Abstract
Contractile forces can be measured in situ and in vitro. To maintain metabolic viability with sufficient diffusion of oxygen, established guidelines for in vitro skeletal muscle preparations recommend use of relatively thin muscles (≤1.25 mm thick). Nevertheless, forces of thin extraocular muscles vary substantially between studies. Here, we examined parameters that affect force measurements of in situ and in vitro preparations, including blood supply, nerve stimulation, direct muscle stimulation, muscle size, oxygenated or non-oxygenated buffer solutions, and the time after interruption of vascular circulation. We found that the absolute forces of extraocular muscle are substantially lower when examined in vitro. In vitro preparation of 0.58 mm thick extraocular muscle from 3-week old birds underestimated contractile function, but not of thinner (0.33 mm) muscle from 2-day old birds. Our study shows that the effective criteria for functional viability, tested in vitro, differ between extraocular and other skeletal muscle. We conclude that contractile force of extraocular muscles will be underestimated by between 10 and 80%, when measurements are made after cessation of blood supply (at 5 – 40 min). The mechanisms responsible for the declining values for force measurements are discussed, and we make specific recommendations for obtaining valid measurements of contractile force.
Keywords: gastrocnemius muscle, eye muscle, contractile force, in situ preparation, in vitro preparation, nerve stimulation, development, bird
Introduction
Extraocular muscles require precise muscle force regulation to align the eyes for coordination of functional pursuit movements and binocular vision. Methods to accurately examine extraocular muscle function are crucial for oculomotor research. Measurement of contractile force utilizes force transducers with the muscle stimulated indirectly (via nerve) or directly, and tested either in situ or in vitro. To date, in situ and in vitro approaches for assessment of extraocular muscle function have not been directly compared. This is likely due to the assumption that the relatively thin extraocular muscles remain fully functional during in vitro incubations. Here we provide evidence that the in situ experimental approach is superior to the in vitro method. In addition, we describe a new animal (chicken) model for the accurate and optimal assessment of extraocular muscle contractile force via both indirect (nerve) and direct muscle stimulation. Use of this avian model is more accessible and technically easier than previously used mammalian model systems/protocols.
In situ and in vitro preparations have been widely used to study the contractile properties of extraocular muscles. While both methods attach the insertion of the muscle to a force transducer, the muscle preparation differs between the two approaches. In situ methods with indirect (nerve) stimulation require extensive surgery and are technically more difficult. Yet, these methods are advantageous because the muscle’s origin is preserved, its orientation is largely conserved, and an intact blood supply maintains the viability of the muscle (Bach-y-Rita and Ito, 1966; Barmack et al., 1971; Hanson and Lennerstrand, 1977; Shall and Goldberg, 1992; Dimitrova et al., 2002; Shall et al., 2003). The relative ease of in vitro preparations for the determination of extraocular muscle force is reflected by their extensive use (Close and Luff, 1974; Luff, 1981; Chiarandini, 1980, 1987; Asmussen and Gaunitz, 1981; Jacoby et al., 1989; Chen and von Bartheld, 2004; McLoon et al., 2006; Anderson et al., 2006). While in vitro methods have the advantage of being technically less demanding and do not require general anesthesia, they may not be optimal for obtaining accurate contractile measurements of extraocular muscle. There are three primary concerns for their use: 1) damage to the muscle during dissection, 2) disruption of muscle orientation, and 3) damage to muscle due to hypoxia. Force discrepancies between in situ and in vitro methods are known from the literature. In adult Wistar rats, the in vitro stimulation of the inferior rectus muscle was found to be 0.66 g (Close and Luff, 1974), which was significantly less than the 51 mN (~5.2 g) reported for the in situ stimulation of the superior rectus muscle from Sprague-Dawley rats (Frueh et al., 2001). Extraocular muscle force in two-day old hatchling chicks, stimulated in vitro, was reported to be 1.43 mN (~146 mg) for the superior oblique muscle (Chen and von Bartheld, 2004), which was significantly less than the 391 mg reported for the in situ stimulation of the same muscle with blood supply intact (Croes et al., 2007).
Here, we tested the effects of several crucial parameters on the validity of force measurements obtained in situ and in vitro in the post-hatch chicken, including blood supply, nerve stimulation, direct muscle stimulation, muscle thickness, oxygenated vs. non-oxygenated buffer solutions, the time after interruption of vascular circulation, and potential differences in sensitivity between limb skeletal muscle and extraocular muscle. We show that acute ischemia resulted in a rapid and dramatic decrease in contractile force of extraocular muscles and gastrocnemius muscles in both hatchlings and three-week old chickens, regardless of the stimulation method (direct or indirect). Theoretically, at an incubation temperature of 35 °C, hypoxia in vitro should not occur in adequately perfused preparations in muscles thinner than 1.25 mm (Van Breda et al., 1990; Bonen et al., 1994), which applies to the extraocular muscles used in our study. However, while in vitro preparations with oxygenated Krebs buffer were able to maintain contractile force in the thinner (0.33 mm) extraocular muscle of two-day old chicks, the buffer failed to maintain forces of the thicker (0.58 mm) extraocular muscle from 3-week old animals or of the lateral gastrocnemius muscle from 2-day and 3-week old chickens, respectively. Such a surprisingly rapid and dramatic effect of compromised blood supply on contractile force measurements of extraocular muscle has not previously been reported. Our findings and recommendations should assist researchers in their quest to apply the most suitable methods for measurements of muscle contractile parameters.
2. Methods
2. 1. Animals
All experimental procedures described in this study were approved by the Institutional Animal Care and Use Committee of the University of Nevada, Reno. Fertilized White Leghorn chicken eggs were obtained from a local supplier (California Golden Eggs, Inc.) and incubated in a humidified force-draft incubator at 37- 38°C. Date of hatching was designated post-hatch day 0 (P0). A total of about 80 chickens were used for this study. Animals were housed in a brooder with controlled temperature (23–25°C) on a 12 hr light/dark cycle and were provided chicken feed and water ad libitum.
2. 2. Surgical preparation of animals
Force production in extraocular (superior oblique) and limb skeletal muscle (lateral gastrocnemius) was evaluated in situ and in vitro with indirect (nerve) or direct muscle stimulation. Chicks were anesthetized with sodium pentobarbital (Nembutal, 50 mg/kg, intramuscular) as assessed by the absence of withdrawal to digit pinch. Supplemental doses of sodium pentobarbital (50 mg/kg) were given intramuscularly to maintain deep anesthesia. Sodium pentobarbital was selected over ketamine and xylazine because it does not interfere with contractility at the doses administered (Lapointe and Cote, 1999). Body temperature was maintained between 37° and 39° C using a heat lamp. To maintain tissue moisture, Krebs buffer (35°C) was continuously supplied to the orbit or leg during the muscle/nerve preparation and throughout the contractile experiment, unless otherwise stated. Depending on the experimental paradigm the muscle was bathed with either non-oxygenated or oxygenated Krebs buffer that was gassed continuously with 95% O2 and 5% CO2. Krebs buffer contained the following (in mM): NaCl 120.4, KCl 5.9, NaHCO3 15.5, glucose 11.5, MgCl2 1.2, NaH2PO4 1.2, CaCl2 2.5. Oxygenation of the incubating medium generally occurs either by continuous gassing of 95% O2 and 5% CO2 through the medium or by saturating the oxygen content of the buffer. Both methods have been shown to maintain adequate oxygen levels during an in vitro experiment (Newsholme et al., 1986; Van Breda et al., 1990; Bonen et al., 1994). For in vitro experiments, the surgical preparation of both the superior oblique muscle and lateral gastrocnemius muscles were similar to the in situ preparation until blood flow was interrupted. This was done to maintain circulation for as long as possible in order to obtain optimal force output in the in vitro condition.
Muscles for this study were selected based on ease of surgical and experimental manipulation. For an extraocular muscle, the superior oblique was chosen because its sole innervation is the trochlear nerve, which can be stimulated in situ with wire electrodes. Because this nerve innervates only one muscle, interference from contraction of other extraocular and retractor bulbi muscles (for nictitating membrane) is eliminated. In addition, the surgical preparation does not require removal of facial or cranial bones, which has been the usual protocol for indirect (nerve) extraocular muscle stimulation experiments conducted in mammals (Bach-y-Rita and Ito, 1966; Barmack et al., 1971; Hanson and Lennerstrand, 1977; Shall and Goldberg, 1992; Dimitrova et al., 2002). For a limb skeletal muscle, the lateral gastrocnemius was selected because it is composed largely of fast muscle fiber types (Armstrong and Phelps, 1984), which is similar to the high percent of fast muscle fiber types found in extraocular muscle. The lateral gastrocnemius muscle can be easily stimulated in situ with wire electrodes in contact with the lateral tibial nerve and has been used frequently for in situ contractile studies (Ameredes and Provenzano, 1997; Grassi et al., 2002; de Haan, 1998; Ma et al., 2004).
