Abstract
TBC1D1 is a Rab-GTPase-activating protein (GAP) known to be phosphorylated in response to insulin, growth factors, pharmacological agonists that activate 5′-AMP-activated protein kinase (AMPK), and muscle contraction. Silencing TBC1D1 in L6 muscle cells by siRNA increases insulin-stimulated GLUT4 translocation, and overexpression of TBC1D1 in 3T3-L1 adipocytes with low endogenous TBC1D1 expression inhibits insulin-stimulated GLUT4 translocation, suggesting a role of TBC1D1 in regulating GLUT4 translocation. Aiming to unravel the regulation of TBC1D1 during contraction and the potential role of AMPK in intact skeletal muscle, we used EDL muscles from wild-type (WT) and AMPK kinase dead (KD) mice. We explored the site-specific phosphorylation of TBC1D1 Ser237 and Thr596 and their relation to 14-3-3 binding, a proposed mechanism for regulation of GAP function of TBC1D1. We show that muscle contraction increases 14-3-3 binding to TBC1D1 as well as phosphorylation of Ser237 and Thr596 in an AMPK-dependent manner. AMPK activation by AICAR induced similar Ser237 and Thr596 phosphorylation of, and 14-3-3 binding to, TBC1D1 as muscle contraction. Insulin did not increase Ser237 phosphorylation or 14-3-3 binding to TBC1D1. However, insulin increased Thr596 phosphorylation, and intriguingly this response was fully abolished in the AMPK KD mice. Thus, TBC1D1 is differentially regulated in response to insulin and contraction. This study provides genetic evidence to support an important role for AMPK in regulating TBC1D1 in response to both of these physiological stimuli.
Keywords: 5′-AMP-activated protein kinase, wortmannin, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside, AMPK kinase dead, extensor digitorum longus muscle
translocation of the glucose transporter GLUT4 from intracellular storage vesicles to the sarcolemma and T-tubules is important for skeletal muscle to increase glucose uptake in response to various stimuli (31). Although it is well established that both insulin and contraction increase glucose uptake through distinct signaling cascades (10), the exact pathways are yet to be elucidated. TBC1 domain family member 4 (TBC1D4, previously known as AS160), has been identified as a component of the insulin-signaling cascade downstream of Akt (protein kinase B) (15). TBC1D4 contains a Rab-GTPase-activating protein (GAP) domain thought to impede GLUT4 exocytosis by promoting conversion of target Rabs to an inactive GDP-bound form. It is believed that phosphorylation of TBC1D4 directly or indirectly inhibits its Rab-GAP function (16, 23). TBC1D4 has been proposed as a nexus for insulin- and contraction-signaling pathways as it appears to be involved both in insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes and L6 muscle cells (23, 27) and in insulin- and contraction-stimulated glucose uptake in skeletal muscle (16).
Recently, TBC1D1, a candidate gene for development of severe obesity and a closely related paralog of TBC1D4, was identified as an Akt substrate (25). Recombinant congenic mice lacking TBC1D1 were reported to be protected against diet-induced obesity, likely due to interference with both lipid and glucose metabolism (2). Furthermore, silencing TBC1D1 in L6 muscle cells by siRNA increases basal and insulin-stimulated GLUT4 translocation (12), and overexpression of TBC1D1 in 3T3-L1 adipocytes with low endogenous TBC1D1 expression inhibits insulin-stimulated GLUT4 translocation, and this inhibitory effect is reversed by expression of a mutated form of TBC1D1 in which the GAP domain has been inactivated (22). Current data thus suggest that TBC1D1 exerts a regulatory role in metabolism and that this role occurs through regulation of its GAP activity.
TBC1D1 and TBC1D4 are 47% identical in sequences, while their GAP domains share 79% identity (22). The two proteins show the same specificity toward Rabs and share several other structural features (22). They also contain conserved Akt phosphorylation sites, and, like TBC1D4, TBC1D1 is phosphorylated in response to insulin and the AMPK activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) in cultured cells (3, 4). In skeletal muscle TBC1D1 is repeated to be phosphorylated in response to insulin, AICAR, and contraction in skeletal muscle (9, 26). Thus, TBC1D1 is another possible point of convergence between the insulin- and contraction-signaling pathways in skeletal muscle. Both TBC1D1 and TBC1D4 are expressed in skeletal muscle (3, 26). TBC1D4 is predominantly expressed in more oxidative muscles such as soleus, whereas TBC1D1 is expressed in more glycolytic muscles, such as tibialis anterior and extensor digitorum longus (EDL) (26). As AMPK is highly activated during muscle contraction (11, 14, 29), the above findings raise the question whether AMPK activation mediates phosphorylation of TBC1D1 during contraction.
In 3T3-L1 adipocytes, binding of 14-3-3 proteins to phosphorylated TBC1D4 is required for insulin-stimulated GLUT4 translocation (20). Recently, it was reported that activation of AMPK in L6 myotubes increased phosphorylation of Ser237 and 14-3-3 binding to TBC1D1, whereas stimulation with insulin promoted Thr596 phosphorylation but not 14-3-3 binding (4). Hence, these observations may suggest that site-specific phosphorylation of TBC1D1 determines the binding affinity of 14-3-3 proteins and perhaps (by analogy with TBC1D4) its GAP activity. To date, the phosphorylation status of TBC1D1 in skeletal muscle has been evaluated using the phospho-Akt substrate (PAS) antibody, which recognizes phosphorylated Akt substrate motifs within an (R/K)X(R/K)XXS*/T* sequence. To further explore the regulation of TBC1D1, we performed analyses of TBC1D1 phosphorylation using site- and phosphospecific antibodies generated against two established phosphorylation sites (Ser237 and Thr596), together with analyses of 14-3-3 binding capacity to TBC1D1. To delineate the role of AMPK, these studies were performed in muscles from wild-type (WT) mice as well as mice expressing a dominant negative AMPKα2 construct (17).
MATERIALS AND METHODS
Animals.
All experiments were approved by the Danish Animal Experimental Inspectorate and complied with the European Convention for the Protection of Vertebrate Animals Used for Scientific Purposes. The muscle-specific AMPKα2 kinase dead (KD) animals used in these experiments have previously been described (17). Briefly, the animals overexpress a Lys45-to-Arg mutant of the α2 protein, driven by a heart- and skeletal muscle-specific creatine kinase promoter. All animals used in these experiments were 10- to 14-wk-old female littermates and were maintained on a C57BL/6 background. Animals were kept on a 10:14-h light-dark cycle with unlimited access to standard rodent diet and water ad libitum.
Muscle incubations.
Before surgery, all animals were anesthetized by intraperitoneal injection of pentobarbital sodium (6 mg/100 g body wt). For all experiments, EDL muscles were quickly excised and suspended at resting tension (3–4 mN) in incubation chambers (Multi Myograph system; Danish Myo-Technology, Aarhus, DK). All muscles were incubated in prebuffer (standard Krebs-Henseleit-Ringer buffer with addition of 8 mM Mannitol, 2 mM pyruvate, and 0.1% BSA) at 30°C and oxygenated with a gas mixture containing 95% O2 and 5% CO2. For stimulation with insulin, muscles were incubated in prebuffer for 10 min and subsequently in prebuffer containing 60 nM insulin (Actrapid; Novo Nordisk, Bagsværd, Denmark) for 40 min. Muscles stimulated to contract were incubated in prebuffer for 40 min, and muscle contraction was subsequently induced by electrical stimulation with a 10-s train (100 Hz, 0.2 ms impulse, ∼30–40 V) per minute for 10 min. Muscles stimulated with AICAR were incubated in prebuffer for 10 min and subsequently in prebuffer containing 2 mM AICAR (Toronto Research Chemicals, Toronto, ON, Canada) for 40 min. When AICAR and insulin stimulation were combined, muscles were incubated in prebuffer for 10 min and subsequently in prebuffer containing AICAR and insulin (concentrations as above) for 40 min. When effects of insulin or AICAR were studied in combination with muscle contraction, muscles underwent 10 min incubation in prebuffer, 30 min of insulin or AICAR stimulation (as above) and subsequently 10 min of electrical stimulation (as above) in the presence of insulin or AICAR. Incubations with insulin or contraction in the presence or absence of the phosphatidylinositol 3-kinase inhibitor wortmannin dissolved in DMSO [wortmannin (0.5 μM; Sigma), DMSO (0.01%; Bie & Berntsen, Rødovre, Denmark)] were performed as described above. After incubation, muscles were quickly collected and snap-frozen by immersion in liquid nitrogen and stored at −80°C for later processing. For all interventions, muscle were incubated pairwise; i.e., one muscle from an animal was used for stimulation, whereas as the muscle from the contralateral leg was used as control (basal/rest). WT littermates were used as controls for the AMPK KD mice.
