Abstract
Positron emission tomography (PET)-computed tomography (CT) is emerging as a valuable tool for assessing a wide variety of pediatric malignancies, including lymphomas, soft-tissue tumors, and bone sarcomas. PET-CT may provide information that is not apparent on conventional imaging performed to stage these diseases and monitor their response to treatment. The use of PET-CT in children requires an awareness of the technical and logistical issues unique to this patient population. In addition, interpretation of pediatric PET-CT imaging requires familiarity with aspects of pediatric anatomy and physiology that differ from those of adults. In this article, the technical considerations in performing pediatric PET-CT, pitfalls in the diagnostic use of PET-CT in children, and current and emerging applications of PET-CT in pediatric oncology are reviewed.
Keywords: Childhood, cancer, malignancy, positron emission tomography, computed tomography
Introduction
The value of positron emission tomography (PET) and PET combined with computed tomography (CT) imaging in pediatric lymphomas has been fairly well described[1]. However, because pediatric solid tumors are rare, the role of PET in their management is less well established. There is a growing body of literature about the use of PET-CT imaging in the diagnosis and management of pediatric sarcomas, the most common of which are the bone tumors, osteosarcoma (OS) and Ewing sarcoma (EWS), and the soft-tissue malignancy, rhabdomyosarcoma (RMS)[2–5]. Therefore, this review focuses on the value of PET-CT imaging in the assessment of these malignancies.
When PET-CT is performed in children, challenges unique to pediatric patients must be anticipated. These include the need for sedation, pregnancy screening of patients and caregivers, and potential artifact caused by metabolically active brown fat. These technical challenges are discussed briefly and suggestions for managing them are offered. Several pitfalls in interpretation of PET-CT in children with sarcomas are also presented.
Unique technical aspects of PET-CT in children
Accurate anatomic coregistration of PET and CT images requires that the patient remain completely still throughout the procedure. Because PET-CT examinations can be lengthy, sedation or general anesthesia may be required if children are unable to cooperate. The approach to sedation or anesthesia depends on institutional resources and physician preferences. When a sedation team is used, its members should rotate through the PET-CT area to minimize their radiation exposure. The need for sedation or anesthesia will influence patient scheduling and the timing of oral contrast administration when a diagnostic CT is also being performed. Before sedation or anesthesia, our institutional policy mandates a 4-h “nothing passed orally” (NPO) period including 2 h of clear liquids only immediately before the procedure. Therefore, when PET-CT patients must ingest oral contrast material for diagnostic CT, appointments are scheduled to allow adequate time between examinations so that sedation or anesthesia, if needed, can be safely administered[6].
Pregnant guardians of young patients must not be allowed in the room where radionuclide tracer (usually the glucose analogue fluorine-18 fluorodeoxyglucose [FDG]) administration and uptake occur. Therefore, arrangements must be made in advance for the supervision of such patients. Further, before administration of any radioisotope, young female patients must be questioned about the possibility of pregnancy. This delicate subject is best handled by a technologist who is experienced in working with young girls. Before FDG is administered at our institution, a nuclear medicine technologist questions female patients who are10 years of age or older, and all female guardians, about the possibility of pregnancy. When a patient or guardian is unsure of her pregnancy status, a pregnancy test is performed and must be negative before FDG is administered.
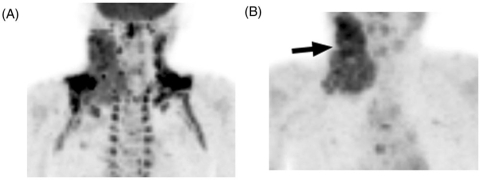
Brown adipose tissue, or brown fat, is metabolically active in pediatric patients, women, and persons with a low body mass index[7–9]. The primary function of brown fat is the production of heat through an anaerobic, glycolytic pathway that results in the uptake of glucose and hence of FDG[7]. Intense FDG activity is often observed within brown fat in the supraclavicular regions, axillae, paraspinal regions of the posterior mediastinum, and adjacent to the adrenal glands. Intense FDG avidity in brown fat can be difficult to distinguish from cervical, supraclavicular, or axillary pathology (which are commonly seen in lymphoma). In such cases, we administer an anxiolytic agent, such as diazepam, the night before and the morning of PET-CT to reduce FDG activity in brown fat and improve the prominence of lesions (Fig. 1).
Figure 1.