2.2.1. Superior oblique muscle, general preparation
Animal surgery was modified from a protocol described for direct muscle stimulation in pigeons (Stelling and McVean, 1988). Each animal was placed on its side and the head immobilized by pinning the beak to a solid support. For each contractile experiment one superior oblique muscle (right side) was used per animal. The distal end of the superior oblique muscle was exposed by removal of the upper eyelid, superior portions of the conjunctiva/Tenons capsule, and nictitating membrane. Visible surface vessels were cauterized. The insertional tendon of the superior oblique muscle was isolated, tied with a 6-0 silk suture, and cut from the sclera. This suture was later used to attach the tendon to the force transducer. Operating space within the orbital cavity was increased by partially deflating the eyeball. This was accomplished by making a small incision (~1 cm) through the cornea and lens which provided a drain for the aqueous humor and a portion of the vitreous humor. A suture was hooked to the sclera near the insertion of the superior rectus muscle and the eyeball was gently pulled downward to expose the trochlear nerve and superior oblique muscle. The trochlear (IV) nerve was carefully trimmed of connective tissue near its entrance to the superior oblique muscle and separated (isolated) from its close proximity with the ophthalmic nerve (Nickel et al., 1977). The ophthalmic nerve was then crushed to prevent possible reflex head movements during stimulation. The suture, attached to the tendon of the superior oblique muscle, was then tied to a calibrated 25 g isometric force transducer (World Precision Instruments, Sarasota, FL), that was secured on a micromanipulator (Narachige, East Meadow, NY). The 25 g isometric force transducer has a resonant frequency of 450 Hz and a resolution of 2 mg. The force transducer arm was positioned with the line of force perpendicular to the animal’s midline and elevated slightly above the horizontal plane of the animal’s head (Christiansen et al., 2003). The configuration of the muscle, suture, and force transducer was adapted to approximate the natural pulling direction of the superior oblique muscle (Frueh et al., 2001) and to yield the maximal contractile force (Fig. 1) when stimulated, as indicated by our preliminary tests. At this point, further preparation of the superior oblique muscle differed depending on whether an in situ or in vitro method was to be used.
Fig. 1.
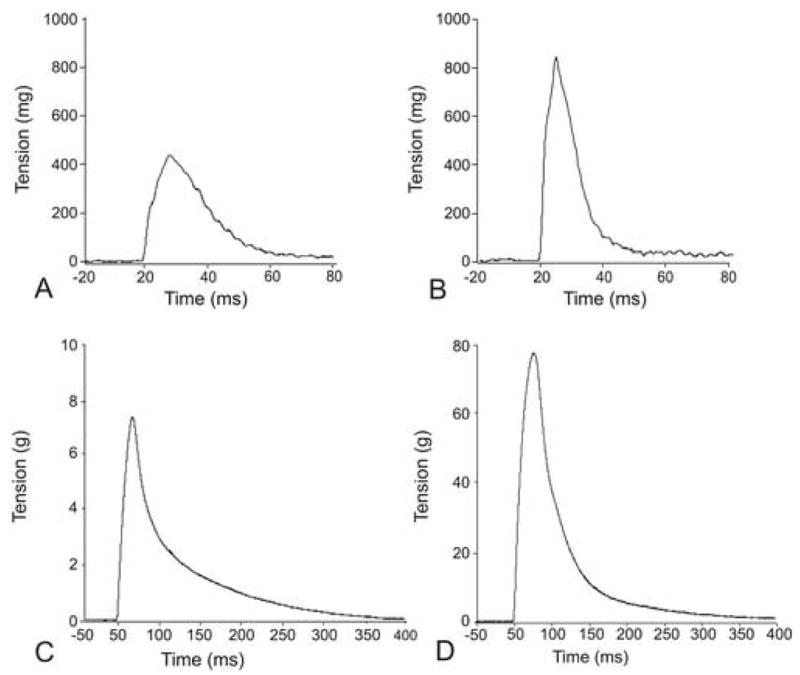
Examples of isometric twitch tension of the chicken superior oblique muscle (A, B) and lateral gastrocnemius muscle (C, D) examined at post-hatch day 2 (P2) and P21. The superior oblique muscle was stimulated via the trochlear nerve and the gastrocnemius muscle was stimulated via the tibial nerve. Force of twitch tension increased with animal age in both muscles, the superior oblique and gastrocnemius.
2.2.1.1 Superior oblique muscle, in situ preparation
Non-oxygenated Krebs buffer, maintained at 35°C, was circulated through the orbital cavity to preserve tissue moisture. Direct and indirect (nerve) stimulation of the extraocular muscle is described in “stimulation procedures.”
2.2.1.2 Superior oblique muscle, in vitro preparation
Depending on the experimental paradigm, oxygenated or non-oxygenated Krebs buffer (maintained at 35°C) was circulated through the orbital cavity. To retain optimal muscle integrity and force production, the muscle’s origin was left intact and its orientation was similar to its natural pulling direction. After surgical preparation was complete and blood supply was still intact, maximum twitch tension was determined by indirect (nerve) or direct muscle stimulation as described in “stimulation procedures.” After a 5 min rest, initial twitch tension (time 0) was determined. Blood supply was interrupted by decapitation and contractile measurements were obtained at 5 min intervals for 40 minutes.
2.2.2. Gastrocnemius muscle, general preparation
Each animal was placed on an operating board in a prone position and the left leg was immobilized. A longitudinal incision was made over the posterior portion of the leg and thigh. The underlying lateral gastrocnemius was freed from its investing external fascia while blood supply was left intact. The insertional tendon (calcaneal) of the lateral gastrocnemius was isolated, tied with a 6-0 silk suture, and cut from its insertion. The sciatic and lateral tibial nerves were exposed by transecting the biceps femoris at its midsection. At this point, further preparation of the gastrocnemius muscle differed, depending on whether an in situ or in vitro method was to be used.
2.2.2.1 Gastrocnemius muscle, in situ preparation
The insertional tendon of the lateral gastrocnemius muscle was attached via the suture to a calibrated 250 g isometric force transducer (Fort 250, World Precision Instruments, Sarasota, FL), that was secured on a micromanipulator (Narachige, East Meadow, NY). The 250 g isometric force transducer has a resonant frequency of 300 Hz and a resolution of 25 mg. The force transducer arm was positioned (via x-, y-, and z-axis micromanipulation) so that the configuration of the muscle and suture were at a 90° angle to the leg. To prevent drying, a 50/50 mixture of Vaseline and mineral oil was used to coat the muscle and non-oxygenated Krebs buffer (maintained at 35°C) flowed over the muscle and provided moisture at the base (insertion) of the muscle where the lateral tibial nerve entered.
2.2.2.2 Gastrocnemius muscle, in vitro preparation
After the lateral gastrocnemius muscle was prepared as previously described (general preparation), the animal was decapitated and the leg was removed by transecting the femur. The leg, with attached lateral gastrocnemius muscle, was immediately placed into a polycarbonate chamber filled with Krebs buffer. The origin of the lateral gastrocnemius muscle was left intact in order to minimize muscle damage due to dissection. Depending on the experimental paradigm, the chamber contained either oxygenated or non-oxygenated Krebs buffer. Both buffer types were continuously circulated through the chamber and maintained at 35°C. The leg was positioned horizontal to the chamber floor and secured by metal hooks to the bottom of the chamber. The suture (attached to the insertional tendon) was tied to a calibrated 25 g or 250 g isometric force transducer. Smaller muscle from the P2 chicks was attached to a 25 g force transducer and larger muscle from the P21 chickens was attached to a 250 g force transducer. The force transducer arm was positioned so that the configuration of the muscle and suture were at a 90° angle to the leg. Muscle preparation from time of decapitation to establishing maximum twitch tension was approximately 10–15 minutes. The protocol for direct and indirect muscle stimulation is described in “stimulation procedures”.
2. 3. Stimulation procedures
In situ and in vitro muscle force measurements of the superior oblique and lateral gastrocnemius muscle were determined in response to indirect (nerve) and direct muscle stimulation at supra maximal intensities. Parallel platinum wire electrodes were used for both indirect and direct muscle stimulation. While platinum wire electrodes are standard for muscle stimulation via the nerve, direct muscle stimulation can be done with either wire (McLoon and Christiansen, 2003) or plate electrodes (Frueh et al., 2001). Initial tests utilizing platinum plate electrodes (width = 4 mm; length = 15 mm; thickness = 0.5 mm), flanked along the length of the superior oblique muscle at a distance of ~1 mm from the muscle (submerged in Krebs solution), resulted in maximum twitch contractile forces that were similar to our twitch tensions elicited by direct contact with wire electrodes (absence of Krebs solution). Thus, to facilitate rapid switching between indirect (nerve) and direct muscle stimulation, we utilized the platinum wire electrodes for experiments involving direct muscle stimulation.
2.3.1. Nerve stimulation
The superior oblique muscle was stimulated through the trochlear (IV) nerve and the gastrocnemius muscle was stimulated through the lateral tibial nerve. Parallel platinum wire electrodes (0.3 mm tip diameter, 2 mm between poles) were positioned so that both electrodes contacted the respective nerve about 5 mm proximal to the nerve’s entry into the target muscle. To ensure that evoked muscle stimulation occurred only through the nerve, Krebs buffer was removed before each stimulus was applied. Optimal muscle length (Lo) and supramaximal stimulation voltage (~15 V for both the tibial and trochlear nerve) were determined from micromanipulations of muscle length and a series of twitch contractions (1 Hz square-wave pulse, 0.2 ms duration) until twitch tension was maximal = maximum isometric twitch force (Pt). Three single-twitch contractions were elicited in 5 s intervals to determine the average Pt at each time point.