Preparation of muscle lysate.
Intact muscles were homogenized in ice-cold buffer [10% glycerol, 20 mM sodium pyrophosphate, 1% NP-40, 2 mM PMSF, 150 mM sodium chloride, 50 mM HEPES, 20 mM β-glycerophosphate, 10 mM sodium fluoride, 1 mM EDTA, 1 mM EGTA, 10 μg/ml aprotinin, 3 mM benzamidine, 10 μg/ml leupeptin, and 2 mM sodium orthovanadate (pH 7.4)] for 20–30 s using a polytron (PT 3100, Brinkman instruments). Homogenates were rotated end over end for 1 h at 4°C and subsequently centrifuged at 16,000 g at 4°C for 20 min. Supernatants were collected and snap-frozen in liquid nitrogen and stored at −80°C for later analysis. Total protein concentrations were analyzed by the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL).
Immunoprecipitation.
Total TBC1D1 protein was immunoprecipitated (IP) from 300 μg of total muscle lysate protein using 1 μg of TBC1D1 antibody, as previously described (4). Protein G-agarose beads (Sigma Aldrich, St. Louis, MO) were washed three times in PBS and added to the muscle lysate. Mixtures of lysate, antibody and protein G beads were rotated end over end overnight at 4°C, and beads were subsequently washed twice in ice-cold PBS and placed in SDS sample buffer for 5 min at 96°C.
SDS-PAGE and western blot analyses.
Muscle lysate proteins were separated by SDS-PAGE and transferred (semidry) to PVDF membranes (Immobilon Transfer Membranes; Millipore, Bagsvaerd, Denmark). Membranes were then blocked for 1 h at room temperature (RT) in TBST + 1% BSA (wt/vol, pH 7.4) for TBC1D1 phosphospecific antibodies and in TBST + 2% skim milk powder (wt/vol, pH 7.4) for all other proteins. Blocked membranes were probed with primary antibodies (see Antibodies) overnight at 4°C and subsequently incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at RT. Thereupon, membranes were probed with ECL (Amersham Biosciences, Piscataway, NJ), and immunocomplexes were visualized using a Kodak Image Station 2000MM. Signals were quantified (Kodak 1D 3.6) and expressed as arbitrary units. Membranes used for 14-3-3 overlays or for detection of phosphorylated Akt or TBC1D1 were stripped with a buffer containing 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris·HCl. Membranes were reprobed with the corresponding total antibody.
Antibodies.
To determine the phosphorylation status of various signaling proteins, the following phosphospecific antibodies were used: AMPK Thr172 (Cell Signaling Technologies, Danvers, MA), acetyl-CoA carboxylase (ACC) Ser227 (Upstate Biotechnologies, Waltham, MA), Akt Thr308 (Upstate Biotechnologies), Akt Ser473 (Cell Signaling Technologies). Phosphorylation of TBC1D1 was detected using either a phospho-Akt substrate (PAS) antibody (Cell Signaling Technologies) or phosphospecific antibodies against Ser237 and Thr596, as previously described (4). The following antibodies were used to determine total protein expression: AMPKα1 and AMPKα2 as previously described (30), Akt total (Upstate Biotechnologies), TBC1D1 as previously described (4), TBC1D4 (Upstate Biotechnologies). HRP-conjugated secondary antibodies were purchased from Dako (Glostrup, Denmark).
14-3-3 Overlay assay.
14-3-3 Binding capacity of TBC1D1 was assessed by a 14-3-3 overlay as previously described (19). In brief, immunoprecipitated TBC1D1 proteins from mouse EDL muscles were subjected to SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in TBST + 1% (vol/wt) BSA (pH 7.4) for 1 h at RT and subsequently probed with digoxigenin (DIG)-labeled recombinant 14-3-3 proteins at 4°C overnight. Membranes were then probed with HRP-conjugated anti-DIG antibody (Roche, Basel, Switzerland) for 1 h at RT and subsequently probed with ECL (Amersham Biosciences). Immunocomplexes were visualized and quantified as described above.
AMPK activity assays.
AMPKα1 and AMPKα2 containing heterotrimeric complexes were sequentially immunoprecipitated from 150 μg of muscle lysate using isoform-specific AMPK antibodies as previously described (30). After an overnight incubation at 4°C, the IP was washed once in IP buffer, once in 480 mM HEPES (pH 7.0) and 240 mM NaCl, and twice in 240 mM HEPES (pH 7.0) and 120 mM NaCl, leaving 10 μl of agarose after the last wash. The reaction ran for 30 min at 30°C in a total volume of 30 μl containing 80 mM HEPES (pH 7.0), 40 mM NaCl, 833 μM DTT, 200 μM AMP, 100 μM AMARA-peptide (Schafer-N, Copenhagen, Denmark), 5 mM MgCl2, 200 μM ATP, and 2 μCi of [γ-33P]ATP (PerkinElmer, Skovlunde, Denmark). The reaction was stopped by adding 25 μl of 1% phosphoric acid to the reaction, after which 50 μl was spotted onto P81 filter paper (Whatman, GE Healthcare, Copenhagen, Denmark), which was then washed four times for 15 min in 1% phosphoric acid. The dried filter paper was analyzed for activity using liquid scintillation (Tri-Carb 2000, Packard Instruments). The α2 activity was analyzed by immunodepleting lysates for α1 heterotrimeric complexes by an overnight α1 IP, after which α2 was immunoprecipitated from the remaining supernatant. Neither one nor two overnight incubations at 4°C had any influence on the phosphorylation state of AMPKα subunits (data not shown). Also, the activity associated with each of the two pools of heterotrimeric complexes was unaffected by either one or two overnight IPs compared with a 4-h IP (data not shown).
Statistics.
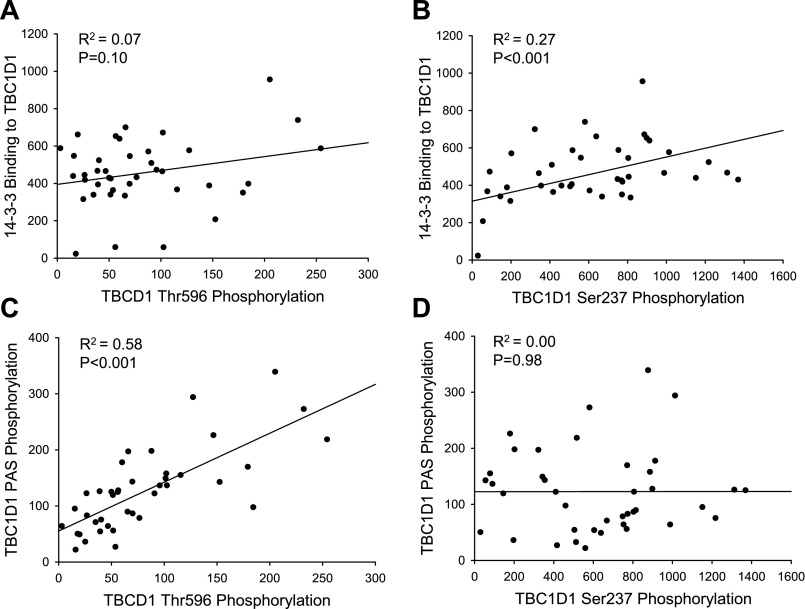
Data are expressed as means ± SE. All statistical analyses were performed in SigmaStat 3.5 (Systat Software, Erkrath, Germany) using a t-test for data in Fig. 1, a two-way ANOVA with repeated measurements for data in Figs. 2, 4, 6, and 7 and Tables 1, 2, and 4, and one-way ANOVA for data in Fig. 3 and Table 3. When ANOVAs revealed significant differences, Tukey's post hoc test was used for multiple comparisons. Correlation analyses in Fig. 5 were performed by obtaining Pearson's product moment correlation coefficient. P < 0.05 was considered statistically significant.
