Abstract
Objective:
Whether total and regional adiposity measured by anthropometry and radiographic studies influences cognitive decline in older adults and whether this association is explained by hormones and inflammatory factors known to be secreted by adipose tissue.
Design:
Prospective cohort study
Setting:
Two clinical centers; Pittsburgh, PA and Memphis, TN
Participants:
3,054 elders enrolled in the Health, Aging and Body Composition Study. Adiposity measures included BMI, waist circumference, sagittal diameter, total fat mass by DEXA, subcutaneous and visceral fat by abdominal CT. We examined the association between baseline body fat measures and change in 3MS score, sequentially adjusting for confounding and mediating variables including comorbid diseases, adipocytokines, and sex hormones.
Main Outcome Measure:
The Modified Mini-Mental State Examination (3MS) administered at the first, 3rd, 5th, and 8th annual clinical examination.
Results:
All baseline adiposity measures varied significantly by sex. In mixed effects models, the association between total and regional adiposity and change in 3MS score varied significantly by sex, with the highest adiposity tertile being associated with greater cognitive declines in men (p<0.05 for each adiposity measure) but not in women (p-for-interaction<0.05). Total fat mass was significantly associated with greater change in 3MS score among men (lowest tertile −1.5, middle tertile −2.4, highest tertile −2.6, p-for-trend=0.005) even after adjusting for mediators.
Conclusions:
Higher levels of all adiposity measures were associated with worsening cognitive function in men after controlling for metabolic disorders, adipocytokines and sex hormone levels. Conversely, there was no association between adiposity and cognitive change in women.
Background
Most nations are facing growing rates of overweight and obesity with latest global projections from the World Health Organization of approximately 1.6 billion overweight and 400 million obese adults1. Well recognized adverse effects of overweight include type 2 diabetes, hypertension, and cardiovascular disease.
Obesity has also been associated with risk of developing of dementia after accounting for cardiovascular risk factors such as hypertension and diabetes2, 3. Overweight has been associated with cerebral atrophy4 and cerebral white matter lesions5. Less is known about the effect of adiposity on rates of cognitive change in non-demented elders. Moreover, most studies use surrogate measures of adiposity such as BMI6, 7 or waist circumference8 which may be less valid in older populations9 than direct measurements of fat mass with whole body DEXA. Abdominal visceral fat measured by CT is closely associated with metabolic disorders such as diabetes, but no prior study has examined radiographically measured regional fat depots and their effect on change in cognitive function. While prior studies have found that inflammatory factors are independently association with cognitive decline10, no studies to date have examined adipose-derived hormones, or adipocytokines, on cognitive function and whether they explain the effect of adiposity on cognitive change. Therefore it is unclear whether the body weight associations observed are due to total fat mass, specific fat depots, or fat-derived hormones.
We analyzed data from participants enrolled in the Health, Aging and Body Composition (Health ABC) study to examine associations between baseline measures of overall and regional adiposity and change in cognitive function. We examined whether adjusting for potentially mediating diseases, adipocytokines, and sex hormones would explain the risk associated with adiposity and cognitive change.
Methods
Participants enrolled in the Health ABC study were well-functioning men and women between the ages of 70 and 79 years who were recruited from April 1997 to June 1998 from Pittsburgh, Pennsylvania and Memphis, Tennessee. To be eligible, participants had to report no difficulty in walking ¼ mile, climbing 10 steps, or performing activities of daily living. Individuals requiring assistive ambulation devices or life-threatening cancers were excluded.
We used the baseline medical history, physical exam measurements, laboratory tests, radiographic assessments and cognitive function tests gathered in 1997-1998. Of the 3,075 participants enrolled in Health ABC, we excluded 21 because they did not have any measurements of total adiposity and another 7 individuals who were missing all 3MS test results at the four time-points resulting in 3,054 in our analytic cohort.
The study was approved by the institutional review boards of the University of California, San Francisco, University of Pittsburgh, and University of Tennessee. All of the study participants provided written informed consent.
