentiated Thyroid Carcinoma Metastases Three to Five Years Later. J Clin Endocrinol Metab. 2005;90:5047–57. doi: 10.1210/jc.2005-0492. [DOI] [PubMed] [Google Scholar]
- 6.Spencer CA. Recoveries Cannot Be Used to Authenticate Thyroglobulin (Tg) Measurements When Sera Contain Tg Autoantibodies. Clin Chem. 1996;42:661–3. [PubMed] [Google Scholar]
- 7.Demers LM, Spencer CA. Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease. Washington, DC: National Academy of Clinical Biochemistry; 2003. pp. 55–65. [DOI] [PubMed] [Google Scholar]
- 8.Spencer CA. Challenges of Serum Thyroglobulin (Tg) Measurement in the Presence of Tg Autoantibodies. J Clin Endocrinol Metab. 2004;89:3702–4. doi: 10.1210/jc.2004-0986. [DOI] [PubMed] [Google Scholar]
- 9.Ericsson UB, Christensen SB, Thorell JI. A High Prevalence of Thyroglobulin Autoantibodies in Adults with and without Thyroid-Disease as Measured with a Sensitive Solid-Phase Immunosorbent Radioassay. Clin Immunol Immunopathol. 1985;37:154–62. doi: 10.1016/0090-1229(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 10.Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, et al. Serum Thyroglobulin Autoantibodies: Prevalence, Influence on Serum Thyroglobulin Measurement, and Prognostic Significance in Patients with Differentiated Thyroid Carcinoma. J Clin Endocrinol Metab. 1998;83:1121–7. doi: 10.1210/jcem.83.4.4683. [DOI] [PubMed] [Google Scholar]
- 11.Okosieme OE, Evans C, Moss L, Parkes AB, Premawardhana L, Lazarus JH. Thyroglobulin Antibodies in Serum of Patients with Differentiated Thyroid Cancer: Relationship between Epitope Specificities and Thyroglobulin Recovery. Clin Chem. 2005;51:729–34. doi: 10.1373/clinchem.2004.044511. [DOI] [PubMed] [Google Scholar]
- 12.Vali M, Rose NR, Caturegli P. Thyroglobulin as Autoantigen: Structure-Function Relationships. Rev Endocr Metab Disord. 2000;1:69–77. doi: 10.1023/a:1010016520778. [DOI] [PubMed] [Google Scholar]
- 13.Preissner CM, O’Kane DJ, Singh RJ, Morris JC, Grebe SK. Phantoms in the Assay Tube: Heterophile Antibody Interferences in Serum Thyroglobulin Assays. J Clin Endocrinol Metab. 2003;88:3069–74. doi: 10.1210/jc.2003-030122. [DOI] [PubMed] [Google Scholar]
- 14.Consiglio E, Acquaviva AM, Formisano S, Liguoro D, Gallo A, Vittorio T, et al. Characterization of Phosphate Residues on Thyroglobulin. J Biol Chem. 1987;262:10304–14. [PubMed] [Google Scholar]
- 15.Gentile F, Ferranti P, Mamone G, Malorni A, Salvatore G. Identification of Hormonogenic Tyrosines in Fragment 1218–1591 of Bovine Thyroglobulin by Mass Spectrometry - Hormonogenic Acceptor Tyr-1291 and Donor Tyr-1375. J Biol Chem. 1997;272:639–46. doi: 10.1074/jbc.272.1.639. [DOI] [PubMed] [Google Scholar]
- 16.Yang SX, Pollock HG, Rawitch AB. Glycosylation in Human Thyroglobulin: Location of the N-Linked Oligosaccharide Units and Comparison with Bovine Thyroglobulin. Arch Biochem Biophys. 1996;327:61–70. doi: 10.1006/abbi.1996.0093. [DOI] [PubMed] [Google Scholar]