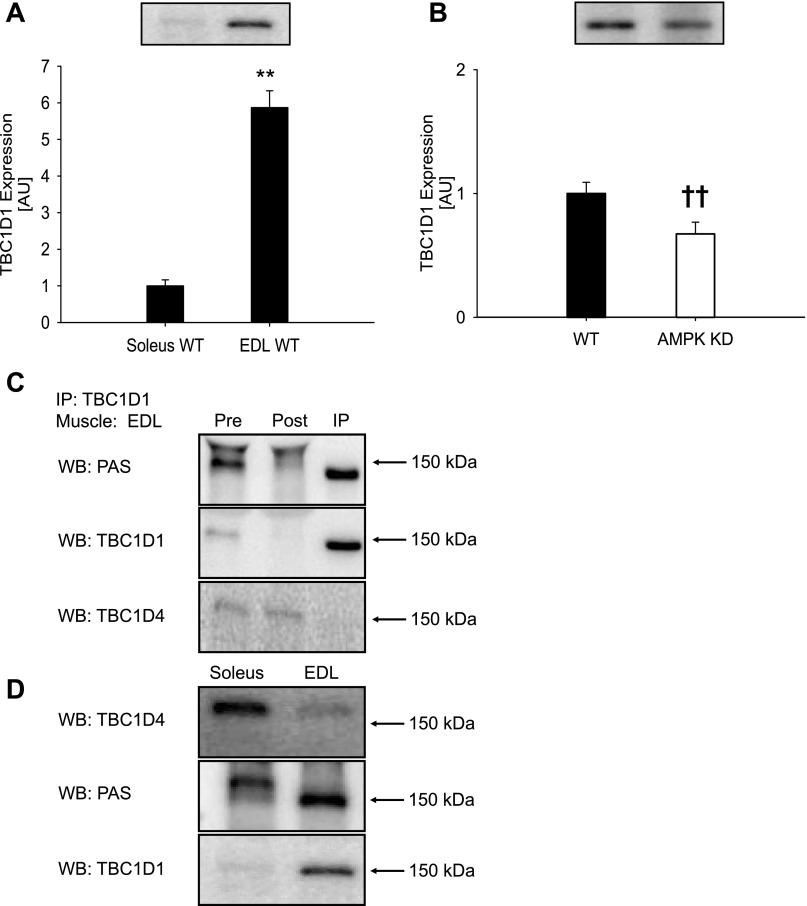
Fig. 1.
TBC1D1 protein expression in muscle from wild-type (WT) and AMPK kinase dead (KD) mice. A: TBC1D1 protein expression in soleus and EDL muscles from WT C57BL/6 mice (n = 8). B: TBC1D1 protein expression in EDL muscles from WT and AMPK KD mice (n = 16). Data are expressed relative to WT soleus and are means ± SE. **Significant difference between soleus and EDL muscle, P < 0.01; ††significant difference between WT and AMPK KD mice, P < 0.01. C: Immunoprecipitation (IP) of TBC1D1 from an EDL muscle lysate from C57BL/6 mice removes almost all signal obtained with the anti-PAS (phospho-Akt substrate) antibody migrating around 150–160 kDa. D: TBC1D4 is predominantly expressed in soleus muscle and migrates around 160 kDa on SDS-PAGE, whereas TBC1D1 is predominantly expressed in EDL muscle and migrates around 150 kDa. Proteins recognized by the anti-PAS antibody migrate at positions corresponding to TBC1D4 in soleus and TBC1D1 in EDL. Taken together, C and D show that the anti-PAS antibody predominantly recognizes TBC1D1 compared with TBC1D4 in mouse EDL muscle and predominantly TBC1D4 compared with TBC1D1 in mouse soleus muscle.
Table 1.
Effects of contraction and insulin on AMPK phosphorylation, activity, and expression and ACC phosphorylation
| WT |
AMPK KD | WT | AMPK KD | |||||
|---|---|---|---|---|---|---|---|---|
| Basal | Contraction | Basal | Contraction | Basal | Insulin | Basal | Insulin | |
| pAMPK Thr172 | 100±9.0 | 188±8.2** | 143±8.9† | 172±11 | 100±11 | 113±9.9 | 142±12(†) | 122±13(†) |
| pACC Ser227 | 100±6.4 | 162±6.4** | 29.8±3.1† | 91.4±17†** | 100±13 | 120±16 | 24.0±4.6†† | 20.0±5.3†† |
| AMPKα1 expression | 100±10 | 94.0±20 | 38.7±7.7†† | 29.7±4.3†† | 100±6.0 | 104±5.1 | 30.0±3.3†† | 24.4±3.0†† |
| AMPKα2 expression | 100±5.8 | 78.3±10* | 236±9.7†† | 221±6.3††* | 100±12 | 100±9.6 | 305±35†† | 289±17†† |
| AMPKα1 activity | 100±7.6 | 272±19** | 90.3±9.9 | 112±5.7†† | ||||
| AMPKα2 activity | 100±11 | 237±40** | 17.5±9.8† | 23.0±10†† | ||||
Data are related to wild-type (WT) basal and expressed as means ± SE; n = 7–8. AMPK Thr172 and acetyl-CoA carboxylase (ACC) Ser227 phosphorylation (p) and AMPKα1 and -α2 protein expression in EDL muscle from WT and AMPK kinase dead (KD) mice in response to contraction or insulin. AMPKα1 and -α2 activity in muscle from WT and AMPK KD mice in response to contraction.
Difference between basal and stimulated muscle, P < 0.05/0.01;
difference between WT and AMPK KD mice, P < 0.05/0.01; (†)differences between WT and AMPK KD mice are borderline significant (P = 0.06).
Table 2.
Effects of contraction and insulin on Akt phosphorylation and expression
| WT |
AMPK KD | WT | AMPK KD | |||||
|---|---|---|---|---|---|---|---|---|
| Basal | Contraction | Basal | Contraction | Basal | Insulin | Basal | Insulin | |
| Akt expression | 100±6.1 | 115±12 | 108±8.3 | 92.6±5.2 | 100±11 | 114±12 | 128±16 | 106±8.3 |
| pAkt Thr308 | 100±8.2 | 103±12 | 117±8.3 | 116±10 | 100±17 | 580±50** | 137±23 | 500±57** |
| pAkt Ser473 | 100±9.3 | 98.1±9 | 109±12 | 102±7.2 | 100±12 | 1152±96** | 163±65 | 958±108** |
Data are related to WT basal and expressed as means ± SE; n = 8. Akt phosphorylation on Thr308 and Ser473 and Akt protein expression in EDL muscles from WT and AMPK KD mice in response to contraction or insulin.
Difference between basal and insulin-stimulated muscle, P < 0.01.
Table 4.
Effects of wortmannin on insulin- and contraction-stimulated Akt Thr308, Akt Ser473, and AMPK Thr172 phosphorylation in WT EDL muscle
| Control |
Wortmannin | Control | Wortmannin | |||||
|---|---|---|---|---|---|---|---|---|
| Basal | Contraction | Basal | Contraction | Basal | Insulin | Basal | Insulin | |
| pAkt Thr308 | 100±25 | 185±24* | 15.4±16†† | 16±7.6†† | 100±32.6 | 2,365±98** | 17.9±7.0†† | 134±32†† |
| pAkt Ser473 | 100±26 | 195±23* | 17.2±5.2†† | 30±6.5†† | 100±37 | 1,835±244** | 51.1±12†† | 253±80†† |
| pAMPK Thr172 | 100±15 | 654±70** | 101±10 | 616±37** | 100±20 | 115±19 | 125±16 | 114±26 |
Data are related to basal control and expressed as means ± SE; n = 8. Akt phosphorylation on Thr308 and Ser473 and AMPK phosphorylation on Thr172 in EDL muscles from WT mice in response to contraction or insulin in the presence or absence of wortmannin.