An 8-year-old boy with Hodgkin lymphoma underwent baseline PET-CT. (A) This maximum intensity projection (MIP) PET image shows intense FDG activity in metabolically active brown fat in the neck, supraclavicular areas and axilla. (B) The examination was repeated after administration of diazepam the evening before and on the morning of the PET-CT scan. Brown fat FDG activity resolved and the site of pathologic uptake in the right neck (arrow) became more apparent.
The emerging role of PET-CT in pediatric oncology
Because the value of PET imaging in lymphoma has been well described, this review focuses on the emerging role of PET-CT in the assessment of children with sarcomas. The conventional staging evaluation of these children includes magnetic resonance imaging (MRI) or CT of the primary tumor and regional nodal beds, chest CT for detection of pulmonary metastases, and technetium-99m methyldiphosphonate ([99mTc]MDP) bone scan for detection of bone metastases.
The Response Evaluation Criteria in Solid Tumors (RECIST), introduced in 2000 as an alternative to the World Health Organization (WHO) criteria, provide a standard, reproducible, and objective method of assessing the efficacy of solid tumor therapies[10]. These criteria incorporate important advances in imaging technology and simplify the WHO scheme, but they still rely on changes in unidimensional tumor measurements to define tumor response or progression. Unfortunately, solid malignancies, such as bone sarcomas and cystic soft-tissue sarcomas, may respond well to chemotherapy without substantially changing in size. Further, sarcomas do not shrink or grow in a uniform manner; therefore, unidimensional measurements may not accurately reflect response or progression. Tumors that respond poorly to therapy may be followed for months before a unidimensional measurement increases significantly. Meanwhile, patients are exposed to toxic but ineffective chemotherapy and are likely to have a diminished probability of survival[11]. FDG-PET has the advantage of revealing viable tumor tissue, which is highly metabolically active and therefore accumulates FDG[12,13]. FDG-PET can assess tumor glucose metabolism with high reproducibility[14]. A decrease in tumor glucose uptake after treatment is correlated with a reduction in the percentage of viable tumor cells[15–17]. Therefore, FDG-PET may offer a more sensitive evaluation of the response or progression of some pediatric solid malignancies than do conventional, anatomic imaging modalities.
Detection of pulmonary disease
In children with bone and soft-tissue sarcomas, the lung is the first site of distant spread and is involved in 20–25% of patients at diagnosis[3–5]. The prognosis is poor for children with OS and pulmonary metastases unless all metastases are surgically resected[3]. The survival of patients with EWS and pulmonary metastases may be improved by whole-lung irradiation[4]. Therefore, identification of pulmonary metastases is crucial to the effective management of these cases.
Chest CT is the gold standard for detection of pulmonary metastases; however, in a retrospective study of 41 children with solid malignancies who underwent biopsy of pulmonary nodules, we found that it has limited accuracy in distinguishing benign from malignant nodule histology[18]. We also found only slight to moderate interreviewer agreement among 3 experienced pediatric radiologists who classified nodules as benign or malignant on the basis of CT features. There is clearly a need to improve the imaging distinction of benign from malignant pulmonary nodules to reduce the frequency of unnecessary thoracotomy.
To assess the ability of PET alone to detect pulmonary metastases of OS or EWS, Franzius and colleagues compared PET to CT, clinical follow-up, and histologic interpretation of resected nodules[19]. Among 30 PET scans performed at diagnosis or recurrence (before initiation of salvage therapy), PET had a sensitivity of 14%, specificity of 91%, and accuracy of 73%. The ability of PET to detect malignant nodules increased with nodule size: 12 of 16 malignant nodules ≥10 mm were identified, whereas none measuring <5 mm were detected (Fig. 2). Their results were supported by the recent work of Völker et al., who investigated the value of PET alone for staging 46 cases of childhood EWS (n = 23), OS (n = 11), and RMS (n = 12)[20]. They found that 21 of 28 lung metastases detected by CT were missed by PET (sensitivity 25%) and that PET-positive nodules were ≥8 mm in diameter whereas PET-negative nodules were <7 mm. Gertha and colleagues showed that the fusion modality PET-CT detected pulmonary EWS metastases better than PET alone in 53 patients[21]. However, because PET-CT is generally performed with a low milliampere-second technique for CT scanning, image quality may be insufficient to detect very small pulmonary nodules. Increasingly smaller nodules can now be detected by using current-generation, multislice helical scanners coupled with picture archiving and communication systems (PACS), whose window and level adjustment features and magnification tools improve image interpretation. We have shown that small pulmonary nodules (<5 mm) are as likely to be malignant as larger nodules in children with solid malignancies[18]. Given the need to aggressively treat pulmonary metastatic disease in children with sarcomas, diagnostic chest CT remains an essential part of the metastatic workup. PET-CT is preferred over PET alone as a potential adjunct to distinguish benign from malignant nodules, but the inherent limitation of spatial resolution of the PET and CT components must be taken into account[19,22]. We have also found that benign processes, such as active infection, can result in substantial FDG uptake within pulmonary nodules, mimicking that seen in metastases (Fig. 3).