Weight was measured on a standard balance beam scale and height was measured by a stadiometer. BMI was calculated as body weight in kg divided by height in meters squared. Total fat mass was measured by whole body Dual X-ray Absorptiometry (DXA) scan (QDR 4500A, Hologic, Waltham, MA) and analyzed by tertile. We evaluated four measures of regional adiposity, two anthropometric and two radiographic. Waist circumference was measured with a flexible tape measure at the level of the largest circumference. Abdominal sagittal diameter was measured by a Holtain–Kahn abdominal caliper while the participant lay supine. The lower blade of the calliper was placed under the small of the back and the upper blade was lowered to a mark midway between the iliac crests. Abdominal visceral and subcutaneous fat area were measured by computed tomography (CT) scans using Somatom Plus 4 (Siemens, Erlangen, Germany), Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH), or a 9800 Advantage scanner (General Electric, Milwaukee, WI). Visceral fat and subcutaneous abdominal fat was measured at the L4-L5 level. Fat areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using ILD development software (RSI Systems, Boulder, CO).
Cognitive function assessment
The Modified Mini-Mental State Examination (3MS) was administered to all participants during the baseline visit (Year 1) and repeated at the 3rd, 5th, and 8th annual examinations. This test is a brief general cognitive test with components for orientation, concentration, language, praxis, and immediate and delayed memory with a maximum score of 10011. The 3MS test is more sensitive than the 30-point Mini-Mental State Examination, especially for mild cognitive change11. We examined cognitive decline by using the change in 3MS score from the baseline examination to the eighth follow-up exam.
Covariates and explanatory factors
Racial group, age, sex, education, and smoking information was obtained. Physical activity was assessed using self-report of walking and exercise assigning kcal/week to activities. Literacy was assessed during the second annual visit using the Rapid Estimate of Adult Literacy in Medicine (REALM); scores <60 were considered as limited literacy12. Depressive symptoms were measured using the Center for Epidemiologic Study Depression Scale (CES-D)13. Each participant had seated systolic blood pressures measured by a manual sphygmomanometer. Diabetes was defined by self-report of diabetes diagnosis, use of diabetes drug, or if fasting plasma glucose ≥126 mg/dl or 2-hour post-challenge glucose ≥200 mg/dl.
Participants had venipuncture after an overnight fast. Serum samples were frozen at −70°C. Fasting lipoproteins (Johnson & Johnson, Vitros chemical methodology), fasting and 2-hour plasma glucose (YSI 2300 Glucose Analyzer, Yellow Springs, OH) were measured. Apolipoprotein E (ApoE) allele genotypes were assessed and coded as presence or not of e4 allele14. Serum creatinine was measured using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY) and estimated glomerular filtration rate (eGFR) was calculated15.
We evaluated four adipocytokines (adiponectin, interleukin (IL)-6, tumor necrosis factor (TNF)-α, and plasminogen activator inhibitor (PAI)-1). Adiponectin was measured in duplicate by radioimmunoassay (Linco Research, St. Charles, MO). IL-6 and TNF-α were measured in duplicate by ELISA (R&D Systems, Minneapolis, MN). PAI-1 was measured by a two-site ELISA (Collen laboratory).
At baseline, total testosterone was measured by a chemiluminescent immunoassay (Diagnostic Products Corporation, Los Angeles, CA). At the third annual examination bioavailable estradiol was measured by radioimmunoassay (EIR, Wurenlingen, Switzerland). All samples were measured in duplicate.
Statistical analyses
We used χ2 and ANOVA to examine whether baseline characteristics were associated with sex-specific total fat mass tertile. We evaluated the distribution of each overall adiposity and regional adiposity measure separately by sex and race.
We determined the unadjusted association between sex-specific tertiles of each adiposity measure with change in 3MS score between baseline and the seventh annual clinical examination and tested for sex and race interaction. Since 3MS scores were negatively skewed, we used the Box-Cox method to find an appropriate transformation16. Since we found an interaction by sex with all adiposity measures, we stratified subsequent models by sex.