Maximum tetanic tension (Po) was evaluated with a pulse duration of 0.2 ms, train duration of 200 ms, and stimulation rates in the range of 350–400 Hz. The stimulation rates that elicit maximum tetanic tension were determined previously (Croes et al., 2007). Three single-tetanic tension contractions were elicited in 5 s intervals to determine the average Po at each time point. Each stimulation train was separated by a 5 second interval to ensure a return to baseline force. The parameters of voltage and optimal length established for maximal isometric twitch were used for maximal tetanic tension measurements. It has been shown that whole-muscle tetanic tensions are obtained at the same muscle lengths as maximal isometric twitch tensions (Barmack et al., 1971). The maximum tetanic tension was seen at or near the fusion frequency of the muscle. Fusion frequency was defined as the stimulation frequency at which individual twitches could not be differentiated at the tension plateau (Goldberg et al., 1998).
2.3.2. Direct muscle stimulation
The lateral gastrocnemius and superior oblique muscles were stimulated directly by contact with platinum electrodes (0.3 mm tip diameter). Parallel platinum electrodes were positioned along each side of the muscle near the mid section. Optimal muscle length (Lo) and supramaximal stimulation voltage (~80 V) were determined from micromanipulations of muscle length and a series of twitch contractions (1 Hz square-wave pulse, 1.0 ms duration) until twitch tension was maximal = maximum isometric twitch force (Pt). Although a 2.0 ms pulse duration approximates the normal excitation duration when occurring through neuromuscular transmission (Fatt and Katz, 1951), we used a 1.0 msec pulse duration to eliminate the chance of multiple twitches (Chervu et al., 1989).
2. 5. Data collection and statistical analysis
In situ muscle force measurements (twitch) of the gastrocnemius muscle and superior oblique muscle were evaluated at post-hatch ages P2 and P21 (4–7 chickens were analyzed per time point for each parameter measured). Maximum twitch tension was measured as the difference between baseline and peak amplitude. For extraocular muscle, in vitro twitch tension was obtained both by indirect (nerve) and direct muscle stimulation in the presence of oxygenated or non-oxygenated Krebs buffer, and data were compared to in situ measurements taken within the same animal. Similarly, in vitro tetanic tension was obtained by indirect (nerve) stimulation in the presence of oxygenated Krebs buffer, and data were compared with initial in situ measurements taken from the same muscle. Results for twitch and tetanic tension are expressed as a percent of the initial values obtained before the onset of ischemia. For lateral gastrocnemius muscle, in vitro twitch tension was obtained by indirect (nerve) and direct muscle stimulation in the presence of oxygenated or non-oxygenated Krebs buffer. For indirect (nerve) measurements, data were compared to in situ measurements taken within the same animal. For direct muscle stimulation measurements, data were compared to the initial measurements taken in vitro within the same animal. Thus, initial measurements for the direct stimulation of the lateral gastrocnemius muscle in vitro were those taken once the leg was removed and placed into the incubation chamber. The difference between indirect and direct stimulation data collection is explained in the results section (3.4.1 Indirect (nerve) stimulation). Muscle stimulation was induced via a Grass S48 stimulator (Quincy, MA). The output of the stimulator was linear and matched the dial setting for stimulation voltages up to 100 volts, indicating that the stimulator generated sufficient current intensity to recruit all the motor units in our preparation. Average resistance for the platinum wire electrodes was 0.11Ω and the average system lead resistance was 0.10Ω . Twitch and tetanic tension were measured with a data-acquisition system (Digidata 1322A; Axon Instruments, Union City, CA). Results are expressed as a percent of the initial values obtained before blood flow was interrupted.
Forces can be given as absolute forces (N), or they can be normalized to the total muscle cross-sectional area (CSA). The CSA can be estimated in various ways that take into account actual myofiber length, myofiber area without extracellular spaces, and myofibrillar content (Close, 1972; Asmussen and Gaunitz, 1981; Taylor and Kandarian, 1994; Frueh et al., 2001), as shown in Table I. Muscle mass, optimal muscle length Lo, muscle fiber length Lf, and Po were used to calculate maximum specific isometric tetanic force (sPo) or maximum Po normalized per total muscle CSA (kN/m2), as described in Frueh et al. (2001). The CSA was estimated in four ways: (i) by the conventional formula that divides muscle mass (mg) by the product of the measured optimal muscle length, Lo, and 1.06 mg/mm3 (the density of mammalian skeletal muscle, Close, 1972); (ii) by correcting for the actual muscle fiber length = mean myofiber length, Lf (estimated to be 45%, Frueh et al., 2001); (iii) by correcting to exclude extracellular spaces in muscle cross sections (Close, 1972; based on data from Baryshnikova et al., 2007); and (iv) by correcting for actual myofibrillar area (Taylor and Kandarian, 1994; based on data from Baryshnikova et al., 2007). Absolute Po values were normalized for muscle CSA using the formula sPo (kN/m2) = Po (mN)/CSA (mm2). Since force normalizations are not relevant to the conclusions of this study, tetanic tensions were normalized only for the P21 superior oblique muscle (Table I), which shows that our measurements are very close to previous data on mammalian and avian eye muscles measured in situ (e.g., Asmussen and Gaunitz, 1981; Stelling and McVean, 1988; Frueh et al., 2001).
Table I.
Properties of the superior oblique muscles of chicken evaluated in situ (time point “0” in Figs. 2,3). P21, posthatch day 21. Note: Twitch and tetanic tension was generated in each group after supramaximal indirect (nerve) stimulation. Values are means ± SEM.
| P21 | n | ||
|---|---|---|---|
| Muscle mass, mg | 13.03 ± 0.34 | 7 | |
| Lo, mm | 9.45 ± 0.16 | 11 | |
| Pt, mN | 8.33 ± 0.69 | 7 | |
| Po, mN | 54.69 ± 2.47 | 3 | |
| CSA, mm2 | 1.30 | ||
| sPo | kN/m2 | N/cm2 | |
| Uncorrected (using formula) | 42.07 ± 1.90 | 4.2 | |
| Corrected for muscle fiber length* | 18.93 ± 0.85 | 1.9 | |
| Corrected for myofiber area** | 28.42 ± 1.28 | 2.8 | |
| Corrected for myofibrillar area*** | |||
| Global Layer | 60.60 ± 2.73 | 6.1 | |
| Orbital Layer | 49.25 ± 2.22 | 4.9 | |
According to Frueh et al., 2001
According to Close, 1972; Asmussen and Gaunitz, 1981; Baryshnikova et al., 2007
According to Close, 1972; Taylor and Kandarian, 1994; Baryshnikova et al., 2007
Current tracings were exported to CorelDraw (version 12, Corel Corporation, Ottawa, Ontario, Canada) for processing of the figures. Muscle thickness was measured at the middle of the muscle at optimal length and determined with a high precision digital caliper (Performance Tool). All statistical analyses were conducted using SigmaStat software (Jandel Corp, San Rafael, CA). The data were reported as the mean ± standard error of the mean (SEM). Statistical significance was evaluated by Student’s paired t-test with a confidence level of p < 0.05.
3. Results
3. 1. Contractile force of superior oblique extraocular muscle: baseline
In order to evaluate the contractile force under either in situ or in vitro conditions we developed an in situ method for stimulating the superior oblique muscle via the trochlear nerve. This extraocular muscle was chosen for its relative ease of surgical manipulation and its innervation. The superior oblique muscle is innervated by the trochlear nerve, which is unique in that it has a sole muscle target. This method enabled us to effectively measure the contractile forces generated by the superior oblique muscle in chickens at 2 days (P2) and 3 weeks (P21) of age in the current study and up to 4 months of age in a previous study (Croes et al., 2007). Representative traces of twitch contractions of the superior oblique muscle via indirect (nerve) stimulation are shown in Fig. 1A (P2) and Fig. 1B (P21). Absolute values for twitch tension and muscle thickness of control (baseline) animals are shown in Table II. Measurements for twitch and tetanic tension were taken at 5 min intervals for 40 minutes. Relatively constant values (see Fig. 2, 3) were seen for both post-hatch ages examined; P2 and P21 (control) muscles. Values in graphs were expressed as a percent of the initial values obtained at time zero (0 min = 100%) with an intact blood supply. Muscle viability was assessed via force output measurements in response to indirect (nerve) and direct muscle stimulation. Although we are not aware of any other studies that measured twitch tension of chicken extraocular muscles in situ, the absolute force values measured for twitch and tetanic tension of our P21 animals are consistent with those obtained for similar muscles previously reported for other avian species of comparable size (Stelling and McVean, 1988).
Table II.
Twitch tension and muscle thickness of superior oblique (Sup. Obliq.) and lateral gastrocnemius (Lat. Gastroc.) muscles of chicken evaluated in situ (time point “0” in Figs. 2,4). P2, posthatch day 2; P21, posthatch day 21.
| Twitch Tension |
||||
|---|---|---|---|---|
| Muscle | Age | Nerve Stimulation, Muscle Force [g] | Direct Stimulation, Muscle Force [g] | Muscle Thickness [mm] |
| Sup. Obliq. | P2 | 0.427 ± 0.03 (6) | 0.883 ± 0.08 (6) | 0.33 ± 0.03 (5) |
| Sup. Obliq. | P21 | 0.850 ± 0.07 (7) | 1.782 ± 0.11 (7) | 0.58 ± 0.05 (5) |
| Lat. Gastroc. | P2 | 8.78 ± 1.06 (4) | 12.60 ± 2.43 (6) | 2.33 ± 0.10 (4) |
| Lat. Gastroc. | P21 | 77.82 ± 9.11 (5) | 105.87 ± 10.85 (7) | 4.30 ± 0.14 (5) |
Note: Twitch tension was generated in each group after supramaximal indirect (nerve) and direct muscle stimulation. Numbers in brackets = “n”. Values are means ± SEM.