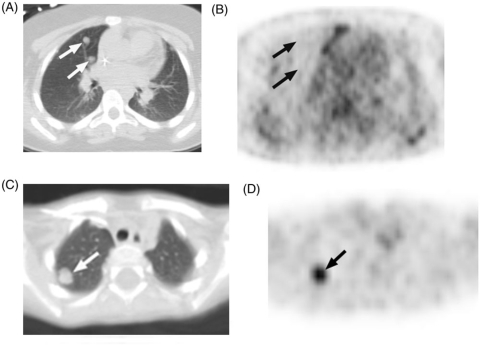
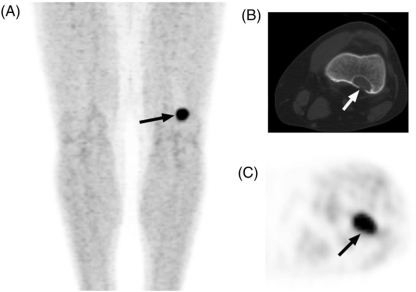
Figure 2.
(A) These 0.8-cm nodules (arrows), metastatic from Ewing sarcoma, seen on CT, show (B) no FDG activity on correlative PET (arrows indicate approximate location of nodules). In contrast, (C) this 1.1-cm nodule (arrow), metastatic from rhabdoid tumor, shows (D) intense FDG activity on correlative PET imaging (arrow). In children, nodule size affects the detection of FDG activity but not the likelihood of malignant histology.
Figure 3.
This 14-year-old boy was suspected of having lymphoma and underwent PET-CT. (A) CT shows an ill-defined, 1.8-cm pulmonary nodule (arrow) that on (B) PET showed intense FDG activity (arrow). Biopsy revealed necrotizing granulomatous disease.
Detection of bone and bone marrow disease
Children with bone or bone marrow metastases of sarcomas have a dismal prognosis. About 10% of OS patients develop bone metastases, with or without concurrent pulmonary metastases[3]. These patients have no chance of cure unless all metastatic disease is surgically resected. As many as 25% of patients with EWS or RMS experience bone or bone marrow involvement. Because EWS is radiosensitive, targeted radiation can be beneficial if these metastases are few and well defined. However, when more than 50% of the marrow is involved, irradiation can cause significant myelosuppression and compound the myelosuppressive toxicity of chemotherapy[4]. Unfortunately, the prognosis of patients with RMS and bone or bone marrow metastases is not improved by surgical metastatectomy or targeted radiotherapy[5].
Because OS metastases often contain calcification or ossification, they can typically be detected by the bone-seeking radioisotope 99mTc MDP. Franzius and colleagues directly compared PET to 99mTc MDP bone scintigraphy for the detection of bone metastases in 32 patients with OS[23]. They found 5 bone metastases in 2 patients; all foci were evident on 99mTc MDP bone scan, whereas none were demonstrated by PET. However, because of their small sample size, the results were inconclusive. Völker et al. found 4 of 12 patients to have 31 bone metastases at the time of diagnosis of OS; the sensitivity of detection was 90% for PET, compared with 81% for 99mTc MDP scintigraphy. However, no statistically significant difference could be demonstrated[20].