Difference between basal and contraction/insulin-stimulated muscle, P < 0.05/0.01.
Difference between control and wortmannin-incubated muscle, P < 0.01.
Table 3.
Effects of AICAR, contraction, and insulin on AMPK, ACC, and Akt phosphorylation in WT EDL muscle
| pAkt Thr308 | pAkt Ser473 | pAMPK Thr172 | pACC Ser227 | |
|---|---|---|---|---|
| Basal | 100±8.9 | 100±11 | 100±29 | 100±11 |
| AICAR | 148±16* | 174±41* | 266±17** | 520±27** |
| Basal | 124±26 | 113±13 | 101±15 | 120±21 |
| Insulin | 1,989±207**‡ | 2,726±215**‡ | 98.3±7.6 | 124±14 |
| Basal | 84.0±15 | 90.1±12 | 96.8±13 | 127±23 |
| AICAR + insulin | 1,962±168** | 2,641±182** | 279±9.8** | 596±35** |
| Basal | 115±20 | 84.8±6.9 | 125±28 | 124±19 |
| Contraction | 246±29* | 287±23** | 489±32**§ | 476±20** |
| Basal | 133±18 | 95.8±18 | 93.4±18 | 112±11 |
| Insulin + contraction | 1,758±159** | 2,413±200** | 486±57** | 469±42** |
| Basal | 102±14 | 77.1±11 | 88.5±14 | 132±18 |
| AICAR + contraction | 274±42* | 336±47* | 454±47**a | 641±38**¤ |
Data are related to basal from AICAR trial and expressed as means ± SE; n = 8. Thr308 and Ser473: increase in phosphorylation was higher in insulin-stimulated muscle than AICAR- or contraction-stimulated muscle (‡P < 0.05). Combining insulin and AICAR or contraction did not increase phosphorylation above insulin levels. pAMPK Thr172: contraction increased phosphorylation to a higher extent than AICAR (§P < 0.05); combining AICAR and contraction increased phosphorylation above AICAR (‡P < 0.05) but not contraction levels. pACC Ser227: AICAR and contraction combined increased phosphorylation to a higher extent than AICAR or contraction alone (aP < 0.05).
P < 0.05/0.01 toward basal.
RESULTS
Protein expression of TBC1D1.
A five- to sixfold higher TBC1D1 protein expression in EDL muscle compared with soleus (Fig. 1) led us to use the former in the present study. Interestingly, TBC1D1 protein expression was 33% (P < 0.01) lower in EDL muscle from the AMPK KD mice compared with WT mice (Fig. 1B). By IP experiments we verified the specificity of the bands detected around 150 kDa using the anti-PAS antibody in Western blotting analyses (Fig. 1, C and D). Thus, these experiments verified a previous observation (26) that almost all signal obtained with the anti-PAS antibody around 150 kDa in mouse EDL muscle can be ascribed to TBC1D1. We furthermore showed that TBC1D1 and TBC1D4 can be separated by SDS-PAGE (Fig. 1, C and D).
Phosphorylation of and 14-3-3 binding to TBC1D1 during contraction requires AMPK.
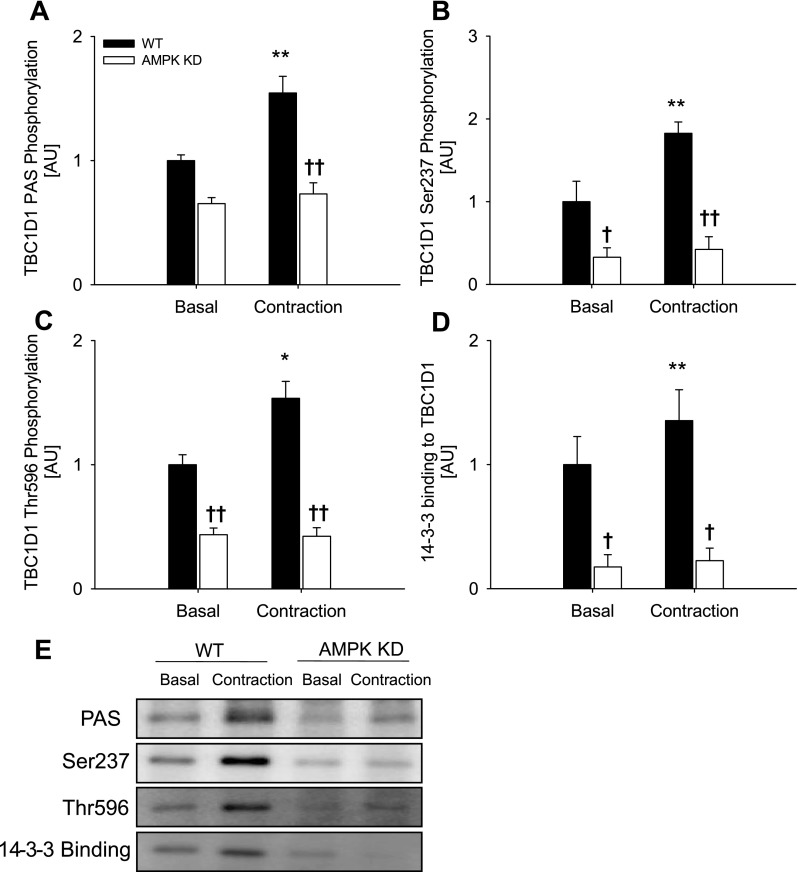
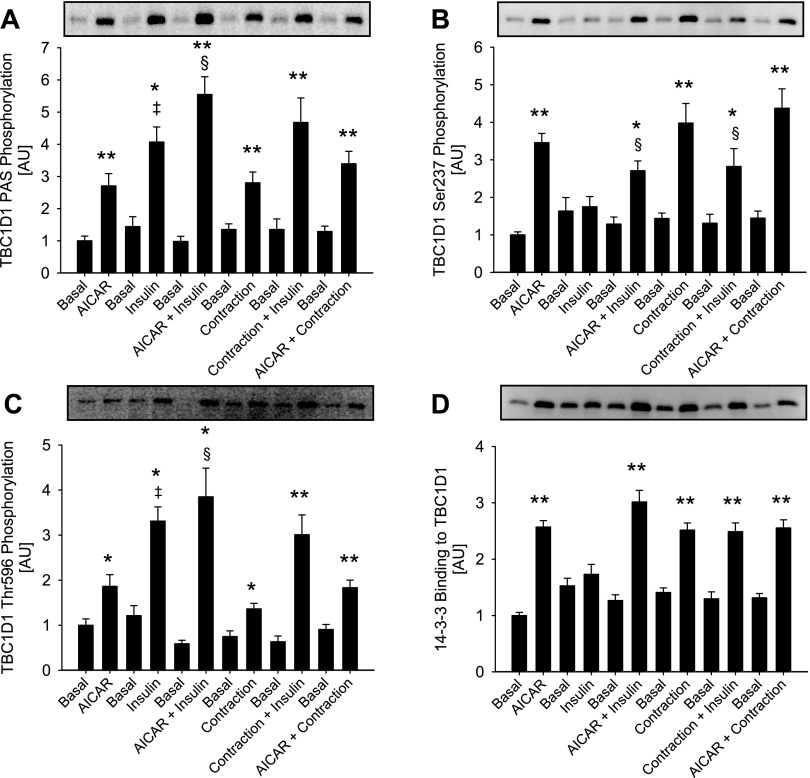
Ex vivo muscle contraction induced phosphorylation of TBC1D1 as detected by the anti-PAS, anti-phospho-Thr596 and anti-phospho-Ser237 antibodies in WT EDL muscle (Fig. 2, A–C). Furthermore, 14-3-3 binding to TBC1D1 increased in response to contraction in WT EDL muscles (Fig. 2D). In nonstimulated EDL muscles from the AMPK KD mice, phosphorylation of, and 14-3-3 binding to, TBC1D1 were markedly decreased (Fig. 2, A–D). After normalization to total TBC1D1 protein, however, only the diminished levels of Ser237 phosphorylation (47%, n = 16, P < 0.01) and 14-3-3 binding (46%, n = 16, P < 0.01) remained evident (data not shown), suggesting that decreased basal PAS and Thr596 phosphorylation merely reflected the lower TBC1D1 protein expression in the EDL muscle of the AMPK KD mouse. Interestingly, in EDL muscle from AMPK KD mice, neither phosphorylation (PAS, Thr596, and Ser237) of, nor 14-3-3 binding to, TBC1D1 were increased by contraction (Fig. 2, A–D). Ex vivo muscle contraction induced phosphorylation of Thr172 of AMPK, and the AMPK activity associated with both the α1 and α2 catalytic subunits was increased in WT EDL muscle (Table 1). Neither of these measures of AMPK signaling was increased by contraction in EDL muscles from the AMPK KD mice. As expected, the basal activity of AMPKα2 was markedly reduced (88%, n = 16, P < 0.01; Table 1) in EDL muscle of the AMPK KD mice. In line with previous reports, phosphorylation of ACC at Ser227 was markedly decreased in unstimulated EDL muscle from AMPK KD mice (16a, 28), and although ACC phosphorylation increased upon contraction [as also seen in WT EDL muscle (Table 1)], the level reached in EDL from AMPK KD mice did not