Fig. 2.
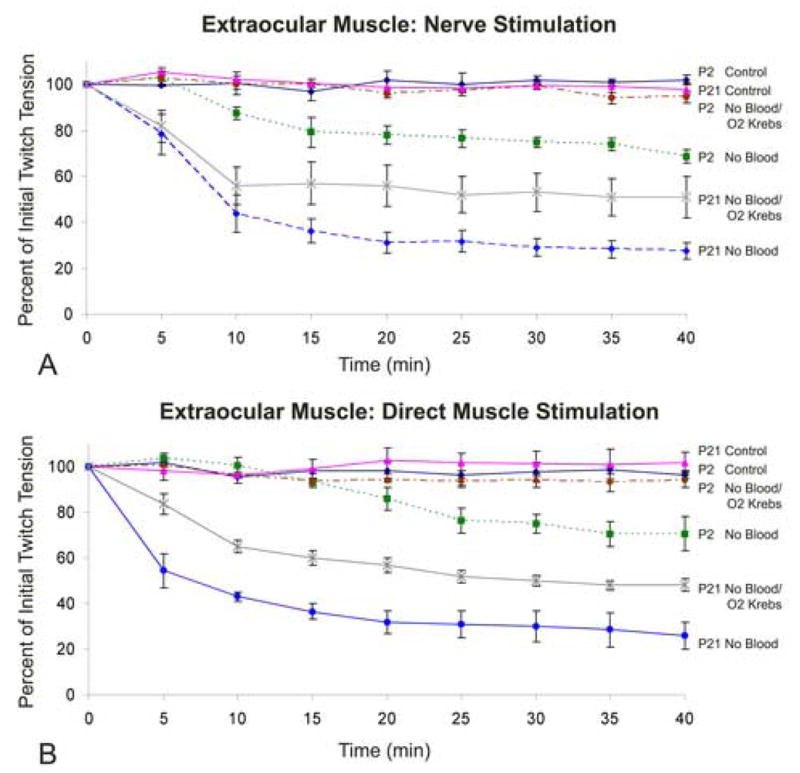
A and B. Influence of age (muscle thickness), method of stimulation, and blood supply on the contractile force of the chicken superior oblique muscle. Time course of twitch tension in superior oblique muscle elicited (A) via the trochlear nerve or (B) by direct muscle stimulation. Animals were examined at post-hatch day 2 (P2) or P21 with blood flow intact (Control) or without blood flow in the presence of non-oxygenated Krebs buffer (No Blood) or oxygenated Krebs buffer (No Blood/O2 Krebs). Note the substantial decrease in force within 10–15 min of ischemia. Error bars = SEM. n = 4–7 animals per data point.
Fig. 3.
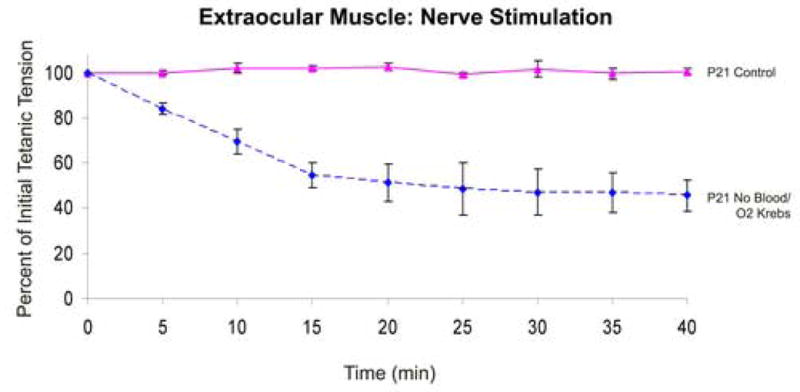
Influence of blood supply on contractile force of the chicken superior oblique muscle. Time course of tetanic tension in superior oblique muscle elicited via the trochlear nerve. Animals were examined at post-hatch day 21 (P21) with blood flow intact (Control) or without blood flow in the presence of oxygenated Krebs buffer (No Blood/O2 Krebs). Note the substantial decrease in force within 15 min of ischemia. Error bars = SEM. n = 3 animals per data point.
3. 2. In vitro incubation: efficacy of oxygenated Krebs buffer to maintain extraocular muscle force
In order to evaluate whether extraocular muscles maintain maximal contractile force during in vitro incubation, we examined the force production of the superior oblique muscle in response to indirect (nerve) and direct muscle stimulation in vitro. We tested whether (i) muscle force decreased while the muscle was incubated in vitro, and if so (ii) at what rate the force decreased. For in vitro preparation, after the initial force was obtained (time 0), blood supply was interrupted (by decapitation) and force measurements were taken every five minutes over the course of 40 minutes. Values in graphs were expressed as a percent of the initial values obtained at time zero (0 min = 100%) with blood supply intact. We then examined the influence of blood supply and in vitro incubation for two different muscle thicknesses (ages P2, P21), using indirect (nerve) and direct stimulation, and in the presence of non-oxygenated or oxygenated Krebs buffer.
3.2.1. Indirect (nerve) stimulation
In the superior oblique muscle of 2 day old chicks (P2) with a muscle thickness of 0.33 ± 0.03 mm, 40 minutes of in vitro incubation with non-oxygenated Krebs buffer resulted in a significant reduction of nerve-induced twitch tension (by 31.3% ± 3.0%, SEM) (Fig. 2A). In the presence of oxygenated Krebs buffer there was no significant difference from control values which indicates muscle viability was maintained. In vitro preparation of older (P21) animals with a muscle thickness of 0.58 ± 0.05 mm, exhibited a more pronounced effect. In the presence of non-oxygenated Krebs buffer, 40 minutes of in vitro incubation resulted in a significant decrease in twitch tension of 71.5% (±3.71%), whereas incubation in oxygenated Krebs resulted only in a 49% (±8.0%) decrease. This indicates that oxygenated Krebs buffer contributes to muscle viability, but can not preserve forces at control values.
In addition to twitch force measurements, tetanic tension can provide further information concerning muscle function. To test the effects of interrupted blood flow on tetanic tension development of the superior oblique muscle, we used post-hatch day 21 (P21) chickens, which have the thicker (0.58 ± 0.05 mm) extraocular muscle. Control animals had an average tetanic tension of 5.58 g (± 0.25 g). Comparable to the twitch response, in vitro preparation of older (P21) animals exhibited a pronounced effect. In the presence of oxygenated Krebs buffer, 40 minutes of incubation resulted in a significant decrease in tetanic tension by 45.7% (± 6.9%) (Fig. 3). This indicates that decreased blood supply, even in the presence of oxygenated Krebs buffer, can significantly alter muscle function as measured by either twitch and tetanic tension development.
3.2.2. Direct muscle stimulation
After 40 min of in vitro incubation in non-oxygenated Krebs buffer, the superior oblique muscle of both younger (P2) and older (P21) animals exhibited a significant reduction in twitch tension (by 29.5% ± 7.5%; 74.0% ± 6.0%, respectively) with older chickens demonstrating a steeper decline in twitch tension than the younger chicks (Fig. 2). In the presence of oxygenated Krebs buffer, muscle viability was maintained throughout the 40 minutes incubation period of P2 animals (muscle thickness, 0.33 ± 0.03 mm), but twitch tension was significantly reduced in the P21 animals (by 48.0% ± 2.70%), muscle thickness of 0.58 mm (± 0.05 mm). However, the reduction of twitch tension of P21 animals in oxygenated Krebs buffer was less severe than the twitch tension generated in the superior oblique muscle incubated in non-oxygenated Krebs.
Thus, extraocular muscle was susceptible to interruption of blood flow, for both indirect (nerve) and direct muscle stimulation. In vitro incubation with oxygenated Krebs buffer maintained muscle force tension of the thinner extraocular muscle in P2 animals, but not of thicker muscle in the P21 animals. This indicates that extraocular muscles are significantly more sensitive to compromised blood flow than previously expected for a relatively thin muscle: a thickness of 0.58 ± 0.05 mm failed to retain viability during an in vitro incubation.
3. 3. Contractile force of gastrocnemius skeletal muscle: baseline
To determine the baseline force (twitch tension) of the gastrocnemius muscle, we used an in situ preparation with blood supply intact, and elicited muscle contractions via indirect (nerve) and direct muscle stimulation. Representative traces of twitch contractions via indirect (nerve) stimulation are shown in Fig. 1C (P2) and Fig. 1D (P21). Absolute values for twitch tension and muscle thickness of control animals are shown in Table II. Measurements were taken at 5 min intervals for 40 minutes. In control animals relatively constant values (see Fig. 4A and B) were seen for both post-hatch ages examined; P2 and P21 muscles. Values in graphs were expressed as a percent of the initial values obtained at time zero (0 min = 100%). We then examined the effectiveness of an in vitro preparation to maintain muscle viability in the lateral gastrocnemius. The lateral gastrocnemius was assessed at P2 and P21, which provided relatively thin (2.33 ± 0.10 mm) and thick (4.30 ± 0.14 mm) muscles, respectively. Muscle viability was assessed via force output measurements in response to indirect (nerve) and direct muscle stimulation.