In patients with EWS, PET is more convincingly superior to 99mTc MDP scintigraphy for the detection of osseous metastases. Franzius et al. found that on 66 paired PET and 99mTc MDP bone scans performed before or after initiation of therapy for EWS, osseous metastases were detected by PET with a sensitivity of 100% (19/19), specificity of 96% (45/47), and accuracy of 97% (64/66), compared with 68% (13/19), 87% (41/47) and 82% (54/66), respectively, for 99mTc MDP scintigraphy[24]. These findings were supported by Völker and colleagues. In 17 EWS patients, 6 of whom had 49 osseous metastases, they found that the sensitivity of PET was 88%, compared with 37% sensitivity for 99mTc bone scan (P < 0.01)[20]. In our practice, we have found PET-CT to be more sensitive than 99mTc bone scan for the detection of osseous metastases in several children with RMS or EWS (Fig. 4)[25]. The greater sensitivity of PET to 99mTc MDP scintigraphy in detecting osseous metastases of EWS and RMS is probably multifactorial. Osseous metastases of these malignancies frequently arise in and infiltrate the bone marrow. FDG-PET is postulated to detect the increased glucose metabolism of bone marrow metastases before an osteoblastic reaction is appreciable. In contrast, detection of bone metastases by 99mTc bone scintigraphy depends on ossification within the metastatic deposit, the degree of associated cortical destruction, and the intensity of osteoblastic activity, all of which are greater in OS bone metastases than in those of EWS or RMS. It is also possible that cellular glucose uptake and metabolism differ in these sarcomas[24].
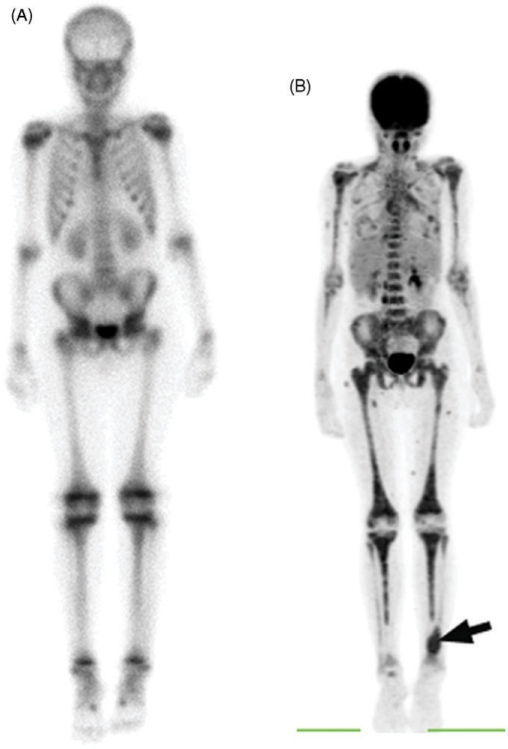
Figure 4.
This 11-year-old girl with alveolar rhabdomyosarcoma underwent baseline 99mTc bone scan. (A) This anterior whole body image shows no obvious bone involvement. (B) This MIP PET anterior image, obtained 1 day later, shows widely metastatic disease throughout the axial and appendicular skeleton. The primary tumor is the focus of intense soft-tissue activity in the lower left calf (arrow). Bone marrow involvement was confirmed by bone marrow aspirate and biopsy. On PET, bone marrow should show FDG activity less than or equal to liver. (Reprinted with permission from AJR 2005; 184: 1293–304.)
In assessing children with malignancies that metastasize to bone, it is important to consider benign processes that can mimic metastatic disease on PET imaging. Akoi and colleagues assessed the value of PET in distinguishing benign (n = 33) from malignant (n = 19) bone tumors in 52 children and adults[26]. They measured the mean standardized uptake value (SUV) within a region of interest (ROI) in the area of tumor with maximum FDG accumulation and found no significant difference between OS and giant cell tumors (P = 0.171), OS and fibrous dysplasia (P = 0.127), or fibrous dysplasia and chondrosarcomas (P = 0.667). Further, they found no SUV threshold value that reliably distinguished benign from malignant bone lesions. We reviewed PET-CT imaging performed to evaluate underlying cancers (n = 13) or aggressive fibromatosis (n = 1) in 14 children who also had benign fibrocortical defects, nonossifying fibromas, or cortical desmoids that were discovered as foci of FDG avidity[27]. In some cases, the intense FDG avidity of these benign lesions mimicked the appearance of bony metastatic disease (Fig. 5). These common, benign lesions are often found incidentally on radiographs of children and young adults. They undergo spontaneous regression over time and are rarely seen after the second decade of life. Because they have characteristic radiographic features that are well described in the literature, the information gained from correlative CT (when PET-CT is performed) or plain radiographs is invaluable in determining whether further imaging or biopsy is necessary.
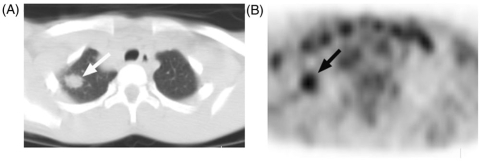
Figure 5.