- 17.Slev PR, Rawlins ML, Roberts WL. Performance Characteristics of Seven Automated CA 15–3 Assays. Am J Clin Pathol. 2006;125:752–7. doi: 10.1309/G6X6-PR75-26FA-KV0E. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Cabaleiro D, Van Uytfanghe K, Stove V, Fiers T, Thienpont LM. Pilot Study for the Standardization of Insulin Immunoassays with Isotope Dilution Liquid Chromatography/Tandem Mass Spectrometry. Clin Chem. 2007;53:1462–9. doi: 10.1373/clinchem.2007.088393. [DOI] [PubMed] [Google Scholar]
- 19.Sapin R. Insulin Immunoassays: Fast Approaching 50 Years of Existence and Still Calling for Standardization. Clin Chem. 2007;53:810–2. doi: 10.1373/clinchem.2006.084012. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn E, Wu J, Karl J, Liao H, Zolg W, Guild B. Quantification of C-Reactive Protein in the Serum of Patients with Rheumatoid Arthritis Using Multiple Reaction Monitoring Mass Spectrometry and 13C-Labeled Peptide Standards. Proteomics. 2004;4:1175–86. doi: 10.1002/pmic.200300670. [DOI] [PubMed] [Google Scholar]
- 21.Bondar OP, Barnidge DR, Klee EW, Davis BJ, Klee GG. Lc-Ms/Ms Quantification of Zn-Alpha2 Glycoprotein: A Potential Serum Biomarker for Prostate Cancer. Clin Chem. 2007;53:673–8. doi: 10.1373/clinchem.2006.079681. [DOI] [PubMed] [Google Scholar]
- 22.Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. Mass Spectrometric Quantitation of Peptides and Proteins Using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA) J Proteome Res. 2004;3:235–44. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- 23.Feldt-Rasmussen U, Profilis C, Colinet E, Black E, Bornet H, Bourdoux P, et al. Human Thyroglobulin Reference Material (CRM 457). 2nd Part: Physicochemical Characterization and Certification. Ann Biol Clin (Paris) 1996;54:343–8. [PubMed] [Google Scholar]
- 24.Barr JR, Maggio VL, Patterson DG, Jr, Cooper GR, Henderson LO, Turner WE, et al. Isotope Dilution--Mass Spectrometric Quantification of Specific Proteins: Model Application with Apolipoprotein A-I. Clin Chem. 1996;42:1676–82. [PubMed] [Google Scholar]
- 25.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, Multiplexed Assays for Low Abundance Proteins in Plasma by Targeted Mass Spectrometry and Stable Isotope Dilution. Mol Cell Proteomics. 2007;6:2212–29. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoofnagle AN, Wener MW. Serum Thyroglobulin: A Model of Immunoassay Imperfection. Clin Lab Int. 2006;8:12–4. [Google Scholar]
- 27.Wunderlich G, Zophel K, Crook L, Smith S, Smith BR, Franke WG. A High-Sensitivity Enzyme-Linked Immunosorbent Assay for Serum Thyroglobulin. Thyroid. 2001;11:819–24. doi: 10.1089/105072501316973064. [DOI] [PubMed] [Google Scholar]
- 28.Whiteaker JR, Zhang H, Zhao L, Wang P, Kelly-Spratt KS, Ivey RG, et al. Integrated Pipeline for Mass Spectrometry-Based Discovery and Confirmation of Biomarkers Demonstrated in a Mouse Model of Breast Cancer. J Proteome Res. 2007;6:3962–75. doi: 10.1021/pr070202v. [DOI] [PubMed] [Google Scholar]
- 29.Gundry RL, Fu Q, Jelinek CA, Van Eyk JE, Cotter RJ. Investigation of an Albumin-Enriched Fraction of Human Serum and Its Albuminome. Proteom Clin Appl. 2007;1:73–88. doi: 10.1002/prca.200600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowther JR. Methods in Molecular Biology. Vol. 149. Totowa, NJ: Humana Press; 2001. The Elisa Guidebook; p. 421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.