exceed the level seen in basal WT EDL muscle (Table 1). In EDL from either WT or AMPK KD mice, contraction did not change Akt phosphorylation of Ser473 or Thr308 (Table 2). Taken together, these data suggest that contraction-stimulated phosphorylation of TBC1D1 on Thr596 and Ser237, as well as 14-3-3 binding, is AMPK dependent, as is Ser237 phosphorylation of, and 14-3-3 binding to, TBC1D1 in resting nonstimulated EDL. In line with these observations, treatment with AICAR also increased phosphorylation (using PAS, Thr596, and Ser237 antibodies) of, and 14-3-3 binding to, TBC1D1 in a manner that was not additive with muscle contraction (Fig. 3, A–D). We next addressed whether AMPK was required for the phosphorylation and 14-3-3 binding of TBC1D1 by using a stimulus that is normally regarded as AMPK independent, i.e., insulin.
Fig. 2.
TBC1D1 phosphorylation and 14-3-3 binding in response to contraction in WT (filled bars) and AMPK KD mice (open bars) and representative blots. TBC1D1 PAS phosphorylation (A), Ser237 phosphorylation (B), Thr596 phosphorylation (C), and 14-3-3 binding capacity (D) in EDL muscle from WT and AMPK KD mice in response to contraction. E: representative blots using corresponding antibodies. Data are related to WT basal and expressed as means ± SE; n = 7–12. **Difference between basal and contracted muscle P < 0.01; †/††difference between WT and AMPK KD mice P < 0.05/0.01.
Fig. 3.
Effects of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), contraction, and insulin on TBC1D1 phosphorylation and 14-3-3 binding. A and C: TBC1D1 PAS and Thr596 phosphorylation. Insulin increased phosphorylation to a higher extent than AICAR and contraction (‡P < 0.05). Combining insulin with AICAR significantly increased phosphorylation above insulin levels (§P < 0.05). Combining insulin with contraction did not increase phosphorylation above insulin levels. B: Ser237: AICAR and contraction increased phosphorylation equally alone and in combination. Combining AICAR or contraction with insulin decreased phosphorylation below AICAR or contraction levels (§P < 0.05). D: 14-3-3 binding: AICAR and contraction alone and combined increased 14-3-3 binding to TBC1D1 to the same extent. Insulin alone did not increase binding, and combining AICAR and contraction with insulin did not alter binding from AICAR and contraction levels alone. The 6 basal groups were treated as independent groups. Data are related to basal from AICAR trial and expressed as means ± SE; n = 8. */**P < 0.05/0.01.
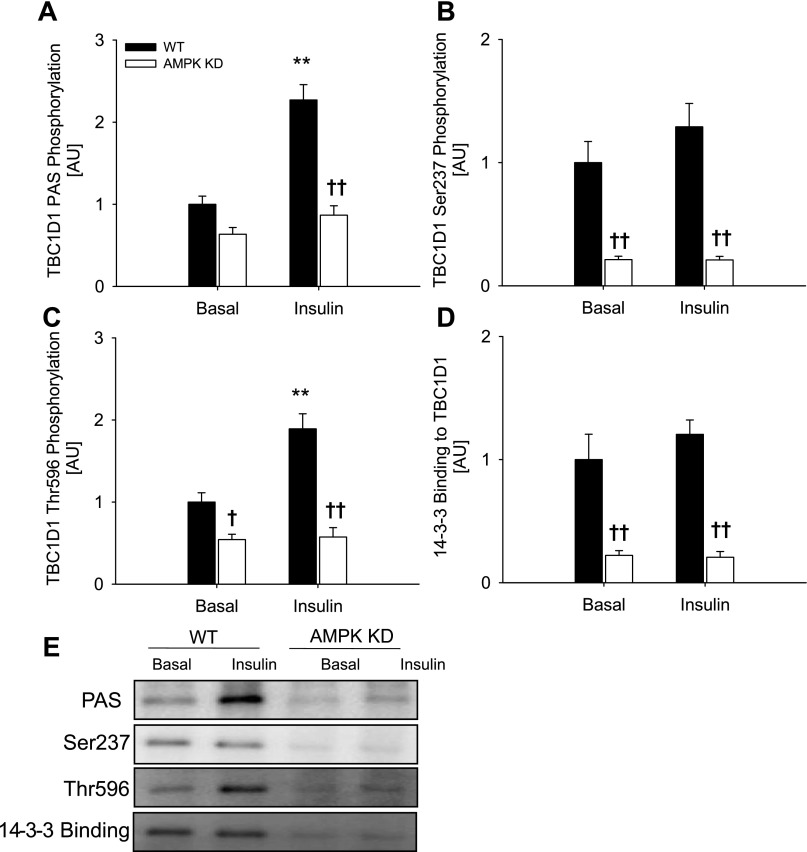
Phosphorylation of Thr596 by insulin is abolished in muscle from AMPK KD mice.
Treatment of incubated WT EDL muscles with insulin, in contrast to contraction, did not induce increased Ser237 phosphorylation of, or 14-3-3 binding to, TBC1D1, whereas marked increases in TBC1D1 phosphorylation detected by both the anti-PAS and anti-Thr596 antibodies were observed (Fig. 4, A–D). Intriguingly, the signals using these two latter antibodies did not increase in response to insulin in EDL muscle from the AMPK KD mice (Fig. 4, A and C). This was observed despite normal insulin-stimulated phosphorylation of Thr308 and Ser473 on Akt in the AMPK KD muscle (Table 2). Insulin did not affect AMPK and ACC phosphorylation in EDL muscle of WT or AMPK KD mice (Table 1). In accordance with the data shown in Fig. 2, all measurements of phosphorylation of, and 14-3-3 binding to, TBC1D1 were diminished in muscle from the AMPK KD mice, and only Ser237 and 14-3-3 binding remained decreased after normalization to TBC1D1 protein expression. The lack of Ser237 phosphorylation and 14-3-3 binding in response to insulin in WT EDL muscle was verified by another independent experiment (Fig. 3, B and D). In that set of experiments, there was a tight correlation between phosphorylation detected with the anti-PAS antibody with that of the anti-Thr596 antibody (R2 = 0.58, P < 0.001, n = 42) but not with that of the anti-Ser237 antibody (R2 = 0.00, P = 0.98, n = 44) (Fig. 5, C and D). This is in agreement with analyses of TBC1D1 mutants in cultured cells, which suggested that the anti-PAS antibody predominantly detects Thr596 (4). Taken together, these data suggest that basal AMPK activity is required for insulin-induced Thr596 phosphorylation of TBC1D1.