This 19-year-old man with metastatic paraganglioma underwent PET-CT. (A) MIP PET image shows a focus of intense activity above the left knee (arrow) that on (B) correlative CT and (C) axial PET images localized to a benign fibrocortical defect (arrows).
Detection of nodal metastases
Local-regional lymph node metastasis is uncommon in OS and EWS but is present at diagnosis in as many as 20% of children with RMS[2–5]. Nodal spread is present at diagnosis in approximately half of children with extremity RMS and occurs more frequently in older boys (≥10 years) with paratesticular RMS and in association with the alveolar RMS subtype. When lymph node metastasis is present, the likelihood of survival can be improved by intensification of chemotherapy and irradiation of the affected region[5]. Therefore, identification of sites of lymph node involvement is crucial to the management and outcome of childhood RMS.
Klem et al. investigated the value of PET in detecting metastases during the baseline staging of 24 patients with RMS by comparing it to CT, MRI, clinical assessment, and histology of resected lesions[28]. The measured SUV of suspicious foci was considered abnormal if it exceeded that of background tissue and did not reflect a normal physiologic process. Six patients had lymph node metastases identified by histologic assessment. All 6 had abnormal SUVs in the involved nodal sites. Although the nodes were seen on conventional imaging, Klem et al. did not report node size or the presence or absence of pathologic enlargement. An additional patient in the study had a lower extremity tumor and a PET interpretation ‘suspicious’ for inguinal lymph node involvement. A CT of the inguinal area was uninformative, and therefore chemotherapy was initiated after resection of the primary tumor, without inguinal node biopsy. Tumor progression in the involved inguinal node subsequently became apparent on physical examination, PET, and MRI and was confirmed by biopsy. The authors concluded that had the nodal involvement been confirmed at diagnosis, the patient might have benefited from more aggressive therapy.
Völker et al. described 20 presumed lymph node metastases identified in 8 of 46 children with bone and soft-tissue sarcomas (1 with OS, 1 with EWS, and 6 with RMS)[20]. Fourteen of the 20 nodal metastases occurred in RMS patients. A lesion-based analysis showed a sensitivity of 95% (19/20) for PET but only 25% (5/20) for CT and MRI. This study was limited by the absence of size criteria defining abnormal lymph nodes on conventional imaging; further, 16 of the 20 presumed nodal metastases were not confirmed by pathology studies.
In a small case series by Ben Arush and colleagues, 3 children with alveolar RMS underwent PET-CT and conventional imaging for baseline staging; 2 had moderately FDG-avid regional lymph nodes from which biopsies were taken[29]. One patient's sampled node was positive for tumor. In the other, there was no evidence of lymph node metastasis. The third patient had mild FDG avidity within a regional node but no biopsy was taken. The patient was started on therapy for high-risk disease; at the time of scheduled follow-up, adenopathy was palpable in the area of the previously mildly FDG-avid node. Subsequent lymph node biopsy revealed alveolar RMS.
Our experience is consistent with these reports. We have found that benign processes, such as reactive hyperplasia, can cause regional node enlargement and FDG avidity on PET imaging in children with sarcomas (Fig. 6). The available evidence suggests that although PET may identify sites of nodal disease not appreciated on conventional imaging, care should be used in interpreting PET findings. Metastatic lymph nodes may demonstrate only mild FDG avidity, whereas nonmetastatic nodes may show intense avidity. Therefore, biopsy of suspicious lymph nodes should be considered when there is discordance between the findings of different imaging modalities or between imaging and clinical findings and when identification of nodal disease will affect patient management and outcome.
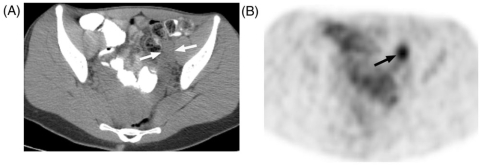
Figure 6.
A 20-year-old woman with malignant peripheral nerve sheath tumor in the left thigh, underwent baseline PET-CT. (A) CT shows enlarged iliac nodes (arrows) ipsilateral to thigh tumor that on (B) PET showed intense FDG activity. Biopsy of these nodes revealed reactive hyperplasia.