We used mixed effects models with random participant-specific intercepts and slopes and an unstructured covariance matrix, allowing the use of all available data without imputation of any missing values. We adjusted for the fixed effects of time in years from the baseline cognitive measurement, potential baseline confounders, and their interactions with time. Baseline cognitive score was included in every model. Cognitive scores were obtained from each model for every time point and tertile and back-transformed to the 3MS scale. Change scores were calculated by subtracting the score at baseline from the score at the final time point, and the standard errors were calculated by bootstrapping the resulting change scores with 5000 replications17. We adjusted for potential confounders in separate stages. First, we adjusted for demographic variables: age, race, education, physical activity, and literacy. We then added chronic risk factors: diabetes and systolic blood pressure. Next, we added adipocytokines to the model to determine whether they would explain the relationship between adiposity and cognitive change. In an exploratory analysis, we further adjusted for endogenous sex hormones.
Lastly, we performed a sensitivity analysis by excluding participants with involuntary weight loss of ≥5% from baseline weight through the 8th clinical examination.
Statistical analyses were performed using Stata (version 9, StataCorp, College Station, TX).
Results
Of the 3,054 participants, men had lower total fat mass (24.2 vs. 29.2 kg, p<0.001), BMI (27.1 vs. 27.7 kg/m2, p<0.001), and subcutaneous fat area (228 vs. 339 cm2, p<0.001) and higher abdominal visceral fat area (155 vs. 131 cm2, p<0.001) compared to women.(Table 1)
Table 1.
Distribution of adiposity measures by sex among the 3,054 Health ABC participants
| Adiposity Measure Tertile* | ||||
|---|---|---|---|---|
| Adiposity Measure | Sex | Lowest tertile | Middle tertile | Highest tertile |
| Overall adiposity measures: mean (SD) | ||||
| Total fat mass (kg) | Men | 16.9 (3.0) | 23.4 (1.6) | 32.2 (5.1) |
| Women | 19.6 (3.6) | 28.3 (2.2) | 39.6 (6.6) | |
| BMI (kg/m2) | Men | 22.9 (1.7) | 27.2 (1.4) | 32.8 (2.6) |
| Women | 22.1 (2.0) | 27.4 (1.4) | 34.5 (3.7) | |
| Regional adiposity measures: mean (SD) | ||||
| Waist circumference (cm) | Men | 89.0 (8.0) | 100.4 (2.5) | 112.9 (6.9) |
| Women | 83.6 (6.6) | 97.8 (3.1) | 113.2 (9.0) | |
| Sagittal diameter (cm) | Men | 19.3 (1.2) | 22.4 (0.8) | 26.2 (2.0) |
| Women | 18.2 (1.4) | 21.7 (0.8) | 25.8 (2.1) | |
| Abdominal visceral (cm2) | Men | 84.6 (24.1) | 144.1 (16.0) | 235.2 (53.3) |
| Women | 71.0 (19.5) | 122.8 (14.1) | 199.9 (43.6) | |
| Abdominal subcutaneous (cm2) | Men | 140.0 (35.6) | 219.3 (20.6) | 326.1 (63.6) |
| Women | 210.5 (52.7) | 327.1 (30.1) | 477.6 (79.7) | |
Sex-specific tertiles for fat mass, waist circumference, sagittal diameter, abdominal visceral and subcutaneous fat. BMI groups are <25, 25-30, and ≥30 kg/m2
Higher total fat mass tertile was associated with higher proportions of Black race, lower educational attainment and low literacy level primarily in women. (Table 2) Diabetes was significantly associated with increasing tertile of fat mass in both men and women. Most adipocytokines in both men and women were significantly associated with fat mass; adiponectin was inversely associated with increased fat mass. Baseline 3MS score was not significantly associated with fat mass tertile in the overall population. However, the association with baseline 3MS and fat mass did vary significantly by sex (p-for-interaction <0.001), with men having a trend toward a positive association (lowest fat mass tertile: mean 3MS score 88.5±9.4, middle: 89.6±8.3, top tertile: 90.4±7.7) and women having an inverse association between fat mass and baseline 3MS score (lowest tertile: 91.2±7.8, middle: 91.2±7.5, top tertile 89.9±7.3).
Table 2.