Fig. 4.
TBC1D1 phosphorylation and 14-3-3 binding in response to insulin in WT (filled bars) and AMPK KD mice (open bars) and representative blots. TBC1D1 PAS phosphorylation (A), Ser237 phosphorylation (B), Thr596 phosphorylation (C), and 14-3-3 binding capacity (D) in EDL muscle from WT and AMPK KD mice in response to insulin. E: representative blots using corresponding antibodies. Data are related to WT basal and expressed as means ± SE; n = 7. **Difference between basal and insulin-stimulated muscle P < 0.01; †/††difference between WT and AMPK KD mice P < 0.05/0.01.
Fig. 5.
Correlations between increases in TBC1D1 site-specific phosphorylation, TBC1D1 PAS phosphorylation, and 14-3-3 binding to TBC1D1. A: TBC1D1 Thr596 phosphorylation vs. 14-3-3 binding (R2 = 0.07, P = 0.10, n = 42). B: TBC1D1 Ser237 phosphorylation vs. 14-3-3 binding (R2 = 0.27, P < 0.001, n = 43). C: TBC1D1 Thr596 phosphorylation vs. TBC1D1 PAS phosphorylation (R2 = 0.58, P < 0.001, n = 42). D: TBC1D1 Ser237 phosphorylation vs. TBC1D1 PAS phosphorylation (R2 = 0.00, P = 0.98, n = 44). Each point represents ▵ values (stimulated − basal) from a single WT mouse. Correlations are calculated from data given in Fig. 3.
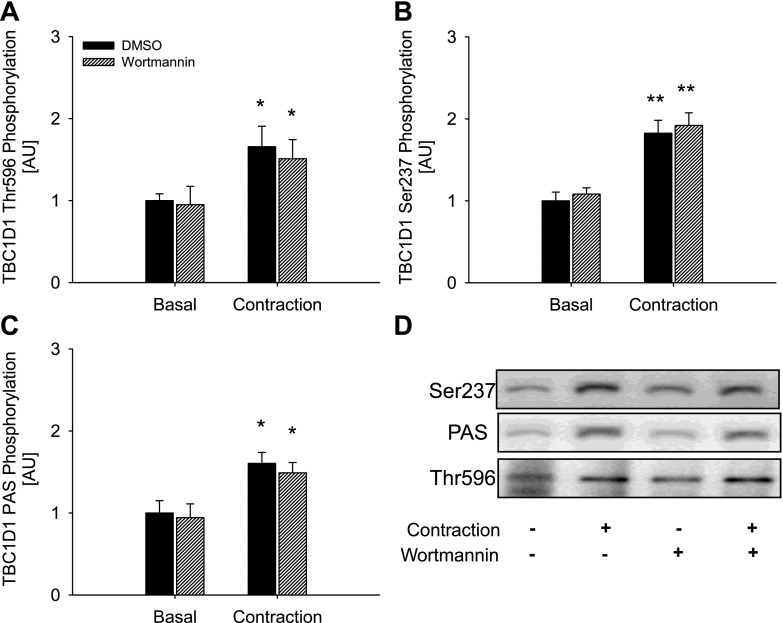
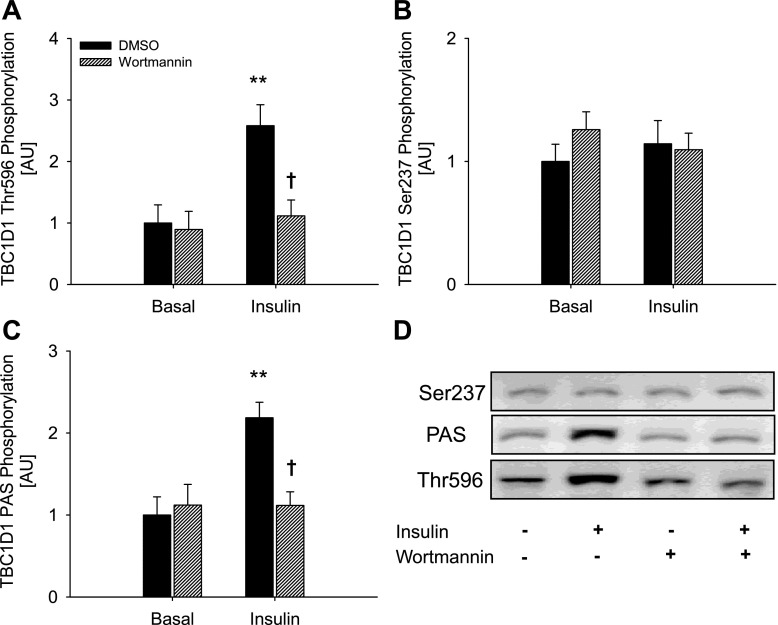
To further explore the role of AMPK, we performed experiments in which treatments that activate AMPK, i.e., muscle contraction and AICAR, were given together with insulin. The increase in phosphorylation detected with the anti-PAS and anti-Thr596 antibody was greater when AICAR and insulin were present together than when present alone (Fig. 3, A and C). Although mean values may appear to indicate similar effects on TBC1D1 PAS phosphorylation when contraction and insulin were combined, this did not reach statistical significance. TBC1D1 Ser237 phosphorylation and 14-3-3 binding were not affected in an additive manner when any of the three stimuli were combined (Fig. 3, B and D). In fact, decreases in Ser237 phosphorylation were seen when insulin was combined with AICAR or contraction (Fig. 3B). In the experiment investigating the additive effect of AICAR, insulin, and contraction, we observed a small, compared with insulin, but nevertheless significant increase in Akt Thr308 and Ser473 phosphorylation in response to both AICAR and contraction (Table 3). Thus, in these experiments Thr596 phosphorylation could have been induced by this activation of Akt. Although such increased phosphorylation of Akt was not evident in the first series of experiments (Table 2), we further clarified this by performing experiments in the presence of the PI3K inhibitor wortmannin. In WT EDL muscle, wortmannin abolished both basal and contraction-stimulated Akt phosphorylation on Thr308 and Ser473 (Table 4). However, neither basal nor contraction-stimulated phosphorylation of TBC1D1, detected using the Ser237, Thr596, or PAS antibodies, were influenced by wortmannin (Fig. 6, A–C), suggesting that contraction-induced TBC1D1 phosphorylation occurs independently of the PI3K. To determine whether the insulin-induced TBC1D1 phosphorylation on Thr596 was mediated through the canonical PI3K-Akt insulin-signaling cascade, we stimulated WT EDL muscle with insulin in the presence or absence of wortmannin. As expected, wortmannin completely prevented both basal and insulin-stimulated phosphorylation of Akt Thr308 and Ser473 (Table 4). This resulted in abolition of insulin-stimulated but not basal Thr596 and PAS phosphorylation of TBC1D1, suggesting that insulin leads to phosphorylation of TBC1D1 Thr596 through a PI3K-dependent mechanism (Fig. 7, A and C).
Fig. 6.
Effects of wortmannin on contraction-stimulated TBC1D1 phosphorylation. Control, filled bars; wortmannin, hatched bars. TBC1D1 Thr596 (A) Ser237 (B), and PAS phosphorylation (C) in WT EDL muscle in response to contraction in the presence or absence of wortmannin. D: representative blots using corresponding antibodies. Data are related to control basal and expressed as means ± SE; n = 8. */**Difference between basal and contraction-stimulated muscle, P < 0.05/0.01.
Fig. 7.
Effects of wortmannin on insulin-stimulated TBC1D1 phosphorylation in WT EDL muscle. Control, filled bars; wortmannin, hatched bars. TBC1D1 Thr596 (A) Ser237 (B), and PAS phosphorylation (C) in WT EDL muscle in response to insulin in the presence or absence of wortmannin. D: representative blots using corresponding antibodies. Data are related to control basal and expressed as means ± SE; n = 8. **Difference between basal and insulin-stimulated muscle, P < 0.01; †difference between Control and wortmannin-incubated muscle, P < 0.05.