Assessment of tumor response to therapy
Patients with nonmetastatic OS and EWS are treated with neoadjuvant therapy before surgical resection of the primary tumor and subsequent adjuvant therapy. Chances of survival are improved when 90% or more of the resected tumor is necrotic and resection margins are void of tumor[3,4]. Factors that predict the histologic tumor response to neoadjuvant therapy might also predict patient outcome and help to identify candidates for limb-sparing surgery versus amputation or early resection[30]. A reliable, noninvasive method for assessing tumor metabolism and viability, such as PET, could allow the oncologist and surgeon to individualize management of tumors that are aggressive, respond poorly, or arise in surgically challenging sites.
Osteogenic sarcoma and EWS are metabolically heterogeneous tumors. Because tumor histologic grade and predicted patient outcome are determined by the percentage of viable tumor in the resected specimen, the maximum tumor SUV is believed to provide the most accurate noninvasive assessment of tumor grade. Several investigators have compared the maximum tumor SUV before and after neoadjuvant therapy with tumor grade and patient outcome[16,17]. In 2002, Hawkins and colleagues reported a comparison of the histologic response of resected tumors (18 OS and 13 EWS) to tumor SUV at diagnosis (SUV1) and at the end of neoadjuvant therapy (SUV2)[16]. In these 31 patients, histologic response was significantly associated with the SUV2 (P = 0.01) and the SUV2/SUV1 ratio (P = 0.01). An SUV2 < 2 had a positive predictive value (PPV) of 93% for identifying tumors that were 90% or more necrotic and a 75% negative predictive value (NPV) for identifying tumors that were <90% necrotic (unfavorable response). When an SUV2/SUV1 cutoff point of 0.5 was used, the PPV and NPV were 78% and 63%, respectively.
In 2005, Hawkins and coworkers reported similar findings in a follow-up study of 36 patients with EWS who had PET imaging before and after neoadjuvant therapy[17]. Among the 32 patients who underwent resection of the primary tumor, an SUV2 < 2.5 had a PPV of 79% for a favorable response and an NPV of 40% for an unfavorable response. The PPV and NPV for an SUV2/SUV1 ratio ≤0.5 were 77% and 33%, respectively. An SUV2 < 2.5 was also associated with greater 4-year progression-free survival (PFS) in all 36 patients (P = 0.006) and in the 24 patients who had no metastatic disease at diagnosis (P = 0.036). Neither the SUV1 nor an SUV2/SUV1 ratio ≤0.5 was associated with PFS. These findings and those of others suggest that the maximum SUV of primary OS and EWS after neoadjuvant therapy can predict tumor response and patient outcome. Further studies are needed to determine whether the SUV can be used to identify unresponsive or poorly responsive tumors earlier in the course of neoadjuvant therapy, so that therapy can be appropriately modified.
Conclusion
PET imaging of children requires an expert team of professionals to manage the unique technical challenges encountered in this age group. Further, the interpreting physician must be aware of the aspects of pediatric anatomy and physiology that differ from those in adults. Whereas the role of PET in the assessment of lymphomas is fairly well established, its role in the management of pediatric solid malignancies is evolving. The growing body of evidence supports the continued investigation of PET imaging in the management of pediatric sarcomas. PET may provide valuable information about pulmonary nodules in children, but accurate interpretation requires awareness of its limitations. Small malignant pulmonary nodules may not be appreciable on PET imaging, whereas large benign nodules may show intense FDG avidity. PET may detect osseous metastases of childhood EWS and RMS earlier than [99mTc]MDP bone scintigraphy, because bone marrow infiltration precedes cortical destruction and an osteoblastic reaction. On the other hand, the bone-seeking radiotracer [99mTc]MDP may offer superior detection of lytic and ossifying bone metastases often associated with OS. When PET is used to detect bone metastasis in children, an awareness of the common benign bone lesions in this age group is essential, as they can mimic metastatic disease. In such cases the correlative CT imaging obtained during PET-CT, or plain-film radiography, can preclude the need for further imaging or biopsy. PET imaging that shows sites of local-regional nodal FDG uptake in children with bone and soft-tissue sarcomas should be interpreted with caution. Biopsy of suspicious lymph nodes is probably indicated when the findings of different imaging modalities or of imaging and physical examination are inconsistent. Because the size of bone tumors does not change substantially in response to neoadjuvant therapy, CT and MRI have limited value for assessment of tumor response. Because FDG-PET reflects the metabolic activity of tumors, which can be semi-quantitatively measured as the SUV, PET holds promise as an alternative method of assessing response in these tumors. Additional studies are needed to better define the optimal timing of PET during neoadjuvant therapy to detect poorly responsive tumors and allow early intervention.