Characteristics of men and women by total fat mass tertile
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Lowest (n=493) |
Middle (n=493) |
Highest (n=493) |
p-value* | Lowest (n=525) |
Middle (n=525) |
Highest (n=525) |
p-value* |
| Age, years | 73.9 (2.9) | 73.9 (2.8) | 73.6 (2.8) | 0.13 | 73.9 (3.0) | 73.5 (2.8) | 73.1 (2.8) | <0.001 |
| Black race, % | 43 | 35 | 33 | 0.002 | 34 | 43 | 61 | <0.001 |
| Education <high school, % | 29 | 27 | 25 | 0.36 | 18 | 20 | 31 | <0.001 |
| REALM level <9th grade, % | 28 | 30 | 27 | 0.69 | 17 | 16 | 26 | <0.001 |
| Self-rated health fair/poor, % | 17 | 14 | 18 | 0.17 | 11 | 16 | 21 | <0.001 |
| Physical activity†, kcal/week | 1411 (2409) | 1460 (2463) | 1388 (2140) | 0.10 | 723 (1069) | 689 (1266) | 703 (1504) | 0.03 |
| Current smoker, % | 16 | 10 | 6 | <0.001 | 14 | 10 | 7 | <0.001 |
| >1 alcoholic drink/day, % | 12 | 12 | 12 | 0.99 | 5 | 3 | 2 | 0.06 |
| Apo E4 carrier, % | 28 | 29 | 26 | 0.58 | 33 | 27 | 31 | 0.10 |
| Estimated GFR, ml/min/1.73m2 | 75 (19) | 73 (16) | 74 (16) | 0.31 | 72 (16) | 71 (15) | 72 (17) | 0.51 |
| Systolic blood pressure, mmHg | 135 (21) | 135 (21) | 135 (21) | 0.89 | 135 (21) | 136 (21) | 138 (21) | 0.03 |
| Comorbid Disorders: % | ||||||||
| Depression score ≥16 | 4 | 2 | 5 | 0.16 | 5 | 7 | 5 | 0.55 |
| Hypertension | 57 | 60 | 62 | 0.25 | 58 | 67 | 74 | <0.001 |
| Diabetes | 21 | 27 | 33 | <0.001 | 14 | 22 | 28 | <0.001 |
| Myocardial infarction | 14 | 18 | 15 | 0.21 | 8 | 9 | 7 | 0.71 |
| Stroke/TIA | 9 | 8 | 7 | 0.71 | 8 | 10 | 7 | 0.14 |
| Adipocytokines† | ||||||||
| Adiponectin, μg/mL | 10.8 (6.3) | 9.1 (5.1) | 8.4 (4.9) | <0.001 | 16.4 (8.0) | 12.3 (6.4) | 11.0 (6.2) | <0.001 |
| PAI-1, ng/mL | 19.7 (17.3) | 26.0 (20.2) | 34.2 (23.5) | <0.001 | 20.8 (20.3) | 31.8 (24.3) | 26.5 (27.5) | <0.001 |
| IL-6, pg/mL | 2.5 (2.1) | 2.2 (1.6) | 2.6 (1.8) | 0.01 | 2.0 (1.8) | 2.2 (1.7) | 2.8 (2.2) | <0.001 |
| TNF-α, pg/mL | 3.5 (1.7) | 3.5 (1.4) | 3.7 (1.6) | 0.08 | 3.2 (1.6) | 3.4 (1.4) | 3.5 (1.5) | <0.001 |
| Sex hormones: | ||||||||
| Total Testosterone, pg/mL† | 8.5 (3.8) | 8.4 (3.7) | 8.2 (4.7) | 0.19 | 3.0 (1.8) | 3.4 (3.1) | 3.6 (2.2) | <0.001 |
| Estradiol, pg/mL† | 32.1 (13.9) | 30.6 (12.8) | 30.8 (16.2) | 0.60 | 20.8 (31.2) | 16.7 (27.0) | 19.9 (23.7) | 0.01 |
| Baseline 3MS score | 88.5 (9.4) | 89.6 (8.3) | 90.4 (7.7) | 0.09 | 91.2 (7.8) | 91.2 (7.5) | 89.9 (7.3) | <0.001 |
values presented are mean (SD) or % when indicated; p-value by chi-square test for dichotomous variables and ANOVA for continuous variables.