DISCUSSION
In this paper, we show that insulin- and contraction-activated signaling pathways converge to cause phosphorylation of TBC1D1 on distinct sites in isolated mouse EDL muscle. Although contraction induced PI3K-independent phosphorylation on both Ser237 and Thr596 and increased 14-3-3 binding to TBC1D1, insulin induced phosphorylation only of Thr596 in a PI3K-dependent manner, with no effects on Ser237 phosphorylation or 14-3-3 binding. Using transgenic mice expressing a mutated AMPKα2 catalytic subunit, we provide genetic evidence that phosphorylation of TBC1D1 on Ser237 and 14-3-3 binding in resting muscles is dependent on AMPK. We propose that heterotrimeric AMPK complexes containing α2 may play important roles in this regulation of TBC1D1, as the activity of these specific complexes, but not the activity of AMPKα1-containing complexes, was downregulated in nonstimulated muscle (Table 1). Intriguingly, both contraction and insulin led to phosphorylation of TBC1D1 through AMPK-dependent mechanisms. Whereas the diminished effect of insulin is likely to be caused by a decreased α2 activity, the effects of contraction and AICAR may be dependent on both α1 and α2, because the activities associated with both of these complexes were increased by these treatments in EDL of the WT (present study and Refs. 14 and 16a) but not the AMPK KD mice (present study and Ref. 16a). In a previous study, using EDL muscles from AMPKα2 KO and KD mice, we also observed markedly reduced phosphorylation by use of the anti-PAS antibody during contraction and AICAR treatment (28). In that study we attributed the anti-PAS signal to AS160 (TBC1D4). However, our data in the present study and those of others (26) strongly suggest that in mouse EDL muscle the majority of the PAS signal arises from phosphorylated TBC1D1 rather than TBC1D4. Thus, we propose that the lack of contraction-induced TBC1D1 phosphorylation in EDL muscles from AMPK KD mice is due to the markedly reduced activity of α2 complexes.
By use of an unspecific AMPK inhibitor (Compound C) (1), it was recently suggested that AICAR- and contraction-induced TBC1D1-PAS phosphorylation might be AMPK dependent in rat skeletal muscle (9). However, the activity of at least 10 kinases is inhibited by Compound C with lower IC50 values than AMPK (1), and several studies have reported inhibition of various biological events by Compound C independently of AMPK inhibition (6, 18). Nevertheless, together with the genetic evidence provided in this study, the support for a role of AMPK becomes stronger. Thus, with the potential caveat of secondary adaptations in the KD mice, we provide genetic evidence to support this role of AMPK affecting both Thr596 and Ser237 phosphorylation as well as 14-3-3 binding of TBC1D1.
The discovery of two homologous proteins, i.e., TBC1D1 and TBC1D4, which are both recognized by the anti-PAS antibody, underlines the importance of identifying the exact polypeptide(s) detected in the present study. Because immunoprecipitating TBC1D1 from lysates of EDL muscle removed the signal obtained with the anti-PAS antibody (Fig. 1 and Ref. 26), and because the signals obtained with the anti-PAS antibody and the anti-TBC1D1 antibody comigrate during SDS-PAGE of EDL muscle lysates (Fig. 1C), we are confident that in EDL muscle from C57BL/6 mice the anti-PAS signal in analyses of lysates originates predominantly from TBC1D1. Furthermore, TBC1D1 and TBC1D4 migrate differently and can be resolved by SDS-PAGE (Fig. 1). Despite this, in the present study we performed analyses on immunopurified TBC1D1 to decrease the risk of cross-contamination.
Phosphorylation of TBC1D1 on Ser237 increased in response to contraction and AICAR treatment in a manner associated with 14-3-3 binding (R2 = 0.27, P < 0.001, n = 43). Such an association was not seen with Thr596 phosphorylation (R2 = 0.07, P = 0.10, n = 42) (Fig. 5, A and B). As insulin promoted substantial Thr596 phosphorylation without increasing 14-3-3 binding, these data indicate that Ser237 phosphorylation plays role in mediating 14-3-3 binding to TBC1D1 in skeletal muscle, extending observations in L6 myotubes (4). Binding of 14-3-3 proteins plays an essential role in TBC1D4-mediated GLUT4 trafficking and thus presumably in regulating the GAP activity of TBC1D4 (20). Whether 14-3-3 binding is also involved in the regulation of the GAP activity of TBC1D1 has not been established, so the physiological significance of 14-3-3 binding to TBC1D1 is unclear. However, due to the close similarity of TBC1D1 and TBC1D4, it is tempting to propose that insulin-induced TBC1D1 Thr596 phosphorylation is insufficient to cause 14-3-3 binding and may also be insufficient to regulate the GAP activity of TBC1D1.
Although it seems well established that TBC1D1 is phosphorylated in response to insulin, muscle contraction, and other stimuli (present study and Refs. 3, 4, 9, 12, 22, 26), the physiological significance of these phosphorylations is yet to be established. Some insights, however, suggest that TBC1D1 does have a regulatory role in metabolism. Recombinant congenic mice lacking TBC1D1 display enhanced whole body and skeletal muscle fat oxidation (2), and skeletal muscle from these mice displays decreased insulin-stimulated glucose transport. As the latter is counterintuitive to the proposed role of TBC1D1 in glucose transport, it is tempting to propose that in this model the decreased insulin-stimulated glucose transport is caused by the reduced GLUT4 expression in skeletal muscle from these mice (2). However, as silencing of TBC1D1 in L6 muscle cells increases basal and insulin-stimulated GLUT4 translocation, and overexpressing TBC1D1 in 3T3-L1 adipocytes inhibits insulin-stimulated GLUT4 translocation, available data indicate a role for TBC1D1 in this process (12, 22).
In the present study, we show that, in EDL muscle from AMPK KD mice, insulin-stimulated phosphorylation of Thr596 on TBC1D1 is completely abolished. Yet, in muscle from these and other similar transgenic mice, maximal (8, 17) and submaximal (unpublished observations) insulin-stimulated glucose uptake is unaltered, as is basal fatty acid oxidation (5). On this basis, it may be suggested either that insulin-stimulated Thr596 phosphorylation of TBC1D1 may have lesser effect on GAP function in muscle than in 3T3-L1 adipocytes or that TBC1D1 is not important for insulin-stimulated glucose uptake in mouse EDL muscle.
In this paper, we provide evidence that AMPK is a key regulator of TBC1D1 phosphorylation and 14-3-3 binding in skeletal muscle in response to contraction. Previous reports indicate that glucose uptake is only partially inhibited in the same AMPK-deficient mouse model in which contraction-induced TBC1D1 phosphorylation is completely abolished (13, 17). Thus, if TBC1D1 phosphorylation at Ser237 and/or Thr596 as well as 14-3-3 binding is involved in contraction-stimulated glucose uptake, other mechanisms must be involved as well. Although acute chemical inhibition of AMPK in rat epitrochlearis muscle inhibits TBC1D1-PAS phosphorylation along with inhibiting contraction- and AICAR-induced glucose transport (9), the inhibitor used, as discussed above, is known to be unspecific (1, 6, 18); thus, these data are inconclusive as to the roles of TBC1D1 and AMPK in AICAR- and contraction-induced glucose transport. Hence, further studies are needed to expand our knowledge on this issue.