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Jadvar H, Connolly LP, Fahey FH, Shulkin BL. PET and PET/CT in pediatric oncology. Semin Nucl Med. 2007;37:316–31. doi: 10.1053/j.semnuclmed.2007.04.001. doi:10.1053/j.semnuclmed.2007.04.001. PMid:17707239. [DOI] [PubMed] [Google Scholar]
- 2.Gurney JG, Young JL, Jr, Roffers SD, Smith MA, Bunin GR. Soft tissue sarcomas. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute; 1999. pp. 111–1223. [Google Scholar]
- 3.Link MP, Gebhardt M, Meyers P. Osteosarcoma. In: Pizzo P, Poplack DG, editors. Principles and practice of pediatric oncology. 5th. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1074–115. [Google Scholar]
- 4.Bernstein M, Kovar H, Paulussen M, et al. Ewing sarcoma family of tumors: Ewing sarcoma of bone and soft tissue and the peripheral primitive neuroectodermal tumors. In: Pizzo P, Poplack DG, editors. Principles and practice of pediatric oncology. 5th. Philadelphia: Lippincott: Williams & Wilkins; 2008. pp. 1002–32. [Google Scholar]
- 5.Wexler L, Meyer W, Helman L. Rhabdomyosarcoma and the undifferentiated sarcomas. In: Pizzo P, Poplack D, editors. Principles and practice of pediatric oncology. 5th. Williams & Wilkins: Lippincott; 2006. pp. 971–1001. [Google Scholar]
- 6.Kaste SC, McCarville MB. How to image a child by PET-computed tomography. In: Charron M, , editor. Practical pediatric PET imaging. 1st. Toronto: Springer; 2006. pp. 121–34. doi:10.1007/0-387-34641-4_9. [Google Scholar]
- 7.Himms-Hagen J. Brown adipose tissue thermogenesis: interdisciplinary studies. FASEB J. 1990;4:2890–8. [PubMed] [Google Scholar]
- 8.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, Von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging. 2002;29:1393–8. doi: 10.1007/s00259-002-0902-6. doi:10.1007/s00259-002-0902-6. PMid:12271425. [DOI] [PubMed] [Google Scholar]
- 9.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–6. [PubMed] [Google Scholar]
- 10.Therasse P, Arbuck S, Eisenhauer E, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. doi:10.1093/jnci/92.3.205. PMid:10655437. [DOI] [PubMed] [Google Scholar]
- 11.Fournier LS, Cuenod CA, Clement O, Siauve N, Frija G. Imaging of response to treatment in oncology. J Radiol. 2007;88:829–43. doi: 10.1016/s0221-0363(07)89885-4. doi:10.1016/S0221-0363(07)89885-4. PMid:17652977. [DOI] [PubMed] [Google Scholar]
- 12.Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305–32. doi: 10.1148/radiol.2312021185. doi:10.1148/radiol.2312021185. PMid:15044750. [DOI] [PubMed] [Google Scholar]
- 13.Kostakoglu L, Agress H, Jr, Goldsmith SJ. Clinical role of FDG PET in evaluation of cancer patients. Radiographics. 2003;23:315–40. doi: 10.1148/rg.232025705. doi:10.1148/rg.232025705. PMid:12640150. [DOI] [PubMed] [Google Scholar]
- 14.Schuetze SM, Baker LH, Benjamin RS, Canetta R. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist. 2008;13(Suppl 2):32–40. doi: 10.1634/theoncologist.13-S2-32. doi:10.1634/theoncologist.13-S2-32. PMid:18434637. [DOI] [PubMed] [Google Scholar]
- 15.Franzius C, Sciuk J, Brinkschmidt C, Jurgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med. 2000;25:874–81. doi: 10.1097/00003072-200011000-00004. doi:10.1097/00003072-200011000-00004. PMid:11079583. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins DS, Rajendran JG, Conrad EU, III, Bruckner JD, Eary JF. Evaluation of chemotherapy response in pediatric bone sarcomas by [F-18]-fluorodeoxy-D-glucose positron emission tomography. Cancer. 2002;94:3277–84. doi: 10.1002/cncr.10599. doi:10.1002/cncr.10599. PMid:12115361. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins DS, Schuetze SM, Butrynski JE, et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol. 2005;23:8828–34. doi: 10.1200/JCO.2005.01.7079. doi:10.1200/JCO.2005.01.7079. PMid:16314643. [DOI] [PubMed] [Google Scholar]