transformed for statistical comparison
In Table 3, we show the predicted change in 3MS score between the baseline and eighth annual examination by each adiposity measure in tertiles separately for men and women. In unadjusted analyses, higher tertiles of total fat mass, sagittal diameter and subcutaneous fat were associated with greater change in 3MS scores in men (total fat tertile 1: −1.5; tertile 2: −2.5; tertile 3: −2.6; p-for-trend 0.009). Conversely, among women, higher tertiles of total fat mass and BMI were associated with less change in 3MS score. But higher waist circumference tertiles was also associated with greater change in 3MS score in women. In analyses adjusted for age, race, education, literacy, hypertension, diabetes, IL-6, TNF-α, PAI-1 and adiponectin, there were no significant associations between baseline adiposity measures and change in 3MS score in women. However higher tertiles of total fat mass, BMI, waist circumference, sagittal diameter and subcutaneous fat were all associated with significantly greater change in 3MS score in men even after adjustment for covariates and potential mediators. There was a similar trend towards greater change in 3MS score with increasing tertile of abdominal visceral fat in men. Race did not modify the association between adiposity measures and change in 3MS score.
Table 3.
Predicted change in 3MS scores between baseline and eighth annual examination in men and women
| MEN | WOMEN | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome: | Model | Low Mean (SE*) |
Middle Mean (SE*) |
High Mean (SE*) |
p† | Low Mean (SE*) |
Middle Mean (SE*) |
High Mean (SE*) |
p† |
| Total fat mass, kg | Unadjusted | −1.61 (0.14) | −2.28 (0.13) | −2.66 (0.14) | 0.009 | −3.11 (0.18) | −2.39 (0.15) | −2.35 (0.16) | 0.033 |
| MV-adjusted‡ | −1.55 (0.15) | −2.35 (0.16) | −2.72 (0.16) | 0.004 | −2.88 (0.2) | −2.22 (0.16) | −2.05 (0.15) | 0.054 | |
| Fully adjusted | −1.65 (0.17) | −2.19 (0.16) | −2.69 (0.17) | 0.006 | −2.87 (0.24) | −2.13 (0.19) | −2.2 (0.18) | 0.612 | |
| BMI, kg/m2 | Unadjusted | −1.83 (0.14) | −2.25 (0.12) | −2.53 (0.15) | 0.138 | −3.03 (0.19) | −2.37 (0.14) | −2.37 (0.16) | 0.015 |
| MV-adjusted‡ | −1.76 (0.17) | −2.31 (0.14) | −2.6 (0.18) | 0.077 | −2.71 (0.2) | −2.29 (0.15) | −2.04 (0.17) | 0.013 | |
| Fully adjusted** | −1.77 (0.17) | −2.2 (0.16) | −2.64 (0.19) | 0.041 | −2.76 (0.22) | −2.2 (0.18) | −2.12 (0.19) | 0.155 | |
| Waist | Unadjusted | −1.75 (0.14) | −2.12 (0.13) | −2.68 (0.15) | 0.029 | −2.52 (0.17) | −2.25 (0.12) | −3.04 (0.17) | 0.673 |
| circumference, cm | MV-adjusted‡ | −1.73 (0.16) | −2.14 (0.14) | −2.75 (0.18) | 0.031 | −2.32 (0.19) | −2.06 (0.13) | −2.73 (0.19) | 0.937 |