Fig. 4.
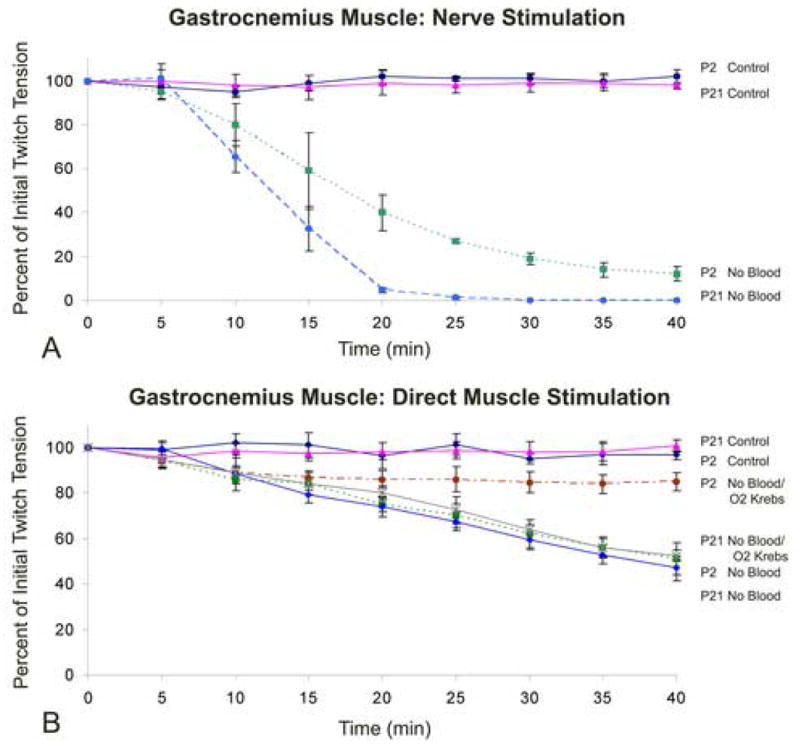
A and B. Influence of age (muscle thickness), blood supply, oxygenated and non-oxygenated Krebs buffer, and method of stimulation on the contractile force of the chicken lateral gastrocnemius muscle. Time course of twitch tension in lateral gastrocnemius muscle elicited (A) via the tibial nerve or (B) by direct muscle stimulation. Animals were examined at post-hatch day 2 (P2) or P21 with blood flow intact (Control) or without blood flow in the presence of non-oxygenated Krebs buffer (No Blood) or oxygenated Krebs buffer (No Blood/O2 Krebs). Note the substantial decrease in force with nerve stimulation after 15–20 min of ischemia. Error bars = SEM. n = 4–7 animals per data point.
3. 4. In vitro incubation: efficacy of oxygenated Krebs buffer to maintain gastrocnemius muscle force
To determine the ability of oxygenated Krebs buffer to maintain limb skeletal muscle viability in vitro, vascular flow to the gastrocnemius muscle was interrupted and the muscle was bathed in either oxygenated or non-oxygenated Krebs buffer depending on the experimental paradigm. The leg was secured within the incubation chamber and maximum twitch tension was determined approximately 10 min after blood flow was interrupted. After maximum twitch tension was determined the muscle was allowed to rest for 5 minutes and then an initial measurement (time point = 0) of twitch tension was obtained. We tested whether (i) force declines in an in vitro preparation, and if this was the case, (ii) at what rate the force declined over 40 min. This was examined for two different muscle thicknesses (ages P2, P21) and in the presence of oxygenated or non-oxygenated Krebs buffer.
3.4.1 Indirect (nerve) stimulation
The lateral gastrocnemius muscles of P2 and P21 chickens had an average thickness of 2.33 ± 0.10 mm and 4.30 ± 0.14 mm, respectively. Initially, the leg was removed and placed into an incubation chamber with oxygenated Krebs buffer, as described previously in methods. The procedure took approximately 15–20 minutes before measurements could be obtained, which was slightly longer than for direct muscle stimulation. Additional time was required to prepare the lateral tibial nerve for stimulation. For P21 animals the initial twitch tension was 19.4 ± 2.4%, of control (in situ) values. This was substantially less than expected. To reveal the functional progression of ischemia on muscle force starting at the time of blood flow interruption we measured twitch tension immediately after blood flow termination. After initial twitch tension was determined in situ, blood flow was interrupted by decapitation and severing the ishiadica externa artery and femoral artery. The ishiadica externa artery is the major vessel that supplies arterial blood to the posterior leg muscles of the chicken (Koch, 1973). The vessel was severed to prevent blood circulation due to continued heart function after decapitation. Twitch tension measurements were then taken every 5 minutes for 40 minutes (Fig. 4A). For P2 animals in the presence of non-oxygenated Krebs buffer, 40 min of hind-limb ischemia resulted in a significant reduction (by 88.7% ± 3.3%, SEM) of twitch tension induced by nerve stimulation. The lateral gastrocnemius of P21 animals exhibited complete failure of muscle contraction via indirect (nerve) stimulation. After 30 minutes of ischemia the muscle did not respond to nerve-induced stimuli and thus twitch tension data was “0” or not measurable beyond 30 minutes for this treatment group (Fig. 4A). The significant decrease in twitch tension seen during the initial 20 minutes of ischemia explained the relatively low twitch forces obtained when the leg was removed and placed into the bath. By the time (15 – 20 min) the muscle and nerve were positioned to elicit contractions, the viability of the nerve/muscle preparation had already significantly decreased. The severity of the decrease for twitch tension was greater than that observed with direct muscle stimulation (see below) indicating a more detrimental ischemic effect on the nerve and/or neuromuscular junction.
3.4.2. Direct stimulation
For lateral gastrocnemius muscle of P2 chicks in the presence of non-oxygenated Krebs buffer, 40 min of hind-limb ischemia resulted in a significant reduction (by 49.3% ± 6.9%, SEM) in twitch tension (Fig. 4B). Twitch tension was also significantly reduced (by 15.0% ± 4.1%, SEM) in the presence of oxygenated Krebs buffer, but the reduction was less severe.
For P21 chickens, the lateral gastrocnemius muscle incubated in the presence of non-oxygenated or oxygenated Krebs buffer, 40 min of hind-limb ischemia resulted in a significant reduction in twitch tension, by 53.0% (± 5.4%, SEM) and 47.5% (± 2.5%) respectively (Fig. 4B). When comparing muscle responses to non-oxygenated or oxygenated Krebs buffer of P21 animals, there were no significant differences between the treatment groups. Thus, the thicker skeletal muscle of P2 and P21 animals was highly susceptible to interruption of blood flow and an in vitro oxygenated environment was not able to maintain muscle viability at control values.
3.5 Differences in contractile force between direct muscle stimulation and indirect (nerve) stimulation
Contractile force generated by direct muscle stimulation was significantly greater than that of indirect (nerve) stimulation for both limb skeletal and extraocular muscle (Table II). While this occurrence has been well documented for limb skeletal muscle (Pagala et al., 1984), our study presents the first experimental evidence for extraocular muscle. These differences in muscle force are likely due to a more synchronous activation of all the muscle fiber types by direct stimulation than by nerve stimulation, which, due to differences in conduction velocity and synaptic delay, produces less synchronous activation of muscle fibers (Sandow and Isaacson, 1966; Pagala et al., 1984).
4. Discussion
Our study provides important new information on the accuracy and validity of contractile force measurements. Comparison of results obtained from extraocular muscles examined in situ or in vitro shows that an in vitro incubated muscle preparation has serious limitations for investigations to determine absolute muscle force. While in vitro studies with isolated muscles are technically easier, the use of extraocular muscle of even a relatively small size or thickness results in compromised muscle function and the measured force does not reflect true maximal forces. In situ methods are technically more difficult, but they offer the closest approximation to the in vivo situation while permitting considerable experimental control. Here we identify crucial parameters that compromise contractile force measurements in vitro and we discuss the benefits of utilizing the chicken as an animal model for the study of extraocular muscle forces.
4.1 Parameters that compromise contractile force measurements in extraocular muscles
Extraocular muscle viability in vitro may be compromised even when the preparation follows the criteria established for other skeletal muscle. A number of studies have provided detailed parameters for successful in vitro viability of skeletal muscle (Ellsworth and Pittman, 1984; Hummel et al., 1988). An important criterion in maintaining muscle viability is muscle thickness. For skeletal muscle the critical muscle thickness or diameter at different incubation temperatures is well established (Segal and Faulkner, 1985; Segal et al., 1986; Bonen et al., 1994). Based on these criteria, an incubation temperature of 35°C, as used in our study, requires that the skeletal muscle thickness should be ≤ 1.25 mm in order to maintain adequate oxygenation (Segal and Faulkner, 1985). The extraocular muscles used in our study were from two different age groups and thus two different muscle thicknesses. Two-day old (P2) and 21-day old (P21) chickens had an average muscle thickness of 0.33 mm (±0.03 mm) and 0.58 mm (±0.05 mm), respectively. Based on these muscle thicknesses and incubation temperature, the in vitro preparation of both groups should have theoretically remained viable over the 40 minute incubation period used in our study (Segal and Faulkner, 1985; Van Breda et al., 1990). However, while the extraocular muscle of P2 animals maintained maximal forces, the muscle function of the P21 group declined substantially. This was an unexpected result that raises serious questions about the suitability of in vitro methods for assessing extraocular muscle function.