- 18.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239:514–20. doi: 10.1148/radiol.2392050631. doi:10.1148/radiol.2392050631. PMid:16641356. [DOI] [PubMed] [Google Scholar]
- 19.Franzius C. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol. 2001;12:479–86. doi: 10.1023/a:1011111322376. doi:10.1023/A:1011111322376. PMid:11398879. [DOI] [PubMed] [Google Scholar]
- 20.Volker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. 2007;25:5435–41. doi: 10.1200/JCO.2007.12.2473. doi:10.1200/JCO.2007.12.2473. PMid:18048826. [DOI] [PubMed] [Google Scholar]
- 21.Gerth HU, Juergens KU, Dirksen U, Gerss J, Schober O, Franzius C. Significant benefit of multimodal imaging: PET/CT compared with PET alone in staging and follow-up of patients with Ewing tumors. J Nucl Med. 2007;48:1932–9. doi: 10.2967/jnumed.107.045286. doi:10.2967/jnumed.107.045286. PMid:18006618. [DOI] [PubMed] [Google Scholar]
- 22.Iagaru A, Quon A, McDougall IR, Gambhir SS. F-18 FDG PET/CT evaluation of osseous and soft tissue sarcomas. Clin Nucl Med. 2006;31:754–60. doi: 10.1097/01.rlu.0000246846.01492.31. doi:10.1097/01.rlu.0000246846.01492.31. PMid:17117068. [DOI] [PubMed] [Google Scholar]
- 23.Franzius C, Bielack S, Flege S, Sciuk J, Jurgens H, Schober O. Prognostic significance of (18)F-FDG and (99m)Tc-methylene diphosphonate uptake in primary osteosarcoma. J Nucl Med. 2002;43:1012–7. [PubMed] [Google Scholar]
- 24.Franzius C, Sciuk J, Daldrup-Link HE, et al. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med. 2000;27:1305–11. doi: 10.1007/s002590000301. doi:10.1007/S002590000301. [DOI] [PubMed] [Google Scholar]
- 25.McCarville MB, Christie R, Daw NC, Spunt SL, Kaste SC. PET/CT in the evaluation of childhood sarcomas. Am J Roentgenol. 2005;184:1293–304. doi: 10.2214/ajr.184.4.01841293. [DOI] [PubMed] [Google Scholar]
- 26.Aoki J, Watanabe H, Shinozaki T, et al. FDG PET of primary benign and malignant bone tumors: standardized uptake value in 52 lesions. Radiology. 2001;219:774–7. doi: 10.1148/radiology.219.3.r01ma08774. [DOI] [PubMed] [Google Scholar]
- 27.Goodin GS, Shulkin BL, Kaufman RA, McCarville MB. PET/CT characterization of fibroosseus defects in children: 18F-FDG uptake can mimic metastatic disease. Am J Roentgenol. 2006;187:1124–8. doi: 10.2214/AJR.06.0171. (Erratum in Am J Roentgenol 2006; 187: 1146). doi:10.2214/AJR.06.0171. PMid:16985165. [DOI] [PubMed] [Google Scholar]
- 28.Klem ML, Grewal RK, Wexler LH, Schoder H, Meyers PA, Wolden SL. PET for staging in rhabdomyosarcoma: an evaluation of PET as an adjunct to current staging tools. J Pediatr Hematol Oncol. 2007;29:9–14. doi: 10.1097/MPH.0b013e3180307693. doi:10.1097/MPH.0b013e3180307693. PMid: [DOI] [PubMed] [Google Scholar]
- 29.Ben Arush MW, Bar SR, Postovsky S, et al. Assessing the use of FDG-PET in the detection of regional and metastatic nodes in alveolar rhabdomyosarcoma of extremities. J Pediatr Hematol Oncol. 2006;28:440–5. doi: 10.1097/01.mph.0000212949.12856.02. doi:10.1097/01.mph.0000212949.12856.02. PMid: [DOI] [PubMed] [Google Scholar]
- 30.Malawer MM, Link MP, Donaldson SS. Philadelphia: Lippincott Williams & Wilkins; 2001. Cancer. Principles and practice of oncology. 6th ed. ; pp. 1891–935. [Google Scholar]