| Fully adjusted** | −1.79 (0.18) | −2.05 (0.15) | −2.67 (0.18) | 0.032 | −2.15 (0.22) | −2.02 (0.16) | −2.94 (0.22) | 0.273 | |
| Sagittal | Unadjusted | −1.75 (0.13) | −2.2 (0.14) | −2.62 (0.15) | 0.092 | −3.01 (0.19) | −2.49 (0.15) | −2.22 (0.14) | 0.013 |
| diameter, cm | MV-adjusted‡ | −1.7 (0.16) | −2.23 (0.16) | −2.71 (0.16) | 0.058 | −2.92 (0.21) | −2.28 (0.16) | −1.84 (0.15) | 0.002 |
| Fully adjusted** | −1.73 (0.17) | −2.11 (0.16) | −2.68 (0.18) | 0.031 | −2.81 (0.24) | −2.26 (0.19) | −1.96 (0.17) | 0.116 | |
| Visceral fat, cm2 | Unadjusted | −2 (0.16) | −2.07 (0.14) | −2.47 (0.13) | 0.079 | −3.07 (0.18) | −2.27 (0.15) | −2.35 (0.15) | 0.212 |
| MV-adjusted‡ | −2.07 (0.18) | −2.07 (0.16) | −2.48 (0.14) | 0.064 | −2.95 (0.2) | −2.12 (0.17) | −1.98 (0.14) | 0.055 | |
| Fully adjusted** | −2.11 (0.19) | −1.98 (0.17) | −2.37 (0.15) | 0.095 | −2.91 (0.24) | −2.03 (0.2) | −2.12 (0.17) | 0.521 | |
| Subcutaneous fat, | Unadjusted | −1.21 (0.12) | −2.13 (0.14) | −3.23 (0.17) | <0.001 | −2.74 (0.19) | −2.27 (0.13) | −2.66 (0.15) | 0.246 |
| cm2 | MV-adjusted‡ | −1.24 (0.14) | −2.1 (0.15) | −3.36 (0.19) | <0.001 | −2.55 (0.2) | −2.12 (0.14) | −2.33 (0.19) | 0.226 |
| Fully adjusted** | −1.31 (0.16) | −2.03 (0.16) | −3.23 (0.21) | <0.001 | −2.48 (0.24) | −2.05 (0.17) | −2.51 (0.22) | 0.910 | |
Standard error, estimated by bootstrap sampling with 1000 replications.
p for trend across tertiles.
MV (multivariate) adjustment for age, race, education, literacy (in women), physical activity, diabetes, systolic blood pressure
Further adjusted the multivariate model for IL-6, TNF-α, PAI-1, adiponectin
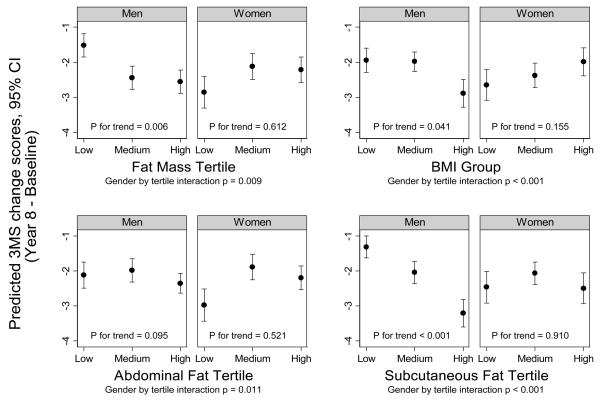
The figure shows the adjusted change in 3MS score between baseline and eighth annual examination for each of the four adiposity measures. Sex significantly modified the association between adiposity and change in 3MS score (p-for-interaction<0.001 for total fat mass, BMI, and abdominal subcutaneous fat; p=0.01 for visceral fat). To determine whether endogenous sex hormones may explain this sex interaction, we further adjusted for total testosterone or bioavailable estradiol. The interaction between sex and each adiposity measure remained robust (p-for-interaction=0.003 for total fat mass and BMI; p=0.005 for abdominal subcutaneous fat; and p=0.05 for visceral fat).
Figure.
Tertiles of each adiposity measure by predicted 3MS change scores (adjusted for age, race, education, literacy (in women), physical activity, systolic blood pressure, diabetes, IL-6, TNF-α, PAI-1, adiponectin)
Finally, the exclusion of 676 individuals who lost a significant amount of body weight unintentionally, did not affect our results.