Discrepancies in extraocular force measurements between in vitro and in situ methods are known from the literature. In a previous comparison, Frueh and colleagues (2001) found that muscle force (force per cross-sectional area, kN/m2) measured in situ were about six times greater than the highest of those reported previously for extraocular muscles in vitro (Close and Luff, 1974; Asmussen and Gaunitz, 1981; Luff, 1981; Asmussen et al., 1994; Frueh et al., 1994). Our own lab has reported force measurements of the superior oblique muscle obtained in situ to be 3 to 6 times greater than that of the same muscle in vitro (Chen and von Bartheld, 2004; Croes et al., 2007).
Differences in contractile force have been previously attributed to damage inflicted during the dissection and/or improper orientation of the muscle. Since the extraocular muscles do not have a tendon at their origin, the complete removal of the muscle causes damage to both its origin and insertion and such trauma may contribute to disparities in contractile strength (Frueh et al., 2001). Similarly, a variable time delay between excising the muscle, mounting it, and resuming oxygenation could result in hypoxic damage. Muscle orientation can also have significant effects on contractile force when measured in situ. In the natural state, extraocular muscles such as the rectus muscles arch over the eye from their origin to their insertion. Frueh and colleagues (2001) found that any deviation from the optimal angle (angle that is closest to the natural anatomical orientation) resulted in significant reductions in muscle force. The current study goes beyond this previous investigation by demonstrating that extraocular muscle thickness, which was previously assumed to be adequately thin for in vitro preparations, is a significant factor in determining muscle viability in vitro. We show that an intact blood supply is crucial to obtain maximal contractile force measurements.
Here, our in vitro method was designed to rule out effects of muscle damage/or orientation by preparing the muscle identical to our in situ method with blood supply being the only effective variable. To minimize muscle damage the muscle’s origin remained intact and the blood supply was not compromised during the muscle preparation. Muscle orientation was preserved by positioning the muscle similar to its natural pulling direction, equivalent to our in situ procedure. By keeping all parameters identical between our in vitro and in situ preparations, except an intact blood supply, we demonstrate that an in vitro method is limited in its maintenance of extraocular muscle viability, resulting in a significant reduction in muscle force over a relatively short (10 – 40 min) time period. Contractile force of extraocular muscle would be underestimated by between 10% (at 5–10 min) and 50–80% (at 20–40 min) when measurements are made after cessation of blood supply even in the presence of oxygenated Krebs buffer. This indicates that measurements of precise absolute muscle force are practically impossible to ascertain in vitro for extraocular muscles with thicknesses of 0.58 mm or greater unless force measurements (twitch and/or tetanic) can be taken within 5 minutes of blood flow interruption. Thus, we recommend that an in situ method be chosen for extraocular muscle analysis when accurate absolute force measurements are required. The criterion for successful in vitro preparation of extraocular muscle cannot be solely deduced by extrapolation from data of other skeletal muscle, but rather has to be determined empirically.
It is currently controversial whether in vitro measurements of extraocular muscle contractile force should be replaced by the in situ approach, or whether in vitro data still have an important – albeit limited – role, for example when relative rather than absolute forces are compared between experimental groups (Frueh et al., 2001)
4.2 Differences between skeletal (limb) muscle and extraocular muscle contractile force measured in vitro
To obtain relevant results that are similar to the in vivo situation, it is essential that the physiological viability and integrity of the muscle is maintained in vitro. Oxygen and other substrates enter the muscle exclusively via diffusion from the incubation medium. Thus, muscle thickness must be suitable to permit diffusion of oxygen into all myofibers in order to prevent hypoxic damage at the muscle core. Skeletal muscles vary histologically in their fiber size, type, and metabolic requirements, and associated with these differences are variations in oxygen and substrate solubilities (Ellsworth and Pittman, 1984). Oxygen may diffuse at different rates depending on the composition of the barriers through which it must pass (Maltin and Harris, 1985; Bonen et al., 1994). Our results of the lateral gastrocnemius muscle support these expectations. In both the P2 and P21 animals, twitch tension (via direct stimulation) of the lateral gastrocnemius muscle was significantly reduced over the 40 minute incubation period regardless of whether oxygenated or non-oxygenated Krebs buffer was present. However, the relatively thin (2.33 ± 0.10 mm) gastrocnemius muscle of the P2 animals in the presence of oxygenated Krebs buffer retained forces longer than in non-oxygenated Krebs buffer, indicating an increased ability to maintain muscle viability.
Extensive research has been conducted on the biochemical viability of limb skeletal muscle in vitro, but little is known about the in vitro effects on extraocular muscle. Although extraocular muscles are classified as skeletal muscle, they differ in many respects from skeletal (limb) muscle, including their diversity of myofiber types (Spencer and Porter, 1988; Baryshnikova et al., 2007, submitted), their functional properties (Nelson et al., 1986), their susceptibility to diseases (Ruff, 2002), and they have a particularly strong vascular supply (Wooten and Reis, 1972; Cheng et al., 2004). These substantial differences between limb skeletal and extraocular muscle may relate to the fact that extraocular muscle viability is not maintained as predicted for other skeletal muscle. It will be of interest to explore the precise biochemical differences between extraocular muscle and skeletal muscle that are responsible for this discrepancy.
4.3. Effects of acute ischemia on muscle contractile force: Potential mechanisms
The structures within the neuromuscular system that may be susceptible to ischemia include the peripheral nerve (Chervu et al., 1989; Hoch et al., 1991), skeletal muscle (Dyck, 1989; Korthals et al., 1985) and the neuromuscular junction (Lundborg, 1970; Hatzipantelis et al., 2001). Our results for the gastrocnemius muscle support the notion that the initial and most detrimental site of ischemic injury is due to nerve involvement, although it is unclear whether axonal injury or presynaptic terminal damage is primarily responsible. While we did not directly test the function of the axon, based on previous studies that have shown that peripheral nerve function can remain intact for 1 to 3 hours of ischemia (Zollman et al., 1991; Hatzipantelis et al., 2001), we propose that, under the conditions of this study, the neuromuscular junction is the site most susceptible to acute ischemia in skeletal muscle.
What are the mechanisms by which ischemic conditions cause the components of the nerve/neuromuscular junction to cease functioning? One possible explanation is a decrease in the number of synaptic vesicles released at the synaptic cleft. Increased lactate production (acidosis) inhibits calcium channels within the nerve terminal (Harris et al., 1986). The acidosis that results from ischemia is thought to cause a reduction in the time that calcium channels remain open. This ultimately causes a reduction in the number of synaptic vesicles released and thus inhibits muscle contraction (Haralambie, 1973; Badonic, 1981).
The decrease in contractile force (twitch tension) would intuitively seem to involve the lack of ATP production, since the energy for muscle contraction is generated by ATP hydrolysis. However, the concentration of ATP in skeletal muscle was shown to remain stable after 80 minutes of ischemia (Harris et al., 1986; Walker, 1991). Acidosis has been proposed to be responsible for the ischemia-induced reduction of twitch tension in response to direct muscle stimulation (Harris et al., 1986). The ischemia-induced acidosis reduces the time that calcium channels of the sarcoplasmic reticulum remain open, and coupled with competition between hydrogen and calcium for the binding to troponin this ultimately decreases the amount of calcium that binds troponin and thus reduces twitch tension (Hudlicka et al., 1973; Harris et al., 1986; Walker, 1991).
For extraocular muscle, our results indicate that both indirect (nerve) and direct muscle stimulation resulted in similar contractile responses in vitro and thus components of the neuromuscular junction were not the limiting factor as seen in the gastrocnemius muscle. The difference in viability of the neuromuscular junction within extraocular muscles may be the result of larger nerve terminals/endplates and an increased safety factor (Croes and von Bartheld, 2005), which could compensate for reduced nerve terminal function. Several aspects contribute to the safety factor, including the quanta of acetylcholine (ACh) released, ACh receptor (AChR) conduction properties, AChR density, density of voltage gated sodium channels, and acetylcholine esterase activity (Boonyapisit et al., 1999). The firing rates of extraocular muscle myofibers are higher than those of limb myofibers, which places an additional electrical stress on the extraocular muscle synapses. The increased electrical stress results in a greater influence on sodium channel inactivation, AChR desensitization, and affects the efficiency of neuromuscular transmission (Ruff and Lennon, 1998; Ruff, 2002). Thus, the benefit of a relatively large nerve terminal and endplate size of en plaque fibers of extraocular muscle may be that it increases the safety factor for neuromuscular transmission and provides protection for the neuromuscular junction in ischemic conditions.