Insulin increased the phosphorylation of TBC1D1 (detected using the PAS and Thr596 antibodies) independently of any changes in AMPK activity as measured by phosphorylation of Thr172 on AMPK and Ser227 on ACC. However, lack of AMPK resulted in significant reductions in basal TBC1D1 phosphorylation and 14-3-3 binding and in total abolition of insulin-induced TBC1D1 phosphorylation and 14-3-3 binding. Furthermore, inhibiting basal Akt Thr308 and Ser473 phosphorylation with wortmannin had no effect on basal phosphorylation of TBC1D1 detected using the Ser237, Thr596, or PAS antibodies. Thus, we propose that a basal priming phosphorylation of Ser237, Thr596, or other uninvestigated residues (e.g., Ser489, Ser505, Ser559, Ser660, or Ser700) (26) by AMPKα2-containing complexes may be required for insulin to exert its effects on TBC1D1 PAS and Thr596 phosphorylation. This interaction between AMPK and insulin signaling is in line with observations in 3T3-L1 adipocytes in which AICAR treatment reduces the inhibitory effect of TBC1D1 overexpression on insulin-induced GLUT4 translocation (3). Speculatively, the functional significance of this interaction in skeletal muscles could be related to the enhanced insulin action following prior AMPK activation as seen in response to AICAR (7) as well as muscle contraction (21). Alternatively, it has been shown that TBC1D1 colocalizes with GLUT4 in response to the AMPK activator A-769662 in L6 myotubes (4), suggesting that TBC1D1 translocates in response to AMPK activation. Thus, in mice lacking basal TBC1D1 phosphorylation by AMPKα2, such as in the AMPK KD mice, TBC1D1 may be localized differently and may be unavailable for Akt when these muscles are stimulated with insulin.
In cell-free systems, prior Akt-induced phosphorylation of TBC1D1 on Ser235 either prevents AMPK-induced phosphorylation of Ser237 or decreases recognition of the anti-Ser237 antibody (4). Although, it is not established whether Ser235 is a physiological phosphorylation site, our data indicate the presence of a similar mechanism in intact skeletal muscle, as insulin partly inhibited both contraction- and AICAR-induced phosphorylation of Ser237 (Fig. 3B).
In conclusion, AICAR, contraction, and insulin increase phosphorylation of distinct sites on TBC1D1 in mouse EDL muscle, but only AICAR and contraction induce enhanced 14-3-3 binding to TBC1D1. We provide genetic evidence that AMPK (most likely the α2 isoform) is required for contraction to induce these changes at TBC1D1 as well as for basal Ser237 phosphorylation and 14-3-3 binding to TBC1D1. Finally, our data show that AMPK is also required for insulin to induce TBC1D1 Thr596 phosphorylation on TBC1D1. Our data suggest that TBC1D1 is a point of convergence for PI3K-dependent insulin-stimulated signaling pathways and AMPK-dependent contraction-induced signaling pathways in skeletal muscle.
GRANTS
This work was supported by the Danish Medical Research Council, the Novo Nordisk Foundation, the Danish Diabetes Association, the Copenhagen Muscle Research Centre, the EXGENESIS consortium of the European Commission (LSHM-CT-2004-005272), Diabetes UK, and the UK Medical Research Council. J. F. P. Wojtaszewski was supported by a Hallas Møller fellowship from the Novo Nordisk Foundation.
Acknowledgments
We are especially grateful to Prof. Morris J. Birnbaum (Howard Hughes Medical Institute and University of Pennsylvania School of Medicine) for providing the AMPK KD mice.
REFERENCES
- 1.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadt A, Leicht K, Deshmukh A, Jiang LQ, Scherneck S, Bernhardt U, Dreja T, Vogel H, Schmolz K, Kluge R, Zierath JR, Hultschig C, Hoeben RC, Schurmann A, Joost HG, Al-Hasani H. Tbc1d1 mutation in lean mouse strain confers leanness and protects from diet-induced obesity. Nat Genet 40: 1354–1359, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Chavez JA, Roach WG, Keller SR, Lane WS, Lienhard GE. Inhibition of GLUT4 translocation by Tbc1d1, a Rab GTPase-activating protein abundant in skeletal muscle, is partially relieved by AMP-activated protein kinase activation. J Biol Chem 283: 9187–9195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, MacKintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J 409: 449–459, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Dzamko N, Schertzer JD, Ryall JG, Steel R, Macaulay SL, Wee S, Chen ZP, Michell BJ, Oakhill JS, Watt MJ, Jørgensen SB, Lynch GS, Kemp BE, Steinberg GR. AMPK-independent pathways regulate skeletal muscle fatty acid oxidation. J Physiol 586: 5819–5831, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emerling BM, Viollet B, Tormos KV, Chandel NS. Compound C inhibits hypoxic activation of HIF-1 independent of AMPK. FEBS Lett 581: 5727–5731, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher JS, Gao J, Han DH, Holloszy JO, Nolte LA. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab 282: E18–E23, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Fujii N, Seifert MM, Kane EM, Peter LE, Ho RC, Winstead S, Hirshman MF, Goodyear LJ. Role of AMP-activated protein kinase in exercise capacity, whole body glucose homeostasis, and glucose transport in skeletal muscle—insight from analysis of a transgenic mouse model. Diabetes Res Clin Pract 77, Suppl 1: S92–S98, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Funai K, Cartee GD. Inhibition of contraction-stimulated AMPK inhibits contraction-stimulated increases in PAS-TBC1D1 and glucose transport without altering PAS-AS160 in rat skeletal muscle. Diabetes 58: 1096–1104, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi T, Wojtaszewski JF, Goodyear LJ. Exercise regulation of glucose transport in skeletal muscle. Am J Physiol Endocrinol Metab 273: E1039–E1051, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Hutber CA, Hardie DG, Winder WW. Electrical stimulation inactivates muscle acetyl-CoA carboxylase and increases AMP-activated protein kinase. Am J Physiol Endocrinol Metab 272: E262–E266, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Ishikura S, Klip A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am J Physiol Cell Physiol 295: C1016–C1025, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Jensen TE, Rose AJ, Jørgensen SB, Brandt N, Schjerling P, Wojtaszewski JF, Richter EA. Possible CaMKK-dependent regulation of AMPK phosphorylation and glucose uptake at the onset of mild tetanic skeletal muscle contraction. Am J Physiol Endocrinol Metab 292: E1308–E1317, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279: 1070–1079, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Kane S, Sano H, Liu SC, Asara JM, Lane WS, Garner CC, Lienhard GE. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J Biol Chem 277: 22115–22118, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Kramer HF, Witczak CA, Taylor EB, Fujii N, Hirshman MF, Goodyear LJ. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem 281: 31478–31485, 2006. [DOI] [PubMed] [Google Scholar]
- 16a.Lefort N, St-Amand E, Morasse S, Côte CH, Marette A. The α-subunit of AMPK is essential for submaximal contraction-mediated glucose transport in skeletal muscle in vitro. Am J Physiol Endocrinol Metab 295: E1447–E1454, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Mu J, Brozinick JT Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7: 1085–1094, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Nam M, Lee WH, Bae EJ, Kim SG. Compound C inhibits clonal expansion of preadipocytes by increasing p21 level irrespectively of AMPK inhibition. Arch Biochem Biophys 479: 74–81, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Pozuelo RM, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, MacKintosh C. 14-3-3-Affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem J 379: 395–408, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramm G, Larance M, Guilhaus M, James DE. A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J Biol Chem 281: 29174–29180, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Enhanced muscle glucose metabolism after exercise: modulation by local factors. Am J Physiol Endocrinol Metab 246: E476–E482, 1984. [DOI] [PubMed] [Google Scholar]
- 22.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on GLUT4 translocation of the Rab GTPase-activating protein Tbc1d1. Biochem J 403: 353–358, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem 278: 14599–14602, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Stone S, Abkevich V, Russell DL, Riley R, Timms K, Tran T, Trem D, Frank D, Jammulapati S, Neff CD, Iliev D, Gress R, He G, Frech GC, Adams TD, Skolnick MH, Lanchbury JS, Gutin A, Hunt SC, Shattuck D. TBC1D1 is a candidate for a severe obesity gene and evidence for a gene/gene interaction in obesity predisposition. Hum Mol Genet 15: 2709–2720, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP, Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem 283: 9787–9796, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thong FS, Bilan PJ, Klip A. The Rab GTPase-Activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 56: 414–423, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jørgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55: 2051–2058, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem 272: 13255–13261, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Woods A, Salt I, Scott J, Hardie DG, Carling D. The alpha1 and alpha2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett 397: 347–351, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 6: 924–928, 2000. [DOI] [PubMed] [Google Scholar]