Comment
In this cohort of well-functioning older adults, higher tertiles of radiographically measured total fat mass and subcutaneous fat were associated with worsening cognitive function after 7 years among men alone. This association remained significant even after adjusting for potential explanatory links between adiposity and cognitive function including metabolic risk factors and adipocytokines. We found a striking paradoxical sex interaction with increasing adiposity measures showing trends towards less cognitive change among women but with greater cognitive change among men. This sex interaction was consistent with all adiposity variables and remained significant even after adjustment for metabolic variables and sex hormone levels.
Several studies have examined the effect of overweight on cognitive function2, 6, 7. Some longitudinal studies have found that higher BMI is associated with increased risk of developing dementia18-21 while others have found no association22-24. Fewer studies have evaluated the association between adiposity and cognitive function or decline in non-demented adults. We found two prospective studies that evaluated the effect of BMI on cognitive function6, 7. The first followed 1,423 individuals in the Framingham Heart Study for 4-6 years and found that higher BMI was associated with worse cognitive function scores in men alone6, 25, with a significant interaction between obesity and sex (p<0.02). The second study was performed in 5,607 Danish postmenopausal women followed for 7 years and examined baseline body weight, yearly change in weight and central fat mass by DXA7. They found a protective association of body fat mass with cognitive impairment in elderly women and showed that women who lost the most weight had the worst cognitive performance at follow-up7. Neither of these studies had baseline measures of cognitive function or more precise measures of regional adiposity. Our findings that men with higher total fat mass have greater cognitive decline is consistent with the Framingham results. And our finding that women have a trend towards inverse associations with total fat mass and cognitive change is consistent with the Danish study. We have extended the literature by finding that total body fat and subcutaneous abdominal fat are the two specific adiposity measures that have the strongest effect on cognitive change in men.
Less literature exists evaluating the effects of regional adiposity on change in cognitive function. Two longitudinal studies have examined the effect of central adiposity using either waist-hip ratio (WHR) or sagittal abdominal diameter with cognition. The first from the Framingham Offspring Study found that the individuals in the uppermost quartile of WHR had significantly poorer performance on executive function and visuomotor skills testing 12 years later8. The second study followed 6,583 members of Kaiser Permanente over 36 years and found that those in the highest quintile of sagittal abdominal diameter had a three-fold increased risk of dementia independently of BMI and other cardiovascular risk factors3. The present study is the first to more closely examine regional adiposity measured radiographically. Surprisingly, our direct measure of visceral fat which has been most closely tied to poor metabolic outcomes was only of borderline significance in association with cognitive change. The anthropometric measures of visceral fat in our study (waist and sagittal diameter) showed stronger associations with cognitive change in men alone. Since the previous studies have found associations only in the highest quartile or quintile of each anthropometric measurement, it is possible that their findings correspond to higher levels of overall obesity rather than visceral adiposity per se.
We were able to evaluate the effect of many potential mediators that may lie in the causal pathway between adiposity and change in cognition. We adjusted for diabetes and blood pressure and novel fat secreted hormones and inflammatory factors26. The observed sex difference appeared strong and consistent for all fat measures, and was only slightly attenuated with additional adjustment for adipocytokines and metabolic variables such as HDL, insulin, and triglycerides. Endogenous sex hormones did not mediate this sex interaction. Other unmeasured metabolic and disease differences such as the severity of hepatic or peripheral insulin resistance, intramyocellular steatosis, or newer adipocyte hormones may provide further mechanistic links to explain this sex interaction.
There are several possible biologic mechanisms that link adipose tissue to cognitive impairment. Some have proposed that adiposity in fetal development may influence cerebrovascular function and dementia risk27, 28. Secondly, adipose tissue hormones that cross the blood-brain barrier may influence brain function and health by impacting energy balance mechanisms and memory29, 30. Another possible mechanism includes intrinsic differences in brain structure and function that can influence adiposity through energy homeostasis, reward, and other behavioral pathways28.
While our study stands apart from others with radiographically measured adiposity, adipocytokines, and repeat measures of cognitive function, we cannot determine if there would be different effects on tests of other cognitive domains. We are unable to determine whether the cognitive change that occurred was due to underlying Alzheimer's disease or vascular dementia processes. While we did not exclude participants with clinical dementia for the Health ABC study, our cohort may represent healthy survivors since they had no evidence of significant physical disability during the baseline examination, and it is possible that effect of adiposity with cognition may differ in other more frail individuals.