4.4. The chicken as a model system: technical aspects of nerve access and stimulation
Previous oculomotor model systems utilizing cats (Bach-y-Rita and Ito, 1966; Hanson and Lennerstrand, 1977; Shall and Goldberg, 1992; Dimitrova et al., 2002) and monkeys (Goldberg et al., 1998; Dimitrova et al., 2003) require removal of orbital bones to expose select extraocular muscles, craniotomy, and the use of stereotaxic parameters for the proper positioning of electrodes. A major disadvantage in using stereotaxic parameters is that one cannot directly visualize the nuclei or nerve that is to be stimulated. Complete visualization of the oculomotor nerves necessitates a more invasive surgical protocol. Frueh and colleagues (2001), utilizing rats, developed a method in which they remove the calvarium and delicately excise the anterior portion of the brain. This procedure allows the researcher to expose and stimulate cranial nerves III-VI that run along the floor of the cranial cavity. In situ preparations for direct extraocular muscle stimulation are technically less demanding than those of indirect (nerve) stimulation. Exposure of the distal portion of the extraocular muscle can be done by removal of the upper or lower eyelids, conjunctiva and, in some protocols, removal of the eyeball. Muscle contractions can then be elicited by placing bipolar electrodes on the distal portion of the muscle (Stelling and McVean, 1988; McLoon and Christiansen, 2003). Here, we have demonstrated that accurate force measurements of extraocular muscle, stimulated indirectly (nerve) or directly, can be obtained by a procedure that is relatively simple and less invasive than previously described for mammals. Chickens are particularly well suited for these measurements because they have relatively large eyes and large extraocular muscles (Baryshnikova et al., 2007, submitted), coordinated ocular movements with precise estimation of target (grain) location (Uchiyama, 1989), high visual acuity (Schmid and Wildsoet, 1998), and chickens have an evolutionarily conserved oculomotor system (Maier et al., 1972; Heaton and Wayne, 1983; Porter et al., 1995). In addition, experiments utilizing chickens in developmental studies are faster and more cost-effective than comparable ones in mammals, allowing for adequate statistical sample sizes (“n” values). Although chickens are lateral-eyed animals, they are a valuable experimental model to obtain precise information on extraocular muscle force (Croes et al., 2007; Baryshnikova et al., 2007, submitted). Thus, the chick oculomotor system is a well-suited model for assessing contractile properties in developing and mature extraocular muscles and to evaluate potential therapeutic interventions at the pre-clinical stage.
Acknowledgments
We thank Larisa Baryshnikova for sharing unpublished data, and Sean Ward, William Gerthoffer, Jim Kenyon and Richard Carlsen for critical comments. Our work was supported by NIH grant EY 12841 (CSvB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ameredes BT, Provenzano MA. Regional intramuscular pressure development and fatigue in the canine gastrocnemius muscle in situ. J Appl Physiol. 1997;83:1867–1876. doi: 10.1152/jappl.1997.83.6.1867. [DOI] [PubMed] [Google Scholar]
- Anderson BC, Christiansen SP, Grandt S, Grange RW, McLoon LK. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47:2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Asmussen G, Gaunitz U. Mechanical properties of the isolated inferior oblique muscle of the rabbit. Pflugers Arch. 1981;392:183–190. doi: 10.1007/BF00581270. [DOI] [PubMed] [Google Scholar]
- Asmussen G, Beckers-Bleukx G, Marechal G. The force-velocity relation of the rabbit inferior oblique muscle; influence of temperature. Pflugers Arch. 1994;426:542–547. doi: 10.1007/BF00378532. [DOI] [PubMed] [Google Scholar]
- Badonic T. Electron microscopic changes of synapses after occlusion of the abdominal aorta. Folia Morphol (Praha) 1981;29:136–138. [PubMed] [Google Scholar]
- Barmack NH, Bell CC, Rence BG. Tension and rate of tension development during isometric responses of extraocular muscle. J Neurophysiol. 1971;34:1072–1079. doi: 10.1152/jn.1971.34.6.1072. [DOI] [PubMed] [Google Scholar]
- Baryshnikova LM, Croes SA, von Bartheld CS. Classification and development of myofiber types in chicken extraocular muscles. Anat Rec A Discov Mol Cell Evol Biol. 2007 doi: 10.1002/ar.20614. (submitted) [DOI] [PubMed] [Google Scholar]
- Bach-y-Rita P, Ito F. In vivo studies on fast and slow muscle fibers in cat extraocular muscles. J Gen Physiol. 1966;49:1177–1198. doi: 10.1085/jgp.0491177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A, Clark MG, Henriksen EJ. Experimental approaches in muscle metabolism: hindlimb perfusion and isolated muscle incubations. Am J Physiol. 1994;266:E1–16. doi: 10.1152/ajpendo.1994.266.1.E1. [DOI] [PubMed] [Google Scholar]
- Boonyapisit K, Kaminski HJ, Ruff RL. Disorders of neuromuscular junction ion channels. Am J Med. 1999;106:97–113. doi: 10.1016/s0002-9343(98)00374-x. [DOI] [PubMed] [Google Scholar]
- Chen J, von Bartheld CS. Role of exogenous and endogenous trophic factors in the regulation of extraocular muscle strength during development. Invest Ophthalmol Vis Sci. 2004;45:3538–3545. doi: 10.1167/iovs.04-0393. [DOI] [PubMed] [Google Scholar]
- Cheng G, Merriam AP, Gong B, Leahy P, Khanna S, Porter JD. Conserved and muscle-group-specific gene expression patterns shape postnatal development of the novel extraocular muscle phenotype. Physiol Genomics. 2004;18:184–195. doi: 10.1152/physiolgenomics.00222.2003. [DOI] [PubMed] [Google Scholar]
- Chervu A, Moore WS, Homsher E, Quinones-Baldrich WJ. Differential recovery of skeletal muscle and peripheral nerve function after ischemia and reperfusion. J Surg Res. 1989;47:12–19. doi: 10.1016/0022-4804(89)90041-3. [DOI] [PubMed] [Google Scholar]
- Chiarandini DJ. Curare-like effect of propranolol on rat extraocular muscles. Br J Pharmacol. 1980;69:13–19. doi: 10.1111/j.1476-5381.1980.tb10877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarandini DJ. Effects of epinephrine on resting and tonic tensions of rat extraocular muscles. Curr Eye Res. 1987;6:741–746. doi: 10.3109/02713688709034840. [DOI] [PubMed] [Google Scholar]
- Christiansen SP, Becker BA, Iaizzo PA, McLoon LK. Extraocular muscle force generation after ricin-mAb35 injection: implications for strabismus treatment. J AAPOS. 2003;7:1–6. doi: 10.1016/S1091-8531(03)00056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close RI, Luff AR. Dynamic properties of inferior rectus muscle of the rat. J Physiol. 1974;236:259–270. doi: 10.1113/jphysiol.1974.sp010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croes SA, von Bartheld CS. Development of the neuromuscular junction in extraocular muscles of white Leghorn chicks. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:110–119. doi: 10.1002/ar.a.20155. [DOI] [PubMed] [Google Scholar]
- Croes SA, Baryshnikova LM, Kaluskar SS, von Bartheld CS. Acute and long-term effects of botulinum neurotoxin on the function and structure of developing extraocular muscles. Neurobiol Dis. 2007;25:649–664. doi: 10.1016/j.nbd.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan A. The influence of stimulation frequency on force-velocity characteristics of in situ rat medial gastrocnemius muscle. Exp Physiol. 1998;83:77–84. doi: 10.1113/expphysiol.1998.sp004093. [DOI] [PubMed] [Google Scholar]
- Dimitrova DM, Shall MS, Goldberg SJ. Short-term effects of botulinum toxin on the lateral rectus muscle of cat. Exp Brain Res. 2002;147:449–455. doi: 10.1007/s00221-002-1265-8. [DOI] [PubMed] [Google Scholar]
- Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol. 2003;90:3809–3815. doi: 10.1152/jn.00622.2003. [DOI] [PubMed] [Google Scholar]
- Dyck PJ. Hypoxic neuropathy: does hypoxia play a role in diabetic neuropathy? The 1988 Robert Wartenberg lecture. Neurology. 1989;39:111–118. doi: 10.1212/wnl.39.1.111. [DOI] [PubMed] [Google Scholar]
- Ellsworth ML, Pittman RN. Heterogeneity of oxygen diffusion through hamster striated muscles. Am J Physiol. 1984;246:161–167. doi: 10.1152/ajpheart.1984.246.2.H161. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951;115:320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh BR, Hayes A, Lynch GS, Williams DA. Contractile properties and temperature sensitivity of the extraocular muscles, the levator and superior rectus, of the rabbit. J Physiol. 1994;475:327–336. doi: 10.1113/jphysiol.1994.sp020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh BR, Gregorevic P, Williams DA, Lynch GS. Specific force of the rat extraocular muscles, levator and superior rectus, measured in situ. J Neurophysiol. 2001;85:1027–1032. doi: 10.1152/jn.2001.85.3.1027. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Greenhaff PL, Hamann JJ, Kelley KM, Aschenbach WG, Constantin-Teodosiu D, Gladden LB. Oxygen uptake on-kinetics in dog gastrocnemius in situ following activation of pyruvate dehydrogenase by dichloroacetate. J Physiol. 2002;538:195–207. doi: 10.1113/jphysiol.2001.012984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–10639. doi: 10.1523/JNEUROSCI.18-24-10629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Lennerstrand G. Contractile and histochemical properties of the inferior oblique muscle in the rat and in the cat. Acta Ophthalmol (Copenh) 1977;55:88–102. doi: 10.1111/j.1755-3768.1977.tb06098.x. [DOI] [PubMed] [Google Scholar]