In conclusion, increasing levels of total fat mass, BMI, waist circumference, sagittal diameter and subcutaneous abdominal fat are strongly associated with worsening cognitive function in men after controlling for metabolic disorders and adipocytokines. A more direct measure of visceral fat was not significantly associated with cognitive change. Women have trends towards inverse associations with higher levels of adiposity being associated with less cognitive change. Traditional metabolic factors, adipocytokines and sex hormones do not explain this sex difference. Future studies should confirm these longitudinal associations with adiposity and cognitive change and investigate why adiposity has inverse associations for men and women.
Acknowledgments
Dr. Alka Kanaya had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Alka Kanaya was funded by K23-HL080026 and R21-DK068608. Kristine Yaffe was supported in part by R01-AG021918. The Health ABC study was funded via contracts with the National Institute on Aging contract #s: N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and included substantial involvement of NIA staff in data collection, analysis, interpretation, review, and approval of the manuscript.
Footnotes
Potential Conflict of Interest: none
References
- 1.World Health Organization Obesity and Overweight (WHO Fact Sheet No. 311) http://www.who.int/mediacentre/factsheets/fs311/en/index.html. Accessed December 18, 2007.
- 2.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007 Jan;36(1):23–29. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008 doi: 10.1212/01.wnl.0000306313.89165.ef. Epub March 26, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Gustafson D, Lissner L, Bengtsson C, Bjorkelund C, Skoog I. A 24-year follow-up of body mass index and cerebral atrophy. Neurology. 2004 Nov 23;63(10):1876–1881. doi: 10.1212/01.wnl.0000141850.47773.5f. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson DR, Steen B, Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int Psychogeriatr. 2004 Sep;16(3):327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- 6.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003 Feb;27(2):260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 7.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes Res. 2004 Sep;12(9):1519–1526. doi: 10.1038/oby.2004.189. [DOI] [PubMed] [Google Scholar]
- 8.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007 Apr;4(2):111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 9.Harris TB, Visser M, Everhart J, et al. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women. The Health, Aging and Body Composition Study. Ann N Y Acad Sci. 2000;904:462–473. doi: 10.1111/j.1749-6632.2000.tb06501.x. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004 Aug 24;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 11.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 12.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 13.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;(1):385–401. [Google Scholar]
- 14.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 15.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 16.Box GEP, Cox DR. An analysis of transformations. . (Series B).Jo Royal Statist Soc. 1964;26:211–243. [Google Scholar]
- 17.Efron B. Bootstrap methods: Another look at the jackknife. Annals of Statistics. 1979;7(1):1–26. [Google Scholar]
- 18.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr., Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. Bmj. 2005 Jun 11;330(7504):1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosengren A, Skoog I, Gustafson D, Wilhelmsen L. Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med. 2005 Feb 14;165(3):321–326. doi: 10.1001/archinte.165.3.321. [DOI] [PubMed] [Google Scholar]
- 20.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch Intern Med. 2003 Jul 14;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 21.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000 Oct;20(10):2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 22.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005 Oct;62(10):1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 23.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003 Jan 14;60(1):117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 24.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45(6):1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 25.Elias MF, Elias PK, Sullivan LM, Wolf PA, D'Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005 Dec;26(Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 27.Finch CE. Developmental origins of aging in brain and blood vessels: an overview. Neurobiol Aging. 2005 Mar;26(3):281–291. doi: 10.1016/j.neurobiolaging.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol. 2008 May 6;585(1):163–175. doi: 10.1016/j.ejphar.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 29.Goossens GH, Blaak EE, van Baak MA. Possible involvement of the adipose tissue renin-angiotensin system in the pathophysiology of obesity and obesity-related disorders. Obes Rev. 2003 Feb;4(1):43–55. doi: 10.1046/j.1467-789x.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- 30.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005 Dec 15;86(5):731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]