- Haralambie G. Importance of humoral changes to physical performance. In: Keul J, editor. Limiting factors of physical performance. Stuttgart: Thieme; 1973. [Google Scholar]
- Harris K, Walker PM, Mickle DA, Harding R, Gatley R, Wilson GJ, Kuzon B, McKee N, Romaschin AD. Metabolic response of skeletal muscle to ischemia. Am J Physiol. 1986;250:H213–220. doi: 10.1152/ajpheart.1986.250.2.H213. [DOI] [PubMed] [Google Scholar]
- Hatzipantelis KP, Natsis K, Albani M. Effect of acute limb ischaemia on neuromuscular function in rats. Eur J Surg. 2001;167:831–838. doi: 10.1080/11024150152717661. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Wayne DB. Patterns of extraocular innervation by the oculomotor complex in the chick. J Comp Neurol. 1983;216:245–252. doi: 10.1002/cne.902160303. [DOI] [PubMed] [Google Scholar]
- Hoch JR, Stevens RP, Keller MP, Silver D. Recovery of neuromuscular function during reperfusion of the ischemic extremity: effect of mannitol and superoxide dismutase. Surgery. 1991;110:656–563. [PubMed] [Google Scholar]
- Hudlicka O, Pette D, Staudte H. The relation between blood flow and enzymatic activities in slow and fast muscles during development. Pflugers Arch. 1973;343:341–356. doi: 10.1007/BF00595821. [DOI] [PubMed] [Google Scholar]
- Hummel RP, 3rd, Hasselgren PO, James JH, Warner BW, Fischer JE. The effect of sepsis in rats on skeletal muscle protein synthesis in vivo and in periphery and central core of incubated muscle preparations in vitro. Metabolism. 1988;37:1120–1127. doi: 10.1016/0026-0495(88)90187-4. [DOI] [PubMed] [Google Scholar]
- Jacoby J, Chiarandini DJ, Stefani E. Electrical properties and innervation of fibers in the orbital layer of rat extraocular muscles. J Neurophysiol. 1989;61:116–125. doi: 10.1152/jn.1989.61.1.116. [DOI] [PubMed] [Google Scholar]
- Koch T. In: Anatomy of the Chicken and Domestic birds. Skold Bernard H, Louis DeVries., editors. The Iowa State University Press; Ames, Iowa: 1973. Illustrated by Erwin Rossa. [Google Scholar]
- Korthals JK, Maki T, Gieron MA. Nerve and muscle vulnerability to ischemia. J Neurol Sci. 1985;71:283–290. doi: 10.1016/0022-510x(85)90066-8. [DOI] [PubMed] [Google Scholar]
- Lapointe BM, Cote CH. Anesthetics can alter subsequent in vitro assessment of contractility in slow and fast skeletal muscles of rat. Am J Physiol. 1999;277:917–921. doi: 10.1152/ajpregu.1999.277.3.R917. [DOI] [PubMed] [Google Scholar]
- Luff AR. Dynamic properties of the inferior rectus, extensor digitorum longus, diaphragm and soleus muscles of the mouse. J Physiol. 1981;313:161–171. doi: 10.1113/jphysiol.1981.sp013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G. Ischemic nerve injury: experimental studies on intraneural microvascular pathophysiology and nerve function in a limb subjected to temporary circulatory arrest. Scand J Plast Reconstr Surg. 1970;6(Suppl):3–113. [PubMed] [Google Scholar]
- Ma J, Elsaidi GA, Smith TL, Walker FO, Tan KH, Martin E, Koman LA, Smith BP. Time course of recovery of juvenile skeletal muscle after botulinum toxin A injection: an animal model study. Am J Phys Med Rehabil. 2004;83:774–780. doi: 10.1097/01.phm.0000137315.17214.93. [DOI] [PubMed] [Google Scholar]
- Maier A, Eldred E, Edgerton VR. Types of muscle fibers in the extraocular muscles of birds. Exp Eye Res. 1972;13:255–265. doi: 10.1016/0014-4835(72)90107-8. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Christiansen SP. Increasing extraocular muscle strength with insulin-like growth factor II. Invest Ophthalmol Vis Sci. 2003;44:3866–3872. doi: 10.1167/iovs.03-0223. [DOI] [PubMed] [Google Scholar]
- McLoon LK, Anderson BC, Christiansen SP. Increasing muscle strength as a treatment for strabismus: sustained release of insulin-like growth factor-1 in rabbit extraocular muscle. J AAPOS. 2006;10:424–429. doi: 10.1016/j.jaapos.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltin CA, Harris CI. Morphological observations and rates of protein synthesis in rat muscles incubated in vitro. Biochem J. 1985;232:927–930. doi: 10.1042/bj2320927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JS, Goldberg SJ, McClung JR. Motoneuron electrophysiological and muscle contractile properties of superior oblique motor units in cat. J Neurophyiol. 1986;35:715–726. doi: 10.1152/jn.1986.55.4.715. [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Leighton B, Challiss RA, Lozeman FJ. Assessment of biochemical viability of isolated incubated muscle preparations. Biochem J. 1986;238:621–622. doi: 10.1042/bj2380621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E. In: Anatomy of the Domestic Birds. Siller WG, Wight PAL, translators. Berlin; Hamburg: Parey; 1977. [Google Scholar]
- Pagala MK, Namba T, Grob D. Failure of neuromuscular transmission and contractility during muscle fatigue. Muscle Nerve. 1984;7:454–464. doi: 10.1002/mus.880070607. [DOI] [PubMed] [Google Scholar]
- Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39:451–484. doi: 10.1016/s0039-6257(05)80055-4. [DOI] [PubMed] [Google Scholar]
- Ruff RL. More than meets the eye: extraocular muscle is very distinct from extremity skeletal muscle. Muscle Nerve. 2002;25:311–313. doi: 10.1002/mus.10063. [DOI] [PubMed] [Google Scholar]
- Ruff RL, Lennon VA. End-plate voltage-gated sodium channels are lost in clinical and experimental myasthenia gravis. Ann Neurol. 1998;43:370–379. doi: 10.1002/ana.410430315. [DOI] [PubMed] [Google Scholar]
- Sandow A, Isaacson A. Topochemical factors in potentiation of contraction by heavy metal cations. J Gen Physiol. 1966;49:937–961. doi: 10.1085/jgp.49.5.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid KL, Wildsoet CF. Assessment of visual acuity and contrast sensitivity in the chick using an optokinetic nystagmus paradigm. Vision Res. 1998;38:2629–2634. doi: 10.1016/s0042-6989(97)00446-x. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985;248:265–270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA, White TP. Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol. 1986;61:660–665. doi: 10.1152/jappl.1986.61.2.660. [DOI] [PubMed] [Google Scholar]
- Shall MS, Goldberg SJ. Extraocular motor units: type classification and motoneuron stimulation frequency-muscle unit force relationships. Brain Res. 1992;587:291–300. doi: 10.1016/0006-8993(92)91010-c. [DOI] [PubMed] [Google Scholar]
- Shall MS, Dimitrova DM, Goldberg SJ. Extraocular motor unit and whole-muscle contractile properties in the squirrel monkey. Summation of forces and fiber morphology. Exp Brain Res. 2003;151:338–345. doi: 10.1007/s00221-003-1506-5. [DOI] [PubMed] [Google Scholar]
- Spencer RF, Porter JD. Structural organization of the extraocular muscles. In: Buttner-Ennever JA, editor. Neuroanatomy of the oculomotor system. New York: Elsevier Science; 1988. pp. 33–79. [PubMed] [Google Scholar]
- Stelling J, McVean A. The contractile properties and movement dynamics of pigeon eye muscle. Pflugers Arch. 1988;412:314–321. doi: 10.1007/BF00582514. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Kandarian SC. Advantage of normalizing force production to myofibrillar protein in skeletal muscle cross-sectional area. J Appl Physiol. 1994;76:974–978. doi: 10.1152/jappl.1994.76.2.974. [DOI] [PubMed] [Google Scholar]
- Uchiyama H. Centrifugal pathways to the retina: influence of the optic tectum. Vis Neurosci. 1989;3:183–206. doi: 10.1017/s0952523800009950. [DOI] [PubMed] [Google Scholar]
- Van Breda E, Keizer HA, Glatz JF, Geurten P. Use of the intact mouse skeletal-muscle preparation for metabolic studies. Evaluation of the model. Biochem J. 1990;267:257–260. doi: 10.1042/bj2670257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PM. Ischemia/reperfusion injury in skeletal muscle. Ann Vasc Surg. 1991;5:399–402. doi: 10.1007/BF02015307. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Reis DJ. Blood flow in extraocular muscle of cat. Arch Neurol. 1972;26:350–352. doi: 10.1001/archneur.1972.00490100080008. [DOI] [PubMed] [Google Scholar]
- Zollman PJ, Awad O, Schmelzer JD, Low PA. Effect of ischemia and reperfusion in vivo on energy metabolism of rat sciatic-tibial and caudal nerves. Exp Neurol. 1991;114:315–320. doi: 10.1016/0014-4886(91)90157-8. [DOI] [PubMed] [Google Scholar]


